Abstract
Influenza infection induces an increase in the level of indoleamine 2,3-dioxygenase (IDO) activity in the lung parenchyma. IDO is the first and rate-limiting step in the kynurenine pathway where tryptophan is reduced to kynurenine and other metabolites. The depletion of tryptophan, and production of associated metabolites, attenuates the immune response to infection. The impact of IDO on the primary immune response to influenza virus infection was determined using the IDO inhibitor 1-methyl-d,l-tryptophan (1MT). C57BL/6 mice treated with 1MT and infected with A/HKx31 influenza virus had increased numbers of activated and functional CD4+ T-cells, influenza-specific CD8+ T-cells and effector memory cells in the lung. Inhibition of IDO increased the Th1 response in CD4+ T-cells as well as enhanced the Th17 response. These studies show that inhibition of IDO engenders a more robust T-cell response to influenza virus, and suggests an approach for enhancing the immune response to influenza vaccination by facilitating increased influenza-specific T-cell response.
Introduction
Influenza virus is a worldwide health concern, particularly for persons at the extremes of age, i.e. the young and elderly (Fiore et al., 2010). While protection from influenza virus is mediated by a neutralizing antibody response (Bridges et al., 2008), a potent T-cell response is required for elimination of virally infected cells and protection from heterologous virus infection as T-cells recognize conserved viral epitopes (Doherty et al., 1997). Numerous studies have shown the importance of T-cell memory in protection from disease in mice (Taylor & Askonas, 1986; Thomas et al., 2006). Although the significance of the T-cell response is less understood for humans, there are studies showing that influenza infection is associated with increased frequencies of memory influenza-specific CD8+ T-cells in the lungs compared with the circulating CD8+ T-cells (de Bree et al., 2005; Oshansky & Thomas, 2012), and evidence that memory CD4+ T-cells can reduce disease severity (Wilkinson et al., 2012). Recently, several studies have focused on developing vaccines to elicit potent T-cell responses in an attempt to bypass the need for yearly vaccination by providing cross-protective immunity (Guillonneau et al., 2009; Mueller et al., 2010).
Several studies have addressed mechanisms that may facilitate the host response to immunity, and have shown that inhibition of indoleamine 2,3-dioxygenase (IDO) has the potential to increase host responses (Dai & Dai, 2008; Liu et al., 2007). IDO is an intracellular enzyme in the kynurenine pathway that catabolizes tryptophan into kynurenine (Grohmann et al., 2003) and was initially shown to provide protection from fetal rejection mediated through T-cells (Munn et al., 1998). This protection was attributed to reduction in tryptophan levels causing immune cells to arrest in the cell cycle and T-cells to become anergic (Fallarino et al., 2006; Munn et al., 2005). Furthermore, IDO activity has been shown to skew CD4+ T-cells toward a Treg phenotype over a proinflammatory response (Baban et al., 2009). IDO activity is linked to immune attenuation and maintaining an immunosuppressive environment; therefore inhibition of IDO has the potential to reverse these effects as seen in the alloantigen memory T-cell response (Grohmann et al., 2003; Terness et al., 2002). Several studies have focused on the role of IDO in the maintenance of cancerous cells and tumours and resistance to T-cell cytotoxicity (Sharma et al., 2007; Wainwright et al., 2012). In some studies, inhibition of IDO using 1-methyl-tryptophan (1MT) was able to reduce the size and growth of tumours, but was unable to provide complete tumour elimination alone (Uyttenhove et al., 2003; Yang et al., 2010).
IDO expression is upregulated through IFN-γ signalling (Bianchi et al., 1988), suggesting IDO may modulate the immune response to viral infection. During influenza virus infection in mice, IDO activity has been shown to increase over time with peak activity coinciding with the peak number of T-cells within the respiratory tract (Yoshida et al., 1979). Thus, while coordinate expression of IDO may serve to regulate the duration of immunity by modulating the T-cell response, it is also likely that IDO-mediated immune attenuation may hinder the quantity and/or quality of the T-cell response. Recent studies have shown IDO to have an attenuating role in the immune response to human immunodeficiency virus (HIV; Andersson et al., 2005), Leishmania major (Makala et al., 2011) and Toxoplasma gondii (Murakami et al., 2012).
IDO inhibition during combined natural killer (NK) T-cell activation and influenza vaccination has been shown to boost protective immunity (Fallarini et al., 2008; Guillonneau et al., 2009); however, it remains unclear how IDO impacts the immune response to influenza virus infection. To address this in this study, 1MT was used to pharmacologically inhibit IDO activity in mice infected with HKx31 (X31). The T-cell response to infection was evaluated in lung airways. The results show that IDO inhibition allowed for an enhanced Th1 response, increased the functional influenza virus-specific CD8+ T-cell response, and produced higher quantities of effector memory cells.
