Key Points
DLBCL patients with MYC/BCL2 coexpression demonstrate inferior prognosis and high-risk gene expression signatures.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is stratified into prognostically favorable germinal center B-cell (GCB)–like and unfavorable activated B-cell (ABC)–like subtypes based on gene expression signatures. In this study, we analyzed 893 de novo DLBCL patients treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). We show that MYC/BCL2 protein coexpression occurred significantly more commonly in the ABC subtype. Patients with the ABC or GCB subtype of DLBCL had similar prognoses with MYC/BCL2 coexpression and without MYC/BCL2 coexpression. Consistent with the notion that the prognostic difference between the 2 subtypes is attributable to MYC/BCL2 coexpression, there is no difference in gene expression signatures between the 2 subtypes in the absence of MYC/BCL2 coexpression. DLBCL with MYC/BCL2 coexpression demonstrated a signature of marked downregulation of genes encoding extracellular matrix proteins, those involving matrix deposition/remodeling and cell adhesion, and upregulation of proliferation-associated genes. We conclude that MYC/BCL2 coexpression in DLBCL is associated with an aggressive clinical course, is more common in the ABC subtype, and contributes to the overall inferior prognosis of patients with ABC-DLBCL. In conclusion, the data suggest that MYC/BCL2 coexpression, rather than cell-of-origin classification, is a better predictor of prognosis in patients with DLBCL treated with R-CHOP.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 4250.
Disclosures
The authors, Associate Editor A. Keith Stewart, and CME questions author Charles P. Vega, Associate Professor and Residency Director, Department of Family Medicine, University of California-Irvine, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Assess genetic abnormalities associated with diffuse large B-cell lymphoma (DLBCL).
Analyze the prevalence and survival impact of MYC and BCL2 co-expression in the current study.
Distinguish the relationship between MYC/BCL2 co-expression and other negative prognostic variables in the current study.
Evaluate the relative effect of MYC/BCL2 co-expression on survival in the context of DLBCL subtypes.
Release date: May 16, 2013; Expiration date: May 16, 2014
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma and has heterogeneous clinicopathological, immunophenotypic, and genetic features. According to the results of gene expression profiling (GEP) studies, DLBCL can be stratified into germinal center B-cell (GCB)–like or activated B-cell (ABC)–like subtypes, and patients with the ABC subtype of DLBCL have an inferior prognosis.1 The GCB and ABC subtypes have distinctive gene expression signatures. GCB-DLBCL expresses many genes selectively and/or highly expressed by normal GCBs, such as CD10 and BCL6. In contrast, ABC-DLBCL has a gene signature similar to peripheral blood B cells activated in vitro. Notably, genes upregulated in ABC-DLBCL include MYC, BCL2, MUM1, CD44, FLIP, and cyclin D2 as well as many other genes. It is believed that constitutive nuclear factor κB (NF-κB) activation in ABC-DLBCL drives the expression of this array of genes and contributes to the ABC phenotype.2 The high NF-κB activity is attributable to a variety of molecular and genetic mechanisms. Mutations of multiple genes have recently been identified that encode proteins involved in the signaling of the B-cell receptor and members of the tumor necrosis factor receptor superfamily, as well as those involving NF-κB regulation.2,3 Despite the identification of many deregulated target genes in ABC-DLBCL, it remains unknown which gene products at the protein level contribute most significantly to the inferior prognosis of patients with ABC-DLBCL.
Although the GCB and ABC subtypes convey general trends regarding clinical outcome, these subtypes do not reliably predict the prognosis of individual patients. Furthermore, it is impractical to routinely perform GEP in the clinical setting. Immunohistochemistry (IHC) studies using various antibody panels and algorithms have been proposed as surrogates for predicting the GCB vs non-GCB subtype.4-10 The results, however, have been controversial as the concordance with GEP results is imperfect to varying degrees and, in some studies, IHC results do not correlate with prognosis.11-13 Furthermore, both the GCB and ABC subtypes of DLBCL as defined by GEP are heterogeneous and contain biological subgroups that have different prognoses and may require different therapeutic approaches. Therefore, a stratification of DLBCL patients into subgroups that are biologically homologous and prognostically meaningful, and that are more predictive than the overall categories of GCB and ABC is needed, thereby facilitating therapeutic decisions.
Double-hit B-cell lymphoma is defined as a B-cell lymphoma associated with chromosomal breaks targeting the MYC gene located at chromosome 8q24 in combination with additional rearrangement affecting another gene, such as BCL2 or BCL6.14 By far, the most studied type of double-hit B-cell lymphoma has concurrent MYC and BCL2 breaks (ie, MYC/BCL2 double-hit). There is a general consensus that patients with MYC/BCL2 double-hit lymphomas have an extremely aggressive clinical course.14-22 Despite their clinical aggressiveness, almost all cases of MYC/BCL2 double-hit lymphoma are of the GCB subtype, a generally favorable prognostic group, illustrating an important discordance between clinical behavior and cell-of-origin (COO) subtypes.14,16,17
More recently, others have extended the concept of MYC/BCL2 double-hit lymphoma by assessing for MYC and BCL2 protein expression by IHC, the logic being that protein expression, regardless of mechanisms, may have prognostic significance. In 2 studies, Green et al and Johnson et al showed that DLBCL patients with MYC/BCL2 coexpression, with or without MYC or BCL2 gene rearrangements, have a poorer prognosis.23,24 These studies were possible because of the recent availability of anti-MYC antibodies suitable for IHC staining in paraffin-embedded tissues.
In this study, we used IHC to assess the prognostic value of MYC and BCL2 expression, and particularly MYC/BCL2 coexpression, in a large cohort of 893 de novo DLBCL patients treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy. Our results show that MYC/BCL2 coexpression is associated with a poor prognosis and is more common in ABC-DLBCL. We further suggest that MYC/BCL2 coexpression explains the poorer prognosis of patients with ABC-DLBCL and may be a better predictor of prognosis than COO classification.
Materials and methods
Patient selection
We studied 700 cases of de novo DLBCL from patients who were treated with R-CHOP chemotherapy, including 466 cases in a training set and 234 cases in a validation set (validation set 1). These cases were organized as a part of the International DLBCL Rituximab-CHOP Consortium Program study.10,25 All cases were diagnosed according to World Health Organization (WHO) classification criteria. Cases were excluded if patients had a history of low-grade B-cell lymphoma, AIDS/HIV infection, primary cutaneous DLBCL, primary central nervous system DLBCL, and Epstein-Barr virus–positive DLBCL. Only cases with successful MYC and BCL2 staining were included for further study. We also used a separate, previously reported validation set (validation set 2) of 193 cases of de novo DLBCL.23 Therefore, total 893 de novo DLBCL cases were included and analyzed in this study. The study was reviewed and approved by the institutional review boards and material transfer agreement established and approved with each of the participating centers.
