Abstract
Since 1990, Boron Neutron Capture Therapy (BNCT) has been used for over 400 cancer patients at the Kyoto University Research Reactor Institute (KURRI). After BNCT, the patients are radioactive and their 24Na and 38Cl levels can be detected via a Na-I scintillation counter. This activity is predominantly due to 24Na, which has a half-life of 14.96 h and thus remains in the body for extended time periods. Radioactive 24Na is mainly generated from 23Na in the target tissue that is exposed to the neutron beam in BNCT. The purpose of this study is to evaluate the relationship between the radioactivity of blood 24Na following BNCT and the absorbed gamma ray dose in the irradiated field. To assess blood 24Na, 1 ml of peripheral blood was collected from 30 patients immediately after the exposure, and the radioactivity of blood 24Na was determined using a germanium counter. The activity of 24Na in the blood correlated with the absorbed gamma ray doses in the irradiated field. For the same absorbed gamma ray dose in the irradiated field, the activity of blood 24Na was higher in patients with neck or lung tumors than in patients with brain or skin tumors. The reasons for these findings are not readily apparent, but the difference in the blood volume and the ratio of bone to soft tissue in the irradiated field, as well as the dose that leaked through the clinical collimator, may be responsible.
Keywords: radioactivity of blood, Boron Neutron Capture Therapy, neutron irradiation, 24Na, germanium detector
INTRODUCTION
Boron Neutron Capture Therapy (BNCT) has been used to treat more than 400 cancer patients in the Kyoto University Research Reactor Institute (KURRI). BNCT is used to treat brain [1], head and neck [2], skin [3], and lung tumors [4]. The principle underlying BNCT is that tumor cells can be destroyed efficiently by the 10B (n, α) 7Li reaction. The radiation dose in tumor tissue is higher than in the adjacent normal tissue, which takes up boron compounds less efficiently than the target tumor tissue. Although the dose in the adjacent normal tissue is lower than in the target tumor region, the possible radioactive damage in the normal tissue is a limiting factor for BNCT, and delayed effects are also a concern when considering how to best protect patients from radiation. In addition, the patients also receive radiation exposure from the induced radionuclides, mainly radio-sodium induced by the reaction of 23Na (n, γ) 24Na. The radiation activation of 24Na in BNCT patients also causes radiation exposure in the clinical staff. However, little is known about the induced radioactivity in BNCT patients. In this study, in order to obtain fundamental knowledge about radiation protection in BNCT patients, we measured the radioactivity of 24Na in the blood of BNCT patients after neutron irradiation, investigated the relationship between the physical radiation doses in the irradiated fields and the radioactivity of blood 24Na, and estimated the effective dose from the induced activity.
MATERIALS AND METHODS
Blood samples
From November 2010 to February 2012, peripheral blood samples were obtained from 30 cancer patients immediately after they completed BNCT. Among the 30 patients, 17, 7, 3 and 3 patients received neutron irradiation at the brain, lung, neck and skin regions, respectively. The boron compounds included borocaptate sodium (BSH) and para-boronophenylalanine (BPA).
Ethical status of BNCT at KURRI
The ethics for each case of BNCT was approved by the ethical committee of KURRI. Informed consent was obtained from each patient.
The KURRI Heavy Water Neutron Irradiation Facility and neutron irradiation
The heavy water column of KURRI was used for BNCT. The neutron beam provided by this facility consists of thermal, epithermal, fast neutrons and gamma rays. The relative absorbed doses are summarized in Table 1.
Table 1.
