Abstract
Esophageal cancer patients are often associated with multiple primary cancers (MPC). The aim of this study is to evaluate the effect of MPC on prognosis in esophageal cancer patients treated by radiotherapy. Between 2001 and 2008, esophageal cancer patients treated by definitive radiotherapy at Gunma Cancer Center were retrospectively reviewed. Exclusion criteria were preoperative or postoperative radiotherapy, palliative radiotherapy, follow-up of <6 months, radiation dose of <50 Gy and no information on MPC. We analyzed 167 esophageal cancer patients and 56 (33.5%) were associated with MPC. Gastric cancer was the most frequent tumor (38.2%), followed by head and neck cancer (26.5%). Median follow-up time was 31.5 months (range 6.1–87.3 months). Patients with MPC included more stage I/II esophageal cancer than those without MPC (66.1% vs. 36.9%, P < 0.01). The 5-year overall survival rate for esophageal cancer with MPC was relatively better than those without MPC (46.1% vs. 26.7%), although the difference did not reach statistical significance in univariate analysis (P = 0.09). Stage I/II esophageal cancer patients had a significantly better overall survival than stage III/IV patients (P < 0.01). Among esophageal cancer patients with MPC, there was no difference in overall survival between antecedent and synchronous cancer (P = 0.59). Our study indicated that the prognosis of esophageal cancer patients treated by radiotherapy was primarily determined by the clinical stage itself, but not the presence of MPC.
Keywords: esophageal cancer, radiotherapy, multiple primary cancers, synchronous cancer, antecedent cancer
INTRODUCTION
Esophageal cancer remains a disease with a poor prognosis, despite advances in treatment [1]. In Japan, esophageal cancer is the sixth most common cancer in men [2]. Several risk factors, including smoking and alcohol, have been shown to be strongly associated with esophageal cancer [3]. These risk factors lead to upper aerodigestive tract cancers, such as oral cavity, oropharyngeal and laryngeal cancers [4–7]. The concept that common carcinogenic agents can lead to multiple cancers in adjacent regions is well-known as ‘field cancerization’ [8]. Recently, the number of esophageal cancer patients with multiple primary cancers (MPC) has been increasing [9], and it is often difficult for clinicians to determine the therapeutic strategy.
Surgery has been the mainstay of treatment for esophageal cancer patients, although the morbidity rate is still high [10, 11]. The presence of synchronous cancers or previously treated antecedent cancers can make surgery more complicated [12]. In the last two decades, radiotherapy, including chemoradiotherapy, has been recognized as a reliable, non-surgical strategy [13]. Radiotherapy has been widely performed for esophageal cancer in Japan and Western countries [14, 15]. Some studies have shown that outcomes of esophageal cancer patients treated by radiotherapy are comparable with surgery [16, 17], which is expected as a less invasive treatment for medically inoperable patients. However, it is still unclear whether the presence of MPC affects the prognosis of esophageal cancer patients treated by radiotherapy. We searched PubMed from 1980 through 2012 for relevant publications and there have been no retrospective or prospective studies, because most clinical trials of esophageal cancer excluded patients with MPC [18, 19].
Therefore, we retrospectively evaluated the effect of MPC on the prognosis of esophageal cancer patients treated by radiotherapy.
MATERIALS AND METHODS
Patient population
Between 2001 and 2008, esophageal cancer patients treated by definitive radiotherapy at the Gunma Cancer Center were retrospectively reviewed. All patients were histologically confirmed with esophageal cancer. Exclusion criteria included preoperative or postoperative radiotherapy, palliative radiotherapy, follow-up of < 6 months, radiation dose of < 50 Gy and no information on MPC. Palliative radiotherapy was defined as the treatment for local disease to reduce dysphagia in esophageal cancer patients with distant or multiple lymph nodes metastases. The institutional review board of our facility approved this study.
