Key Points
Stem cell gene therapy results in enhanced virus-specific immunity and recovery of CD4+ T cells in a nonhuman primate model of AIDS.
Gene therapy–mediated protection of stem cells results in a disease state similar to that observed in long-term nonprogressors.
Abstract
Despite continued progress in the development of novel antiretroviral therapies, it has become increasingly evident that drug-based treatments will not lead to a functional or sterilizing cure for HIV+ patients. In 2009, an HIV+ patient was effectively cured of HIV following allogeneic transplantation of hematopoietic stem cells (HSCs) from a CCR5−/− donor. The utility of this approach, however, is severely limited because of the difficulty in finding matched donors. Hence, we studied the potential of HIV-resistant stem cells in the autologous setting in a nonhuman primate AIDS model and incorporated a fusion inhibitor (mC46) as the means for developing infection-resistant cells. Pigtail macaques underwent identical transplants and Simian-Human Immunodeficiency Virus (SHIV) challenge procedures with the only variation between control and mC46 macaques being the inclusion of a fusion-inhibitor expression cassette. Following SHIV challenge, mC46 macaques, but not control macaques, showed a positive selection of gene-modified CD4+ T cells in peripheral blood, gastrointestinal tract, and lymph nodes, accounting for >90% of the total CD4+ T-cell population. mC46 macaques also maintained high frequencies of SHIV-specific, gene-modified CD4+ T cells, an increase in nonmodified CD4+ T cells, enhanced cytotoxic T lymphocyte function, and antibody responses. These data suggest that HSC protection may be a potential alternative to conventional antiretroviral therapy in patients with HIV/AIDS.
Introduction
Highly active antiretroviral therapy (HAART) has greatly reduced viral loads and reduced morbidity and mortality from AIDS1; however, the side effects of prolonged use can often lead to severe disease,2 and the emergence of drug resistance strains remains a major public health concern. HIV proviral DNA remains present in lymphoid cell populations regardless of prolonged HAART, and patients that have terminated treatment, either because of intolerance or noncompliance, experience a rapid resurgence of viral burden to pretreatment levels.3-5 Even in long-term compliant patients, the emergence of resistant mutant viruses has been documented.6,7 Thus, novel therapeutic strategies are needed to better control or eradicate the latent reservoir and potentially cure HIV.3,8
Hematopoietic stem cell (HSC)-based therapy for HIV infection recently garnered the interest of the scientific community when an AIDS patient with acute myeloid leukemia was effectively cured of HIV upon the allogeneic transplant of CD34+ cells from a homozygous CCR5Δ32 donor.9 Donor cells returned the patient’s CD4+ T-cell population to normal levels and controlled HIV replication to levels undetectable for more than 4 years following the cessation of HAART.10 Although these results demonstrate the potential benefits of stem cell–based therapies, obtaining sufficient numbers of matched CCR5Δ donors and the risks posed by allogeneic transplants make this approach unfeasible for most patients.11 The ex vivo modification and infusion of gene-modified, autologous HSCs would overcome several of these obstacles.
Recent studies have demonstrated that ex vivo genetic modification of both mature T cells and CD34+ HSCs could significantly alter the course of disease progression in humanized mouse models and confer a selective advantage of gene-modified cells.12-14 Although the direct genetic modification of CD4+ T cells has shown promising results, the genetic modification of HSCs has significant advantages, including the protection of both lymphoid and myeloid lineages, both of which are susceptible to HIV infection.15 In addition, modification of HSCs is likely to result in a long-lived source of lineage-specific progenitors, some of which may be targets for infection themselves.16 Transplanting autologous protected HSCs could lead to long-term protection against further infection and, potentially, the eradication of viral reservoirs.
The targeted disruption of the CCR5 gene locus using zinc-finger nucleases was recently shown to be highly effective at reducing viral loads and maintaining a selective advantage for CD4+ T cells upon HIV challenge in a humanized mouse model system.12 An alternative strategy to CCR5 gene disruption is expression of a previously identified short peptide corresponding to a 46-amino acid sequence of gp41 that potently inhibits viral entry when fused to a membrane anchor (mC46) and expressed on the surface of normally susceptible target cells.17 Recent studies by Kimpel et al demonstrated that mC46 expression in primary human CD4+ T cells led to the positive selection of transduced cells in a xenotransplant mouse model after challenge with a pathogenic HIV strain.13 These studies, however, did not show any protection or selective advantage of unmodified or unprotected CD4+ T cells. The mC46 peptide has been shown to significantly inhibit a broad range of viral isolates including dual tropic and CXCR4 tropic HIV and Simian-Human Immunodeficiency Virus (SHIV) isolates.13 Hence, there is a reduced risk of escape variants arising or that ×4-tropic variants will have a selective advantage over R5 tropic variants.
In this study, macaques transplanted with HIV/SHIV resistant HSCs were used to demonstrate the potential of this therapy, primarily to allow for the positive selection of mC46-expressing cells in vivo using chemotherapeutic agents. Hence, following transplantation of pigtailed macaques with autologous gene-modified CD34+ cells expressing both the mC46 fusion inhibitor and the chemoresistant gene MGMTP140K, chemotherapeutic agents were used to select gene-modified cells.18-20 In vivo selection was deemed necessary in order to obtain a sufficient percentage of gene-modified cells before SHIV challenge and enabling an initial comparison of the percentage of gene-modified cells vs the degree of viral pathogenesis. Here, we evaluated whether mC46 expression would lead to in vivo control of SHIV89.6P-MN, a dual-tropic SHIV strain previously shown to be highly pathogenic in the pigtail macaque model.21,22 This is the first study in a nonhuman primate model to demonstrate the protective effect of transplanted gene modified, infection-resistant HSCs against SHIV challenge.
Materials and methods
Ethics statement
Healthy juvenile pigtailed macaques (Macaca nemestrina) were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Study protocols were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board and the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee. Animals are monitored closely and animal welfare was assessed on a daily basis and, if necessary, several times per day. If animals experienced pain, they received pain medications. If pain cannot be relieved, or if veterinary examination reveals signs of suffering that cannot be relieved by analgesics, antiemetics, or antibiotic therapy, animals are killed.