Physical outline of the neutron beam used for BNCT
| Neutron flux (cm−2s−1) |
Absorbed dose rate (Gy/h) |
|||||
|---|---|---|---|---|---|---|
| Thermal–0.5 eV | Epithermal 0.5 eV–10 keV | Fast 10 keV– | Thermal–0.5 eV | Epithermal 0.5 eV–10 keV | Fast 10 keV– | Gamma ray |
| 3.0E + 07 | 7.3E + 08 | 4.7E + 07 | 2.4E − 03 | 2.2E − 01 | 1.6E + 00 | 6.2E − 01 |
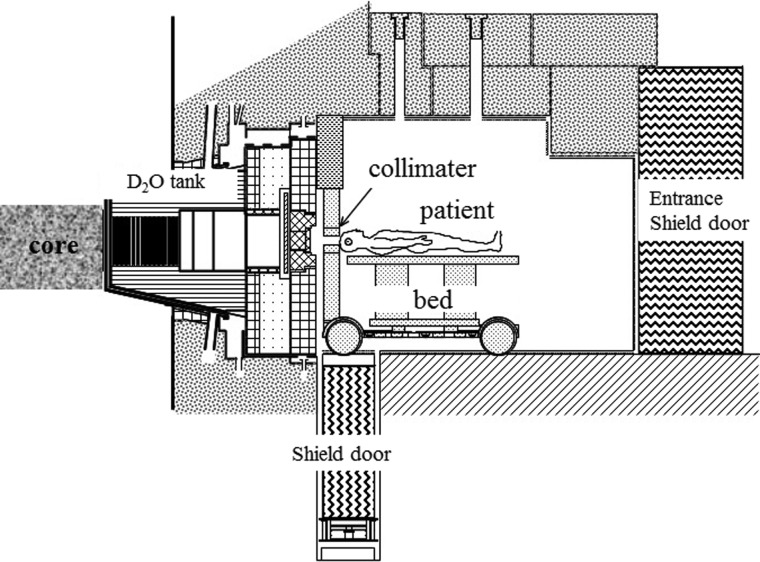
The layout of the KURRI clinical irradiation system is shown in Fig. 1. A clinical collimator and a medical bed were placed on a remote-control carrier, and they moved with the patient on the rail between the inside and outside of the irradiation room. Therefore, the irradiation is localized to specific regions and the radiation dose can be limited to low levels of exposure, except for the irradiated field.
Figure 1.
Layout of the KURRI clinical irradiation system. BNCT patients are irradiated with the collimated neutron beam.
Measurement of radioactivity in the blood and the absorbed gamma ray dose at the skin surface
Blood samples were collected from patients after BNCT, placed in a polypropylene vessel, and then measured for 10 000 seconds using a p-type high-purity germanium detector (model IGC3019, Princeton Gamma Tech) with a multi channel analyzer (mode 7600, SEIKO EG&G). The gamma ray counting efficiency of the detector was determined by constructing a relative gamma ray counting efficiency curve using a certified mixed radionuclide gamma ray reference source (5054QB) containing 57Co(122.1 keV), 137Cs(661.7 keV), and 60Co(1173 and 1332 keV), which was normalized to the 1460 keV gamma ray peak from a 40K in KCl solution placed in the same type of vessel.
The gamma ray doses, including secondary capture gamma rays, at the skin surface of the tumor lesion were measured with BeO thermoluminescence detectors (TLDs). These TLDs were located on the skin surface directly beneath the beam line. We typically use special-ordered TLDs that are encapsulated in quartz glass, which does not contain 10B. The thermal neutron fluence of 8 × 1012 n/cm2 is approximately equal to a 1 cSv gamma ray dose. We generally used a TLD with gold foil for the neutron-sensitivity correction. A Panasonic TLD reader (UD-512P) was used for this measurement. When determining the gamma ray dose rate, the uncertainty is caused mainly by the measurement error of the TLD, which is approximately 20%.
RESULTS
The activities of blood 24Na and the absorbed gamma ray doses are shown in Table 2. The activity of 24Na indicates the value for each 1 ml of blood. The activities of blood 24Na were 6.0–21.2, 9.1–22.6, 17.0–27.3, and 7.6–21.4 Bq/ml for brain, skin (inguinal and foot), neck, and lung tumor patients, respectively. These activities were normalized to the time-point at the end of BNCT-mediated irradiation. In addition to 24Na, other induced radionuclides, such as 38Cl, were also detected (data not shown).
Table 2.
