Abstract
The efficacy and toxicity of three-fraction CyberKnife radiotherapy were evaluated in patients with brain metastases in critical areas. One hundred and fifty-nine metastases in 145 patients including tumors >10 cm3 were treated with three-fraction CyberKnife radiotherapy with a median marginal dose of 27 Gy at a median prescribed isodose of 60%. Changes in the neurological manifestations, local tumor control and adverse effects were investigated after treatment. The surrounding brain volumes circumscribed with 23.1 Gy (single dose equivalence of 14 Gy: V14) were measured to evaluate the risk of adverse effects. Neurological manifestations, such as motor weakness, visual disturbances and aphasia improved in 26 of 97 patients (26.8%). Local tumor control was obtained in 137 of 143 metastases (95.8%) during a median follow-up of 7 months. Nine patients had symptomatic edema and three of them (2.1%) required surgical resection because of radiation necrosis. The V14 of these patients was 4.6–31.5 cm3. There were 35 lesions with a V14 of 7 cm3 or more and three of them developed extensive brain edema due to radiation necrosis. None of the patients with a V14 of <7 cm3 exhibited edema requiring an operation. We therefore conclude that a high rate of local tumor control and low rates of complications are obtained after three-fraction CyberKnife radiotherapy for metastases in critical areas. The V14 of the surrounding brain therefore seems to be a useful indicator for the risk evaluation of radiation necrosis in patients with larger metastases.
Keywords: brain metastases, hypofractionated radiotherapy, three-session radiosurgery, radiation necrosis, V14
INTRODUCTION
Symptomatic brain metastases may occur in 8–10% of cancer patients, although the incidence is known to increase in parallel with the aging population, improvements in cranial imaging and longer survival periods owing to recent advances in cancer treatments [1–2]. The majority of brain metastases occur in patients with advanced stages of primary cancer, and brain metastases may decrease the patient's quality of remaining life because of such symptoms as hemiparesis, aphasia, hemianopia, dementia and disturbances of consciousness, especially in patients with brain metastases in critical areas including the brainstem. The survival period of patients with untreated brain metastases is reportedly 1–3 months [3]. The radiation therapy oncology group-recursive partitioning analysis (RTOG-RPA) of prognostic factors has shown the best survival (median 7.1 months) of patients in class 1: less than 65 years of age with a Karnofsky performance status (KPS) of at least 70, and a controlled primary cancer with the brain demonstrating only metastases; however, the worst survival (median 2.3 months) is found in class 3 patients, with a KPS of less than 70 [4]. The KPS is especially low in patients with a dysfunction in critical areas, such as the motor cortex, visual pathways and brainstem. The optimal treatment of brain metastases in these areas may improve the KPS and quality of remaining life for such advanced cancer patients.
The therapeutic approaches for brain metastases include surgery, whole-brain radiotherapy (WBRT), radiosurgery and chemotherapy. Many patients are treated with a combination of these approaches, depending on the clinical stage of primary lesions and the number, size and site of the brain metastases. However, treating brain metastases in critical areas effectively is not easy, especially for larger tumors [2]. Surgery has the risks of inducing a functional deterioration and single fraction radiosurgery has dose limitations for the surrounding brain. WBRT and chemotherapy are sometimes unable to control larger brain metastases. Fractionation or multisession radiosurgery is an option for treating brain metastases in critical areas to reduce the adverse effects on surrounding structures, as reported for the treatment of gliomas and perioptic lesions [5–6]. Three-fraction radiotherapy is intended to treat brain metastases in critical areas to avoid causing any dysfunction of the surrounding brain and maintain sufficient treatment doses for malignant lesions.
This report presents the efficacy and toxicity of three-fraction CyberKnife radiotherapy performed at this institution as a useful treatment option for brain metastases in critical areas, including larger metastases.