Macaque transplant and ex vivo modification of enriched CD34+ HSCs
Animal transplantation and ex vivo modification were conducted as previously described.23,24 Briefly, animals were administered recombinant human granulocyte colony-stimulating factor at 100 µg/kg and also given recombinant human stem cell factor at 50 µg/kg before bone marrow harvest. CD34+ cells were enriched by magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions with purity levels ranging from 85% to 99% CD34+ HSCs. A minimum of 107 CD34+ HSCs per kilogram of body weight were transduced twice with lentiviral vectors at a multiplicity of infection of 10 during a 48-hour ex vivo culture period. The average percentage of gene-modified HSCs grown in liquid culture was 44% at day 4 and 17.2% at day 11 posttransduction. All animals received myeloablative total body irradiation, were infused with a minimum of 107 HSCs/kg, and received recombinant human granulocyte colony-stimulating factor and standard supportive care following transplantation. Lentiviral constructs expressing mC46 (experimental only), MGMTP140K and GFP, ex vivo CD34+ transduction, and in vivo selection of gene-modified HSC-derived lineages were previously described.18,19 In all cases, macaques were allowed to recover and stabilize following chemotherapy administration (typically >3 months) before SHIV challenge. The elapsed time from transplantation to SHIV challenge was variable and dependent on the number of chemotherapy doses required to obtain a predetermined set point of gene-modified cells.
Immunological assays and viral titer analysis
Viral load was assayed as previously described.25 Antibody titer against disrupted whole Simian Immunodeficiency Virus (SIV) or HIV envelope (Env) was determined by enzyme-linked immunosorbent assay (ELISA). Western blot analysis of serum samples was conducted using an SIVmac western blot kit from ZeptoMetrix. Interferon (IFN)-γ enzyme-linked immunospot assay was conducted in accordance with the manufacturer’s protocol (R&D Systems). Neutralization assay was performed as previously described.21 Fluorescence-activated cell sorter (FACS)-based quantification of SHIV-specific responders was performed following overnight recovery and a brief 6-hour pulse with viral peptide pools obtained from the AIDS Research and Reference Reagent Program following overnight recovery. Cryopreserved samples from 2, 4, and 6 months post-SHIV challenge were analyzed. The percentage of Gag and Pol responders was determined for all samples; additional analysis of Env and Nef responders was conducted as indicated. SEB was used as positive control. Responses 3 times greater than dimethylsulfoxide treatment alone were considered positive.
Results
C46 expression results in reduced plasma viremia and recovery of CD4+ T cells following the acute phase of infection
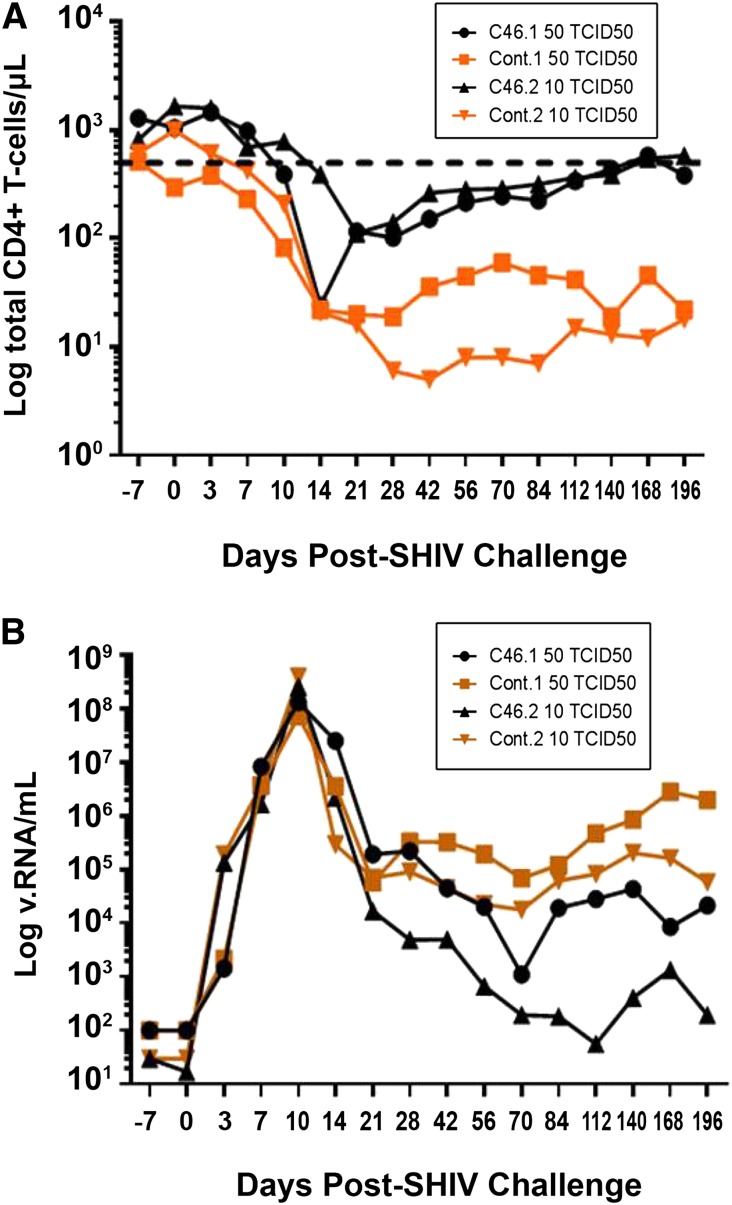
The goal of these studies was to determine the protective effect of transplanting genetically modified, infection-resistant autologous HSCs before SHIV challenge. Briefly, 4 pigtailed macaques underwent identical pre- and posttransplant conditioning regimens, in vivo selection, and SHIV challenge, with the only variation being the lentiviral vector used to transduce HSCs ex vivo following CD34+ isolation. Control macaques received autologous HSCs transduced with a green fluorescent protein (GFP)-expressing lentiviral vector while experimental macaques were transplanted with HSCs expressing a membrane bound fusion inhibitor (mC46) in addition to GFP. Experimental and control macaques were then challenged with SIV/HIV89.6P-MN, a dual-tropic, highly pathogenic chimeric HIV/SIV strain. The first set of control and experimental macaques were challenged with 50 Tissue Culture Infectious Dose (TCID) 50 (mC46.1 and control.1, respectively), while the second set was challenged with 10 TCID50 (mC46.2 and control.2, respectively). Twenty-one days following SHIV challenge, CD4+ T-cell counts were reduced by 26- and 38-fold in control.1 and control.2, respectively (Figure 1A). Similarly, a 44-fold decrease was observed in mC46.1, whereas mC46.2 exhibited a more modest 14-fold reduction. An initial decrease in CD4+ T-cell counts was anticipated; although genetically modified T cells would be resistant to direct infection, they are still susceptible to bystander-mediated apoptosis. In comparing mC46.1 with control.1, a peak reduction in plasma viremia of approximately 320-fold was observed at day 168; for mC46.2 and control.2, a reduction of 1477-fold was observed at day 20 (Figure 1B; P = .02). Remarkably, plasma viremia in mC46.2 decreased to below 200 viral RNA copies/mL by day 112 postinfection. Of interest, the persistent plasma viremia detected in mC46.1 did not result in any deleterious effects on CD4+ T-cell levels as the overall number of CD4+ T cells continued to recover (Figure 1A-B; P = .004). By day 140, CD4+ T cells were within the normal range observed in uninfected pigtailed macaques in both mC46-expressing macaques. These results indicate a direct correlation between the engraftment levels of gene-modified cells and both viral titer and CD4+ T-cell levels following the acute phase of infection.