Results
Influenza infection increases IDO activity in the lungs
The presence of IDO is associated with attenuated T-cell responses to infectious agents (Boasso et al., 2007; Makala et al., 2011). As these pathogens drive expression of IFN-γ, IDO activity is upregulated in dendritic cells, macrophages and epithelial cells (Bianchi et al., 1988; Hwu et al., 2000; Munn et al., 1999). IDO activity has been previously shown to be upregulated in the lungs of influenza-infected mice (Yoshida et al., 1979); however, the effect IDO has on the quantity and quality of the anti-influenza T-cell response has not been examined. In this study, we address the hypothesis that inhibition of IDO activity during T-cell priming will augment the magnitude and duration of the pulmonary T-cell response to influenza virus infection. To test this, IDO expression was inhibited using 1MT, a competitive inhibitor of IDO (Cady & Sono, 1991). 1MT was administered via drinking water 3 days prior to infection, and the mice remained on 1MT throughout the course of the study. IDO activity was measured in mice receiving 1MT or vehicle (sweetened water) by evaluating the ratio of kynurenine (kyn) to tryptophan (trp) in lung homogenates and serum of influenza-infected mice at 0, 2, 4, 6, 8, 10, 12 and 14 days post-infection (p.i.) (Fig. 1). The kyn/trp ratio directly measures IDO activity by comparing the concentrations of metabolite to substrate produced. The serum kyn/trp ratios in mice receiving 1MT or vehicle control were similar to day 10 p.i.; however, there was a significant (P<0.05) difference in kyn/trp ratios in the serum of control versus 1MT-treated mice on day 12 p.i. (Fig. 1a). Although the kyn/trp ratios were similar to day 10 p.i., there was a trend towards increased kyn/trp ratios in control compared with 1MT-treated mice. In comparison, the kyn/trp ratios were substantially higher in the lungs following influenza infection compared with control-treated mice, suggesting that infection modulates IDO activity (Fig. 1b), and at day 4 p.i., there was a small but significant (P<0.05) difference in IDO activity in the lungs (Fig. 1b). Notably, at day 10 p.i., a significant (P<0.01) increase in the kyn/trp ratios was evident in the lungs of control compared with 1MT-treated mice (Fig. 1b). These findings are consistent with a previous report showing that IDO activity peaks between day 10 and 11 p.i. in the lungs after influenza infection (Yoshida et al., 1979), and also demonstrates a temporal pharmacological inhibition of influenza-induced IDO activity through the administration of 1MT. Since IDO activity peaked at day 10, we evaluated the cellular response at this time point for the remainder of the study.
Fig. 1.
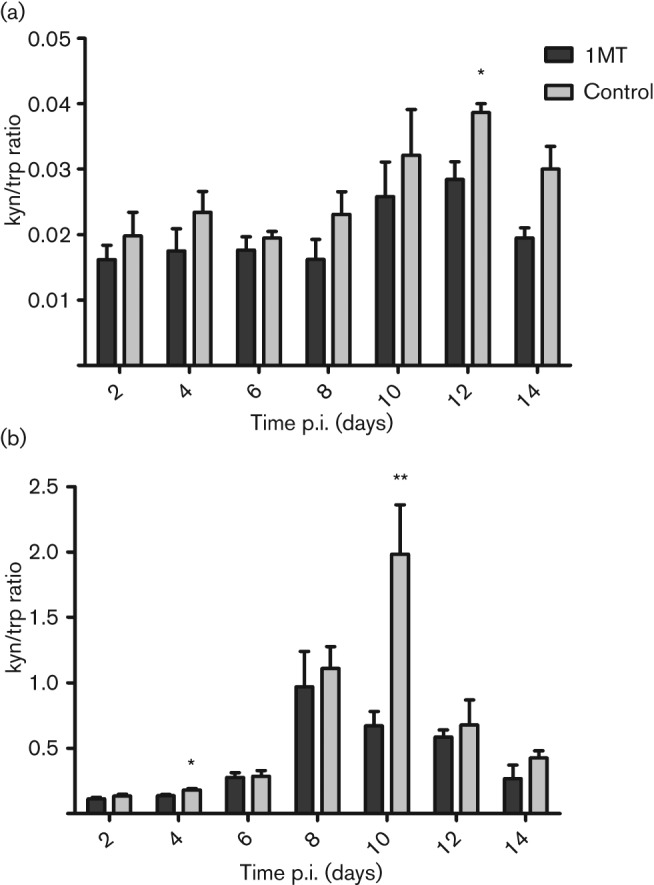
Influenza infection increases IDO activity in the lungs and sera. Mice were treated with 1MT or control 3 days prior to infection. On day 0, i.e. 3 days after treatment, mice were intranasally (i.n.) infected with 103 p.f.u. X31 in PBS. (a) Serum and (b) lung homogenates were collected every other day from day 0 (uninfected animals) until day 14 p.i. Each time point represents the mean and sem of 3–5 mice per group and shows two independent experiments. (**P value <0.01 and *P value <0.05).
IDO inhibition does not affect leukocyte infiltration or viral clearance
Since IDO has been shown to increase apoptosis and reduce cell proliferation (Lee et al., 2002; Munn et al., 1999), we sought to determine if IDO affected pulmonary leukocyte numbers. To examine the effects of IDO inhibition on the overall frequency of cells responding to virus infection, the number of leukocytes in the bronchoalveolar lavage (BALs) and draining mediastinal lymph nodes (MLNs) were determined at day 10 p.i. It is known that following influenza virus infection in mice, NK cells can be detected in the airways at day 3 p.i., peaking by day 5 p.i., whereby influenza-specific T-cells begin to accumulate at detectable frequencies in the lung airways at day 5 p.i. with their overall numbers peaking at day 10 p.i. (Culley, 2009; Flynn et al., 1998; Kreijtz et al., 2011). In this study, 1MT treatment did not have any substantial effect on the overall level or kinetics of pulmonary leukocyte recruitment (Fig. 2a). Similarly, 1MT treatment did not have any substantial effect on MLN cell numbers (Fig. 2b).
Fig. 2.
1MT treatment does not affect total frequency of T-cells infiltrating the lungs. Mice were treated with 1MT or control 3 days prior to infection and subsequently infected with 103 p.f.u. X31 i.n. (a) BAL and (b) MLN cell numbers from day 10 p.i. Number of CD8+ and CD4+ T-cells in the (c) BALs and (d) MLNs. Representative data from one experiment are shown from three independent experiments. (*P value <0.05).
It was important to determine if IDO was selectively affecting the frequency and/or function of specific pools of respondent leukocytes, as modulation of local tryptophan levels has been shown to affect the survival and function of T-cells (Fallarino et al., 2002). Examination of the CD4+ and CD8+ T-cell subpopulations isolated from the BALs of 1MT-treated mice showed no significant difference as compared with vehicle treated mice (Fig. 1c), while there was an increase in the CD4+ T-cell population in the MLNs in the control-treated mice (Fig. 1d).