PATIENTS AND METHODS
Eight hundred and eighty-six patients with brain metastases were treated with single fraction radiosurgery or hypofractioned radiotherapy at the Kanto Neurosurgical Hospital between March 2005 and March 2012. Hypofractioned treatment was performed for 473 lesions in 339 patients. This report analyzed a consecutive series of three-fraction treatment for lesions in critical areas used for 159 brain metastases in 145 patients including tumors > 10 cm3. The median age of the patients was 61 years old, and 54 patients (37.2%) were 65 years old or older. The patients’ primary cancers were the lung (n = 56), breast (n = 45), gastrointestinal tract (n = 21), kidney (n = 5), uterus (n = 4), larynx (n = 3) and other regions (ovary, pancreas, parotid, etc.). Sixty-three patients (43.4%) had metastases to other organs and 109 patients (75.2%) had multiple brain metastases. Tumors treated with the three-fraction protocol were situated in the frontal lobe (close to the optic pathway, Broca's area and motor cortex), parietal lobe (sensory cortex and dominant angular cortex), temporal lobe (close to the optic pathway and Wernicke's area), occipital lobe (visual cortex), thalamus, basal ganglia, brainstem or cerebellum close to the brainstem. Fifteen patients (10.3%) had previously received WBRT. Ninety-seven patients (66.9%) had neurological manifestations due to the lesions that we intended to treat using three-fraction radiotherapy. Neurological manifestations observed before treatment included motor weakness in 43 patients (29.7%), visual disturbances in 21, unsteady gait in 16, aphasia in 9, numbness in 7, and focal seizure, agraphia, dysphagia and dementia in 1 patient each. Fifty-seven patients (39.3%) had a KPS of < 70 and all patients were in RTOG-RPA class 2 or 3. The initial tumor volume was measured using the MultiPlan (Accuray, Sunnyvale, CA, USA) software program, which determines the treatment volume based on enhanced T1-weighted magnetic resonance imaging (MRI). The median tumor volume was 6.9 cm3 (range 0.04–25.9 cm3). Thirty-two were tumors > 10 cm3. Table 1 shows the patient characteristics.
Table 1.
Patient characteristics treated with three-fraction CyberKnife radiotherapy
| Number of patients | 145 | Location of tumor | |
| Median age (years) (range) | 61 (37–85) | Cerebral hemisphere | 102 |
| Age ≥65 | 54 | Cerebellum | 29 |
| Age <65 | 91 | Brainstem | 28 |
| Sex | Neurological manifestation in 97 patients | ||
| Male | 62 | Motor weakness | 43 |
| Female | 83 | Visual disturbances | 21 |
| Primary cancer | Unsteady gait | 16 | |
| Lung | 56 | Aphasia | 9 |
| Breast | 45 | Numbness | 7 |
| Gastro-intestinal tract | 21 | Others | 4 |
| Kidney | 5 | Median KPS score | 70 (40–100) |
| Uterus | 4 | KPS ≥70 | 88 |
| Larynx | 3 | KPS <70 | 57 |
| Others | 11 | Tumor volume (cm3) (range) | 6.9 (0.04–25.9) |
| Multiple vs. single | >10.0 | 32 | |
| Multiple metastases | 109 | 5.0–10.0 | 71 |
| Single metastasis | 36 | <5.0 | 56 |
| Metastases to other organ | 63 | Image follow-up period (months) (range) | 7 (1–30) |
| Whole brain radiotherapy | 15 | Survival period (months) (range) | 7 (1–39) |
Three-fraction radiotherapy
All patients evaluated in this study were treated consecutively with three-fraction radiotherapy over three sequential days using a CyberKnife (Accuray). Patients with perifocal brain edema and progressing symptoms were treated with the concomitant intravenous administration of glycerol and beta-methasone (osmo-steroid therapy). All treatment procedures were performed under computed tomography (CT) and MRI (1.5-T or 3.0-T) guidance in a frameless system. Critical areas such as the optic pathway, brain stem and other cranial nerves were identified using CISS (heavy T2) images (MR cisternography). Gross tumor volume (GTV) was demarcated for only enhanced lesions from fusion images of enhanced CT and MR using 1-mm-thick axial images. The clinical target volume (CTV) was identical to the GTV (CTV = GTV) for treatment planning to measure the exact surrounding brain volume within the isodose line. More than 90% or 95% volume of the GTV was intended to cover with the same 50–70% isodose line as single fraction radiosurgery, instead of the 80–90% isodose line used in usual hypofractioned radiotherapy. The intended prescribed marginal dose was 27–30 Gy depending on the tumor volume and surrounding critical structures. The maximum dose to the optic pathway (optic nerve, chiasm and tract) was intended to be < 12.8 Gy instead of 15 Gy (normal tissue dose constraint for the optic pathway in three-fraction treatment, an equivalent dose of 8 Gy in a single fraction treatment) [7] to reduce the amount of any adverse effects.