Figure 1.
Protection of CD4+ T cells and reduced viremia in SHIV-infected macaques previously engrafted with mC46-expressing HSCs. (A) Absolute number of CD4+CD3+ T cells/μl peripheral blood of SHIV-infected macaques engrafted with control (squares and diamonds) or mC46-expressing (circles and triangles) CD34+ HSCs, as determined by FACS analysis. The lower limit of the normal CD4+ T-cell range is indicated by the dotted line. (B) Levels of SHIV RNA detected in peripheral blood of macaques previously engrafted with control (squares) or mC46-expressing (circles) CD34+ HSCs. Macaques were challenged with a viral dose equivalent to 10–50 TCID50. The limit of quantification of the viral load real-time polymerase chain reaction assay was 102 viral RNA copies/mL.
Positive selection of modified CD4+ T cells during the acute phase of infection and recovery of unmodified CD4+ T cells
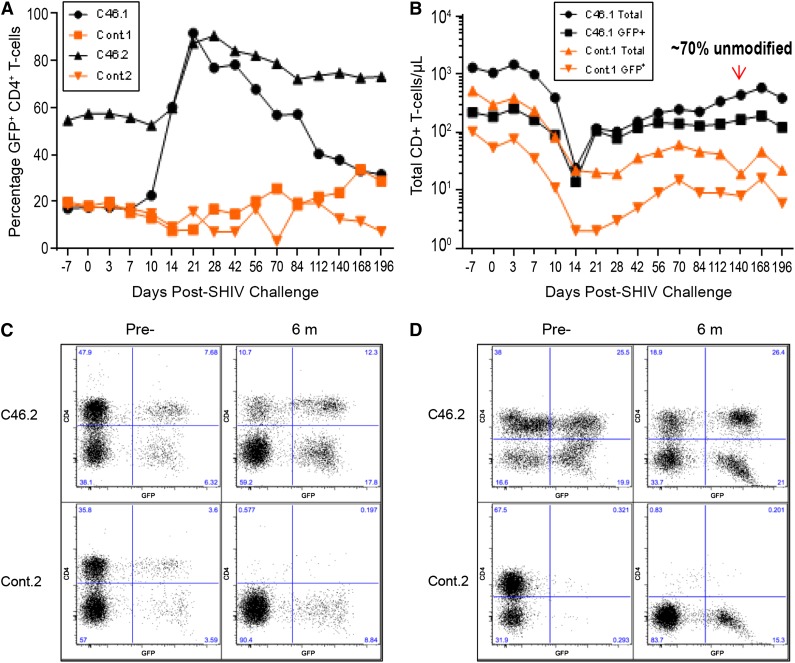
Before SHIV challenge, the percentage of gene-modified CD4+ T cells in the mC46-macaques was ∼20% (mC46.1) and ∼55% (mC46.2), respectively, whereas levels of gene-modified cells in both control macaques was ∼20% (control.1 and control.2). Twenty-one days following SHIV challenge, the percentage of gene-modified CD4+ T cells rapidly increased to >90% of the total CD4+ T-cell population in the peripheral blood of both experimental macaques (Figure 2A; solid symbols). Conversely, the percentage of gene-modified CD4+GFP+ T cells in the control macaques exhibited no selective advantage during the acute or chronic phase of infection (Figure 2A; open symbols). Unexpectedly, gene-modified CD4+ T cells appeared to exhibit a positive bystander effect on unmodified CD4+ T cells in the mC46-macaques. As indicated in Figure 2B, the reduction in the percentage of gene-modified cells was not due to a decrease in the absolute number of gene-modified CD4+ T-cells, but a gradual, yet significant recovery of unmodified CD4+ T cells. By day 140, unmodified cells accounted for ∼70% of all CD4+ T cells in mC46.1. A more moderate, yet appreciable recovery of unmodified CD4+ T cells was observed in mC46.2. Given the high percentage of gene-modified CD4+ T cells before infection, the modest recovery of unprotected cells appeared less pronounced. Positive selection of gene-modified CD4+ T cells was observed in all CD4+ T-cell subsets examined and in CD4+ T cells isolated from gastrointestinal (GI) and lymph node (LN) biopsies in mC46.1 and mC46.2 (Figure 2C-D; supplemental Figures 1 and 2). As indicated in supplemental Figure 3, proviral DNA content in mC46-expressing macaques was 103 to 104 lower than in control macaques. Proviral DNA content in sorted GFP+ and GFP− populations was approximately 4 and 27 copies, respectively, in mC46-expressing macaques. Consistent with the recovery of CD4+ T cells in peripheral blood, CD4+ T-cell levels in both GI and LN biopsies made a remarkable recovery in mC46-macaques 6 months following SHIV challenge (Figure 2C-D). In contrast, CD4+ T cells were virtually undetectable in control macaques with levels that were equivalent to those observed in GI and LN biopsies following the acute phase of infection. Similar to results obtained in peripheral blood, gene-modified CD4+ T cells isolated from GI and LN biopsies exhibited a selective advantage and accounted for the majority of CD4+ T cells in both tissues. The overall percentage of CD4+CD3+CCR5+ T cells in GI biopsies decreased by 2.3-fold and 68-fold in the mC46-macaques and control macaques, respectively. The same CD4+ T-cell population in LN was reduced by only 25% in mC46-expressing macaques, whereas an 11-fold reduction was observed in control macaques. Overall, positive selection of gene-modified CD4+ T cells was observed in all tissues and subsets examined during the acute phase of infection; however, unmodified cells made a gradual yet substantial recovery during the chronic phase of infection in mC46-expressing macaques. Of note, positive selection was only observed in CD4+ T cells, whereas the percentage of gene-modified cells in other HSC-derived lineages remained relatively unchanged throughout the course of these studies.
Figure 2.