Previous studies have shown that inhibition of IDO reduces the pathogen load during Leishmania infections (Makala et al., 2011). Thus, we wanted to determine if 1MT treatment had any effect on lung virus clearance. No differences in virus were evident between 1MT- and control-treated mice by TCID50 (Fig. S1a, available in JGV Online) or M gene expression (Fig. S1b). As differences in IDO activity were not detected until day 10 p.i. in lung homogenate (Fig. 1a), and there was no substantial difference in pulmonary CD8+ T-cell numbers (Fig. 2c), it is not surprising that 1MT had no detectable effect on virus clearance.
Inhibition of IDO activity enhances the Th1 cytokine response
The CD4+ T-cell response to influenza infection in mice has been characterized as a Th1-type response (Cella et al., 2000; Doherty et al., 1997); however, IDO has been shown to activate regulatory T-cells and block their conversion into Th17-like cells (Baban et al., 2009). Thus, the effects of IDO on the differentiation of Th1- or Th2-type CD4+ T-cells were determined in the BALs. Cytokines representative of Th1-, Th2- and Th17-type responses were determined, i.e. IFN-γ, IL-4 and IL-6, respectively. The proportion and frequency of CD4+ T-cells expressing IFN-γ, IL-6 or IL-4 was determined at day 10 p.i. (Fig. 3). There was a significant increase (P<0.05) in the percentage (Fig. 3b) and number (Fig. 3c) of CD4+ T-cells expressing IFN-γ. There was also a significant increase in the percentage of CD4+ T-cells expressing IL-6 (Fig. 3d), although there was only a slight increase in the number of CD4+ T-cells expressing IL-6 (Fig. 3e). There was no change in the CD4+ IL-4-expressing population (Fig. 3f, g), showing a specific role of IDO in the suppression of a Th1/Th17 response. Together, these findings indicate that IDO inhibition through 1MT treatment enhances the Th1-type response indicated by higher numbers of BAL CD4+ T-cells expressing IFN-γ following infection with influenza virus.
Fig. 3.
1MT treatment enhances the Th1 response. Mice were treated with 1MT or control, as described, and infected with 103 p.f.u. X31 i.n. Ten days p.i., BAL cells were stimulated for 6 h with UV-inactivated influenza virus and subsequently stained for intracellular expression of IFN-γ, IL-6 and IL-4. (a) Representative dot plots of CD4+ T-cells expressing IFN-γ, IL-6 and IL-4. (b, d, f) Proportion and (c, e, g) frequency of CD4+ T-cells expressing (b, c) IFN-γ, (d, e) IL-6 and (f, g) IL-4. Representative data from one experiment are shown from three independent experiments. (**P value <0.01 and *P value <0.05).
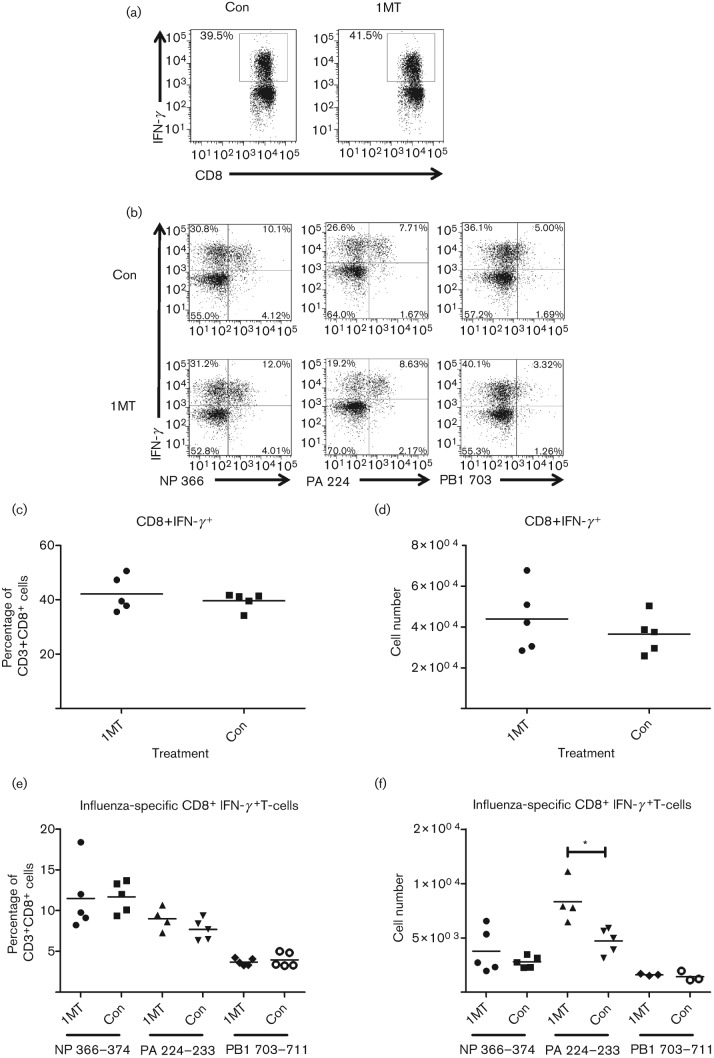
IDO inhibition is associated with increased numbers of influenza-specific CD8+ T-cells
Total numbers of CD8+ T-cells in the BALs of 1MT-treated mice were not affected (Fig. 2c); thus virus-specific CD8+ T-cell frequencies were determined following 1MT or vehicle treatment. CD8+ T-cells from the BALs were collected at day 10 p.i. and stained with tetramers detecting reactivity to the influenza nucleoprotein (NP) (H-2DbNP366–374), acid polymerase (PA) (H-2DbPA224–233) or basic polymerase 1 (PB1) (H-2KbPB1703–711) (Fig. 4). NP and PA have been shown to be the dominant CD8+ T-cell epitopes in response to influenza, with PB1 being subdominant to NP and PA (Crowe et al., 2003, 2006; Zhong et al., 2003). While there was no difference in the proportion of influenza-specific CD8+ T-cells between 1MT- and control-treated mice (Fig. 4a), treatment with 1MT increased the number of CD8+ T-cells for each immune dominant influenza-specific epitope (Fig. 4b). There was a significant (P<0.05) increase in PA-specific CD8+ T-cells (Fig. 4b) and a trend toward higher numbers of NP- and PB1-specific CD8+ T-cells (Fig. 4b). There were no substantial differences in the frequency of influenza-specific CD8+ T-cells in the MLNs in the 1MT- and control-treated groups (data not shown), suggesting that this increase occurs at the site of infection.