Evaluation of brain volume around lesions involved in isodose line
The isodose volume of surrounding brain (excluding the GTV) circumscribed with a 23.1-Gy dose line was measured and recorded in each patient to compare the risk of adverse effects on the surrounding brain. Instead of 21.9 Gy (normal tissue dose constraint for the cauda equine in three-fraction treatment, an equivalent dose of 14 Gy in a single fraction treatment) [7] 23.1 Gy was used as the isodose of three-fraction treatment in order to compare the findings with other hypofractioned treatments [8]. The V14 (surrounding brain volume circumscribed with a single dose equivalence (SDE) of 14 Gy) as well as tumor volumes included within the prescribed marginal isodose line were calculated from integral dose–volume histograms (DVHs) as shown in Fig. 1. The V14 of each patient was evaluated in relation with the toxicity (brain edema and necrosis) to the surrounding brain and critical areas such as the brainstem and cerebellum close to the brainstem.
Fig. 1.
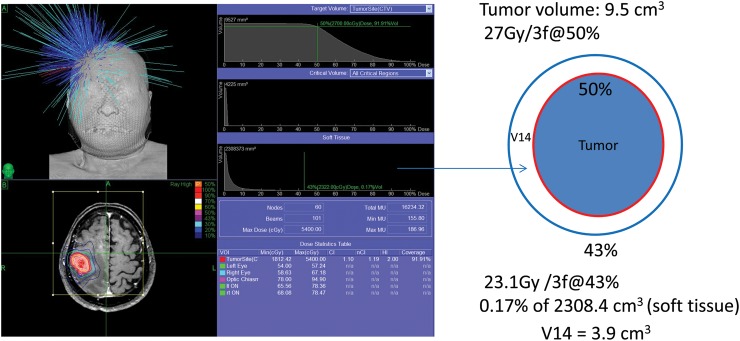
The measurement of the surrounding brain volume circumscribed with a single dose equivalence (SDE) of 14 Gy in three-fraction CyberKnife radiotherapy (V14) from dose–volume histograms.
Example: Brain metastasis (volume 9.5 cm3) in the premotor cortex was planned to be treated with a marginal dose of 27 Gy at 50% isodose in three-fraction radiotherapy. The isodose (%) of an SDE of 14 Gy (23.1 Gy in three-fraction) was obtained from a dose–volume histogram of soft tissue as 43%. The volume circumscribed with the 23.1 Gy (43%) line was 0.17% of 2309.4 cm3. As a result, the V14 was calculated as 3.9 cm3.
Follow-up evaluations and patient data
Changes in the neurological symptoms, such as paresis, sensory disturbances, aphasia and visual disturbances were examined after treatment, in patients with metastases in critical areas causing related symptoms. The severity of symptoms was divided into four groups based on the activities of daily living using the medical care accreditation criteria: grade 0, no trouble (able to do without help); grade 1, slightly impaired (able to do with some difficulty); grade 2, moderately affected (need partial support); and grade 3, severely affected (useless in daily life and need total support). The improvement of symptoms was defined as changes of one grade up or more.
Serial imaging studies (MRI or CT) with thin sections (1–2 mm thickness) were requested 6 weeks after treatment and every 2–3 months thereafter. The patients who lived far from the center were examined by their referring physicians. Contrast-enhanced imaging studies were used to define the tumor response and local control. The tumor volumes were calculated using the geometric method using the diameter of three dimensions (x, y and z) of the ellipse obtained from axial and coronal slices of serial imaging studies [9]. The calculated volume was within a 15% error of the volume data obtained using the MultiPlan software. The tumor response was then divided into four groups: almost disappeared (tumor volume decreased >95%), reduced (tumor volume decreased 15–95%), stable (tumor volume change within ±15%) and enlarged (tumor volume increased >15%) [2]. The incidence of brain edema and necrosis was examined in relation to the V14 of the surrounding brain.
Statistical analysis
Differences between the groups were evaluated using Student's t-test. The cumulative incidence was estimated according to the Kaplan–Meier method, and was examined for significance with a log rank test and a generalized Wilcoxon test. All analyses used the conventional P < 0.05 level of significance.