Pretreatment evaluations included physical examination, gastrointestinal endoscopy, computed tomography (CT) and barium esophagography. The diagnosis of MPC was based on criteria described by Warren: (i) the tumors must be clearly separated at histological examination; (ii) the tumors must be separated by normal mucosa; and (iii) the possibility that the second tumor represents a metastasis must be excluded [20]. Synchronous cancer was defined if it occurred within 6 months of the esophageal cancer diagnosis [21], whereas multiple esophageal cancers were not included. Antecedent cancer was defined if it was diagnosed > 6 months before esophageal cancer [21]. Other primary cancer diagnosed > 6 months after esophageal cancer was not included as antecedent cancer in this study.
Radiotherapy
All patients provided informed consent before treatment. The details of radiation techniques have been reported previously [22, 23]. Briefly, radiotherapy was delivered as 1.8–2.0 Gy/fraction with 10-MV equipment. The initial 40–46 Gy was delivered using anterior–posterior opposed fields and an additional 10–20 Gy with a shrinking field was delivered using oblique parallel-opposed fields to avoid the spinal cord. The prescribed dose was delivered at the center of the radiation field. The clinical target volume included the primary tumor plus a 3.0-cm craniocaudal margin and the metastatic lymph nodes plus a 1.0-cm margin. The planning target volume included the clinical target volume plus a 0.5–1.5-cm margin. Elective nodal irradiation of cervical, mediastinal and perigastric lymph nodes were not performed.
Follow-up
After treatment, patients were seen every 3 months for the first year and every 6 months thereafter. Each follow-up consisted of physical examination, CT and gastrointestinal endoscopy.
Statistical analysis
Student's t-test and chi-square tests were performed to evaluate differences of characteristics between groups. The endpoint of this study was overall survival that was calculated from the first date of radiotherapy to the date of death. Overall survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. A P value <0.05 was used to indicate statistical significance. All analyses were performed by SPSS 11.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
We retrospectively reviewed 280 consecutive esophageal cancer patients treated with radiotherapy in Gunma Cancer Center between 2001 and 2008. Patients were excluded for the following reasons: 37 patients were treated with palliative radiotherapy, 32 with preoperative or postoperative radiotherapy, 21 without information on MPC, 17 with a radiation dose of < 50 Gy and 6 with < 6 months follow-up. A total of 167 patients were analyzed in this study and their clinical characteristics are summarized in Table 1. Fifty-six esophageal cancer patients (33.5%) were associated with MPC. Median follow-up time was 31.5 months (range 6.1–87.3 months). The median age was 69 years (range 46–94 years), 94.0% of patients were male and 78.4% had a performance status of 0 or 1. Regarding histological subtypes, squamous cell carcinoma was present in the majority (93%), and there were 10 patients with adenocarcinoma (6.0%), 1 basaloid carcinoma (0.6%) and 1 carcinosarcoma (0.6%). Patients with MPC included more with stage I/II esophageal cancer than those without MPC (66.1% vs. 36.9%, P < 0.01). Significant differences were not observed in other clinical factors, such as age, gender, performance status and histologic subtypes, between the two groups.
Table 1.
