Abstract
This study was performed to explore and compare the dosimetric variance caused by respiratory movement in the breast during forward-planned IMRT after breast-conserving surgery. A total of 17 enrolled patients underwent the 3DCT simulation scans followed by 4DCT simulation scans during free breathing. The treatment planning constructed using the 3DCT images was copied and applied to the end expiration (EE) and end inspiration (EI) scans and the dose distributions were calculated separately. CTV volume variance amplitude was very small (11.93 ± 28.64 cm3), and the percentage change of CTV volumes receiving 50 Gy and 55 Gy between different scans were all less than 0.8%. There was no statistically significant difference between EI and EE scans (Z =–0.26, P = 0.795). However, significant differences were found when comparing the Dmean at 3DCT planning with the EI and EE planning (P = 0.010 and 0.019, respectively). The homogeneity index at EI, EE and 3D plannings were 0.139, 0.141 and 0.127, respectively, and significant differences existed between 3D and EI, and between 3D and EE (P = 0.001 and 0.006, respectively). The conformal index (CI) increased significantly in 3D treatment planning (0.74 ± 0.07) compared with the EI and EE phase plannings (P = 0.005 and 0.005, respectively). The V30, V40, V50 and Dmean of the ipsilateral lung for EE phase planning were significantly lower than for EI (P = 0.001–0.042). There were no significant differences in all the DVH parameters for the heart among these plannings (P = 0.128–0.866). The breast deformation during respiration can be disregarded in whole breast IMRT. 3D treatment planning is sufficient for whole breast forward-planned IMRT on the basis of our DVH analysis, but 4D treatment planning, breath-hold, or respiratory gate may ensure precise delivery of radiation dose.
Keywords: breast neoplasms, intensity-modulated radiotherapy, four-dimensional computed tomography, dosimetric parameters
INTRODUCTION
Breast-conserving treatment (BCT) is the standard treatment for patients with early breast cancer, and radiotherapy is the important component of BCT. For radiotherapy of the breast, whole breast irradiation (WBI) is the basic mode [1–3]. Whole breast intensity-modulated radiotherapy (IMRT) increased the dose homogeneity to the treated breast, meanwhile improving normal tissue-sparing [4]. In addition, the long-term follow-up results showed that WBI delivered by IMRT not only decreased acute radiation dermatitis, but also decreased the late breast fibrosis, chronic pulmonary and cardiac toxicities [5]. As the IMRT dose distributions are highly conformal to the target, the target motions during setup and respiration may lead to a change in the dose distributions.
With the development of the image-guided online and offline setup verification and correction techniques, interfraction setup error has been reduced significantly during delivery of irradiation for breast cancer patients [6, 7]. However, respiration-induced motion during radiation delivery becomes one of the main geometrical uncertainties, which may affect the treatment accuracy. So the dosimetric variances of the target resulting from the intrafraction motion become relatively more significant.
For the measurement of respiration-induced motion in the breast target and the chest wall, passive markers placed on the skin (skin marker), and implanted clips placed in the lumpectomy cavity could be considered as the surrogates for the breast [8–11]. Several image-guided therapy techniques have been applied to breast radiotherapy, such as electronic portal image devices (EPID), cone beam computed tomography (CBCT), four-dimensional computed tomography (4DCT) combined with real-time position management system (RPM), and so on [9, 12, 13]. The respiration-induced motion amplitude of the breast during free breathing was generally < 4 mm in all directions [8, 9]. Chopra et al. [8] found that during normal breathing, breast movement judged by five surface markers was 1.07, 1.94 and 1.86 mm in the mediolateral, superoinferior and anteroposterior dimensions, respectively. Qi et al. [9] examined the respiratory motion for the target between the two extreme phases, and the maximum centroid movement ranged from 1.1–3.9 mm for the treated breast. The respiration-induced motion was small, but the effect of the dose distribution resulted from respiration-induced motion within the breast tissue and the organ at risk (OAR) were inconsistently reported [9, 14–17].
On the other hand, IMRT based on three-dimensional computed tomography (3DCT) simulation is the current main mode in most countries of the world. However, the variability of the specified dosimetric parameters between 4DCT and 3DCT IMRT planning for WBI is unknown. The purpose of this study was to evaluate the respiratory motion-induced change in the specified dosimetric parameters of the irradiated breast and OARs during free breathing, in the tangential field technique with static multileaf collimator segments (SMLC) IMRT planning, and to compare the specified dosimetric changes between 4DCT and 3DCT IMRT plannings.