Activities of 24Na in 1 ml of blood and the absorbed doses of BNCT patients
| The irradiated part | The activity of 24Na | The absorbed gamma ray dose of the irradiated field | The area of the irradiated field | |
|---|---|---|---|---|
| Bq/ml | Gy | cm2 | ||
| 1 | skin (inguinal) | 9.1 ± 0.6 | 1.3 | 180 |
| 2 | skin (inguinal) | 12.1 ± 0.7 | 2.3 | 144 |
| 3 | skin (foot) | 22.6 ± 1.5 | 3.9 | 126 |
| 4 | head | 6.0 ± 0.3 | 1.5 | 144 |
| 5 | head | 7.7 ± 0.3 | 1.2 | 144 |
| 6 | head | 8.0 ± 0.3 | 0.9 | 99 |
| 7 | head | 8.2 ± 0.5 | 1.5 | 225 |
| 8 | head | 8.3 ± 0.3 | 1.6 | 195 |
| 9 | head | 8.9 ± 0.3 | 1.1 | 120 |
| 10 | head | 8.9 ± 0.3 | 1.8 | 144 |
| 11 | head | 9.8 ± 0.7 | 1.3 | 144 |
| 12 | head | 10.1 ± 0.4 | 1.5 | 121 |
| 13 | head | 10.1 ± 0.9 | 1.7 | 144 |
| 14 | head | 12.4 ± 0.5 | 2.2 | 283 |
| 15 | head | 12.5 ± 0.4 | 1.8 | 144 |
| 16 | head | 13.3 ± 1.2 | 1.3 | 144 |
| 17 | head | 14.2 ± 1.0 | 1.9 | 144 |
| 18 | head | 14.7 ± 0.6 | 2.0 | 144 |
| 19 | head | 16.1 ± 0.4 | 2.5 | 225 |
| 20 | head | 21.2 ± 0.8 | 2.5 | 270 |
| 21 | lung | 7.6 ± 0.3 | 1.0 | 400 |
| 22 | lung | 8.6 ± 0.4 | 0.3 | 400 |
| 23 | lung | 12.0 ± 0.5 | 1.0 | 400 |
| 24 | lung | 16.4 ± 1.2 | 0.8 | 225 |
| 25 | lung | 18.8 ± 0.5 | 1.1 | 400 |
| 26 | lung | 20.9 ± 0.8 | 1.4 | 225 |
| 27 | lung | 21.4 ± 0.8 | 0.8 | 225 |
| 28 | neck | 17.0 ± 0.4 | 1.2 | 144 |
| 29 | neck | 17.8 ± 0.4 | 1.5 | 225 |
| 30 | neck | 27.3 ± 0.7 | 1.0 | 225 |
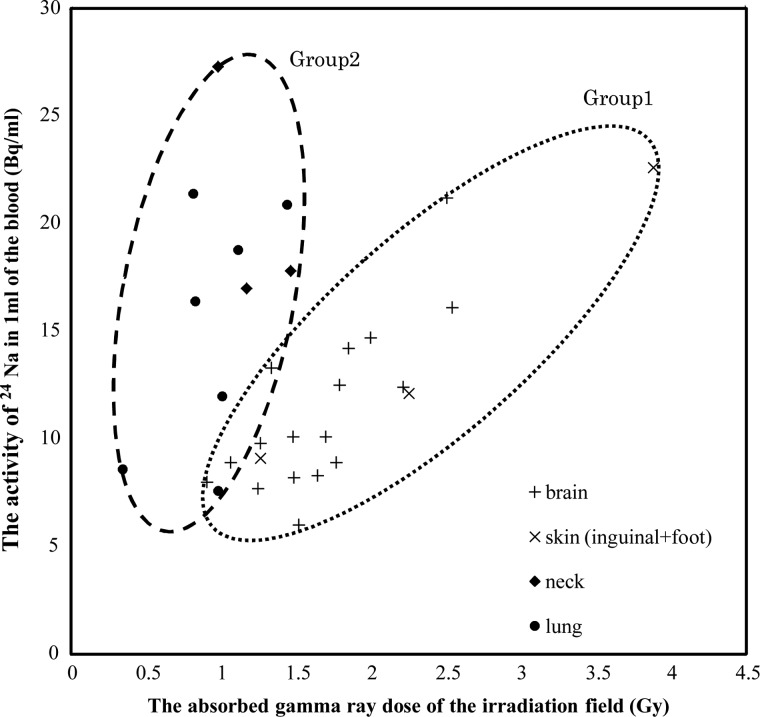
The absorbed gamma ray dose at the skin surface of the irradiated field was 1.7 (0.9–2.5), 2.5 (1.3–3.9), 1.2 (1.0–1.5), and 0.9 (0.3–1.4) Gy for brain, skin (inguinal and foot), neck, and lung tumor patients, respectively. Approximately 70% of the gamma ray dose that was absorbed at the skin surface in the irradiated field came from the 1H (n, γ) 2H reaction in the body [5]. The absorbed gamma ray dose for neck and lung tumor patients tended to be lower compared to brain and skin tumor patients. The activities of blood 24Na versus the absorbed gamma ray doses in the irradiated field are presented as a scatter plot (Fig. 2). The activity of blood 24Na increased as the absorbed gamma ray dose increased. Interestingly, these datasets could be classified into two groups. The datasets for the BNCT patients with brain or skin (inguinal or foot) tumors were categorized as Group 1, while datasets for BNCT patients with neck or lung tumors were classified as Group 2 (Fig. 2).