Patient characteristics
| Characteristics | Esophageal cancer with multiple primary cancers | Esophageal cancer without multiple primary cancers | |
|---|---|---|---|
| n = 56 (%) | n = 111 (%) | P value | |
| Age (years) | |||
| Median | 71.5 | 69 | |
| Range | 48–89 | 46–94 | 0.24 |
| Gender | |||
| Male | 52 (92.9%) | 105 (94.6%) | |
| Female | 4 (7.1%) | 6 (5.4%) | 0.66 |
| Performance status | |||
| 0/1 | 47 (83.9%) | 84 (75.7%) | |
| ≥2 | 9 (16.1%) | 27 (24.3%) | 0.22 |
| Histologic subtypes | |||
| Squamous cell carcinoma | 55 (98.2%) | 100 (90.1%) | |
| Others | 1 (1.8%) | 11 (9.9%) | 0.15 |
| Clinical stage | |||
| I/II | 37 (66.1%) | 41 (36.9%) | |
| III/IV | 19 (33.9%) | 70 (63.1%) | <0.01 |
| Treatment | |||
| Chemoradiotherapy | 29 (51.8%) | 75 (67.6%) | |
| Radiotherapy | 27 (48.2%) | 36 (32.4%) | 0.06 |
Chemoradiotherapy was performed in 104 patients (62.3%) and most were treated using 5-fluorouracil and platinum compounds, including cisplatin and nedaplatin. Radiation alone was performed in 63 patients (37.7%), which included 13 patients (7.8%) treated by brachytherapy after external beam irradiation. Chemoradiotherapy was performed in 51.8% of patients with MPC and in 67.6% of those without MPC, respectively (P = 0.06). The median radiation dose was 60 Gy (range 50–74 Gy). There were no significant differences in treatment types and radiation doses between esophageal cancer patients with and without MPC.
Treatments for other primary cancer
Characteristics of MPC are given in Table 2. Among 56 patients with MPC, there were 12 patients with triple cancers and 68 tumors observed in several organs. Gastric cancer was the most frequent tumor (38.2%), followed by head and neck cancer (26.5%). Surgery was performed for 32 tumors, radiotherapy for 17, endoscopic mucosal resection (EMR) for 9 gastric cancers, hormonal therapy for 4 prostate cancers, radiofrequency ablation (RFA) for 2 hepatocellular carcinomas (HCC), chemotherapy for 1 metastatic gastric cancer and 1 lymphoma, transurethral resection of bladder tumor (TURBT) for 1 bladder cancer and best supportive care for 1 lung cancer. Surgery was performed less frequently in synchronous cancer than antecedent cancer (21.2% vs. 71.4%, P < 0.01). In contrast, radiotherapy was performed more frequently in synchronous cancer than antecedent cancer (39.4% vs. 11.4%, P < 0.01). There were no significant differences in other treatments between two groups. All antecedent cancers were treated by several treatments with curative intent, which maintained stable disease. Stage I/II and III/IV synchronous cancers were 21 and 12, respectively. Among synchronous cancers, 1 metastatic gastric cancer was treated by chemotherapy with palliative intent and 1 lung cancer was treated with best supportive care due to poor performance status.
Table 2.
Type of cancer and treatments in antecedent and synchronous cancers
| Variables | Antecedent cancer | Synchronous cancer | Total |
|---|---|---|---|
| n = 35 (%) | n = 33 (%) | n = 68 (%) | |
| Type of cancer | |||
| Gastric cancer | 15 (43%) | 11 (33%) | 26 (38%) |
| Head and Neck | 9 (26%) | 8 (24%) | 17 (27%) |
| Colon Cancer | 3 (9%) | 4 (12%) | 7 (10%) |
| Prostate cancer | 4 (11%) | 2 (6%) | 6 (9%) |
| Lung cancer | 1 (3%) | 3 (9%) | 4 (6%) |
| HCC | 1 (3%) | 2 (6%) | 3 (4%) |
| Bladder cancer | 0 (0%) | 2 (6%) | 2 (3%) |
| Cervical cancer | 1 (3%) | 0 (0%) | 1 (2%) |
| Breast cancer | 1 (3%) | 0 (0%) | 1 (2%) |
| Lymphoma | 0 (0%) | 1 (3%) | 1 (2%) |
| Treatment | |||
| Surgery | 25 (71%) | 7 (21%) | 32 (47%) |
| Radiotherapy | 4 (11%) | 13 (39%) | 17 (25%) |
| EMR | 2 (6%) | 7 (21%) | 9 (13%) |
| Hormonal therapy | 3 (9%) | 1 (3%) | 4 (6%) |
| RFA | 1 (3%) | 1 (3%) | 2 (3%) |
| Chemotherapy | 0 (0%) | 2 (6%) | 2 (3%) |
| TURBT | 0 (0%) | 1 (3%) | 1 (2%) |
| Best supportive care | 0 (0%) | 1 (3%) | 1 (2%) |
HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; EMR, endoscopic mucosal resection; TURBT, transurethral resection of bladder tumor.