MATERIALS AND METHODS
Patient selection and instruction
A total of 17 breast cancer patients who were prescribed to receive adjuvant whole breast radiotherapy (WBRT) after breast-conserving surgery were enrolled in this study between June 2009 and May 2011. Patients with restricted arm movement after surgery and poor pulmonary function were excluded. Of these 17 patients, 10 had right-sided breast cancer, and the remaining 7 had left-sided breast cancer. Written informed consents were obtained from all the patients, and the study was approved by the institutional research ethics board of the hospital.
3DCT and 4DCT data acquisition
The patients were immobilized in supine position on a breast board using an arm support (with both arms above the head to expose the breast adequately) and a knee support. The 3DCT and 4DCT data sets were acquired from the 17 patients on a 16-slice CT scanner (Philips Brilliance Bores CT, Netherlands) during free breathing. Three laser alignment lines were marked on the patient before CT acquisition.
The 3DCT scan, in which 12 contiguous slices with a thickness of 2 mm were produced per gantry rotation (1 s) and interval (1.8 s) between rotations was acquired in sequential mode, and the 4DCT scan was acquired in helical mode with the scanning pitch between 0.09 and 0.15. The respiratory signal was recorded with the Varian real-time position management (RPM) System (Varian Medical Systems, Palo Alto, CA), by measuring the displacement of the infrared markers placed on the epigastric region of the patient's abdomen. GE Advantage 4D software (GE Healthcare, Waukesha, WI) sorted the reconstructed 4DCT images into 10 respiratory phases labeled as 0–90% on the basis of triggered signal. Phase 0% denoted the maximum end inspiration (EI) and phase 50% denoted the maximum end expiration (EE). The 4DCT images were reconstructed using a thickness of 2 mm and then transferred to the Eclipse treatment planning system (TPS) (Eclipse 8.6, Varian Medical Systems, Palo Alto, CA) for structure delineation and treatment planning generation.
Treatment planning and dosimetric evaluation
The whole breast (CTV), ipsilateral lung (IPSL) and heart were delineated on the image data sets separately from 3DCT images, EE and EI phase of 4DCT images. The delineation of the CTV and OARs were done by one clinician using the same window and level setting. The CTV was delineated based on the visible glandular breast tissue seen on the CT images, taking into consideration the anatomic references, which were defined as medially at the sternal-rib junction, inferiorly at the inframammary fold, superiorly at the inferior edge of the medial head of the clavicle, and laterally at mid-axillary line typically. The anterior margin of the CTV was shrunk by 5 mm below the skin surface, and the posterior margin was the junction of the breast tissue and the pectoralis muscles. The planning target volume (PTV) was generated using a 5-mm margin around the CTV and shrunk by 5 mm below skin surface.
Treatment planning was established at the 3DCT images, using the tangential field technique with static multileaf collimator segments (SMLC) IMRT, with two parallel opposed tangential fields. To reduce the lung volume within the treatment field and the volumes of hot spots in the treatment field, 2–5 segmented fields were set up in each direction. The prescription dose was 50 Gy in 25 fractions (2 Gy per fraction) to the PTV using 6 MV photon beams, which was defined as the 90% isodose line. The criteria of the SMLC-IMRT planning was to ensure at least 95% of the PTV volume received the prescription dose. The segmented MLCs were manipulated to shield the areas of PTV receiving a dose >103% of the isodose line, and to keep the dose delivered to OARs such as the IPSL and heart within normally accepted tolerances.
The treatment planning designing based on the 3DCT images was copied and applied to the EI and EE phase images with the same gantry angles, collimator angles, primary field size, monitor units delivered per beam, and so on.