Positive selection of gene-modified CD4+ T cells following SHIV challenge. (A) The percentage of gene-modified CD4+ T cells in peripheral blood was determined by FACS analysis based on GFP expression in the control (squares and diamonds) and mC46 macaques (circles and triangles). (B) The absolute number of CD4+CD3+ (circles and triangles) and total CD4+CD3+ GFP+ T cells (squares and diamonds) was determined by FACS analysis for control and mC46 macaque set 1. The area between the respective lines of each macaque represents nonmodified CD4+ T cells. As indicated, 70% of CD4+ T cells at day 140 post-SHIV challenge are nonmodified. (C-D) FACS plots demonstrating the relative percentage of total CD4+CD3+ T cells and CD4+CD3+GFP+ T cells in mixed cell populations isolated from (C) duodenal biopsies and (D) LN biopsies before and 6 months after SHIV challenge. See supplemental Figure 2 for additional analysis at days 7 and 21 following SHIV challenge and averages of both sets of macaques.
CD4+ to CD8+ T-cell ratio is significantly improved in mC46 macaques
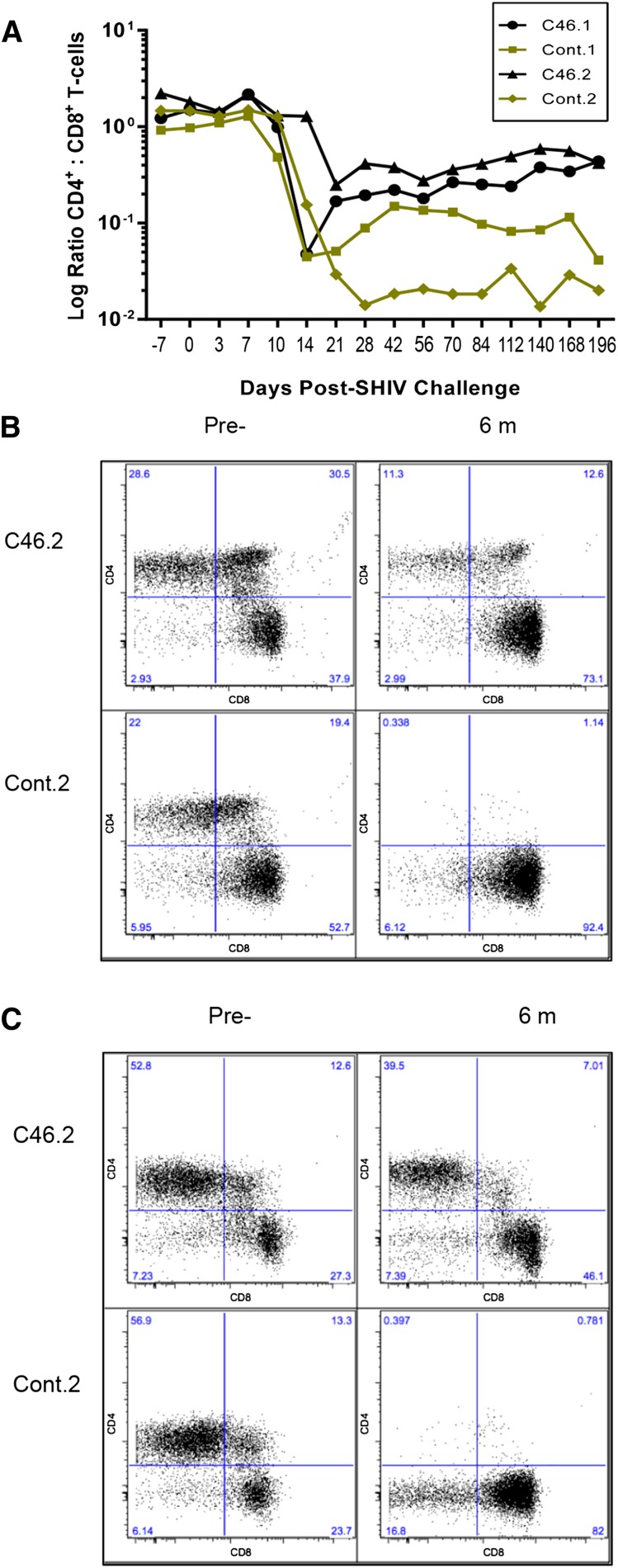
Twenty-one days following SHIV challenge, the CD4+ to CD8+ T-cell ratio decreased by nearly 2 logs in control.1, control.2, and mC46.1; ∼1-log decrease was observed in mC46.2, the macaque with the highest level of mC46-expressing CD4+ T cells. Corresponding with the increase in CD4+ T cells observed in both mC46-expressing macaques, the CD4+/CD8+ T-cell ratio increased following the acute phase of infection in peripheral blood samples (Figure 3A; P = .009). Similarly, the ratio of CD4+ T cells to CD8+ T cells in the lymphocyte population isolated from GI and LN biopsies 6 months following SHIV challenge had nearly recovered to normal levels, with a minimal 4.75- and 1.46-fold decrease observed relative to preinfection levels in mC46-expressing macaques (Figure 3B-C). In comparison, the CD4+ to CD8+ T-cell ratio in control macaques was reduced by 196- and 383-fold in cells isolated from GI and LN biopsies, respectively. Hence, although a significant drop in CD4+/CD8+ T-cell ratio is observed in mC46-expressing macaques early after SHIV challenge, the gradual recovery of CD4+ T cells leads to a substantial improvement in the ratio during the chronic phase of infection. Additionally, the double-positive CD4+CD8+ T-cell subset appeared to benefit from mC46 expression in the experimental macaques (Figure 3B-C).
Figure 3.
Improved ratio of CD4+ to CD8+ T cells in mC46 macaques. (A) The ratio of CD4+CD3+ T cells over CD8+CD3+ T cells was determined by FACS analysis throughout the course of these studies. Results shown are for both mC46-expressing macaques (circles and triangles) and both control macaques (squares and diamonds). (B-C) The relative percentage of CD4+ T cells and CD8+ T cells in mixed-cell populations isolated from (B) GI and (C) LN samples was determined by FACS analysis. Results for second set are shown before SHIV challenge and after 6 months; averages from both sets are shown in supplemental Figure 2 in addition to time points taken at days 7 and 21 post-SHIV challenge.