Treatment outcome
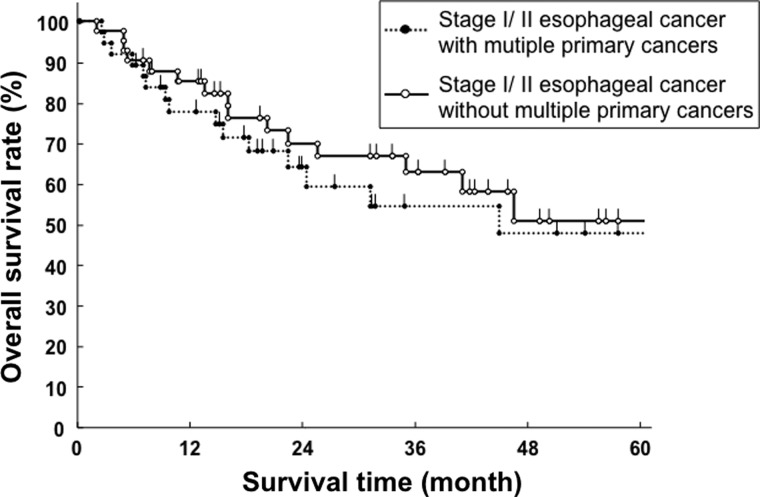
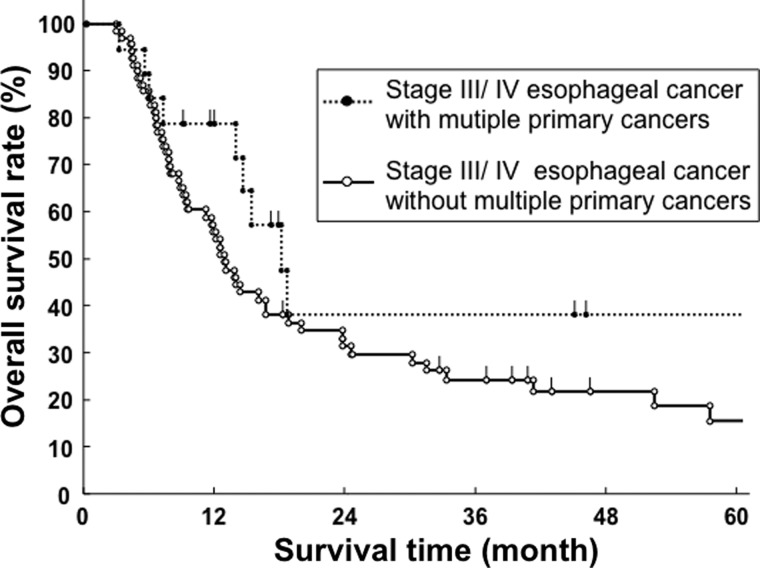
For all patients, 73 patients (43.7%) were alive and 94 (56.3%) died during follow-up. Of 56 patients with MPC, 19 patients (33.9%) died from esophageal cancer, 4 (7.1%) another disease, 2 (3.6%) synchronous cancer, 1 (1.7%) treatment-related death and 0 (0%) antecedent cancer. There were 5 synchronous cancer patients who had progressive disease. Of 111 patients without MPC, 56 patients (50.4%) died from esophageal cancer, 9 (8.1%) another disease and 3 (2.7%) treatment-related death. Clinical characteristics, including the presence of MPC, were analyzed by univariate analysis (Table 3). The 5-year overall survival for esophageal cancer with MPC was relatively better than for those without MPC (46.1% vs. 26.7%), although the difference did not reach statistical significance (P = 0.09, Fig. 1). Stage I/II esophageal cancer patients had a significantly better overall survival rate than stage III/IV patients (P < 0.01). In stage I/II esophageal cancer, the 5-year overall survival rates for patients with and without MPC were 47.6% and 50.7%, respectively (P = 0.62, Fig. 2). In stage III/IV esophageal cancer, the 5-year overall survival rates for patients with and without MPC were 38.3% and 15.8%, respectively (P = 0.14, Fig. 3). Performance status was also significantly associated with overall survival (P < 0.01). Other clinical factors, such as age, gender, histological subtypes and types of treatment were not associated with overall survival. Among patients with esophageal cancer with MPC, there was no difference in overall survival rate between antecedent and synchronous cancers (P = 0.59, Fig. 4). Seven esophageal cancer patients synchronously associated with head and neck cancers were treated by radiotherapy in the same time. The 5-year overall survival rate of these patients was 47.6%, which was not worse than other patients with 31.8% ( P = 0.49).