The dose distribution was calculated separately in all the three treatment plannings, and dose–volume histogram (DVH) parameters for the CTV, PTV, IPSL and heart were calculated for each treatment planning in all patients. The CTVs were evaluated on the basis of the volumes receiving 50 Gy and 55 Gy. The parameters such as mean dose (Dmean), homogeneity index (HI), and conformal index (CI) were evaluated in PTV. HI was defined as
in which D2 and D98 represent the dose covered 2% and 98% of the target volume, and DT is the prescription dose [18]. CI is defined as
where PTVref represents the volume of PTV that is covered by prescription dose, VPTV is defined as the planning target volume and Vref represents the volume enclosed by the prescribed isodose [19, 20]. OARs were evaluated using Dmean and the volumes receiving ≥ 5, 10, 20, 30, 40, or 50 Gy (V5, V10, V20, V30, V40, V50).
Statistical analysis
Statistical analysis was performed with the SPSS statistical analysis software package. The Wilcoxon Signed Ranks Test was used for each dosimetric parameter. Data were regarded as statistically significant at P < 0.05.
RESULTS
The mean CTV volume based on EI, EE and 3DCT images were 680.05 ± 251.55 cm3, 678.88 ± 256.28 cm3 and 668.12 ± 241.93 cm3, respectively. The mean volume of the maximum volumetric difference among the three CTVs of these patients was only 11.93 ± 28.64 cm3 (1.42 ± 3.79 %).
The mean percentage volume of CTV receiving 50 Gy was 98 ± 1.15%, 98.49 ± 1.07% and 98.76 ± 0.66% for EI, EE and 3DCT treatment planning, respectively. The percentage volume of CTV (mean ± SD) receiving 55 Gy in the EI, EE and 3DCT treatment planning was 7.90 ± 4.19%, 7.83 ± 4.18%, and 7.78% ± 4.02%, respectively. For the SMLC-IMRT technique, only a minor difference was observed: the percentage differences in the volume of CTV receiving 50 Gy and 55 Gy among different scans were all < 0.8%.
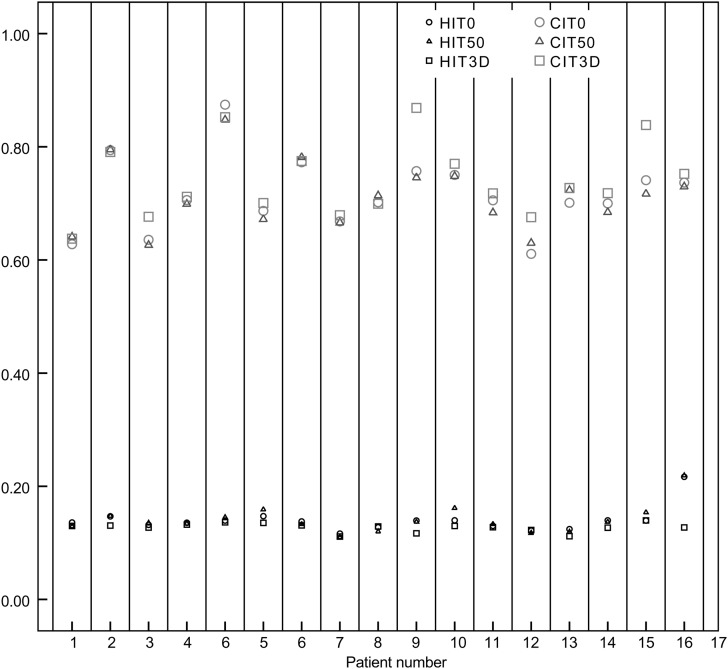
The mean PTV doses were 5303.95 cGy (SD: 32.36) and 5306.69 cGy (SD: 34.89) for the EI phase planning and EE phase planning, respectively (Z =–0.26, P = 0.795). However, the Dmean at 3DCT planning for PTV was 5314.12 cGy (SD: 27.97), and compared with the EI and EE phases, significant differences were found (Z =–2.58,–2.34; P = 0.010, 0.019). For the PTV, the variations in CI and HI for the 17 patients were shown in Fig. 1. The HI was 0.139 ± 0.02 for the EI phase planning and 0.141 ± 0.02 for the EE phase planning (Z =–0.02, P = 0.981). The CI was 0.716 ± 0.07 for the EI phase planning, and 0.712 ± 0.06 for the EE phase planning (Z =–0.17, P = 0.287). There was no significant difference between the EE and EI phase plannings for either CI or HI. Significant differences did exist for the HI between the 3DCT planning (0.127 ± 0.01) and the EI and EE phase plannings (Z =–3.34,–2.77; P = 0.001, 0.006). The CI increased significantly more in 3DCT scan planning (0.74 ± 0.07) compared with the EI and EE phase plannings (Z =–2.817,–2.817; P = 0.005, 0.005).