RESULTS
The prescription isodoses ranged from 49–83% (median 60%) for the GTV. The marginal dose ranged from 21–36 Gy (median 27 Gy) and the maximum dose ranged from 31.9–64.3 Gy (median 46.9 Gy) delivered in three fractions. Eight patients received osmo-steroid therapy during three-fraction CyberKnife radiotherapy for symptoms due to perifocal edema.
Follow-up evaluation
Neurological manifestations observed in 97 patients before treatment improved in 26 patients from several weeks to several months after treatment, accompanied by the tumor response. Motor weakness was improved by more than one grade in 14 patients. Five of seven patients with grade 2 paresis (with support) recovered to almost normal function and two patients improved to grade 1 (without support). All seven patients with grade 1 paresis recovered to normal function. Aphasia improved in five patients, unsteady gait in four, visual disturbances in three, numbness in four, and dysphagia, seizure and dementia in one patient each (Table 2). The KPS improved in 26.8% of the patients after treatment as a result of neurological improvements that changed the performance status. Three patients were able to return to their workplace, and three patients were able to resume their work as housewives (KPS: 90 or 100). There were no new neurological deficits from the direct damage of the brainstem or functional areas, though recurrences of symptoms appeared in seven patients due to adverse effects (brain edema and necrosis) on the surrounding brain.
Table 2.
Results of three-fraction CyberKnife radiotherapy
| Median prescribed isodose (%) (range) | 60 (49–83) |
| Median marginal dose (Gy) (range) | 27 (21–36) |
| Improved neurological manifestation (no. of grade 0, 1, 2, 3) | 26/97 |
| Motor weakness | 14/43 |
| before radiotherapy | (0, 7, 7, 0) |
| after radiotherapy | (11, 3, 0, 0) |
| Aphasia | 5/9 |
| before radiotherapy | (0, 4, 1, 0) |
| after radiotherapy | (5, 0, 0, 0) |
| Unsteady gait | 4/16 |
| before radiotherapy | (0, 2, 2, 0) |
| after radiotherapy | (3, 1, 0, 0) |
| Visual disturbances | 3/21 |
| Before radiotherapy | (0, 3, 0, 0) |
| after radiotherapy | (3, 0, 0, 0) |
| Numbness | 4/7 |
| before radiotherapy | (0, 2, 2, 0) |
| after radiotherapy | (3, 1, 0, 0) |
| Other symptoms | 3/4 |
| before radiotherapy | (0, 2, 1, 0) |
| after radiotherapy | (3, 0, 0, 0) |
| Tumor response (n = 143) | |
| Almost disappeared (volume decrease >95%) | 6 |
| Reduced (volume decrease 15–95%) | 133 |
| Stable (volume change ±15%) | 3 |
| Enlarged (volume increase >15%) | 1 (cyst expansion) |
| Tumor recurrence | 5/143 (3.5%) |
| Adverse effects | |
| Radiation edema | 6 |
| Radiation necrosis | 3 |
Grade 0: no trouble (able to do without help). Grade 1: slightly impaired (able to do with some difficulty) Grade 2: moderately affected (need partial support). Grade 3: severely affected (need total support).
Tumor response and local control after treatment
One hundred and forty-three lesions in 132 patients were subjected to sequential imaging studies 1–30 months (median 7 months) after treatment. Imaging studies for the volume evaluation were not available in 13 patients because of improper examinations or because there were no cranial examinations due to acute deterioration of the primary cancers. All 143 lesions with the exception of four (three stable and one enlarged due to cyst expansion) showed tumor regression on follow-up images (Fig. 2A–B). Five lesions showed marginal recurrence 5–12 months after radiotherapy and required additional treatment. The local tumor control rate was 95.8% (Table 2).
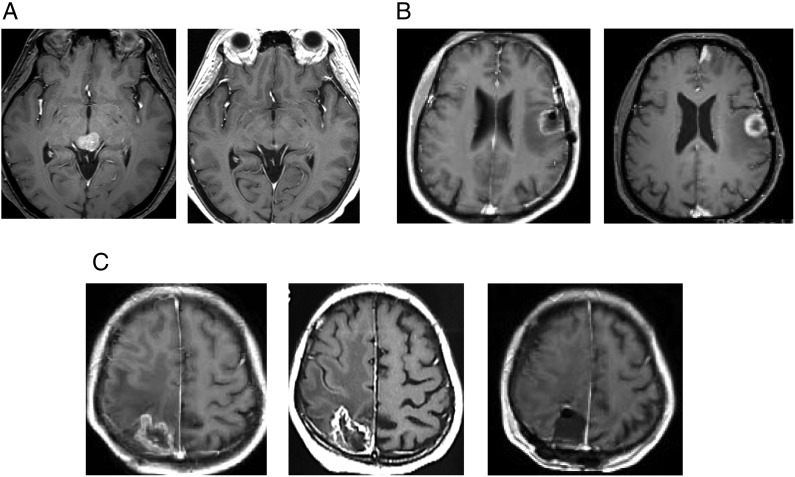
Fig. 2.