mC46-expressing macaques maintain gene-modified SHIV-specific CD4+ T cells
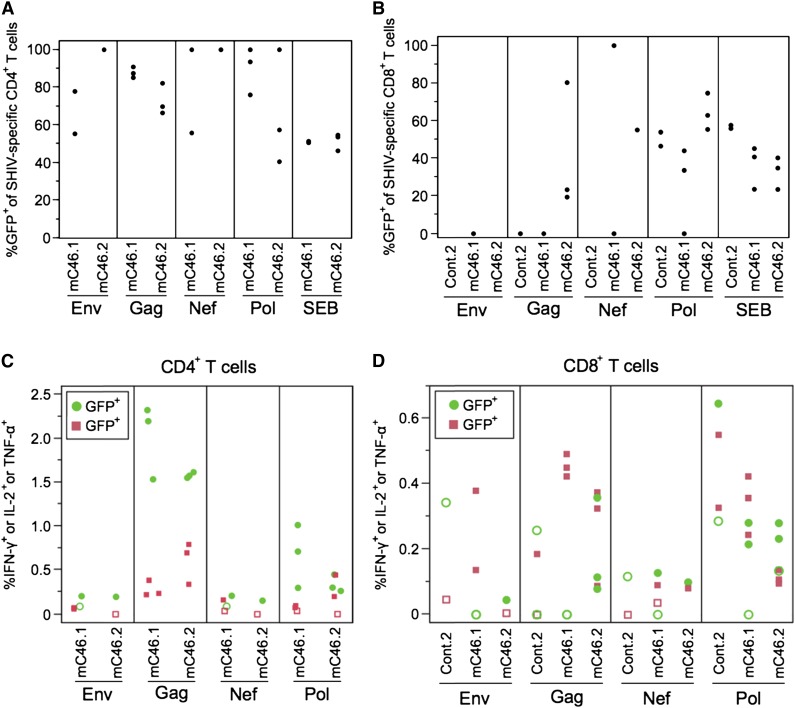
To identify potential mechanisms involved in the recovery of unmodified CD4+ T cells in the presence of gene-modified cells, antigen-specific immune responses were examined. As demonstrated in Figure 1, although CD4+ T-cell levels decreased rapidly following the acute phase of infection, a gradual rebound in CD4+ T-cell levels was observed in the mC46 macaques. This led to the hypothesis that mC46-expressing macaques may maintain elevated levels of SHIV-specific CD4+ T cells, thereby leading to an enhanced immunological response against the challenge virus. To test this hypothesis, autologous PBMCs collected at specific time points throughout this study were stimulated in the presence of peptide pools corresponding to SIV-Gag, -Pol, -Nef, and HIV89.6-Env. Consistent with the near absence of CD4+ T cells in peripheral blood, no antigen specific CD4+ T-cell responders were detected in the control macaques. However, in mC46 macaques, significant levels of CD4+ T-cell responders were detected by FACS following intracellular staining (Figure 4A-C). In particular, Gag and Pol peptides elicited robust immunological responses as indicated by the relatively high percentage of responders in the CD4+ T-cell population. Upon further examination of SHIV-specific CD4+ T-cell responders, it was determined that the vast majority of responders were gene-modified (Figure 4A-C). In some instances, 100% of the responders were gene-modified, whereas, overall, ∼85% of SHIV-specific responders were gene-modified. These results indicate that SHIV-specific, infection-resistant CD4+ T cells are maintained throughout the course of infection as no recovery of unmodified SHIV-specific, CD4+ T-cell responders was observed at any of the time points examined.
Figure 4.
SHIV-specific, mC46-expressing CD4+ T cells are maintained in mC46-expressing macaques. (A-B) PBMC isolated from postchallenge macaques were stimulated with SHIV peptide for 6 hours and SHIV-specific cells were identified using intracellular cytokine staining. Boolean gating was used to identify the frequencies of CD4+ (A) and CD8+ (B) cells expressing 1 or more of the following cytokines: IFN-γ, IL-2, and tumor necrosis factor (TNF)-α. To calculate percentages, all negative values after background subtracting were made equal to 0. CD4+ T-cell responders from control (cont.)2 data were omitted because all data failed the count >1000 filter. (C-D) The same Boolean gating data were used to observe the frequencies of SHIV-specific CD4+ and CD8+ cells within the GFP+ and GFP− subsets. Frequencies of GFP+ cells are displayed as green circles; frequencies of GFP− cells are displayed as red squares; data that failed positivity calls are depicted as open symbols. One to 3 samples per macaque was examined per peptide pool depending on viability and total number of gated CD4+ or CD8+ T cells. Cryopreserved samples from 2, 4, and 6 months after SHIV challenge were used for these experiments.
As expected, no selective advantage was observed in CD8+ T cells following SHIV challenge (Figure 4B). To determine if the increase in CD4+ T-cell levels in the mC46 macaques may enhance the overall cytotoxic T lymphocyte (CTL)-mediated immune responses against SHIV antigens, IFN-γ enzyme-linked immunospot assays were conducted by combining freshly isolated peripheral blood mononuclear cells (PBMCs) with autologous skin fibroblasts transduced with lentiviral vectors expressing SIVMAC239-GAG or HIV89.6-Env. As expected, IFN-γ production was detected in both control and mC46 macaques (supplemental Figure 4). However, a more robust T-lymphocyte response was observed in the C46 macaques. A 5.3- to 5.4-fold increase in IFN-γ production was detected in the mC46 macaques, whereas a modest 2.1- and 2.2-fold increase was detected in PBMCs isolated from the control macaques. Although these results do not indicate direct killing of antigen-expressing target cells, these results warranted further investigation and characterization of the T-lymphocyte immune responses against the challenge virus. To quantify the number of SHIV-specific CD8+ T cells in peripheral blood samples, PBMCs were cocultured with peptide pools as previously indicated. Overall, the percentage of CD8+ T-cell responders against SIV antigens were higher and broader in mC46 macaques based on our exclusion criteria detailed in the “Materials and methods” section (Figure 4D and supplemental Figures 5 and 6). Overall, these results indicate that there is a significant difference in the generation and presence of SHIV-specific CD4+ T cells between mC46 macaques and control macaques.