Fig. 1.
The homogeneity index (HI) and conformal index (CI) for the 17 patients in the three different treatment plannings.
Table 1 showed the variation of the specified dose and volume parameters for the ipsilateral lung and heart for the three treatment plannings. A comparison of these parameters for the treatment planning based on the three different CT images is detailed in Table 2. In our observation, during free respiration cycle, changes in the lung volume followed respiratory patterns and the smaller lung volume was most likely to be associated with a lower dose to the IPSL. For the percent volume receiving a high dose (V30, V40 and V50), significant differences were observed between EE phase and EI phase plannings. Average lung volume varied by up to 8.72%, which suggested that the IPSL volume change during respiration might needed to be considered. For the 7 left-sided breast cancer patients, no differences in the percentage volume receiving the specified dose and Dmean were observed between the EI, EE and 3DCT plannings, and the heart volume varied by only ≤ 2.68% during the whole respiration cycle.
Table 1.
Dosimetric and volume parameters of the ipsilateral lung and heart for each CT scan planning
| V5 (%) | V10 (%) | V20 (%) | V30 (%) | V40 (%) | V50 (%) | Dmean cGy | V cm3 | |
|---|---|---|---|---|---|---|---|---|
| IPSL | ||||||||
| EI | 31.45 ± 4.38 | 22.60 ± 4.55 | 17.97 ± 4.62 | 15.47 ± 4.55 | 12.67 ± 4.46 | 3.69 ± 3.02 | 1016.39 ± 211.35 | 1204.09 ± 275.45 |
| EE | 31.39 ± 4.63 | 22.40 ± 4.65 | 17.56 ± 4.73 | 14.98 ± 4.69 | 12.13 ± 4.56 | 3.15 ± 3.08 | 997.60 ± 217.72 | 1099.14 ± 280.12 |
| 3D | 31.39 ± 4.55 | 22.50 ± 4.69 | 18.04 ± 4.47 | 15.30 ± 4.69 | 12.45 ± 4.66 | 3.54 ± 2.98 | 1010.96 ± 219.67 | 1178.79 ± 282.13 |
| HT | ||||||||
| EI | 24.08 ± 7.13 | 17.74 ± 7.25 | 14.59 ± 6.22 | 12.42 ± 5.32 | 10.44 ± 5.82 | 4.92 ± 3.04 | 858.57 ± 285.89 | 518.37 ± 99.49 |
| EE | 25.02 ± 7.12 | 18.44 ± 7.19 | 15.16 ± 6.24 | 12.91 ± 5.39 | 10.86 ± 4.94 | 5.11 ± 3.22 | 887.74 ± 287.67 | 505.97 ± 99.23 |
| 3D | 24.29 ± 6.57 | 17.82 ± 6.80 | 14.69 ± 6.00 | 12.53 ± 5.27 | 10.53 ± 4.87 | 5.03 ± 3.31 | 864.21 ± 276.97 | 504.49 ± 90.33 |
IPSL = ipsilateral lung, HT = heart, EI = end inspiration, EE = end expiration.
Table 2.