Tissue microarray immunohistochemical studies
Hematoxylin-eosin–stained slides from all DLBCL cases were reviewed, and representative areas with the highest percentage of tumor cells were selected for tissue microarray (TMA) construction. IHC studies for a variety of markers were performed using a streptavidin-biotin complex technique on 4-μm TMA sections. MYC (clone Y69; Epitomics) expression showed a distinct nuclear pattern and BCL2 (clone 124; DAKO) expression exhibited a cytoplasmic pattern. A cutoff value for each marker was established from analysis of receiver-operating characteristic curves to achieve maximum specificity and sensitivity as described previously.10 Cutoff values of 40% for MYC and 70% for BCL2 were established. These values were similar to those in a previous study, in which median values were used as the cutoff values.23 For all other markers assessed in this study, cutoff values have been described previously.10
FISH for MYC and BCL2 and sequencing of TP53
Fluorescence in situ hybridization (FISH) analysis was performed using formalin-fixed paraffin-embedded (FFPE) tissue sections of all 466 cases in the training set using BCL2 dual-color breakapart probes (Vysis) as described previously.10 MYC was assessed by FISH using locus-specific IGH/MYC/CEP8 tricolor dual-fusion probes and locus-specific MYC dual-color breakapart probes (Vysis). Cases were considered for evaluation if at least 200 tumor cell nuclei per core displayed positive signals in the TMA sections. For TP53 sequencing, genomic DNA and total RNA were extracted from FFPE tissue of all cases in the training set and processed as previously described.25
Gene expression profiling
Total RNA was extracted from FFPE tissue samples of 451 cases in the training set using the HighPure Paraffin RNA Extraction Kit (Roche Applied Science) and subjected to GEP as described previously.10 For data analysis and classification, the microarray DQN signals were generated and normalized to the quantiles of β distribution with parameters P = 1.2 and q = 3. DQN is an ideal expression algorithm used for expression microarray analysis and represents the non-central trimmed mean of differences between perfect match and mismatch intensities with quantile normalization. A Bayesian model was also used to determine the classification probability.26 The GEP classification method developed from this study was validated with an independent Leukemia Lymphoma Molecular Profiling Program dataset in the Gene Expression Omnibus database GSE 1084627 with 181 CHOP-treated and 233 R-CHOP–treated DLBCL patients and achieved over 97% concordance rate for the classification of 2 subtypes (GCB and ABC).
COO classification
COO classification was achieved by combining GEP and IHC data, with the GEP data considered the “gold standard.” Briefly, IHC was performed in all cases in the training set and validation set 1. GEP was performed in 451 cases in the training set and 411 were classified as GCB or ABC; 40 (9%) were unclassifiable. The classification of these 411 cases was based on the GEP results regardless of IHC results. The 40 cases not classifiable by GEP and 15 additional cases in the training set, for which GEP was not performed, as well as all cases in validation set 1 were classified by IHC methods according to both the Visco-Young and Choi algorithms (supplemental Figure 6, available on the Blood Web site).10 The COO classification of validation set 2 was previously reported.23
Statistical analysis
Statistical analyses were performed as described in supplemental Materials and methods. The outcome analyses were based on the entire training set of 466 cases. The outcome analysis results limited to the 411 cases stratified by GEP were strikingly similar to those derived from the entire training set and are shown in supplemental Figures 1-5.
Results
The clinical and pathological features of 466 cases in the training set are listed in Table 1. Two hundred forty-one (52%) cases were classified as the GCB subtype and 225 (48%) were classified as the ABC subtype (Table 1). The median follow-up time for this study cohort was 57 months.
Table 1.
Clinicopathological characteristics and outcome of DLBCLs treated with R-CHOP
| Overall | DP | Non-DP | P value | |||
|---|---|---|---|---|---|---|
| N (%) | OS, P | PFS, P | N (%) | N (%) | ||
| Patients | 466 (100) | 157 (100) | 309 (100) | |||
| Gender | ||||||
| Male | 272 (58) | .7477 | .4730 | 90 (57) | 182 (59) | .7445 |
| Female | 194 (42) | 67 (43) | 127 (41) | |||
| Age | ||||||
| ≤60 | 194 (42) | .0004 | .0016 | 49 (31) | 145 (47) | .0011 |
| >60 | 272 (58) | 108 (69) | 164 (53) | |||
| B symptoms* | ||||||
| Absence | 276 (68) | .0015 | .0014 | 88 (62) | 188 (72) | .0541 |
| Presence | 127 (32) | 53 (38) | 74 (28) | |||
| ECOG performance status* | ||||||
| <2 | 350 (88) | <.0001 | <.0001 | 111 (83) | 239 (90) | .0453 |
| ≥2 | 50 (12) | 23 (17) | 27 (10) | |||
| Stage* | ||||||
| I-II | 219 (49) | <.0001 | <.000l | 50 (33) | 169 (57) | <.0001 |
| III-IV | 228 (51) | 100 (67) | 128 (43) | |||
| Extranodal sites* | ||||||
| <2 | 346 (78) | <.0001 | <.000l | 106 (72) | 240 (82) | .0160 |
| ≥2 | 96 (22) | 42 (28) | 54 (18) | |||
| Lactate dehydrogenase* | ||||||
| Normal | 168 (40) | .0003 | <.000l | 51 (36) | 117 (42) | .2908 |
| Elevated | 252 (60) | 89 (64) | 163 (58) | |||
| IPI risk group* | ||||||
| 0-2 | 263 (64) | <.000l | <.0001 | 70 (51) | 193 (70) | .0001 |
| 3-5 | 148 (36) | 67 (49) | 81 (30) | |||
| Tumor size, cm* | ||||||
| <7.5 | 253 (77) | .0100 | .0172 | 81 (73) | 172 (79) | .2587 |
| ≥7.5 | 77 (23) | 30 (27) | 47 (21) | |||
| Treatment response | ||||||
| CR | 354 (76) | <.000l | <.000l | 103 (66) | 251 (84) | <.0001 |
| Others | 112 (24) | 54 (34) | 48 (16) | |||
| COO classification | ||||||
| GCB | 241 (52) | .0080 | .0075 | 53 (34) | 188 (61) | <.0001 |
| ABC | 225 (48) | 104 (66) | 121 (39) | |||
| Ki-67* | ||||||
| <7O | 158 (34) | .2998 | .3434 | 41 (26) | 117 (38) | .0086 |
| ≥70 | 304 (66) | 116 (74) | 188 (62) | |||
| TP53 mutations | ||||||
| Absence | 357 (77) | 117 (75) | 240 (78) | .4480 | ||
| Presence | 109 (23) | .0005 | .0004 | 40 (25) | 69 (22) | |
DP, MYC/BCL2 double positive by IHC; ECOG, Eastern Cooperative Oncology Group; Non-DP, non-MYC/BCL2 double positive.