mC46 macaques exhibit enhanced SHIV-specific B-lymphocyte function
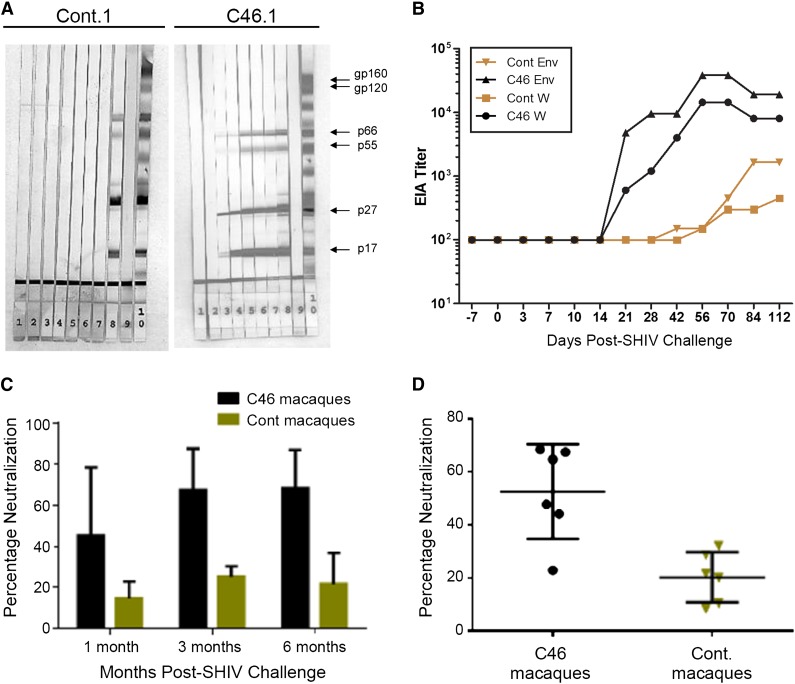
Previous studies have demonstrated a relatively inept antibody response in naive pigtailed macaques challenged with SHIV89.6P-MN.26,27 As indicated in Figure 5A, serum samples obtained from mC46.1, which were incubated with strips containing SIV lysates, could readily be used to detect viral antigens (see supplemental Figure 7 for results from second set of macaques). Using an ELISA-based method, a significant increase in antibody titers directed against the whole SIVmac239 and HIV89.6 Env (>105) was detected 28 days after SHIV challenge in the mC46 macaque (Figure 5B). In contrast, a limited increase in SIV-specific antibody titers was detected by ELISA in the control macaques. To determine whether the increase in antibody production resulted in increased neutralization of viral infectivity, serum samples collected at 1, 3, and 6 months post-SHIV challenge were incubated with HIV89.6 pseudotyped lentiviral vectors encoding GFP. These samples were added to TZMbl cells and, after 48 hours, FACS analysis was performed to determine the percentage neutralization. As indicated in Figure 5C-D, neutralizing activity was significantly enhanced in mC46-expressing macaques at each time point and over the duration of these studies. Six months following SHIV challenge, neutralizing activity increased by >4-fold in mC46 macaques vs controls. Based on the detection of enhanced neutralizing antibody responses and improved SHIV-specific lymphocyte function, we conclude that mC46-expressing macaques exhibit substantially improved immunological responses against the challenge virus.
Figure 5.
mC46-fusion inhibitor enhances B-lymphocyte responses against HIV/SIV antigens. (A) Western blot analysis was used to visualize the production of anti-SHIV antibodies in the control (left) and mC46-macaque (right) using premade strips from SIVmac239 lysates. Serum samples and control strips are as follows: lanes 1-7, serum samples from weeks 0, 2, 4, 6, 8, 10, and 12; lane 8, SIVmac239-positive control; lane 9, negative control; lane 10, SHIV positive control. (B) ELISA was used to determine the antibody titer against whole SIV and HIV Env (mC46 macaques: circles and triangle; control [cont.] macaques: squares and diamonds). (C-D) Diluted serum samples collected at 1, 3, and 6 months after SHIV challenge were incubated with VSV-G or HIV89.6 env pseudotyped lentiviral vectors encoding GFP for 1.5 hours at 37°C before addition to TZMbl cells. FACS analysis was performed 48 hours following transduction. Antibody titer and neutralization assays were performed in duplicate for each serum sample. (C-D) Results shown are averages observed for controls and mC46 macaques (C: P < .0137; D: P < .0035). Two-tailed t test was used for statistical analysis between each subset (P < .05 is considered significant). Western blot analysis for the second set of macaques is shown in supplemental Figure 7.
Discussion
Here, we demonstrate that autologous transplantation of genetically modified HIV/SHIV-protected HSCs results in in vivo protection of mC46-expressing CD4+ T cells and reduced viral pathogenesis in a clinically relevant nonhuman primate model of AIDS. During the acute phase of infection, a pronounced selective advantage of gene-modified CD4+ T cells was observed in all tissues examined. Unlike previous findings in murine models, our study is the first to show a significant recovery of unmodified, unprotected CD4+ T cells, which resulted in close to normal CD4+ T-cell levels in the mC46-protected macaques. We observed an enhanced immune response with maintenance of SHIV-specific CD4+ T cells and significantly improved CTL and antibody responses directed against the SHIV challenge virus in the mC46-expressing macaques. Furthermore, disease progression was not observed in the mC46 macaques in the absence of antiretroviral treatment, whereas control macaques developed AIDS with CD4+ T-cell levels declining rapidly following the acute phase of infection.
The initial drop in CD4+ T-cell levels following acute infection was anticipated because the vast majority of CD4+ T cells residing within the gut-associated lymphoid tissue undergo apoptosis either by direct infection or due to bystander-mediated apoptosis.28-31 Although mC46 expression would limit infection of gene-modified CD4+ T cells, these cells are still susceptible to activation-induced cell death and pro-apoptotic ligands. Nonetheless, the rapid rebound in mC46-expressing CD4+ T-cell levels observed shortly after the initial drop in CD4+ T-cell counts suggests that these protected cells may play an important role not only in the development of an adaptive immune response, but also in the expansion of CD4+ T-cell populations following the acute phase of infection. Interestingly, our analysis further indicated that both mC46-expressing macaques maintained elevated levels of pro-T lymphocyte expansion cytokines, including interleukin (IL)-12, which is critical for the development of Th1 cell–mediated immune responses (supplemental Figure 8). These findings suggest that, in addition to increased CD4+ T-cell function, the functional activity of other potential target cells may also be elevated because IL-12 is typically produced by phagocytic and antigen- presenting cells including dendritic cells and macrophages.32 In addition, the maintenance of protected CD4+ T cells may further contribute to IL-12 production through T-cell–APC interactions.
Typically, HIV-specific CD4+ T cells are rapidly depleted because of antigen-dependent activation and subsequent heightened susceptibility to infection.33 To date, the possible benefits of rendering CD4+ T cells resistant to HIV infection during the course of infection remains largely unknown. Because of their central role in the development and maintenance of an adaptive immune response, CD4+ T-cell depletion causes widespread immune dysregulation.34 In addition to CD4+ T cells playing a central role in the initial acute phase of viral infection, they are also critical for maintaining functionality and diversity of CTL responses during chronic stages of disease.35 The results obtained in this study demonstrate that both SHIV-specific CD8+ and CD4+ T cells are maintained over the course of infection and that both populations are readily detectable at higher frequencies in macaques transplanted with autologous mC46-expressing HSCs than in control animals.