Table 3.
Univariate analysis of overall survival
| Variables | No. patients | 5-year overall survival rate | P value |
|---|---|---|---|
| Multiple primary cancers | |||
| Presence | 56 | 46.1% | |
| Absence | 111 | 26.7% | 0.09 |
| Age (years) | |||
| ≥70 | 81 | 34.5% | |
| <70 | 86 | 31.7% | 0.98 |
| Gender | |||
| Male | 157 | 31.4% | |
| Female | 10 | 50.0% | 0.49 |
| Performance status | |||
| 0/1 | 131 | 39.0% | |
| ≥2 | 36 | 10.2% | <0.01 |
| Histologic subtypes | |||
| Squamous cell | 155 | 31.9% | |
| carcinoma | |||
| Others | 12 | 50.5% | 0.68 |
| Treatment | |||
| Chemoradiotherapy | 104 | 32.2% | |
| Radiotherapy | 63 | 19.4% | 0.73 |
| Clinical stage | |||
| I/II | 78 | 49.3% | |
| III/IV | 89 | 19.8% | <0.01 |
Fig. 1.
Overall survival curve of esophageal cancer patients with and without multiple primary cancers (MPC).
Fig. 2.
Overall survival curve of stage I/II esophageal cancer patients with and without multiple primary cancers (MPC).
Fig. 3.
Overall survival curve of stage III/IV esophageal cancer patients with and without multiple primary cancers (MPC).
Fig. 4.
Overall survival curve of antecedent and synchronous cancers.
DISCUSSION
Esophageal cancer patients are often associated with MPC. Although a few surgical studies analyzed the prognosis of these patients [24, 25], there have been no reports of radiotherapy. To our knowledge, this is the first report to describe the clinical characteristics and overall survival rates of esophageal cancer patients treated by radiotherapy with and without MPC.
In our study, the incidence of MPC in esophageal cancer patients was 33.5%, which was relatively higher than previous studies (range 9.5–32.2%) [9, 24–26]. One possibility is that esophageal cancer patients with MPC tended to receive radiotherapy in our institution. We showed that patients with MPC were significantly associated with an earlier stage of esophageal cancer than those without MPC (P < 0.01). This finding was in accordance with previous reports of esophageal cancer patients with MPC treated by surgery [24, 25]. Meticulous follow-up after antecedent cancer or clinical surveillance for synchronous cancer may allow the early detection of esophageal cancer.
The presence of MPC has been generally considered to have an unfavorable prognosis in esophageal cancer patients. However, some studies showed that there was no significant difference in overall survival rate between esophageal cancer patients with and without MPC [24, 26]. Furthermore, one surgical study showed that patients with MPC had a significantly better overall survival rate than those without MPC (P = 0.02) [25]. In our study, patients with esophageal cancer with MPC tended to have better overall survival rates than those without MPC (5-year overall survival rate; 46.1% and 26.7%, P = 0.09). One possible reason is that patients with MPC were significantly associated with an earlier stage of esophageal cancer than those without MPC as described above. Indeed, univariate analysis showed that an earlier stage of esophageal cancer was significantly associated with better overall survival in our study (P = 0.01). These results suggest that the prognosis of esophageal cancers is primarily determined by the clinical stage itself, but not the presence of MPC.