Fig. 4.

IDO inhibition enhances the influenza-specific response. Mice were treated with 1MT or control, as described, and infected with 103 p.f.u. X31 i.n. On day 10 p.i., BAL cells were analysed for virus-specific CD8+ T-cell numbers as determined by tetramers specific to NP366–374, PA224–233 or PB1703–711. (a) Proportion and (b) frequency of CD8+ T-cells are shown. Representative data from one experiment is shown from three independent experiments. (*P value <0.05).
Since IDO has a role in dampening the T-cell response, and there were increases in the number of influenza-specific CD8+ T-cells in the BALs, the T-cell receptor (TCR) Vβ diversity was examined in 1MT-treated and control mice at days 0, 6, 8, 10, 12 and 14 p.i. Splenocytes were stained for TCR Vβ 2, 6, 7, 8 and 8.1/8.2. No substantial variation was detected in TCR Vβ usage among influenza-specific CD8+ T-cells from that previously shown (La Gruta et al., 2006) (data not shown). These results support the finding that IDO inhibition increased the numbers of pulmonary CD8+ T-cells that are influenza virus-specific.
IDO affects influenza-specific CD8+ T-cell functionality and the effector memory population
As IDO treatment was associated with an enhanced Th1-type response determined by increased numbers of CD4+ T-cells expressing IFN-γ (Fig. 3b), and there were increases in the number of PA-specific CD8+ T-cells in the BALs (Fig. 4b), the effect of IDO treatment on CD8+ T-cell activation was evaluated. To determine if IDO inhibition was linked to a concomitant increase in virus-specific CD8+ T-cells, the percentage and number of CD8+ T-cells and influenza-specific CD8+ T-cells expressing IFN-γ collected from the BALs was analysed 10 days p.i. (Fig. 5a, c, d). In contrast to the increased numbers of CD4+ T-cells expressing IFN-γ in the BALs of 1MT-treated mice (Fig. 3), inhibition of IDO did not change the percentage of CD8+ T-cells expressing IFN-γ (Fig. 5c, d). There were slightly higher numbers of BAL CD8+ T-cells expressing IFN-γ in 1MT-treated mice compared with control mice (Fig. 5d). CD8+ T-cells were stained with H-2DbNP366–374, H-2DbPA224–233 and H-2KbPB1703–711 tetramers and for intracellular IFN-γ to evaluate activation (Fig. 5b, e, f). Of the CD8+ T-cells expressing IFN-γ, there was a significant (P<0.05) increase in the number of PA-specific T-cells, while NP and PB1 CD8+ T-cells showed no difference between the treatments (Fig. 5f). These findings indicate that in the absence of IDO, the activated PA-specific CD8+ T-cells are increased over the NP and PB1. These results suggest that IDO modifies the CD8+ T-cell frequency, and IDO inhibition increases the number of functional influenza virus-specific CD8+ T-cells, in particular a co-dominant epitope, at the site of infection.
Fig. 5.
IDO inhibition increases the frequency of functional PA-specific CD8+ T-cells. Mice were treated with 1MT or control, as described, and infected with 103 p.f.u. X31 i.n. Ten days p.i., single cells from the BALs were stimulated for 4 h with influenza immunodominant peptides and subsequently stained for intracellular expression of IFN-γ. (a, b) Representative dot plots of (a) CD8+ T-cells and (b) influenza-specific CD8+ T-cells expressing IFN-γ. (c) Percentage and (d) frequency of CD8+ T-cells expressing IFN-γ. (e) Percentage and (f) frequency of influenza-specific CD8+ T-cells expressing IFN-γ. Representative data from one experiment are shown from three independent experiments. (*P value <0.05).
Given the increase in CD4+ and CD8+ T-cell activity with IDO inhibition, the level of effector and central memory T-cell populations were evaluated. CD4+ and CD8+ T-cells were phenotyped for expression of CD44 and CD62L, with CD44hiCD62Llo expression being indicative of effector memory cells and CD44hiCD62Lhi expression representing central memory cells (Roberts et al., 2005). Ten days post-infection there was an increased frequency of CD8+ effector memory cells in the absence of IDO activity (Fig. 6a, b). While there was no difference between the CD4+ T-cell effector memory populations (Fig. 6c, d), there was a significant increase in the central memory population in control compared with 1MT-treated mice (Fig. 6d). These results support a role of IDO in the reduction of the production of the effector memory population, particularly the CD8+ T-cell population.
Fig. 6.
Inhibition of IDO activity increases the presence of CD8+ effector memory cells. Mice were treated with 1MT or control 3 days prior to infection. On day 0, mice were i.n. infected with 103 p.f.u. X31. (a, b) CD8+ and (c, d) CD4+ T-cells collected 10 days p.i. from the BALs were stained for the presence of CD44 and CD62L. Representative data from one experiment is shown from two independent experiments. (*P value <0.05).
Discussion
The findings from this study show that IDO has an immune dampening role in the response to influenza virus infection where IDO inhibition resulted in an overall enhancement in the number of activated T-cells in the lungs. IDO dampening of the IFN-γ response appeared greatest for the CD4+ T-cell compartment with an enhanced Th1 and Th17 response, although IFN-γ expression by CD8+ T-cells was also affected. In the BALs, the most abundant functional CD8+ T-cell response in the absence of IDO was directed to the PA epitope (PA224–233) compared with the control-treated mice. These findings suggest that IDO might alter CD8+ T-cell frequency while there is no detectable shift in the TCR Vβ usage of the CD8+ T-cells. This could possibly be attributed to enhance trafficking of PA-specific T-cells to the lungs related to a survival advantage.