Gd-enhanced T1-weighted MR images. (A) Breast cancer brain metastases in a 55-year-old female. A tectum tumor treated with a marginal dose of 30 Gy in three fractions at 60% isodose (left). A significant tumor response and no adverse imaging effects found 11 months after three-fraction CyberKnife radiotherapy (right). (B) Lung cancer brain metastasis in a 64-year-old male. A tumor in the speech area with perifocal edema treated with a marginal dose of 25 Gy in three fractions at 60% isodose (left). A tumor response found 1 year after three-fraction radiotherapy (right). (C) Breast cancer brain metastasis in a 37-year-old female. A tumor in the sensory area with perifocal edema treated with a marginal dose of 30 Gy in three fractions at a 59% isodose (left). A tumor response was found 11 months after treatment. However, both clinical and radiological deterioration were noted (center) and surgical resection was required after osmo-steroid therapy. The surgical specimen confirmed as radiation necrosis and the hemiparesis improved after surgery (right).
Adverse effects (brain edema and necrosis)
Nine patients (6.2%) had recurrent symptoms or symptoms of increased intracranial pressure and exhibited extensive brain edema required osmo-steroid therapy on admission. Six of them showed both clinical and radiological deterioration 1–8 weeks after treatment; however, the symptoms and edema rapidly improved after osmo-steroid therapy. They received further oral administration of steroids at the outpatient clinic. Three patients (2.1%) showed symptoms from 10–18 months after treatment and required surgical resection because osmo-steroid therapy insufficiently reduced the symptoms. The surgical specimens confirmed radiation necrosis. The extensive edema rapidly decreased within several days (Fig. 2C) and almost disappeared within 2 weeks after surgery. The V14 values of the patients with brain edema and necrosis were 4.6–7.1 cm3 and 7.3–31.5 cm3, respectively. No symptomatic adverse effects occurred in patients with either brainstem metastases and/or patients with metastases close to the optic pathway.
The V14 of the surrounding brain and adverse effects
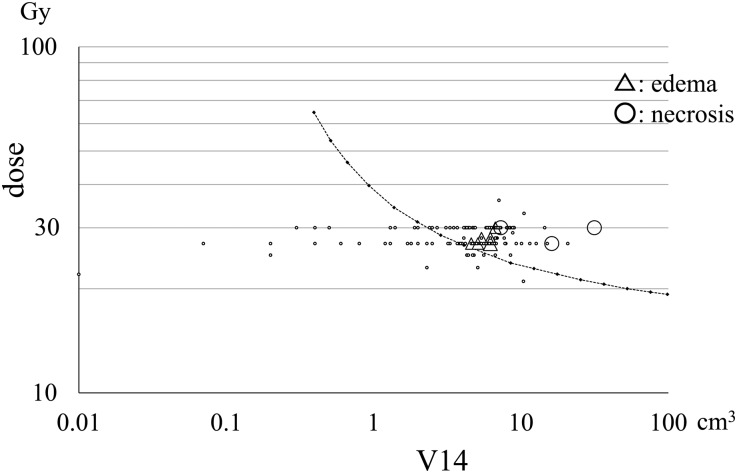
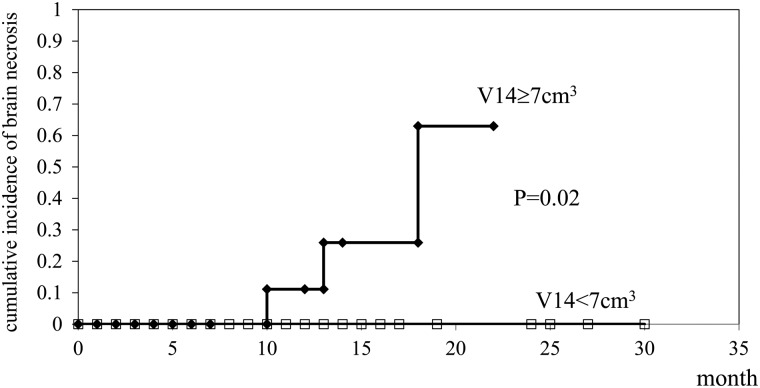
The V14 was calculated in all patients and plotted in relation to the marginal dose (Fig. 3). The median tumor volume of brainstem metastases was 1.0 cm3 and the median V14 in patients with brainstem metastases was 1.0 cm3. No adverse radiation imaging effects on the brainstem were observed. Median tumor volumes of cerebellar and cerebral metastases were 7.3 cm3 and 8.1 cm3, respectively, and the median V14 values in patients with cerebellar and cerebral metastases were 5.6 cm3 and 6.1 cm3, respectively. Six patients with cerebral metastases developed brain edema and two patients with cerebral metastases had radiation necrosis thus requiring surgical removal (Table 