Figure 2:
The activity of 24Na in the blood of patients after BNCT plotted against the absorbed gamma ray dose by TLD measurement. The activity of 24Na in the blood of BNCT-treated patients was classified into two groups. BNCT-treated patients with brain and skin (inguinal and foot) tumors are included in Group 1, and BNCT-treated patients with neck and lung tumors are included in Group 2. The solid line of Group 1 represents the best fit obtained by a linear regression analysis (R2 = 0.69).
In Group 1, the activities of blood 24Na correlated well with the absorbed gamma ray doses. The proportional constant was 0.0644, and the correlation coefficient (R2) was 0.69. However, the data for Group 2 differed from the data for Group 1. In Group 2, the correlation between the blood 24Na concentration and the absorbed gamma ray dose was not clear (R2 = − 0.06), and the activity of blood 24Na versus the absorbed gamma ray dose in the irradiated field was generally higher compared to Group 1.
DISCUSSION
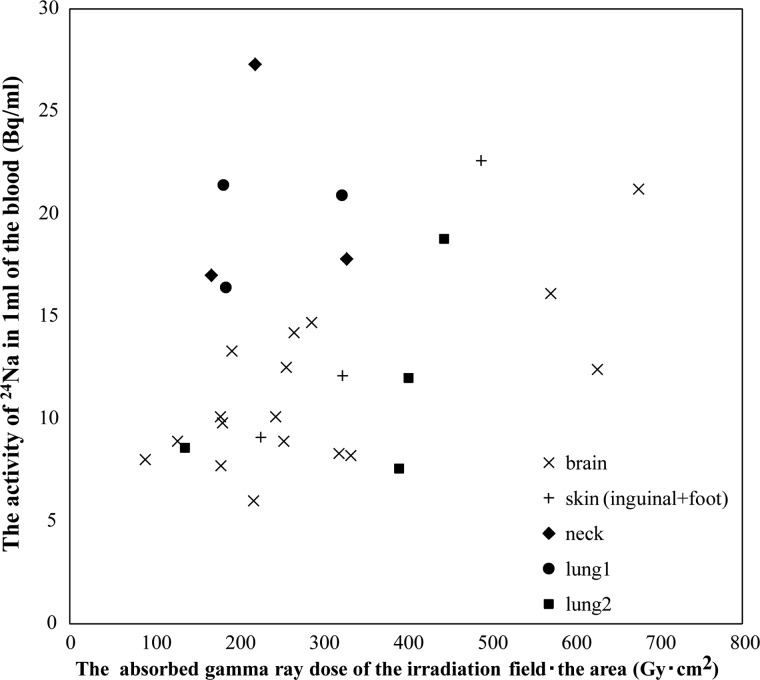
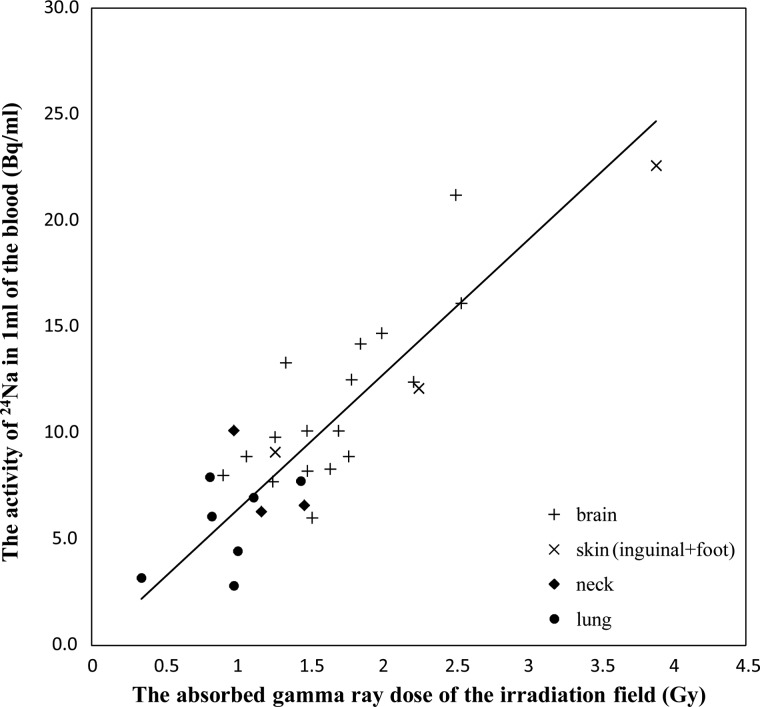
When the patients were irradiated with neutrons, the reaction of 23Na (n, γ) 24Na and 1H (n, γ) 2H that occurred in the body was predominantly due to thermal neutrons. This is evident because the blood 24Na concentration correlated with the absorbed gamma ray dose, especially in Group 1. However, it is not readily apparent why the datasets fell into these two groups or why the activity of blood 24Na was relatively higher in Group 2. The area of irradiation, which is collimator size, differed in each patient and was 99–283, 126–180, 144 or 225, and 225 or 400 cm2 for brain, skin (inguinal and foot), neck, and lung tumor patients, respectively. We also considered the absorbed gamma ray dose in relation to the area of the irradiation. However, the blood 24Na concentration did not correlate with the absorbed gamma ray dose within the area of irradiation (Fig. 3). There was no correlation between the absorbed gamma ray dose and the activity of the 24Na in Group 1(R2 = 0.062) or Group 2 (R2 =–1.40). We confirmed the relationship between the blood 24Na concentration and the blood 10B concentration, but the blood 24Na concentration did not correlate with the blood 10B concentration (data not shown). The irradiated field in Group 2 includes a greater proportion of hematological organs than Group 1, and hematological organs may have a larger blood volume because they make blood. Therefore, we can speculate that there may have been a greater proportion of hematological organs with a larger blood volume included in the irradiated field in Group 2 compared to Group 1. Moreover, the activity of the 24Na might be affected by the blood volume in the irradiation field in the irradiation time. The circulating blood volume of the lung is about five times or more than that of the brain [6]. A previous study reported that the density of 23Na, which is the origin of 24Na, is 2.7 times larger in bone than in soft tissues [7]. These values, which can be generated by dividing the activities of blood 24Na in Group 2 by 2.7, versus the absorbed