Surgery is the established strategy for esophageal cancer, although morbidity rates are still high. The presence of synchronous or antecedent cancers may add further difficulties in surgery. Among esophageal cancer patients with MPC who have received surgery, it has been shown that postoperative mortality rates were relatively high (range 8.5–9.3%) [24, 27]. In our study, the percentage of treatment-related deaths for patients with and without MPC were 1.7% and 2.7%, respectively. Due to its low mortality rate, radiotherapy can be considered as a relatively safe treatment for esophageal cancer, even if patients are associated with MPC.
It is difficult to determine the therapeutic strategy for synchronous cancer patients, because several treatments are required for multiple cancers during the same periods. Considering the clinical stage of the esophageal cancer and MPC, performance status, age and complication, personalized treatments for each patient should be determined by a comprehensive team including radiation oncologists, medical oncologists and thoracic surgeons. Previously, there have been few reports showing treatment details for MPC in esophageal cancer patients. In our study, radiotherapy was more frequent in patients with synchronous cancers compared with those with antecedent cancers (P < 0.01). In contrast, surgery was less common in patients with synchronous cancers compared with those with antecedent cancers (P < 0.01). Given the burden on patients who need to receive several cancer treatments during the same period, radiotherapy can be chosen for both esophageal cancer and synchronous cancer. Furthermore, we showed that overall survival rates for synchronous and antecedent cancers were similar (P = 0.59) and the deaths from synchronous cancer were uncommon (only two patients). These finding indicate that our therapeutic strategy for esophageal cancer patients with synchronous cancer is effective, which encourages those patients.
Although we showed the prognosis of esophageal cancer patients with and without MPC, there are several limitations such as the retrospective nature of the study and selection bias. In this study, all antecedent cancer patients had stable disease, and most synchronous cancer patients were treated with curative intent. From the beginning, esophageal cancer patients with progressive antecedent cancers or highly advanced synchronous cancers might not be referred from medical oncologists and thoracic surgeons. Therefore, our conclusion may apply particularly to esophageal cancer patients with stable antecedent cancers or synchronous cancers that can receive definitive treatments. It has been unclear which esophageal cancer patients with MPC benefit from radiotherapy. From a clinical point of view, esophageal cancer patients with highly advanced cases of other primary cancers developing fatal disease should not be treated by radical radiotherapy. Further investigations for esophageal cancer with MPC are warranted to establish the therapeutic strategy, including radiotherapy.
In conclusion, our study showed that the prognosis of esophageal cancer patients treated by radiotherapy was primarily determined by the clinical stage itself, but not the presence of MPC. Radiotherapy was effective and tolerable for esophageal cancer patients with MPC, as well as those without MPC.