Information not available in some cases.
MYC/BCL2 protein coexpression predicts poor prognosis in DLBCL
Using cutoff values of 40% and 70% positive tumor cells for MYC and BCL2, respectively, 300 (64%) were positive for MYC and 233 (50%) cases were positive for BCL2. One hundred fifty-seven (34%) were positive for both MYC and BCL2 and 90 (19%) were negative for both.
MYC and BCL2 protein coexpression in DLBCL had a significant adverse impact on patient survival (Figure 1A-B). The 5-year overall survival (OS) of DLBCL patients with MYC/BCL2 coexpression vs all other patients was 30% vs 75% (P < .0001); the 5-year progression-free survival (PFS) was 27% vs 73% (P < .0001). When assessed separately, patients with MYC+ or BCL2+ DLBCL had significantly inferior OS (Figure 1C,E) and PFS (data not shown) compared with patients with MYC-negative or BCL2-negative DLBCL, respectively. However, the prognostic impact of MYC or BCL2 protein expression was apparently due to the confounding effect of cases with MYC/BCL2 coexpression. When all cases with MYC/BCL2 coexpression were excluded, neither MYC nor BCL2 protein expression significantly impacted OS (Figure 1D,F) and PFS. Similarly, MYC or BCL2 protein expression did not correlate with COO subtypes (data not shown).
Figure 1.
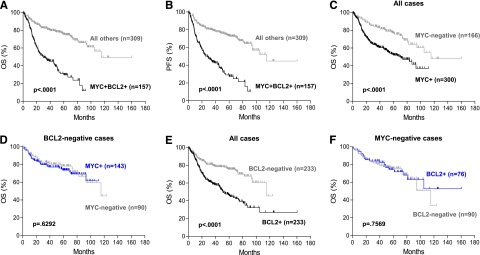
Prognostic impact of MYC/BCL2 coexpression in DLBCL. (A-B) OS (A) and PFS (B) of patients with DLBCL with MYC/BCL2 coexpression (MYC+BCL2+) in the training set. (C-D) OS of patients with MYC+ DLBCL in the presence (C) or absence (D) of BCL2 coexpression in the training set. (E-F) OS of patients with BCL2+ DLBCL in the presence (E) or absence (F) of MYC coexpression in the training set.
Stratifying all patients into GCB and ABC subtypes, patients with DLBCL with MYC/BCL2 coexpression had significantly worse OS and PFS within both COO subtypes (Figure 2A-D). The prognostic impact of MYC/BCL2 coexpression in DLBCL was further assessed according to various clinical parameters. The significantly worse OS and PFS conferred by MYC/BCL2 coexpression were observed in both low- and high-risk subgroups of DLBCL stratified by International Prognostic Index (IPI) scores (Figure 2E-F) and other individual clinical parameters (supplemental Figure 7).
Figure 2.
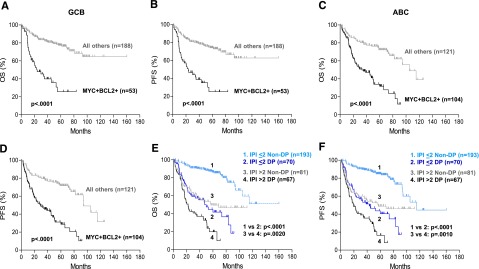
Prognostic impact of MYC/BCL2 coexpression in DLBCL risk-stratified according to clinicopathologic parameters. (A-B) OS (A) and PFS (B) of patients with MYC+BCL2+ DLBCL of the GCB subtype in the training set. (C-D) OS (C) and PFS (D) of patients with MYC+BCL2+ DLBCL of the ABC subtype in the training set. (E-F) OS (E) and PFS (F) of patients with MYC+BCL2+ DLBCL risk-stratified according to IPI risk scores in the training set. DP, MYC/BCL2 double-positive; Non-DP, non–double positive.
The adverse prognostic impact of MYC/BCL2 coexpression in DLBCL and its COO subtypes was validated in an independent set of 234 cases (validation set 1) of de novo DLBCL treated with R-CHOP (supplemental Figure 8). In multivariate analysis, controlling for other clinicopathological parameters, MYC/BCL2 coexpression remained a strong independent predictor of OS (P < .0001) and PFS (P < .0001) in DLBCL patients (Table 2).
Table 2.
Multivariate analysis of clinicopathological parameters in DLBCLs treated with R-CHOP
| OS | PFS | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| B symptoms | 1.47 | 1.04-2.09 | .0310 | 1.45 | 1.03-2.03 | .0314 |
| Tumor size, ≥7.5 cm | 1.22 | 0.87-1.71 | .2467 | 1.21 | 0.86-1.69 | .2708 |
| IPI risk, >2 | 2.38 | 1.67-3.38 | <.0001 | 2.22 | 1.59-3.11 | <.0001 |
| COO classification, ABC | 1.17 | 0.79-1.72 | .4329 | 1.18 | 0.82-1.71 | .3750 |
| TP53 mutation | 1.72 | 1.17-2.52 | .0057 | 1.63 | 1.12-2.37 | .0105 |
| MYC/BCL2 coexpression | 2.52 | 1.73-3.67 | <.0001 | 2.45 | 1.71-3.51 | <.0001 |
CI, confidence interval; HR, hazard ratio.
MYC/BCL2 coexpression is associated with high-risk clinical parameters
Various clinicopathological parameters were compared between patients with DLBCL with or without MYC/BCL2 coexpression (Table 1). Patients with DLBCL with MYC/BCL2 coexpression had multiple adverse prognostic factors included in the IPI risk stratification, including advanced age (P = .0011), high-stage disease (P < .0001), poor performance status (P = .0453), and multiple extranodal sites of disease (P = .0160). Consequently, more patients with DLBCL with MYC/BCL2 coexpression had an intermediate-high to high IPI score (P = .0001). Patients with DLBCL with MYC/BCL2 coexpression were also associated with a lower rate of complete remission (P < .0001) and a higher proliferation index (P = .0086). There was no significant difference in gender, serum lactate dehydrogenase level, tumor size, or frequency of TP53 mutations between DLBCL patients with or without MYC/BCL2 coexpression.