Changes in the frequency have implications on the diversity of the T-cell population directed at influenza. There are multiple possibilities of how IDO affects the influenza-specific CD8+ T-cell population. One potential mechanism is through changes in antigen expression in antigen presenting cells (APCs). NP is commonly expressed by most APCs including dendritic cells and non-dendritic cells, while the PA peptide is almost exclusively expressed on dendritic cells and this has been shown to affect the peptide dominance between acute and secondary influenza infection (Crowe et al., 2003). The expression pattern for PB1, however, has not been established. Since both macrophages and dendritic cells can express IDO through stimulation with IFN-γ (Hwu et al., 2000), there is potential for a differential expression pattern of influenza epitopes. This could be driven through the immunoproteasome, which is also upregulated through IFN-γ (Tanoka & Kasahara, 1998). The immunoproteasome is responsible for the cleavage of proteins during infection for peptide presentation through the MHC class I pathway (Kloetzel, 2001) and IDO may be influencing the expression of epitopes. Furthermore, it has been shown that immunodominance is affected by the recruitment and expansion of CD8+ T-cells (La Gruta et al., 2010). With reduced IDO activity, there was increased activation of the influenza-specific CD8+ T-cells, suggesting prolonged exposure to antigen or continued activation of T-cell arriving ‘late’ to the immune response. The continued activation can be due in part to the reduced production of Treg cells as a result of IDO inhibition (Baban et al., 2009).
Although inhibition of IDO increased the amount of virus-specific cells expressing IFN-γ, surprisingly 1MT treatment did not affect lung viral titres as noted for other pathogens (Makala et al., 2011). One explanation may relate to the tempo of IDO expression. Accordingly, there are likely two phases of IDO activity in response to influenza virus infection, the first during initial infection of respiratory epithelial cells, followed by a larger induction related to IFN-γ produced by CD4+ and CD8+ T-cells. IFN-γ is an extremely potent activator of IDO (Bianchi et al., 1988), and the second wave of IDO activity is more robust and can be easily detected by examination of kyn levels or the ratio of kyn/trp. As the first induction of IDO likely occurs during infection of respiratory epithelial cells, this lower level of IDO activity has limited effects on the early response to infection, and thus no immediate impact on virus clearance. Another possibility may be linked to the location of T-cell activation. Findings from our lab show IDO to be active in the MLNs of influenza-infected mice. It is possible that the T-cell response is affected by the expression of IDO in the lymph node, rather than in the lungs, which would help explain the lack of differences in viral titres. Furthermore, influenza infection of epithelial cells induces IDO (Jacoby & Choi, 1994); however, the role of IDO in lung epithelial cells has not been thoroughly examined. IDO may be involved in cell survival, as it does have antioxidant properties which could reduce the damage caused by superoxides produced during infection (Hirata & Hayaishi, 1975). In addition, increased tryptophan availability has been shown to aid in pathogen proliferation (Gupta et al., 1994), and this may also facilitate influenza replication.
Interestingly, there was no difference in the level of serum IgG titres between 1MT-treated and control mice (data not shown), and the immunoglobulin isotype was the same between the two groups (data not shown). These findings suggest that IDO has no detectable effect on the B cell response to infection. This is consistent with an earlier study that showed IDO treatment of peripheral blood-derived B-cells had no effect on B-cells or their proliferation (Frumento et al., 2002). Furthermore, treatment with 1MT did not change the expression of IFN-γ from splenocytes 21 days p.i. by ELISPOT detection following influenza peptide stimulation compared with control-treated mice (data not shown). Since there was an increase in the CD8+ T-cell effector memory population, the effect in the memory population may be more evident in the resident lung cells (not evaluated in this study).
In this study, increased IDO activity was induced following influenza virus infection, a feature that had a dampening effect on the immune response. Specifically, 1MT-treated mice had higher numbers of Th1-CD4+ T-cells and effector memory CD8+ T-cells. IDO activity peaked in the lungs at day 10 p.i., a finding consistent with an earlier study (Yoshida et al., 1979). Early leukocyte recruitment to the lungs was not substantially affected by IDO inhibition (data not shown). There are several possibilities for this that relate to trp catabolism, particularly in the MLNs. IDO metabolites have been shown to induce apoptosis of T-cells (Hayashi et al., 2007) and to sway the CD4+ T-cells to a regulatory phenotype (Favre et al., 2010). The production of regulatory T-cells in the MLNs could suppress the pulmonary T-cell recruitment. Preliminary work in our lab shows that 1MT treatment decreases the number of cytotoxic Treg cells in the BALs. The lack of Treg suppression and production of trp metabolites may contribute to altered leukocyte trafficking, proliferation and increased survival of cells in the airways. Another possibility that is related to Treg production is the inability of inhibitory molecules to produce an effect on the system. The PD-1/PD-L pathway has been linked to IDO expression and activity (Baban et al., 2011). Tregs activated by IDO upregulate the expression of PD-L1/PD-L2 on dendritic cells (Sharma et al., 2007). The lack of PD-L expression will increase the activation and survival of the effector T-cell population (Brown et al., 2003).
The results from this study show that regulating IDO can enhance aspects of the adaptive immune response to influenza infection. This is attractive as regulating IDO activity during vaccination may facilitate vaccine efficacy. These observations support the concept that controlling IDO activity during vaccination with the live, attenuated influenza virus may be a means to augment vaccine efficacy and the robustness of the T-cell response, an attribute that could potentially facilitate heterosubtypic immunity.
Methods
Mice, virus propagation and infection.
Female C57BL/6 mice (6–8 weeks old) were purchased from Charles River Laboratories (Raleigh, NC) and all animal studies were approved by the Animal Care and Use Committee of the University of Georgia. A/HKx31 (X31, H3N2) influenza virus was propagated in the allantoic cavity of 9-day-old embroynated chicken eggs at 37 °C for 72 h. Titres were determined by an influenza plaque assay (Matrosovich et al., 2006). All mice were anaesthetized by intraperitoneal injection of Avertin (2,2,2-tribromoethanol) followed by intranasal infection with 103 p.f.u. X31 in 50 µl PBS at 8–10 weeks old.