gamma ray doses at the irradiated field are presented in a scatter plot (Fig. 4). Groups 1 and 2 showed a similar tendency. Of course, the irradiated area includes some bone, and the ratio of bone by comparison with soft tissue in the irradiated field should be calculated. The ratio of the density of bone to soft tissue of lung is higher than brain, because the density of lung substance is about ten times lower than that of brain. Consequently, we also believe that the ratio of bone to soft tissue in the irradiated area is one of the factors to consider with this treatment. Furthermore, the dose that leaked though the collimator due to the setting position in BNCT might have contributed to the high activity of blood 24Na in Group 2. These points await further experimentation and analysis.
Figure 3:
The activity of 24Na in the blood of patients after BNCT plotted against the absorbed gamma ray dose based on TLD measurement (Gy) multiplied by the irradiated area (cm2).
Figure 4:
The activities of blood 24Na in Group 2 divided by 2.7 plotted against the absorbed gamma ray dose based on TLD measurement. The solid line represents the best fit obtained by a linear regression analysis (R2 = 0.73).
The highest 24Na concentration was 27.3 Bq/ml of blood in patients with neck cancers. We assume that 24Na is distributed uniformly throughout the body, although this is apparently an overestimation because each patient received a locally limited neutron exposure. The total body burden of 24Na was calculated using 1.9 × 105 Bq/70 kg. According to the International Commission on Radiological Protection (ICRP) model, this body burden will give 0.1 mSv for a committed effective dose, indicating that the radiation dose of patients who received BNCT-induced radioactivity is quite small compared to patients who received direct irradiation from a neutron beam.
The medical staff take care of the patients 20–30 min after neutron irradiation and the radiation exposure of the staff is under observation. The ambient dose rate of patients after BNCT is less than 10 µSv/h at a 30 cm distance. In cases of BNCT for multiple liver tumors and lung tumors [8], which often require a large irradiated field in the torso area, the radioactivity of 24Na might increase and thus safety measures for radiation protection are required.
The induced activity of 24Na is a good parameter to estimate the dose (dose re-construction) after accidental neutron exposure, and was used to estimate the absorbed dose in the JCO Co., Ltd Tokai Plant (JCO) criticality accident [9–11]. However, it is generally difficult to estimate the absorbed dose based on 24Na because the state of the neutron spectra and gamma ray ratio is uncertain, and the irradiated field of the accident victim is also undetermined. In the present paper, we determined the blood 24Na concentrations in patients receiving BNCT, where the exposure parameters (energy spectra of neutron, irradiation region, etc.) are well defined. Accumulating these data will provide the necessary parameters to more precisely estimate doses (re-construction) using 24Na after neutron exposure accidents.