MYC/BCL2 coexpression shows ABC predominance and contributes to the inferior prognosis of ABC-DLBCL
The presence of MYC/BCL2 coexpression correlated significantly with the ABC subtype (P < .0001) (Table 1). Of 157 cases of DLBCL with MYC/BCL2 coexpression, 104 (66%) were ABC-DLBCL (Figure 3A and Table 1). By contrast, only 121 of 309 (39%) of DLBCL without MYC/BCL2 coexpression were ABC-DLBCL (Table 1). Approximately 46% (104 of 225) of ABC-DLBCL had MYC/BCL2 coexpression compared with 22% (53 of 241) of GCB-DLBCL with MYC/BCL2 coexpression (P < .0001) (Figure 3B). In cases only stratified by GEP, 49% of ABC-DLBCL and 19% of GCB-DLBCL showed MYC/BCL2 coexpression (supplemental Figure 3). Considering BCL2 and MYC protein expression individually, ABC-DLBCL had a significantly higher frequency of BCL2 (61% vs 40%; P < .0001) and MYC (72% vs 57%; P = .0009) expression than GCB-DLBCL (Figure 3B).
Figure 3.
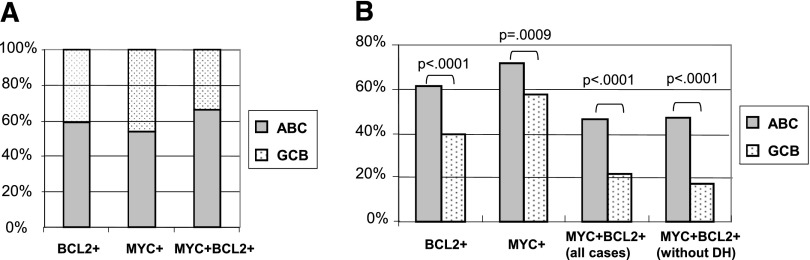
Frequency of BCL2 and MYC expression in COO subtypes of DLBCL. (A) Relative frequency of the ABC vs GCB subtype in DLBCL positive for BCL2 expression, MYC expression, or MYC/BCL2 coexpression in the training set. (B) Frequency of BCL2 expression, MYC expression, or MYC/BCL2 coexpression (in the presence or absence of MYC/BCL2 corearrangements, DH) in DLBCL of the ABC and GCB subtypes in the training set. DH, double hit.
In the training set, ABC-DLBCL was associated significantly with inferior OS (P = .0080) and PFS (P = .0075) (Figure 4A-B). However, after excluding all cases with MYC/BCL2 coexpression, the prognosis of patients with ABC-DLBCL was similar to that of patients with GCB-DLBCL (OS: P = .3163; PFS: P = .4291) (Figure 4C-D). This result was validated in a previously reported independent cohort (supplemental Figure 9). Considering only patients with DLBCL with MYC/BCL2 coexpression, there was no significant difference in OS (P = .4114) or PFS (P = .7020) between the ABC and GCB subtypes (Figure 4E-F).
Figure 4.
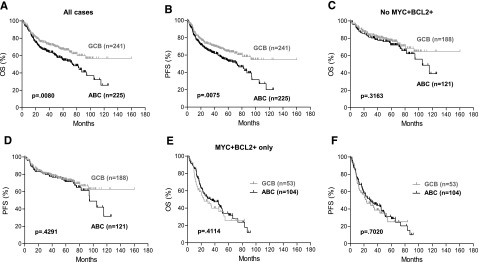
MYC/BCL2 coexpression contributes to the inferior prognosis of ABC-DLBCL. (A-B) OS (A) and PFS (B) of the ABC vs GCB subtype of DLBCL in the entire training set. COO classification of 411 cases was based on GEP results and 55 cases based on IHC results. (C-D) OS (C) and PFS (D) of the ABC vs GCB subtype of DLBCL after all MYC+BCL2+ cases were excluded. (E-F) OS (E) and PFS (F) of the ABC vs GCB subtype in MYC+BCL2+ DLBCL.
When analysis was limited to the 411 cases classified by GEP data, similar results were observed (supplemental Figure 4). Consistent with these results, in multivariate analysis, after controlling for MYC/BCL2 coexpression, the ABC subtype was not a significant prognostic predictor of OS (P = .4329) or PFS (P = .3750) (Table 2). These data support the notion that the inferior clinical outcome of patients with ABC-DLBCL is attributable to a significantly higher frequency of cases with MYC/BCL2 coexpression.
MYC/BCL2 coexpression confers an adverse prognostic impact independent from MYC/BCL2 corearrangement and TP53 mutation status
Approximately 3% (10 of 394) of DLBCL cases in the training set had concurrent MYC and BCL2 rearrangements; 9 of them were of the GCB subtype. DLBCL with MYC/BCL2 corearrangement was associated with markedly poor OS (P < .0001) and PFS (P < .0001) (Figure 5A-B). By IHC, 8 of these cases exhibited MYC/BCL2 coexpression and the other 2 cases were positive for either MYC or BCL2.
Figure 5.
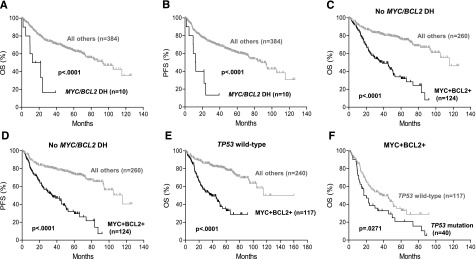
Prognostic impact of MYC/BCL2 coexpression in DLBCL is independent of MYC/BCL2 corearrangement and TP53 mutation status. (A-B) OS (A) and PFS (B) of patients with MYC/BCL2 double-hit DLBCL. (C-D) OS (C) and PFS (D) of patients with MYC+BCL2+ DLBCL in the absence of MYC/BCL2 double hit. (E) OS of patients with MYC+BCL2+ DLBCL in the absence of TP53 mutation. (F) Prognostic impact of TP53 mutation in MYC+BCL2+ DLBCL.
The remaining 384 cases (199 ABC and 185 GCB) in the training set lacked concurrent MYC/BCL2 rearrangements; 124 (32%) had MYC/BCL2 coexpression. In the absence of MYC/BCL2 corearrangement, MYC/BCL2 coexpression showed a marked predilection for the ABC subtype: 47% (93 of 199) vs 17% (31 of 185) in the GCB subtype (P < .0001) (Figure 3B) and remained a significant predictor of inferior OS (P < .0001) and PFS (P < .0001) (Figure 5C-D). Similarly, MYC/BCL2 coexpression predicted inferior survival in the absence of TP53 mutations (Figure 5E). However, TP53 mutation remained a significant prognostic factor in patients with DLBCL with MYC/BCL2 coexpression (Figure 5F).