3). Five of 87 lesions with a V14 of 5.0 cm3 or more developed symptomatic brain edema and required osmo-steroid therapy; however, only one of 72 lesions with a V14 of < 5.0 cm3 had symptomatic brain edema requiring treatment. Three of 35 lesions with a V14 of 7.0 cm3 or more developed radiation necrosis and required an operation after treatment (Fig. 3); however, none of 124 lesions with a V14 of < 7.0 cm3 including 16 patients followed more than 12 months after treatment exhibited extensive radiation edema requiring an operation (Table 4). Statistical analysis of the incidence of radiation necrosis using a t-test was significant between two groups with a V14 of < 7.0 cm3 and 7.0 cm3 or more (P = 0.04). The incidence of brain necrosis increased in the long-term survival patients with a V14 of 7.0 cm3 or more, however, none of patients with a V14 of < 7.0 cm3 experienced brain necrosis that required surgical removal (Fig. 4).
Fig. 3.
The risk evaluation of adverse effects (brain edema and radiation necrosis).
The V14 of 159 brain metastases plotted in relation to the marginal doses administered in three-fraction CyberKnife radiotherapy. Symptomatic brain edema developed in six patients (triangle) and radiation necrosis requiring surgical resection appeared in three patients (circle). Kjellberg's 5% necrosis risk line [10] was converted and then drawn for three-fraction radiotherapy (dotted line).
Table 3.
The V14 and adverse effects of three-fraction CyberKnife radiotherapy
| Brainstem | Cerebellum | Cerebrum | |
|---|---|---|---|
| Median tumor volume (cm3) (range) | 1.0 (0.04–2.7) | 7.3 (0.5–22.2) | 8.1 (2.4–25.9) |
| Median prescribed isodose (%) (range) | 63 (49–83) | 60 (50–77) | 59.5 (50–74) |
| Median marginal dose (Gy) (range) | 27 (25–30) | 27 (22–30) | 27 (21–36) |
| Median maximum dose (Gy) (range) | 45.7 (32.5–61.2) | 46.9 (34.9–55.6) | 47.5 (31.9–64.3) |
| Median V14 (cm3) (range) | 1.0 (0.07–2.7) | 5.6 (0.01–16.2) | 6.1 (1.7–31.5) |
| Symptomatic brain edema | 0/28 | 0/29 | 6/102 |
| V14 (cm3) | – | – | 4.6–7.1 |
| Radiation necrosis | 0/28 | 1/29 | 2/102 |
| V14 (cm3) | – | 16.2 | 7.3–31.5 |
Table 4.
The incidence of adverse effects and the V14
| Symptomatic brain edema | V14 <5.0 cm3 | V14 ≥5.0 cm3 | P value |
|---|---|---|---|
| Number of lesions | 1/72 | 5/87 | 0.07 |
| Median tumor volume (cm3) (range) | 3.3 (0.04–18.9) | 8.5 (1.5–25.9) | |
| Median V14 (cm3) (range) | 2.9 (0.01–4.9) | 6.6 (5.0–31.5) | |
| Follow-up period (month) | 1–30 | 1–30 | |
| No. patients survived more than 12 months | 9 | 14 | |
| Radiation necrosis required surgery | V14 <7.0 cm3 | V14 ≥7.0 cm3 | P value |
| Number of patients | 0/124 | 3/35 | 0.04 |
| Median tumor volume (cm3) (range) | 6.0 (0.04–18.9) | 9.6 (3.4–25.9) | |
| Median V14 (cm3) (range) | 4.7 (0.01–6.9) | 8.5 (7.0–31.5) | |
| Follow-up period (month) | 1–30 | 1–26 | |
| No. patients survived more than 12 months | 16 | 7 | |
Fig. 4.