Antigen-specific CD4+ T cells are likely to play multiple and essential roles in the postinfection treatment of HIV+ patients. First, antigen-specific CD4+ T cells may rescue immune exhausted CD8+ T cells, as was previously demonstrated in a chronic cytomegalovirus murine model.36 The reversal of CD8+ T-cell exhaustion was shown to significantly enhance CTL activity as measured by direct killing of infected cells, increased secretion of antiviral cytokines, and a significant decrease in viral load. Second, the presence of antigen-specific CD4+ T cells has been shown to lead to the development of virus-specific memory CD8+ T cells.36 Finally, CD4+ T cells are indispensable in the differentiation of naive B cells into plasma cells that secrete high-affinity virus-specific antibodies.37 In our studies, a strong, early SHIV-specific antibody response was generated against viral antigens, which was subsequently shown to lead to an enhanced neutralization of Env89.6-pseudotyped lentiviruses. These findings predict that the presence of SHIV-specific CD4+ T cells may enable the development of broadly neutralizing antibodies.
Recent data obtained from research on elite controllers and long-term nonprogressors have suggested that HIV-specific CD4+ T-cell function is associated with disease control and the enhanced capacity of HIV-specific CTLs to suppress viral replication.38-40 Hence, the development of HIV/SHIV-resistant CD4+ T-cell populations may lead to the establishment of a functional immune system capable of controlling viral replication in the absence of antiretroviral therapy. Following the transplant of gene-modified cells into a previously infected macaque, it is unclear how the development of a functional immune response will affect viral reservoirs, which typically appear significantly reduced in natural controllers. Several key findings including the significant decrease in viral titers and the gradual, yet substantial increase of unmodified CD4+ T cells were not observed in the mC46/humanized murine model, indicating the importance of conducting similar studies in an autologous nonhuman primate model.13
Previous vaccination studies conducted in pigtailed macaques have also reported recovery of CD4 T cells.25,41,42 Thus, we propose that mC46-mediated protection of CD4+ T cells and other immune cells is a primary mechanism leading to a heightened immune response against the challenge virus. Hence, HSC transplantation of genetically modified cells expressing the mC46-fusion inhibitor gives rise to infection-resistant immune cells that initiate an enhanced immune response against the challenge virus. Importantly, phase 1 clinical trials have previously shown that mC46 expression of gene-modified CD4+ T cells is well-tolerated in HIV+ patients.43
Although a limited number of animals were used in this study, the results indicate that mC46 infection-resistant, HSC-derived CD4+ T cells (and presumably other potential target cells) are resistant to infection in vivo and may lead to an enhanced SHIV-specific immune response resulting in reduced plasma viremia and decreased viral pathogenesis. Although it has been reported that a limited number of rhesus macaques may spontaneously control plasma viremia because of a number of inherent factors, previous studies in pigtailed macaques have indicated that natural controllers are far less frequent (approximately 1 in 10 macaques),22,41 in particular when animals are challenged with SHIV89.6P-MN.44 As determined in both experimental macaques, the vast majority of SHIV-specific CD4+ T cells were indeed gene-modified cells, which is a clear indication of selective advantage of mC46-expressing CD4+ T cells. To our knowledge, this is the first study in a clinically relevant animal model of AIDS to demonstrate the possible benefits of genetically modified, autologous HSCs to treat HIV+ patients.
Supplementary Material
Acknowledgments
The authors thank Veronica Nelson, Christina Ironside, Heather Mack, Taryn Urion, Ryan Wallerstedt, Leon Flanary, Joel Ahrens, LaRene Kuller, Randy Mclain, and Dr Michael Agy for technical support and Helen Crawford, Laura Farren, and Bonnie Larson for their help in preparing this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
The authors are grateful for research funding from the National Institutes of Health (R01 AI080326, U19 AI096111, R01 HL098489, P30 DK056465, and P51 RR00016). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. In addition, H.-P.K. is a Markey Molecular Medicine investigator and the recipient of the Jose Carreras/E.D. Thomas Chair for Cancer Research. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: SHIV 89.6P env Peptides Complete Set, SIVmac 239 Gag (15-mer) Peptides - Complete Set, SIVmac239 Pol (15-mer) Peptides - Complete Set, and SIVmac239 Full Length Nef (15-mer) Peptides - Complete Set.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.-P.K. is the principal investigator of the study and drafted the manuscript with P.M.Y.; P.M.Y., P.P., G.D.T., S.-L.H., and H.-P.K. designed and/or coordinated the animal studies; P.M.Y., J.P.K., C.W.P., N.J.M., N.P.W., and O.H. contributed substantially to data collection and analysis; and P.M.Y., D.V.L., M.P., B.C.B., and S.D. contributed substantially to study conception and design and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for G.D.T. is Washington State University, Pullman, WA.
Correspondence: Hans-Peter Kiem, Fred Hutchinson Cancer Research Center, Mail Stop D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: hkiem@fhcrc.org.