Preparation and administration of 1-methyl-d,l-tryptophan (1MT).
The d,l racemic mixture of 1MT (Sigma-Aldrich) was administered to the mice through drinking water at a concentration of 2 mg ml−1. The treated water was prepared by dissolving 1MT powder in water using NaOH. The pH was adjusted to 7. To coax the mice to drink the water, aspartame was added to the water. The water was filter-sterilized and contained in autoclaved water bottles covered in aluminium foil. Control animals received aspartame-sweetened water only. 1MT-treated water was given to the mice 3 days prior to infection and the animals remained on the treatment throughout the course of the infection. Mice receiving the 1MT treatment were weighed during the 3 days prior to infection to ensure consumption of the water. The water and water bottles were changed every 5–7 days.
Measuring IDO activity through the kyn/trp ratio by HPLC.
Lungs and serum were collected from mice at 2, 4, 6, 8, 10, 12 and 14 days p.i. Lungs were harvested in PBS containing antibiotics and antimycotics and homogenized using a tissue lyser (Qiagen). Clarified lung homogenate and serum were aliquoted and frozen at −80 °C until processing. The concentration of kyn and trp was determined by HPLC analysis using a standard curve (Laich et al., 2002). Briefly, proteins were removed from the clarified lung homogenate and serum using trichloroacetic acid and analysed using a C18 reverse phase column (Restek).
Isolation and phenotyping of lymphocyte populations.
At 10 days p.i., mice were anaesthetized and BALs were collected by instillation of 1 ml PBS into the lungs three times for each mouse. MLNs were removed and placed into HBSS at 4 °C until processing. Single-cell suspensions were prepared from the MLNs using a 100 µm cell strainer (BD Biosciences). Cells were centrifuged and resuspended in complete tumour medium (CTM) prepared as previously described (Zhong et al, 2000) in RPMI-1640 (Hyclone). Cell numbers were determined with a Coulter Counter (Beckman Coulter). Single cell suspensions were plated between 5×104 to 5×105 cells per well. The cells were resuspended in staining wash buffer (SWB) (PBS+1 % BSA+0.09 % NaN3) followed by incubation with Fc Block (BD Pharmingen) at 4 °C for 15 min. Cells were then incubated with anti-CD3e (clone 145-2C11), anti-CD8α (clone 53-6.7), anti-CD4 (clone RM4-5), anti-CD44 (clone IM7) and anti-CD62L (clone MEL-14) (BD Pharmingen) for 30 min at 4 °C. The cells were rinsed with SWB and fixed with 1 % paraformaldehyde in PBS.
For intracellular cytokine staining, cells were stimulated with a cocktail of influenza immunodominant peptides (NP366–374, PA224–233, PB1703–711) (1 µg ml−1), irrelevant peptide (RSV M282–90) (1 µg ml−1) or DMSO for 4 h in CTM at 37 °C followed by surface staining with anti-CD3, anti-CD8α, H2DbNP366–374 tetramer, H2DbPA224–233 tetramer or H2KbPB1703–711 tetramer (NIH Tetramer Core Facility, Emory, Atlanta, GA) for 1 h at room temperature for the IFN-γ response from CD8+ T-cells. Cells were stimulated with UV-inactivated X31 virus (1 : 100) or allantoic fluid for 6 h in CTM at 37 °C followed by surface staining with anti-CD3 and anti-CD4 for intracellular staining of CD4+ T-cells for IFN-γ (clone XMG1.2), IL-6 (clone MP5-32C11) or IL-4 (clone 11B11) (BD Pharmingen). Cultured cells were stained as described above, but were kept in the presence of GolgiStop (BD Biosciences). Following surface staining, cells were rinsed with SWB+GolgiStop and fixed and permeabilized with the Foxp3 fixation/permeabilization solution (eBiosciences). The cells were rinsed with Perm/Wash Buffer (BD Biosciences) and incubated with intracellular markers listed above for 30 min at 4 °C. Cells were washed with Perm/Wash Buffer and resuspended in PBS. All samples were run on a LSRII flow cytometer (BD Biosciences) and analysed using BD FACS Diva software or FloJo (Tree Star). All populations were initially gated on CD3+ cells. Isotype control antibodies and mock stimulation were used to set gates for analysis.
Statistical analysis.
Statistics were performed using GraphPad Prism Version 5.01. The data were analysed using a two-tailed Student’s t-test comparing 1MT treatment with control-treated mice at each time point. Significance was assigned when the P value was <0.05.
Acknowledgements
We thank Dr Phillip Chandler for his technical assistance with 1MT preparation and administration, Spencer Poore and Scott Johnson for assistance with animal work, and the NIH Tetramer Core Facility for generating the tetramers. This work was supported by the National Institutes of Health U01 grant AI083005-01.