The cumulative incidence of brain necrosis in 145 patients treated with three-fraction CyberKnife radiotherapy.
None of the patients with a V14 of <7.0 cm3 developed brain necrosis including those who survived more than 12 months.
DISCUSSION
The prognosis for patients with brain metastases is related to the stage of the primary cancer, age and KPS score [4]. The worst survival (median 2.3 months) is seen in patients with a KPS of < 70 (RTOG-RPA: class 3). This series showed that 39.3% had a KPS of < 70 because of neurological manifestations. The KPS in patients with brain metastases in critical areas improved in 26.8% of the patients after three-fraction treatment. A recovery of motor weakness was found in 32.6% of the 43 patients. The symptoms caused by compression or mass effects usually improved with tumor regression. However, the symptoms caused by large lesions directly involving such areas as the motor cortex, internal capsule, angular cortex and visual cortex persisted even after tumor regression. Three-fraction CyberKnife radiotherapy therefore helps to increase the KPS, at least in patients with symptomatic lesions not directly affecting functional areas.
The role of radiosurgery has been described in 10 institutional studies for patients treated with radiosurgery and WBRT [11]. Radiosurgery also plays a role in the treatment of small multiple brain metastases in advanced cancer patients because of the short treatment time and the absence of the need for general anesthesia. However, the treatment of metastases in the brainstem and around risky organs has some dose limitations in single fraction radiosurgery. Local tumor control rates of 76–100% for brainstem metastases have been reported with single fraction radiosurgery of marginal doses of 13–20 Gy using a Gamma Knife or linear accelerator radiosurgery. The complication rates for brainstem metastases have been reported to range from 0–27% in patients treated with single fraction radiosurgery [12]. The 12-Gy volume of the brainstem is recommended to be decreased to as low as 0.1 cm3 in single fraction radiosurgery to reduce the occurrence of any adverse radiation imaging effects on the brainstem and to avoid new neurological deficits [13]. In the current series, 28 brainstem metastases were treated with three-fraction radiotherapy. The median tumor volume was 1.0 cm3 and the median marginal dose was 27 Gy. All tumors were controlled and no symptomatic adverse effects on the brainstem were found. The median V14 was 1.0 cm3. Three-fraction CyberKnife radiotherapy thus seems to be safe and effective for the treatment of small brainstem metastases.
The three-fraction treatment yielded tumor control rates of 95.8% in patients with tumors in critical areas including larger tumors. The median marginal dose of 27 Gy at a median prescribed isodose of 60% in three fractions seems to be effective for most brain metastases as well as a marginal dose of 20 Gy at the prescribed isodose of 50–60% in single fraction radiosurgery. Hypofractionation or multisession treatments are used to decrease the incidence of complications and adverse effects on the surrounding brain [6, 14]. The development of new technologies for performing frameless radiosurgery has enabled the treatment of larger lesions using multisession treatments or hypofractionation [15–17]. However, the incidence of radiation necrosis is not insignificant for the treatment of large metastases, even when hypofractionation is used [14, 16, 18]. Surgical management after radiosurgery may thus be required in some patients.
The concept of the volume involved within the treatment plans is essential and widely known in radiosurgery. Large lesions are not suitable for single-fraction treatment. The marginal dose is also important for tumor control and protection against adverse effects including radiation necrosis. The marginal dose of 12 Gy used to treat schwannomas is adequate for tumor control and functional preservation. No radiation necrosis is found around lesions treated with 12 Gy even though the lesions are relatively large [19]. The 12-Gy volume of the surrounding brain is recognized as a predictor of complications from arteriovenous malformation (AVM) radiosurgery with a median marginal dose of 15 Gy administered in a single fraction [20]. Lower dose treatment of AVM has been shown to result in a very low rate of complications, while also providing functional preservation after radiosurgery [21]. Meanwhile, a marginal dose of 12–14 Gy instead of 15–16 Gy has been suggested in order to avoid complications after radiosurgery for large meningiomas [22]. A marginal dose of 14–15 Gy is used to treat cavernous sinus lesions and small lesions around the brainstem. No adverse effects on cranial nerves in the cavernous sinus wall or brainstem are found with a marginal dose of 15 Gy; however, treating large lesions with 14–15 Gy may cause radiation necrosis in the surrounding brain, especially in the white matter depending on the volume involved. A lot of data and long-term results of brain metastases including the risk of radiation necrosis are available for single-fraction treatment. Adverse effects, including radiation necrosis, have been shown to occur experimentally in hypofraction treatment depending on the treatment dose and volume [23]. The V14 is a good indicator for comparing adverse effects between radiosurgery and hypofraction radiotherapy.