Dose and volume evaluation of the ipsilateral lung and heart for three different plannings
| Parameters | EI-EE |
EI-3D |
EE-3D |
||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | Z | P value | mean | Z | P value | mean | Z | P value | |
| IPSL | |||||||||
| V5 (%) | –0.04 ± 0.90 | −0.81 | 0.421 | −0.07 ± 1.21 | –1.42 | 0.887 | 0.11 ± 1.24 | –0.26 | 0.795 |
| V10 (%) | 0.20 ± 0.75 | 0.639 | 0.523 | 0.11 ± 1.14 | –1.87 | 0.061 | –0.09 ± 1.26 | –0.24 | 0.813 |
| V20 (%) | 0.41 ± 0.69 | –1.87 | 0.061 | –0.07 ± 0.89 | –0.24 | 0.813 | –0.48 ± 0.95 | –1.87 | 0.061 |
| V30 (%) | 0.49 ± 0.66 | –2.53 | 0.011 | 0.17 ± 1.03 | 0.54 | 0.586 | –0.32 ± 1.11 | –1.54 | 0.124 |
| V40 (%) | 0.54 ± 0.67 | –2.72 | 0.006 | 0.22 ± 0.94 | –1.32 | 0.187 | –0.32 ± 1.05 | –1.35 | 0.177 |
| V50 (%) | 0.54 ± 0.48 | –3.36 | 0.001 | 0.15 ± 0.49 | –0.19 | 0.052 | –0.39 ± 0.55 | –2.49 | 0.013 |
| Dmean (cGy) | –18.78 ± 32.50 | –1.87 | 0.042 | 5.43 ± 49.90 | –0.142 | 0.887 | –13.35 ± 54.27 | 1.30 | 0.193 |
| V (cm3) | 104.95 ± 95.64 | 4.53 | 0.000 | 25.29 ± 107.35 | 0.97 | 0.346 | –79.65 ± 50.24 | –6.54 | 0.000 |
| HT | |||||||||
| V5 (%) | –1.04 ± 1.38 | –1.52 | 0.128 | –0.21 ± 0.96 | 1 | 0.612 | 0.82 ± 1.09 | 1.52 | 0.128 |
| V10 (%) | –0.70 ± 1.18 | –1.18 | 0.237 | –0.07 ± 0.88 | –0.17 | 0.866 | 0.63 ± 1.11 | –1.52 | 0.128 |
| V20 (%) | –0.57 ± 1.03 | –1.18 | 0.237 | –0.10 ± 0.73 | –0.34 | 0.735 | 0.46 ± 0.99 | –1.52 | 0.128 |
| V30 (%) | –0.50 ± 0.94 | –1.18 | 0.237 | –0.11 ± 0.67 | –0.34 | 0.735 | 0.39 ± 0.92 | –1.35 | 0.176 |
| V40 (%) | –0.42 ± 0.83 | –1.18 | 0.237 | –0.09 ± 0.66 | –0.42 | 0.672 | 0.33 ± 0.88 | –1.35 | 0.176 |
| V50 (%) | –0.19 ± 0.60 | –1.01 | 0.672 | –0.11 ± 0.58 | –0.42 | 0.672 | –0.08 ± 0.62 | –0.34 | 0.735 |
| Dmean (cGy) | –29.17 ± 50.00 | –1.18 | 0.237 | –5.64 ± 37.47 | –0.34 | 0.735 | 23.53 ± 48.26 | –1.35 | 0.176 |
| V (cm3) | 12.40 ± 29.07 | 1.23 | 0.302 | 13.89 ± 26.28 | 1.40 | 0.212 | 1.49 ± 13.10 | 0.30 | 0.774 |
DISCUSSION
For whole breast IMRT, the dose distribution was highly conformal to the target in the treatment planning, therefore, part of the target could move out of the treatment field due to patient breathing. Volume variance of the breast treated is one of the geometrical uncertainties affecting treatment. In addition, a small breathing motion may lead to a large change in the dose distribution. There was a small difference between the EI, EE and 3DCT scans in terms of the breast volume, and the mean volume variation of the maximum volumetric difference was generally within 1.42 ± 3.79%. Previous study has suggested that the intraobserver variability in breast target volume delineation decreases according to a standard contouring protocol [21]. In our study, all the delineations were performed by one radiation oncologist referring to the same detailed delineation criteria. Breast volume did not show a significant change throughout free respiration, which indicated that the breast deformation during respiration could be ignored for WBI. Qi et al. [9] reported that, for a series of 18 patients, during normal breathing, the dosimetric impact of respiratory motion was clinically insignificant, with the exception of internal mammary nodes. In our investigation, although there was no significant difference between EE and EI plannings for the Dmean of PTV, significant differences were found between 3DCT planning and EI, and between 3DCT and EE phase plannings. These differences may derive from the variations in the treatment margin, the technique adopted in our study and the non-linear trajectories due to hysteresis during CT datasets acquisition. For the PTV, a 5-mm margin in all directions was added to the CTV and the whole breast treated with SMLC-IMRT. Whereas Qi et al. used conventional 3D planning with tangential beams for the whole breast and boost to the lumpectomy bed PTV. We also demonstrated statistically significant differences between 3DCT planning and EI/EE plannings in CI and HI for PTV. During treatment, the decrease of the target dose homogeneity significantly increased acute skin toxicity [22]. In addition, compared with conventional radiotherapy, hypofractionated whole breast irradiation (HF-WBI) which increasing the prescription dose per fraction delivered has been investigated recently [23–25]. The dose inhomogeneities and inconformities in irradiated volumes strongly influence radiation-induced toxicities, hence the target position during treatment with HF-WBI has to be determined with high precision.