MYC/BCL2 coexpression contributes to different gene expression signatures of GCB and ABC-DLBCL
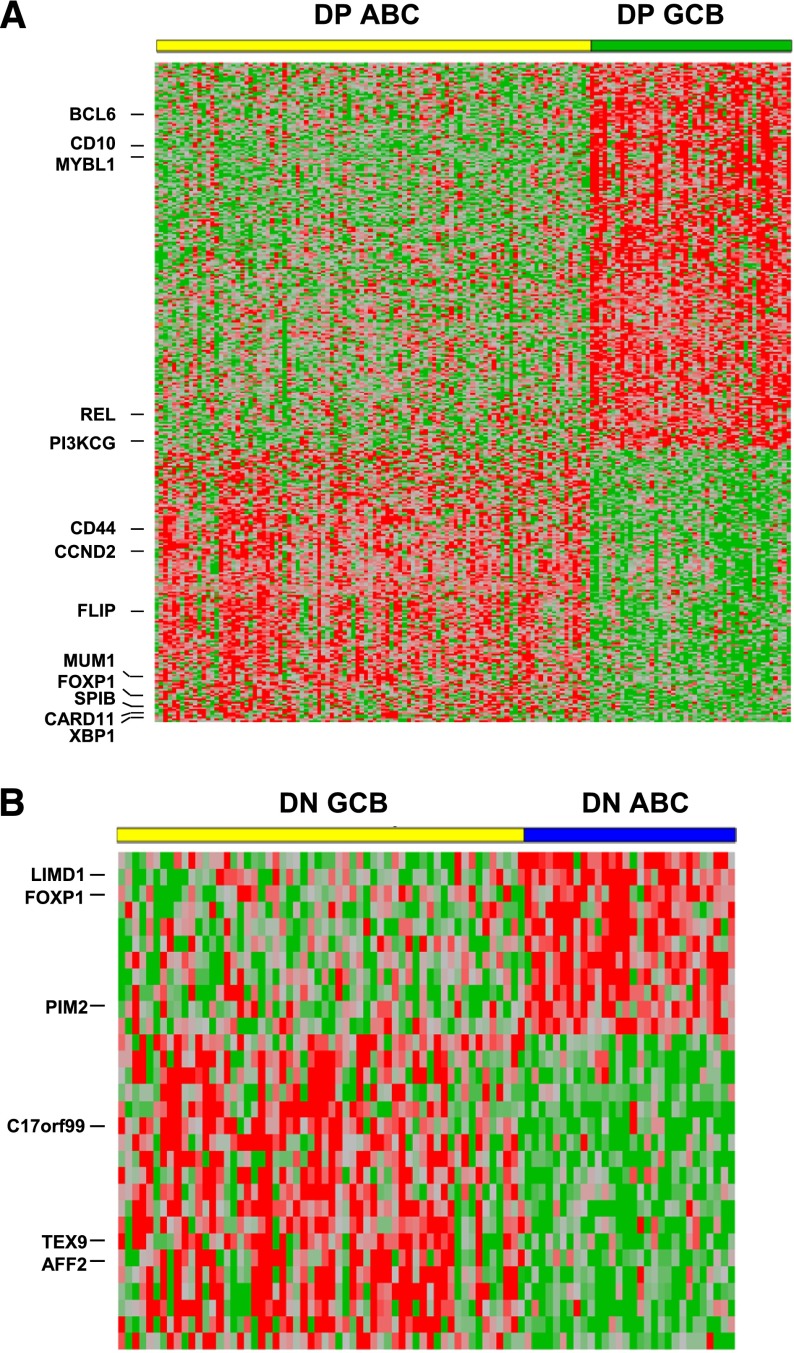
The above data show that the poorer prognosis of patients with ABC-DLBCL can be attributed, in large part, to the higher frequency of cases with MYC/BCL2 coexpression in the ABC subtype. We compared GEP results between the ABC and GCB subtypes with MYC/BCL2 coexpression (Figure 6; supplemental Table 1). A total of 208 genes were differentially expressed (P < .001), including 121 genes highly expressed in the GCB subtype and 87 genes highly expressed in the ABC subtypes. As expected, the gene signatures of the ABC vs GCB subtype of DLBCL with MYC/BCL2 coexpression largely reflected those reported by Alizadeh et al.1 The notable genes included CD10, BCL6, MYBL1, and PI3KCG in the GCB subtype vs MUM1, cyclin D2, FLIP, CD44, and SLAP in the ABC subtype. Additionally, we also identified many genes that have been shown to be differentially expressed between the 2 COO subtypes and confer prognostic impact by others, such as REL and CIITA in GCB-DLBCL, and CARD11, IGHM, FOXP1, and SPIB in ABC-DLBCL (supplemental Table 1).2,28-32
Figure 6.
MYC/BCL2 coexpression contributes to the different gene expression profiles between GCB and ABC subtypes of DLBCL. (A) GEP comparison between the ABC vs GCB subtype of DLBCL with MYC/BCL2 coexpression. Of 157 cases of MYC+BCL2+ DLBCL, GEP was successfully performed in 149 cases (ABC: 102; GCB: 47). DP, MYC/BCL2 double positive. A total of 208 genes corresponding to 365 probe sets were differentially expressed (P < .001). (B) GEP comparison between the ABC (30 cases) vs GCB (58 cases) subtype of DLBCL negative for both MYC and BCL2 protein expression. A total of 20 genes corresponding to 30 probesets were differentially expressed between the 2 COO subtypes (P < .01). DN, MYC/BCL2 double negative.
We further compared GEP results between the ABC and GCB subtypes in cases negative for both MYC and BCL2 expression (Figure 6B; supplemental Table 2). Surprisingly, there were no genes differentially expressed between the 2 subtypes at the same significance level of P < .001 and only a few genes at a significance level of P < .01. A total of 20 genes were differentially expressed between the 2 subtypes, including 12 highly expressed in the GCB and 8 highly expressed in the ABC subtype. Thirteen of these 20 genes were also differentially expressed between the 2 subtypes with MYC/BCL2 coexpression. However, only a few genes (AFF2/FMR2, FOXP1, and PIM2) were among those previously found to be differentially expressed between the 2 COO subtypes.1,32
Gene expression signature of DLBCL with MYC/BCL2 coexpression
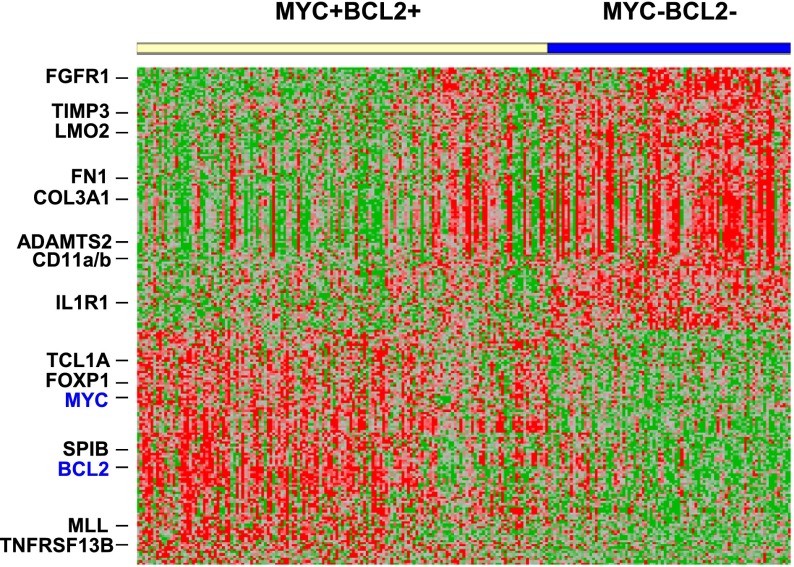
To elucidate the potential molecular basis behind the aggressive clinical course of patients with DLBCL with MYC/BCL2 coexpression, we compared GEP results of DLBCL with MYC/BCL2 coexpression with those of DLBCL negative for both MYC and BCL2 expression (Figure 7; supplemental Table 3). A total of 153 genes were differentially expressed, including 65 genes upregulated and 88 genes downregulated in DLBCL with MYC/BCL2 coexpression (P < .001).