REFERENCES
- 1.Brown LM, Hoover R, Silverman D, et al. Excess incidence of squamous cell esophageal cancer among US black men: role of social class and other risk factors. Am J Epidemiol. 2001;153:114–22. doi: 10.1093/aje/153.2.114. [DOI] [PubMed] [Google Scholar]
- 2.Higuchi K, Koizumi W, Tanabe S, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res. 2009;3:153–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 4.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 5.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–7. [PubMed] [Google Scholar]
- 6.Bosetti C, Gallus S, Peto R, et al. Tobacco smoking, smoking cessation, and cumulative risk of upper aerodigestive tract cancers. Am J Epidemiol. 2008;167:468–73. doi: 10.1093/aje/kwm318. [DOI] [PubMed] [Google Scholar]
- 7.Ansary-Moghaddam A, Huxley RR, Lam TH, et al. The risk of upper aero digestive tract cancer associated with smoking, with and without concurrent alcohol consumption. Mt Sinai J Med. 2009;76:392–403. doi: 10.1002/msj.20125. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Koide N, Komatsu D, Hiraga R, et al. Esophageal cancer associated with other primary cancers—historical comparison of clinicopathologic features in 359 esophageal cancer patients. Hepatogastroenterology. 2010;57:513–18. [PubMed] [Google Scholar]
- 10.Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225–32. doi: 10.1097/00000658-200008000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363–72. doi: 10.1097/SLA.0b013e31814697f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wind P, Roullet MH, Quinaux D, et al. Long-term results after esophagectomy for squamous cell carcinoma of the esophagus associated with head and neck cancer. Am J Surg. 1999;178:251–5. doi: 10.1016/s0002-9610(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 13.Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol. 2007;25:4110–17. doi: 10.1200/JCO.2007.12.0881. [DOI] [PubMed] [Google Scholar]
- 14.Kenjo M, Uno T, Murakami Y, et al. Radiation therapy for esophageal cancer in Japan: results of the Patterns of Care Study 1999–2001. Int J Radiat Oncol Biol Phys. 2009;75:357–63. doi: 10.1016/j.ijrobp.2009.03.073. [DOI] [PubMed] [Google Scholar]
- 15.Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78:1820–8. doi: 10.1002/(sici)1097-0142(19961015)78:8<1820::aid-cncr25>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Hironaka S, Ohtsu A, Boku N, et al. Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T(2-3) N(any) M(0) squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2003;57:425–33. doi: 10.1016/s0360-3016(03)00585-6. [DOI] [PubMed] [Google Scholar]
- 17.Ariga H, Nemoto K, Miyazaki S, et al. Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2009;75:348–56. doi: 10.1016/j.ijrobp.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 18.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 19.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 20.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;51:1358–414. [Google Scholar]
- 21.Cahan WG, Castro EB, Rosen PP, et al. Separate primary carcinomas of the esophagus and head and neck region in the same patient. Cancer. 1976;37:85–9. doi: 10.1002/1097-0142(197601)37:1<85::aid-cncr2820370112>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Shirai K, Tamaki Y, Kitamoto Y, et al. Dose-volume histogram parameters and clinical factors associated with pleural effusion after chemoradiotherapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys. 2011;80:1002–7. doi: 10.1016/j.ijrobp.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Shirai K, Tamaki Y, Kitamoto Y, et al. Comparison of chemoradiotherapy with radiotherapy alone in patients with esophageal adenocarcinoma. J Radiat Res. 2011;52:264–9. doi: 10.1269/jrr.10166. [DOI] [PubMed] [Google Scholar]
- 24.Natsugoe S, Matsumoto M, Okumura H, et al. Multiple primary carcinomas with esophageal squamous cell cancer: clinicopathologic outcome. World J Surg. 2005;29:46–9. doi: 10.1007/s00268-004-7525-y. [DOI] [PubMed] [Google Scholar]
- 25.Kumagai Y, Kawano T, Nakajima Y, et al. Multiple primary cancers associated with esophageal carcinoma. Surg Today. 2001;31:872–6. doi: 10.1007/s005950170025. [DOI] [PubMed] [Google Scholar]
- 26.Poon RT, Law SY, Chu KM, et al. Multiple primary cancers in esophageal squamous cell carcinoma: incidence and implications. Ann Thorac Surg. 1998;65:1529–34. doi: 10.1016/s0003-4975(98)00177-5. [DOI] [PubMed] [Google Scholar]
- 27.Nagasawa S, Onda M, Sasajima K, et al. Multiple primary malignant neoplasms in patients with esophageal cancer. Dis Esophagus. 2000;13:226–30. doi: 10.1046/j.1442-2050.2000.00116.x. [DOI] [PubMed] [Google Scholar]