It is also noteworthy that 1 ml of blood was sufficient to determine the relationship between the absorbed dose at the irradiated field and the radioactivity of 24Na in the blood. Previously, a relatively large blood sample was used, for example 20 ml in the JCO criticality accident [11], and 50–100 ml in the Oak Ridge accident [12]. This small collection volume is advantageous for accident victims because it reduces the burden on victims.
CONCLUSION
Our results indicate that it may be possible to estimate the absorbed gamma ray dose at the BNCT irradiated field from blood 24Na activities. The relationship between the absorbed gamma ray dose and the blood 24Na levels can be categorized into two patterns with respect to the irradiated field. The effective dose based on induced 24Na in patients was very low compared with the direct exposure. In cases of BNCT for lung and liver tumors with a large irradiated field in the torso area, safety measures should be implemented for patients and staff. Our approach will provide fundamental data that can be used to easily estimate emergency radiation neutron exposure accidents.
FUNDING
This work was supported by the KUR Research Program for Scientific Basis of Nuclear Safety, Kyoto University, Japan.
REFERENCES
- 1.Kawabata S, Miyatake S, Hiramatsu R, et al. Phase II clinical study of boron neutron capture therapy combined with X-rayzolomide in patients with newly diagnosed glioblastoma multiforme—Study design and current status report. Appl Radiat Isot. 2011;69:1769–99. doi: 10.1016/j.apradiso.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Fuwa N, Suzuki M, Sakurai Y, et al. Treatment results of boron neutron capture therapy using intra-arterial administration of boron compounds for recurrent head and neck cancer. Br J Radiol. 2008;81:749–52. doi: 10.1259/bjr/65306248. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda H, Hiratsuka J, Kobayashi T, et al. Boron neutron capture therapy (BNCT) for malignant melanoma with special reference to absorbed doses to the normal skin tumor. Australas Phys Eng Sci Med. 2003;26:97–103. doi: 10.1007/BF03178777. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Endo K, Satoh H, et al. A novel concept of treatment of diffuse or multiple pleural tumors by boron neutron capture therapy (BNCT) Radiother Oncol. 2008;88:192–195. doi: 10.1016/j.radonc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai Y, Kobayashi T. The medical-irradiation characteristics for neutron capture therapy at the Heavy Water Neutron Irradiation Facility of Kyoto University Research Reactor. Med Phys. 2002;29:2328–2337. doi: 10.1118/1.1509444. [DOI] [PubMed] [Google Scholar]
- 6.Calzia E, Iványi Z, Radermacher P. Determinants of blood flow and organ perfusion. In: Pinsky MR, Payen D, editors. Function Hemodynamic Monitoring. Berlin: Spriger Berlin Heidelberg; 2005. pp. 19–32. [Google Scholar]
- 7.Sakurai Y, Tanaka H, Suzuki S, et al. An estimation of activation for target volume due to BNCT at Kyoto University Reactor. In: Zonta A, Altieri S, Roveda L, Barth R, editors. Proceedings of the 13th International Congress on Neutron Capture Therapy, Florence: Italian National Agency for New Technologies, Energy and the Environment (ENEA) 2008. pp. 697–9. [Google Scholar]
- 8.Suzuki M, Tanaka H, Sakurai Y, et al. Impact of accelerator-based boron neutron capture therapy (AB-BNCT) on the treatment of multiple liver tumors and malignant pleural mesotherioma. Radiother Oncol. 2009;92:89–95. doi: 10.1016/j.radonc.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi F, Endo A, Yamaguchi Y. Dose assessment from activated sodium within a body in criticality accidents. Radiat Prot Dosimetry. 2003;106:197–206. doi: 10.1093/oxfordjournals.rpd.a006350. [DOI] [PubMed] [Google Scholar]
- 10.Momose T, Tsujimura N, Tasaki T, et al. Dose evaluation based on 24Na activity in the human body at the JCO criticality accident in Tokai-mura. J Radiat Res. 2001;42(Suppl):S95–105. doi: 10.1269/jrr.42.s95. [DOI] [PubMed] [Google Scholar]
- 11.Fujimoto K. Chiba, Japan: National Institute of Radiological Science; 2002. Final report on dose estimation for three victims of JCO accident; NIRS-M-153. [Google Scholar]
- 12.Hurst GS, Ritchie RH, Emerson LC. Accidental radiation excursion at the Oak Ridge Y-12 plant-III: determination of radiation doses. Health Phys. 1959;2:121–33. doi: 10.1097/00004032-195904000-00001. [DOI] [PubMed] [Google Scholar]