Figure 7.
Gene expression signature of DLBCL with MYC/BCL2 coexpression. Comparison of GEPs of DLBCL with MYC/BCL2 coexpression (149 cases) vs DLBCL negative for MYC and BCL2 expression (88 cases). A total of 153 genes corresponding to 219 probe sets were differentially expressed (P < .001).
The most striking finding in DLBCL with MYC/BCL2 coexpression was the downregulation of a large number of genes (33 of 88 or 38%) encoding various extracellular matrix (ECM) proteins or those involving the ECM deposition and remodeling (Table 3; supplemental Table 3). The ECM-encoding genes included those encoding various subtypes of collagen, fibronectin, versican, thrombospondin, SPARC, and biglycan. The ECM remodeling genes included metallopeptidase/serine proteases and their inhibitors and matrix-associated proteins. Those genes involving the production of ECM included FGFR1 and FAP. Another prominent feature was the downregulation of genes (21 of 88, 24%) involved in cell adhesion, motility, and cytoskeletal organization, such as integrins, CD58, cortactin, caldesmon, calponin, and tropomyosin.
Table 3.
Differentially expressed genes in MYC+BCL2+ de novo DLBCL
| Gene functional categories | No. of genes | Representative genes |
|---|---|---|
| Downregulated genes | ||
| ECM, ECM production and remodeling | 33 | COL3A1, VCAN, TNS1, FN1, THBS2, TIMP3, SPARC, SULF1, SPINK2, MMP2, ADAM12, FGFR1, FAP |
| Cell adhesion and cytoskeletal organization | 21 | CD11A/CD11B, CD58, THY1, RFTN1, ANTXR1, RHOB, MICAL2 |
| Cell growth regulation | 16 | LM02, TRAF1, CDK14, SGK1, RGS1, NBL, PDE4D |
| Others, including unknown | 18 | PSAP, LYZ, LOC115110, ZNF662 |
| Upregulated genes | ||
| Cell proliferation | 20 | MYC, BCL2, TCL1A, MLL, FOXP1, SPIB, TCF4, TNFRSF13B, PMDAIP1, GAB1, PLOR3G |
| Cell metabolism | 5 | DCTPP1, CYB5R2, HK2, TMEM97, CYB5R2 |
| Miscellaneous cell functions | 13 | PPIL1, PIGW, FUT8, SPINK5 |
| Unknown | 27 | KIAA0664, C9orf91, ZNF107 |
Of the genes up-regulated in DLBCL with MYC/BCL2 coexpression, 20 encoded proteins involved in cell proliferation, including MYC and BCL2 as expected, as well as TCL1A, MLL, FOXP1, SP1B, TCF4, TNFRSF13B, and those promoting DNA and protein synthesis (Table 3; supplemental Table 3). Chromosomal breakpoints involving the TCL1A and MLL genes define T-prolymphocytic leukemia and a subtype of acute myeloid leukemia, respectively. FOXP1 and SPIB are transcriptional factors amplified in the ABC-DLBCL.32 Mutations of TCF4, a component of the Wnt pathway, have been shown in various types of solid tumors and lymphoma/leukemia. TNFRSF13B signaling activates NF-κB, NF-AT, and AP1. POLR3G and POLR1B are DNA-dependent RNA polymerases.
Discussion
In this study, we show that patients with DLBCL characterized by MYC/BCL2 coexpression have a poor clinical outcome with a 5-year OS and PFS of <30%. MYC/BCL2 coexpression in DLBCL is also a strong predictor of poor prognosis in the 2 COO subtypes. Patients with DLBCL with MYC/BCL2 coexpression have many clinicopathological features associated with adverse prognosis, including older age, advanced stage of disease, multiple extranodal sites of involvement, high IPI score, high proliferation index, and poor treatment response. Approximately one-third of DLBCL demonstrate MYC/BCL2 coexpression, in keeping with the 29% frequency reported in an earlier study by Green et al.23 By contrast, MYC/BCL2 double-hit B-cell lymphoma characterized by chromosomal breaks involving MYC and BCL2 is a rare disease, representing ∼3% of all DLBCL cases in our study. Thus, the findings in this study expand the spectrum of aggressive DLBCL, defined previously at the genetic level, by using the immunohistochemical approach. In our study, we observed an overall MYC+ rate of 64% and MYC+BCL2+ 34% in our training set and an overall MYC+ rate of 54% and MYC+BCL2+ 32% in our validation set, which were in line with the overall MYC+ rate of 54% and MYC+BCL2+ rate of 29% observed by Green et al23 (personal communication and legends of supplemental Figures 8 and 9), but higher than those reported by Johnson et al.24 The cause of the discrepancy is not known.
ABC-DLBCL, as stratified by gene expression signatures, is associated with a poor prognosis compared with GCB-DLBCL.1 The inferior prognosis of patients with the ABC-DLBCL has been attributed to constitutive NF-κB activity, leading to the upregulation of a number of NF-κB target genes, including MYC and BCL2.2 At the protein level, we show here that MYC/BCL2 coexpression is more frequently observed in the ABC subtype. We suggest that the high frequency of MYC/BCL2 coexpression in ABC-DLBCL greatly contributes to the overall poor prognosis of this patient subset. Our data support this statement in 3 ways. First, there was no significant prognostic difference between DLBCL patients with the GCB vs ABC subtype when all cases with MYC/BCL2 coexpression were excluded. Second, when only patients with DLBCL with MYC/BCL2 coexpression were considered, the 2 subtypes did not confer significant prognostic difference either. Third, although there was a striking difference in gene signatures between the ABC and GCB subtypes with MYC/BCL2 coexpression, the difference in gene signatures between the 2 subtypes was minimal in MYC/BCL2 double-negative DLBCL. We found this result somewhat surprising given that the GCB and ABC subtypes of DLBCL are assumed to be derived from B cells at different differentiation stages. We found that MYC/BCL2 double-negative DLBCL had a higher percentage of cases unclassifiable by GEP or that showed a discordance between GEP and IHC-based classification (30% in MYC/BCL2 double-negative group vs 19% in DLBCL with MYC/BCL2 coexpression; P = .07). These borderline cases may blur the boundary between the GCB and ABC signatures. There are at least 5 distinct subsets of mature B cells corresponding to different B-cell differentiation stages33 and it is possible that DLBCL could be derived from each of these subsets. Our results raise the issue as to whether it is appropriate to “force” DLBCL cases into a binary and perhaps overly simplistic classification.