References
- Andersson J., Boasso A., Nilsson J., Zhang R., Shire N. J., Lindback S., Shearer G. M., Chougnet C. A. (2005). The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol 174, 3143–3147 [DOI] [PubMed] [Google Scholar]
- Baban B., Chandler P. R., Sharma M. D., Pihkala J., Koni P. A., Munn D. H., Mellor A. L. (2009). IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 183, 2475–2483 10.4049/jimmunol.0900986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban B., Chandler P. R., Johnson B. A., III, Huang L., Li M., Sharpe M. L., Francisco L. M., Sharpe A. H., Blazar B. R. & other authors (2011). Physiologic control of IDO competence in splenic dendritic cells. J Immunol 187, 2329–2335 10.4049/jimmunol.1100276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M., Bertini R., Ghezzi P. (1988). Induction of indoleamine dioxygenase by interferon in mice: a study with different recombinant interferons and various cytokines. Biochem Biophys Res Commun 152, 237–242 10.1016/S0006-291X(88)80705-8 [DOI] [PubMed] [Google Scholar]
- Boasso A., Herbeuval J. P., Hardy A. W., Anderson S. A., Dolan M. J., Fuchs D., Shearer G. M. (2007). HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood 109, 3351–3359 10.1182/blood-2006-07-034785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. B., Katz J. M., Levandowski R. A., Cox N. J. (2008). Inactivated influenza vaccines. In Vaccines, pp. 259–290 Edited by Potkin S. A., Orenstein W. A., Offit P. A. Philadelphia, PA: Elsevier [Google Scholar]
- Brown J. A., Dorfman D. M., Ma F. R., Sullivan E. L., Munoz O., Wood C. R., Greenfield E. A., Freeman G. J. (2003). Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol 170, 1257–1266 [DOI] [PubMed] [Google Scholar]
- Cady S. G., Sono M. (1991). 1-Methyl-dl-tryptophan, beta-(3-benzofuranyl)-dl-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-dl-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys 291, 326–333 10.1016/0003-9861(91)90142-6 [DOI] [PubMed] [Google Scholar]
- Cella M., Facchetti F., Lanzavecchia A., Colonna M. (2000). Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol 1, 305–310 10.1038/79747 [DOI] [PubMed] [Google Scholar]
- Crowe S. R., Turner S. J., Miller S. C., Roberts A. D., Rappolo R. A., Doherty P. C., Ely K. H., Woodland D. L. (2003). Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J Exp Med 198, 399–410 10.1084/jem.20022151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S. R., Miller S. C., Woodland D. L. (2006). Identification of protective and non-protective T cell epitopes in influenza. Vaccine 24, 452–456 10.1016/j.vaccine.2005.07.090 [DOI] [PubMed] [Google Scholar]
- Culley F. J. (2009). Natural killer cells in infection and inflammation of the lung. Immunology 128, 151–163 10.1111/j.1365-2567.2009.03167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Dai Z. (2008). The role of tryptophan catabolism in acquisition and effector function of memory T cells. Curr Opin Organ Transplant 13, 31–35 10.1097/MOT.0b013e3282f3dee1 [DOI] [PubMed] [Google Scholar]
- de Bree G. J., van Leeuwen E. M., Out T. A., Jansen H. M., Jonkers R. E., van Lier R. A. (2005). Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med 202, 1433–1442 10.1084/jem.20051365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Topham D. J., Tripp R. A., Cardin R. D., Brooks J. W., Stevenson P. G. (1997). Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev 159, 105–117 10.1111/j.1600-065X.1997.tb01010.x [DOI] [PubMed] [Google Scholar]
- Fallarini S., Paoletti T., Panza L., Lombardi G. (2008). Alpha-galactosylceramide modulates the induction of indoleamine 2,3-dioxygenase in antigen presenting cells. Biochem Pharmacol 76, 738–750 10.1016/j.bcp.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Fallarino F., Grohmann U., Vacca C., Bianchi R., Orabona C., Spreca A., Fioretti M. C., Puccetti P. (2002). T cell apoptosis by tryptophan catabolism. Cell Death Differ 9, 1069–1077 10.1038/sj.cdd.4401073 [DOI] [PubMed] [Google Scholar]
- Fallarino F., Grohmann U., You S., McGrath B. C., Cavener D. R., Vacca C., Orabona C., Bianchi R., Belladonna M. L. & other authors (2006). The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 176, 6752–6761 [DOI] [PubMed] [Google Scholar]
- Favre D., Mold J., Hunt P. W., Kanwar B., Loke P., Seu L., Barbour J. D., Lowe M. M., Jayawardene A. & other authors (2010). Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2, 32ra36 10.1126/scitranslmed.3000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore A. E., Uyeki T. M., Broder K., Finelli L., Euler G. L., Singleton J. A., Iskander J. K., Wortley P. M., Shay D. K. & other authors (2010). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 59 (RR-8), 1–62 [PubMed] [Google Scholar]
- Flynn K. J., Belz G. T., Altman J. D., Ahmed R., Woodland D. L., Doherty P. C. (1998). Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8, 683–691 10.1016/S1074-7613(00)80573-7 [DOI] [PubMed] [Google Scholar]
- Frumento G., Rotondo R., Tonetti M., Damonte G., Benatti U., Ferrara G. B. (2002). Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 196, 459–468 10.1084/jem.20020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann U., Fallarino F., Puccetti P. (2003). Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 24, 242–248 10.1016/S1471-4906(03)00072-3 [DOI] [PubMed] [Google Scholar]
- Guillonneau C., Mintern J. D., Hubert F. X., Hurt A. C., Besra G. S., Porcelli S., Barr I. G., Doherty P. C., Godfrey D. I., Turner S. J. (2009). Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci U S A 106, 3330–3335 10.1073/pnas.0813309106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Carlin J. M., Pyati P., Dai W., Pfefferkorn E. R., Murphy M. J., Jr (1994). Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immun 62, 2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Mo J. H., Gong X., Rossetto C., Jang A., Beck L., Elliott G. I., Kufareva I., Abagyan R. & other authors (2007). 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A 104, 18619–18624 10.1073/pnas.0709261104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. (1975). Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem 250, 5960–5966 [PubMed] [Google Scholar]
- Hwu P., Du M. X., Lapointe R., Do M., Taylor M. W., Young H. A. (2000). Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol 164, 3596–3599 [DOI] [PubMed] [Google Scholar]
- Jacoby D. B., Choi A. M. (1994). Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Radic Biol Med 16, 821–824 10.1016/0891-5849(94)90198-8 [DOI] [PubMed] [Google Scholar]