Some studies have found that segmented and wedged IMRT planning were not sensitive to breathing motion, which suggested that 4D treatment planning was not required for daily clinical practice in postoperative segmented or wedged radiotherapy after breast-conserving surgery [15–17]. We explored the different dosimetric impact of treatment planning based on conventional 3DCT scans and the two extreme phases of 4DCT scans. In our study, we have verified that respiratory motion-induced CTV volume variance is minimal; in addition, for the mean PTV dose, a significant difference between the 3DCT scans and both the two extreme scans (EE and EI) was found. This might be induced by the fact that the conventional scans were composed of all phases of the respiratory cycle collected in the fixed interval. Another possible explanation was, in spite of the good compliance and active participation of all patients, the different scanning methods and the target envelope variance during the respiratory cycle might be considered to be the significant relevant factors. For the HI and CI, the differences between the 3DCT planning and EI/EE phase plannings also supported our conclusions that during free breathing dose distributions and target coverage during the intrafraction treatment might be affected by the scanning mode of the simulation CT and the choice of the treatment planning CT. There were significant differences in statistics for some of the PTV parameters between 3DCT and EI, and between 3DCT and EE, but the small absolute differences indicated that the conventional 3D treatment planning using the tangential field technique with SMLC was sufficient for daily radiotherapy, but could decrease the precision of IMRT.
The use of IMRT for whole breast treatment can minimize the volume of lung and heart being irradiated and decrease the acute and late radiation reaction. The volume, V30, V40, V50 and Dmean for IPSL in EI phase were all higher than in EE phase, which showed that although the target movement was small during free breathing, the thorax expansion was significant between the extreme respiratory phases, and thus induced the dose variation for the ipsilateral lung.
The respiratory motion-induced dose variations for heart were not statistically significant, and the absolute difference was rather small. This is, in part, due to the intrinsic cardiac contractions. Qi et al. [9] showed the heart normal tissue complication probability (NTCP) was < 10% for the patients calculated, with approximately 1% of NTCP variations respiratory motion-induced. During free breathing, the dosimetric impact of respiratory motion is clinically insignificant for heart. It should be noted that these findings may not necessarily be extrapolated to all patients due to the minimal number of patients enrolled in this study.
CONCLUSION
Conventional 3D-treatment planning using the tangential field technique with SMLC is sufficient for daily radiotherapy, because 3DCT images are taken disregarding the respiratory cycle during free breathing. The variance of the ipsilateral lung volume receiving high dose irradiation and mean lung dose (MLD) between the EE and EI phase plannings showed that 4D-treatment planning with consideration of breathing motion, breath-hold or respiratory gate would achieve a high-precision treatment delivery, especially for the patients with an irregular breathing pattern.
FUNDING
This work was supported by the National Natural Science Foundation of China (Grant No. 30870742).