References
- 1.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292(2):251–265. doi: 10.1001/jama.292.2.251. [DOI] [PubMed] [Google Scholar]
- 2.Carr A. Toxicity of antiretroviral therapy and implications for drug development [Review]. Nat Rev Drug Discov. 2003;2(8):624–634. doi: 10.1038/nrd1151. [DOI] [PubMed] [Google Scholar]
- 3.Louie M, Hogan C, Di Mascio M, et al. Determining the relative efficacy of highly active antiretroviral therapy. J Infect Dis. 2003;187(6):896–900. doi: 10.1086/368164. [DOI] [PubMed] [Google Scholar]
- 4.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277(5322):112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 5.Autran B. Strategies toward restoration of immunity to HIV. AIDS. 2002;16(10):4–6. [PubMed] [Google Scholar]
- 6.Hirsch MS, Brun-Vézinet F, Clotet B, et al. Antiretroviral drug resistance testing in adults infected with human immunodeficiency virus type 1: 2003 recommendations of an International AIDS Society-USA Panel. Clin Infect Dis. 2003;37(1):113–128. doi: 10.1086/375597. [DOI] [PubMed] [Google Scholar]
- 7.Eshleman SH, Krogstad P, Jackson JB, et al. Analysis of human immunodeficiency virus type 1 drug resistance in children receiving nucleoside analogue reverse-transcriptase inhibitors plus nevirapine, nelfinavir, or ritonavir (Pediatric AIDS Clinical Trials Group 377). J Infect Dis. 2001;183(12):1732–1738. doi: 10.1086/320728. [DOI] [PubMed] [Google Scholar]
- 8.Smith KA. To cure chronic HIV infection, a new therapeutic strategy is needed [Review]. Curr Opin Immunol. 2001;13(5):617–624. doi: 10.1016/s0952-7915(00)00270-3. [DOI] [PubMed] [Google Scholar]
- 9.Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 10.Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117(10):2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 11.van Lunzen J, Fehse B, Hauber J. Gene therapy strategies: can we eradicate HIV? Curr HIV/AIDS Rep. 2011;8(2):78–84. doi: 10.1007/s11904-011-0073-9. [DOI] [PubMed] [Google Scholar]
- 12.Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28(8):839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimpel J, Braun SE, Qiu G, et al. Survival of the fittest: positive selection of CD4+ T cells expressing a membrane-bound fusion inhibitor following HIV-1 infection. PLoS ONE. 2010;5(8):e12357. doi: 10.1371/journal.pone.0012357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez EE, Wang J, Miller JC, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808–816. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases [Review]. Nat Rev Genet. 2010;11(9):636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 16.Carter CC, Onafuwa-Nuga A, McNamara LA, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16(4):446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egelhofer M, Brandenburg G, Martinius H, et al. Inhibition of human immunodeficiency virus type 1 entry in cells expressing gp41-derived peptides. J Virol. 2004;78(2):568–575. doi: 10.1128/JVI.78.2.568-575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trobridge GD, Wu RA, Beard BC, et al. Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection. PLoS ONE. 2009;4(11):e7693. doi: 10.1371/journal.pone.0007693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiem H-P, Wu RA, Sun G, von Laer D, Rossi JJ, Trobridge GD. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2010;17(1):37–49. doi: 10.1038/gt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trobridge GD, Beard BC, Gooch C, et al. Efficient transduction of pigtailed macaque hematopoietic repopulating cells with HIV-based lentiviral vectors. Blood. 2008;111(12):5537–5543. doi: 10.1182/blood-2007-09-115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doria-Rose NA, Pierce CC, Hensel MT, et al. Multigene DNA prime-boost vaccines for SHIV89.6P. J Med Primatol. 2003;32(4-5):218–228. doi: 10.1034/j.1600-0684.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Cleveland B, Klots I, et al. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J Virol. 2008;82(2):638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiem H-P, Heyward S, Winkler A, et al. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90(11):4638–4645. [PubMed] [Google Scholar]
- 24.Trobridge G, Beard BC, Kiem H-P. Hematopoietic stem cell transduction and amplification in large animal models. Hum Gene Ther. 2005;16(12):1355–1366. doi: 10.1089/hum.2005.16.1355. [DOI] [PubMed] [Google Scholar]
- 25.Hu SL, Zarling JM, Chinn J, et al. Protection of macaques against simian AIDS by immunization with a recombinant vaccinia virus expressing the envelope glycoproteins of simian type D retrovirus. Proc Natl Acad Sci USA. 1989;86(18):7213–7217. doi: 10.1073/pnas.86.18.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsburg E, Rose NF, Marx PA, et al. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78(8):3930–3940. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence [Review]. Curr HIV Res. 2008;6(5):388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434(7037):1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 29.Badley AD, Dockrell DH, Algeciras A, et al. In vivo analysis of Fas/FasL interactions in HIV-infected patients. J Clin Invest. 1998;102(1):79–87. doi: 10.1172/JCI2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badley AD, Parato K, Cameron DW, et al. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ. 1999;6(5):420–432. doi: 10.1038/sj.cdd.4400509. [DOI] [PubMed] [Google Scholar]
- 31.Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS [Review]. J Gen Virol. 2003;84(Pt 7):1649–1661. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- 32.Trinchieri G. The two faces of interleukin 12: a pro-inflammatory cytokine and a key immunoregulatory molecule produced by antigen-presenting cell [Review].; Ciba Found Symp; 1995. pp. 203–214. [DOI] [PubMed] [Google Scholar]
- 33.Brenchley JM, Ruff LE, Casazza JP, Koup RA, Price DA, Douek DC. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J Virol. 2006;80(14):6801–6809. doi: 10.1128/JVI.00070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perelson AS, Kirschner DE, De Boer R. Dynamics of HIV infection of CD4+ T cells. Math Biosci. 1993;114(1):81–125. doi: 10.1016/0025-5564(93)90043-a. [DOI] [PubMed] [Google Scholar]
- 35.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aubert RD, Kamphorst AO, Sarkar S, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA. 2011;108(52):21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonjić S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med. 1989;169(4):1199–1212. doi: 10.1084/jem.169.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porichis F, Kaufmann DE. HIV-specific CD4 T cells and immune control of viral replication [Review]. Curr Opin HIV AIDS. 2011;6(3):174–180. doi: 10.1097/COH.0b013e3283454058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5(5):518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 40.Poropatich K, Sullivan DJ., Jr Human immunodeficiency virus type 1 long-term non-progressors: the viral, genetic and immunological basis for disease non-progression [Review]. J Gen Virol. 2011;92(Pt 2):247–268. doi: 10.1099/vir.0.027102-0. [DOI] [PubMed] [Google Scholar]
- 41.Doria-Rose NA, Ohlen C, Polacino P, et al. Multigene DNA priming-boosting vaccines protect macaques from acute CD4+-T-cell depletion after simian-human immunodeficiency virus SHIV89.6P mucosal challenge. J Virol. 2003;77(21):11563–11577. doi: 10.1128/JVI.77.21.11563-11577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorelick RJ, Benveniste RE, Lifson JD, et al. Protection of Macaca nemestrina from disease following pathogenic simian immunodeficiency virus (SIV) challenge: utilization of SIV nucleocapsid mutant DNA vaccines with and without an SIV protein boost. J Virol. 2000;74(24):11935–11949. doi: 10.1128/jvi.74.24.11935-11949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Lunzen J, Glaunsinger T, Stahmer I, et al. Transfer of autologous gene-modified T cells in HIV-infected patients with advanced immunodeficiency and drug-resistant virus. Mol Ther. 2007;15(5):1024–1033. doi: 10.1038/mt.sj.6300124. [DOI] [PubMed] [Google Scholar]
- 44.Ho O, Larsen K, Polacino P, et al. Pathogenic infection of Macaca nemestrina with a CCR5-tropic subtype-C simian-human immunodeficiency virus. Retrovirology. 2009;6:65. doi: 10.1186/1742-4690-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.