- Kloetzel P. M. (2001). Antigen processing by the proteasome. Nat Rev Mol Cell Biol 2, 179–187 10.1038/35056572 [DOI] [PubMed] [Google Scholar]
- Kreijtz J. H., Fouchier R. A., Rimmelzwaan G. F. (2011). Immune responses to influenza virus infection. Virus Res 162, 19–30 10.1016/j.virusres.2011.09.022 [DOI] [PubMed] [Google Scholar]
- La Gruta N. L., Kedzierska K., Pang K., Webby R., Davenport M., Chen W., Turner S. J., Doherty P. C. (2006). A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc Natl Acad Sci U S A 103, 994–999 10.1073/pnas.0510429103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta N. L., Rothwell W. T., Cukalac T., Swan N. G., Valkenburg S. A., Kedzierska K., Thomas P. G., Doherty P. C., Turner S. J. (2010). Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest 120, 1885–1894 10.1172/JCI41538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laich A., Neurauter G., Widner B., Fuchs D. (2002). More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem 48, 579–581 [PubMed] [Google Scholar]
- Lee G. K., Park H. J., Macleod M., Chandler P., Munn D. H., Mellor A. L. (2002). Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 107, 452–460 10.1046/j.1365-2567.2002.01526.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Dai H., Wan N., Wang T., Bertera S., Trucco M., Dai Z. (2007). Suppression of memory CD8 T cell generation and function by tryptophan catabolism. J Immunol 178, 4260–4266 [DOI] [PubMed] [Google Scholar]
- Makala L. H., Baban B., Lemos H., El-Awady A. R., Chandler P. R., Hou D. Y., Munn D. H., Mellor A. L. (2011). Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J Infect Dis 203, 715–725 10.1093/infdis/jiq095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M., Matrosovich T., Garten W., Klenk H. D. (2006). New low-viscosity overlay medium for viral plaque assays. Virol J 3, 63 10.1186/1743-422X-3-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. N., Langley W. A., Carnero E., García-Sastre A., Ahmed R. (2010). Immunization with live attenuated influenza viruses that express altered NS1 proteins results in potent and protective memory CD8+ T-cell responses. J Virol 84, 1847–1855 10.1128/JVI.01317-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H., Zhou M., Attwood J. T., Bondarev I., Conway S. J., Marshall B., Brown C., Mellor A. L. (1998). Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 10.1126/science.281.5380.1191 [DOI] [PubMed] [Google Scholar]
- Munn D. H., Shafizadeh E., Attwood J. T., Bondarev I., Pashine A., Mellor A. L. (1999). Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 189, 1363–1372 10.1084/jem.189.9.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn D. H., Sharma M. D., Baban B., Harding H. P., Zhang Y., Ron D., Mellor A. L. (2005). GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642 10.1016/j.immuni.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Murakami Y., Hoshi M., Hara A., Takemura M., Arioka Y., Yamamoto Y., Matsunami H., Funato T., Seishima M., Saito K. (2012). Inhibition of increased indoleamine 2,3-dioxygenase activity attenuates Toxoplasma gondii replication in the lung during acute infection. Cytokine 59, 245–251 10.1016/j.cyto.2012.04.022 [DOI] [PubMed] [Google Scholar]
- Oshansky C. M., Thomas P. G. (2012). The human side of influenza. J Leukoc Biol 92, 83–96 10.1189/jlb.1011506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. D., Ely K. H., Woodland D. L. (2005). Differential contributions of central and effector memory T cells to recall responses. J Exp Med 202, 123–133 10.1084/jem.20050137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M. D., Baban B., Chandler P., Hou D. Y., Singh N., Yagita H., Azuma M., Blazar B. R., Mellor A. L., Munn D. H. (2007). Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 117, 2570–2582 10.1172/JCI31911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoka K., Kasahara M. (1998). The MHC class I ligand-generating system: roles of immunoproteasomes and the interferon-gamma-inducible proteasome activator PA28. Immunol Rev 163, 161–176 10.1111/j.1600-065X.1998.tb01195.x [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Askonas B. A. (1986). Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology 58, 417–420 [PMC free article] [PubMed] [Google Scholar]
- Terness P., Bauer T. M., Röse L., Dufter C., Watzlik A., Simon H., Opelz G. (2002). Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 196, 447–457 10.1084/jem.20020052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. G., Keating R., Hulse-Post D. J., Doherty P. C. (2006). Cell-mediated protection in influenza infection. Emerg Infect Dis 12, 48–54 10.3201/eid1201.051237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B. J. (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9, 1269–1274 10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- Wainwright D. A., Balyasnikova I. V., Chang A. L., Ahmed A. U., Moon K. S., Auffinger B., Tobias A. L., Han Y., Lesniak M. S. (2012). IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18, 6110–6121 10.1158/1078-0432.CCR-12-2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T. M., Li C. K., Chui C. S., Huang A. K., Perkins M., Liebner J. C., Lambkin-Williams R., Gilbert A., Oxford J. & other authors (2012). Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 18, 274–280 10.1038/nm.2612 [DOI] [PubMed] [Google Scholar]
- Yang H. J., Yen M. C., Lin C. C., Lin C. M., Chen Y. L., Weng T. Y., Huang T. T., Wu C. L., Lai M. D. (2010). A combination of the metabolic enzyme inhibitor APO866 and the immune adjuvant L-1-methyl tryptophan induces additive antitumor activity. Exp Biol Med (Maywood) 235, 869–876 10.1258/ebm.2010.010001 [DOI] [PubMed] [Google Scholar]
- Yoshida R., Urade Y., Tokuda M., Hayaishi O. (1979). Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci U S A 76, 4084–4086 10.1073/pnas.76.8.4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Marshal D., Coleclough C., Woodland D. L. (2000). CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J Immunol 164, 3274–3282 10.1074/jbc.M307417200 [DOI] [PubMed] [Google Scholar]
- Zhong W., Reche P. A., Lai C. C., Reinhold B., Reinherz E. L. (2003). Genome-wide characterization of a viral cytotoxic T lymphocyte epitope repertoire. J Biol Chem 278, 45135–45144 10.1074/jbc.M307417200 [DOI] [PubMed] [Google Scholar]
Footnotes
One supplementary figure and methods are available with the online version of this paper.