REFERENCES
- 1.Sautter-Bihl ML, Budach W, Dunst J, et al. DEGRO practical guidelines for radiotherapy of breast cancer I: breast-conserving therapy. Strahlenther Onkol. 2007;183:661–6. doi: 10.1007/s00066-007-1811-1. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–7. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 4.Kestin LL, Sharpe MB, Frazier RC, et al. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: initial clinical experience. Int J Radiat Oncol Biol Phys. 2000;48:1559–68. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 5.McDonald MW, Godette KD, Butker EK, et al. Long-term outcomes of IMRT for breast cancer: a single-institution cohort analysis. Int J Radiat Oncol Biol Phys. 2008;72:1031–40. doi: 10.1016/j.ijrobp.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 6.White EA, Cho J, Vallis KA, et al. Cone beam computed tomography guidance for setup of patients receiving accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2007;68:547–54. doi: 10.1016/j.ijrobp.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 7.Topolnjak R, Vroegindeweij CV, Sonke JJ, et al. Breast-conserving therapy: radiotherapy margins for breast tumor bed boost. Int J Radiat Oncol Biol Phys. 2008;72:941–8. doi: 10.1016/j.ijrobp.2008.06.1924. [DOI] [PubMed] [Google Scholar]
- 8.Chopra S, Dinshaw KA, Kamble R, et al. Breast movement during normal and deep breathing, respiratory training and set up errors: implications for external beam partial breast irradiation. Br J Radiol. 2006;79:766–73. doi: 10.1259/bjr/98024704. [DOI] [PubMed] [Google Scholar]
- 9.Qi XS, White J, Rabinovitch R, et al. Respiratory organ motion and dosimetric impact on breast and nodal irradiation. Int J Radiat Oncol Biol Phys. 2010;78:609–17. doi: 10.1016/j.ijrobp.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 10.Penninkhof J, Quint S, Boer H, et al. Surgical clips for position verification and correction of non-rigid breast tissue in simultaneously integrated boost (SIB) treatments. Radiother Oncol. 2009;90:110–5. doi: 10.1016/j.radonc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell J, Formenti SC, De Wyngaert JK. Interfraction and intrafraction setup variability for prone breast radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:1571–7. doi: 10.1016/j.ijrobp.2009.07.1683. [DOI] [PubMed] [Google Scholar]
- 12.Fatunase T, Wang Z, Yoo S, et al. Assessment of the residual error in soft tissue setup in patients undergoing partial breast irradiation: results of a prospective study using cone-beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;70:1025–34. doi: 10.1016/j.ijrobp.2007.07.2344. [DOI] [PubMed] [Google Scholar]
- 13.Topolnjak R, Sonke JJ, Nijkamp J, et al. Breast patient setup error assessment: comparison of electronic portal image devices and cone-beam computed tomography matching results. Int J Radiat Oncol Biol Phys. 2010;78:1235–43. doi: 10.1016/j.ijrobp.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Moeckly SR, Lamba M, Elson HR. Respiratory motion effects on whole breast helical tomotherapy. Med Phys. 2008;35:1464–75. doi: 10.1118/1.2841936. [DOI] [PubMed] [Google Scholar]
- 15.Richter A, Sweeney R, Baier K, et al. Effect of breathing motion in radiotherapy of breast cancer: 4D dose calculation and motion tracking via EPID. Strahlenther Onkol. 2009;185:425–30. doi: 10.1007/s00066-009-1980-1. [DOI] [PubMed] [Google Scholar]
- 16.Smith RP, Bloch P, Harris EE, et al. Analysis of interfraction and intrafraction variation during tangential breast irradiation with an electronic portal imaging device. Int J Radiat Oncol Biol Phys. 2005;62:373–8. doi: 10.1016/j.ijrobp.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita R, Shimizu S, Taguchi H, et al. Three-dimensional intrafractional motion of breast during tangential breast irradiation monitored with high-sampling frequency using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys. 2008;70:931–4. doi: 10.1016/j.ijrobp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, Mohan R, Morris M, et al. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys. 2003;56:573–85. doi: 10.1016/s0360-3016(02)04617-5. [DOI] [PubMed] [Google Scholar]
- 19.Baltas D, Kolotas C, Geramani K, et al. A conformal index (COIN) to evaluate implant quality and dose specification in brachytherapy. Int J Radiat Oncol Biol Phys. 1998;40:515–24. doi: 10.1016/s0360-3016(97)00732-3. [DOI] [PubMed] [Google Scholar]
- 20.Oozeer R, Chauvet B, Garcia R, et al. Dosimetric evaluation of conformal radiotherapy: conformity factor. Cancer Radiother. 2000;4:207–16. doi: 10.1016/s1278-3218(00)89096-4. [DOI] [PubMed] [Google Scholar]
- 21.Hurkmans CW, Borger JH, Pieters BR, et al. Variability in target volume delineation on CT scans of the breast. 2001;50:1366–72. doi: 10.1016/s0360-3016(01)01635-2. [DOI] [PubMed] [Google Scholar]
- 22.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–92. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 23.Fehlauer F, Tribius S, Alberti W, et al. Late effects and cosmetic results of conventional versus hypofractionated irradiation in breast-conserving therapy. Strahlenther Onkol. 2005;181:625–31. doi: 10.1007/s00066-005-1404-9. [DOI] [PubMed] [Google Scholar]
- 24.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–20. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 25.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]