Our results show that DLBCL with MYC/BCL2 coexpression is a unique subset of DLBCL with dismal clinical outcome. The potential molecular basis behind the dismal outcome is seen through the gene expression profiles of cases with MYC/BCL2 coexpression: stromal, adhesion, and proliferation signatures. The stromal signature is similar to what was described by Lenz et al (stromal signature 1) although it is more striking in DLBCL with MYC/BCL2 coexpression.27 Both stroma-poor and proliferation signatures are associated with poor prognosis in DLBCL.27,28,34,35 A cell-adhesion signature has not been described in lymphoma to date although its role in the invasion of solid tumors is well-established.36 Presumably, the lack of cell-cell and cell-matrix adhesions might play a role in the high frequency of advanced stage of disease and involvement of multiple extranodal sites in DLBCL with MYC/BCL2 coexpression.
However, we do not wish to imply that there are no other pathogenetic factors that contribute to the inferior prognosis of patients with ABC-DLBCL, nor are we suggesting that the ABC and GCB subtypes of DLBCL with MYC/BCL2 coexpression are biologically homogeneous. Each of these subtypes might include additional subsets with additional predictive prognostic factors that deserve further investigation. Our GEP studies clearly show heterogeneity in the group of DLBCL with MYC/BCL2 coexpression. Instead, we only wish to emphasize that MYC/BCL2 coexpression has significant prognostic value for DLBCL patients and these tumors represent almost half of the ABC-DLBCL cases. In addition, the recognition of DLBCL with MYC/BCL2 coexpression expands the spectrum of aggressive B-cell lymphomas for which novel therapies are needed, and IHC assessment for MYC and BCL2 expression provides a practical approach to effectively stratify DLBCL into prognostically relevant subgroups.
With regard to the prognostic impact of BCL2 expression alone in DLBCL in the era of R-CHOP chemotherapy, previous studies reported inconsistent results.21,23,24,37-44 Most of these studies had small patient cohorts and/or also did not address the confounding effects of other factors, such as MYC expression. In this study, we found that BCL2 expression predicted survival in DLBCL in the overall patient cohort and in the COO subtypes. However, the observed prognostic impact of BCL2 expression was attributable to the subset of DLBCL cases with MYC/BCL2 coexpression. Similarly, MYC protein expression affected prognosis only in the presence of BCL2 coexpression, consistent with the studies by Johnson et al.24 Approximately 60% of cases with BCL2 but without concurrent MYC rearrangement demonstrated MYC protein expression in our cohort. This may explain our previous observation that DLBCL with BCL2 rearrangement showed worse prognosis irrespective of MYC rearrangement status.45
In summary, DLBCL with MYC/BCL2 coexpression characterizes a subset of DLBCL patients with high-risk gene signatures, high-risk clinicopathological features and poor prognosis. DLBCL with MYC/BCL2 coexpression apparently expands the spectrum of MYC/BCL2 double-hit B-cell lymphoma defined genetically and well-recognized to have a poor prognosis. However, it should be emphasized that our data do not show that MYC/BCL2 double-hit B-cell lymphoma and DLBCL with MYC/BCL2 coexpression are equivalent. It is possible that additional molecular abnormalities or levels of MYC and BCL2 protein expression may distinguish these 2 groups. We further show that DLBCL with MYC/BCL2 coexpression occurs in almost half of ABC-DLBCL cases and appears to account, in large part, for the inferior prognosis of patients with ABC-DLBCL. These data also suggest that assessment for MYC and BCL2 protein expression more reliably predicts prognosis than the COO classification.
Supplementary Material
Acknowledgments
The authors thank their consortium program team of pathologists, hematologists, clinicians, and each of the contributing center principal physicians for their support. The DLBCL Rituximab-CHOP Consortium Program has its principal investigation center at The University of Texas MD Anderson Cancer Center (Houston, TX), and includes 29 medical centers for the collaboration. Material transfer agreement and institutional review board protocol were established and approved by each of the participating centers.
S.H. is the recipient of a Hematopathology Research Fellowship Award. Z.Y.X.-M. is a recipient of a Shannon Timmins Leukemia Fellowship Award at The University of Texas MD Anderson Cancer Center. R.D.G. is supported by Cancer Terry Fox program project grant (19001). A.T. is a recipient of the Stiftung zur Krebsbekaempfung Zurich Grant 269 award. K.H.Y. is supported by The University of Texas MD Anderson Cancer Center Institutional R & D Fund, Institutional Research Grant Award, an MD Anderson Lymphoma Specialized Programs of Research Excellence Development Program Award, an MD Anderson Myeloma Specialized Programs of Research Excellence Research Development Program Award, Gundersen Lutheran Medical Foundation Award, and an MD Anderson Collaborative Funds with Roche Molecular System, HTG Molecular Diagnostic, and Daiichi Sankyo Pharm. This work is also partially supported by the National Cancer Institute/National Institutes of Health (R01CA138688, 1RC1CA146299, P50CA136411, and P50CA142509, to Y.L. and K.H.Y.).
Authorship
Contribution: S.H. and K.H.Y. conceived and designed the study and performed research; S.H., Z.Y.X.-M., A.T., T.G., L.W., A.B., W.-m.L., C.V., Y.L., R.N.M., S.M.-M., K.D., A.C., A.O., Y.Z., G.B., K.L.R., E.D.H., W.W.L.C., X.Z., J.H.v.K., Q.H., J.H., W.A., M.P., A.J.M.F., F.Z., M.T., D.V., R.S.G., M.A.P., M.B.M., L.J.M., and K.H.Y. provided critical reagents, technology or method, resources, analysis, study materials or patients under approved institutional review board and material transfer agreement; G.W.S. and R.D.G. provided a dataset of 128 cases for validation discussion; S.H., Z.Y.X.-M., A.T., T.G., C.V., R.N.M., S.M.-M., K.D., A.C., A.O., Y.Z., G.B., K.L.R., E.D.H., W.W.L.C., X.Z., J.H.v.K., Q.H., J.H., W.A., M.P., A.J.M.F., F.Z., M.T., D.V., W.C., R.S.G., M.A.P., M.B.M., L.J.M., and K.H.Y. collected and assembled data under approved institutional review board and material transfer agreement; S.H., L.J.M., and K.H.Y. analyzed and interpreted data and wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ken H. Young, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: khyoung@mdanderson.org.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
References
- 1.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 2.Lim KH, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF-κB in lymphoid malignancies. Immunol Rev. 2012;246(1):359–378. doi: 10.1111/j.1600-065X.2012.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compagno M, Lim WK, Grunn A, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459(7247):717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 5.Muris JJ, Meijer CJ, Vos W, et al. Immunohistochemical profiling based on Bcl-2, CD10 and MUM1 expression improves risk stratification in patients with primary nodal diffuse large B cell lymphoma. J Pathol. 2006;208(5):714–723. doi: 10.1002/path.1924. [DOI] [PubMed] [Google Scholar]
- 6.Natkunam Y, Farinha P, Hsi ED, et al. LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J Clin Oncol. 2008;26(3):447–454. doi: 10.1200/JCO.2007.13.0690. [DOI] [PubMed] [Google Scholar]
- 7.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Leppä S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol. 2009;22(8):1094–1101. doi: 10.1038/modpathol.2009.73. [DOI] [PubMed] [Google Scholar]
- 8.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15(17):5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29(2):200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visco C, Li Y, Xu-Monette ZY, et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia. 2012;26(9):2103-2113. [DOI] [PMC free article] [PubMed]
- 11.Gutiérrez-García G, Cardesa-Salzmann T, Climent F, et al. Grup per l’Estudi dels Limfomes de Catalunya I Balears (GELCAB) Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117(18):4836–4843. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 12.Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol. 2011;29(31):4079–4087. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- 13.Gu K, Weisenburger DD, Fu K, et al. Cell of origin fails to predict survival in patients with diffuse large B-cell lymphoma treated with autologous hematopoietic stem cell transplantation. Hematol Oncol. 2012;30(3):143-149. [DOI] [PMC free article] [PubMed]
- 14.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 15.Kanungo A, Medeiros LJ, Abruzzo LV, Lin P. Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol. 2006;19(1):25–33. doi: 10.1038/modpathol.3800500. [DOI] [PubMed] [Google Scholar]
- 16.Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC rearrangement: a neoplasm of germinal center immunophenotype with poor prognosis. Haematologica. 2007;92(10):1297–1301. doi: 10.3324/haematol.11263. [DOI] [PubMed] [Google Scholar]
- 17.Le Gouill S, Talmant P, Touzeau C, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica. 2007;92(10):1335–1342. doi: 10.3324/haematol.11305. [DOI] [PubMed] [Google Scholar]
- 18.Niitsu N, Okamoto M, Miura I, Hirano M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia. 2009;23(4):777–783. doi: 10.1038/leu.2008.344. [DOI] [PubMed] [Google Scholar]
- 19.Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica. 2009;94(7):935–943. doi: 10.3324/haematol.2008.005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114(11):2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25(1):145–156. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 22.Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34(3):327–340. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 24.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu-Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120(19):3986–3996. doi: 10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100(17):9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz G, Wright G, Dave SS, et al. Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenwald A, Wright G, Chan WC, et al. Lymphoma/Leukemia Molecular Profiling Project. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 29.Roberts RA, Wright G, Rosenwald AR, et al. Loss of major histocompatibility class II gene and protein expression in primary mediastinal large B-cell lymphoma is highly coordinated and related to poor patient survival. Blood. 2006;108(1):311–318. doi: 10.1182/blood-2005-11-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimsza LM, Roberts RA, Campo E, et al. Loss of major histocompatibility class II expression in non-immune-privileged site diffuse large B-cell lymphoma is highly coordinated and not due to chromosomal deletions. Blood. 2006;107(3):1101–1107. doi: 10.1182/blood-2005-04-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruminy P, Etancelin P, Couronné L, et al. The isotype of the BCR as a surrogate for the GCB and ABC molecular subtypes in diffuse large B-cell lymphoma. Leukemia. 2011;25(4):681–688. doi: 10.1038/leu.2010.302. [DOI] [PubMed] [Google Scholar]
- 32.Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008;105(36):13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kjeldsen MK, Perez-Andres M, Schmitz A, et al. Multiparametric flow cytometry for identification and fluorescence activated cell sorting of five distinct B-cell subpopulations in normal tonsil tissue. Am J Clin Pathol. 2011;136(6):960–969. doi: 10.1309/AJCPDQNP2U5DZHVV. [DOI] [PubMed] [Google Scholar]
- 34.Meyer PN, Fu K, Greiner T, et al. The stromal cell marker SPARC predicts for survival in patients with diffuse large B-cell lymphoma treated with rituximab. Am J Clin Pathol. 2011;135(1):54–61. doi: 10.1309/AJCPJX4BJV9NLQHY. [DOI] [PubMed] [Google Scholar]
- 35.Perry AM, Cardesa-Salzmann TM, Meyer PN, et al. A new biologic prognostic model based on immunohistochemistry predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2012;120(11):2290–2296. doi: 10.1182/blood-2012-05-430389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2—associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood. 2003;101(11):4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 38.Iqbal J, Neppalli VT, Wright G, et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(6):961–968. doi: 10.1200/JCO.2005.03.4264. [DOI] [PubMed] [Google Scholar]
- 39.Shivakumar L, Armitage JO. Bcl-2 gene expression as a predictor of outcome in diffuse large B-cell lymphoma. Clin Lymphoma Myeloma. 2006;6(6):455–457. doi: 10.3816/CLM.2006.n.025. [DOI] [PubMed] [Google Scholar]
- 40.Amen F, Horncastle D, Elderfield K, et al. Absence of cyclin-D2 and Bcl-2 expression within the germinal centre type of diffuse large B-cell lymphoma identifies a very good prognostic subgroup of patients. Histopathology. 2007;51(1):70–79. doi: 10.1111/j.1365-2559.2007.02721.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilson KS, Sehn LH, Berry B, et al. CHOP-R therapy overcomes the adverse prognostic influence of BCL-2 expression in diffuse large B-cell lymphoma. Leuk Lymphoma. 2007;48(6):1102–1109. doi: 10.1080/10428190701344881. [DOI] [PubMed] [Google Scholar]
- 42.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Enblad G, Leppä S. Bcl-2 but not FOXP1, is an adverse risk factor in immunochemotherapy-treated non-germinal center diffuse large B-cell lymphomas. Eur J Haematol. 2009;82(5):364–372. doi: 10.1111/j.1600-0609.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 43.Iqbal J, Meyer PN, Smith LM, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17(24):7785–7795. doi: 10.1158/1078-0432.CCR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma [published online ahead of print January 18, 2013]. Blood. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 45.Visco C, Tzankov A, Xu-Monette ZY, et al. Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica. 2013;98(2):255–263. doi: 10.3324/haematol.2012.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.