A dose of 27 Gy was used as the median marginal dose of three-fraction radiotherapy in this series, in order to decrease complications in critical areas. The V14 of the surrounding brain was also calculated to evaluate the risk of radiation necrosis after three-fraction treatment based on the results of low-dose treatment. A dose of 23.1 Gy was used as an SDE of 14 Gy in three-fraction radiotherapy in this study. A dose of 21.9 Gy for the cauda equina, penile bulb and femoral heads is used as an SDE of 14 Gy in three-fraction body radiotherapy and volumes of < 5, 3 and 10 cm3 are recommended to avoid complications, respectively [7]. The exact calculation method to obtain the proper SDE of 14 Gy in three-fraction radiotherapy has still not yet been established [24]. Animal experiments have shown that equivalent single doses for two or three fractions calculated from the L–Q formula were lower than the actual measured doses by 21–31% [25]. However, the accumulation of clinical data regarding the use of the V14, which can be converted for proper SDE in the future, may support the creation of an ideal calculation method for converting to SDE from hypofractionation. The V14 obtained to approximate the SDE is very useful for avoiding adverse effects in hypofraction treatment beyond the need to identify ideal calculation methods. In any case, the volume of risk organs involved within the prescribed isodose should be considered for three-fraction radiotherapy in relation to complications. Three patients with tumors 9.6–25.9 cm3 in volume developed radiation necrosis after three-fraction radiotherapy during the early period (between 2006 and 2009) of the current series. The V14 of these patients ranged from 7.3–31.5 cm3. Surgical operations were performed to remove radiation necrosis and the intended V14 of 7 cm3 has been lowered since 2010 to avoid further complications. The V14 may need to be further reduced when treating tumors situated deep in the white matter and with extensive perifocal edema, especially when retreating such cases, as indicated by Chin et al. who reported their experiences using a 10-Gy volume during single fraction radiosurgery based on the Kjellberg 1% risk line and the Flickinger 3% risk line [26]. They reported the median 10-Gy volumes of the normal brain in patients with and without necrosis to be 19.8 and 7.1 cm3, respectively.
CyberKnife radiotherapy with a prescription isodose of 50–60% has the benefits of decreasing the isodose volume (V14) of the surrounding brain in comparison with conventional treatment or higher prescription isodose treatment of 80–90% in usual hypofractionation, because sharp fall-off of the dose distribution is obtained. The rate of radiation necrosis requiring resection was only 2.1% of the patients in this series, including larger tumors treated with the mean prescription isodose of 60%. The complication rate is therefore expected to further decrease when the V14 of the surrounding brain is restricted to < 7 cm3 (applied since 2010), because no radiation necrosis was found in the current series during the later period. The complication data shown in Fig. 3 are therefore useful for dose selection in three-fraction radiotherapy. For example, when a treatment plan using the prescribed dose of 30 Gy has a V14 > 7.0 cm3, the dose should be lowered to 27 Gy. Another method to decrease the V14 involves decreasing the marginal isodose down to 50% when a treatment plan is made with a marginal isodose of 60% to 70%. Optimal dose fractionation (multisession radiosurgery) is also possible using V14, and an increased number of fractions (sessions) is able to reduce the V14 for large lesions [27].
CONCLUSION
Three-fraction CyberKnife radiotherapy is safe and effective for patients with brain metastases in critical areas including the brainstem. An accurate determination of the isodose volume of the surrounding brain is important for decreasing adverse effects, the same as it is in single fraction radiosurgery. The V14 seems to be a useful indicator for the risk evaluation for adverse effects (brain edema and radiation necrosis) in patients with larger metastases being treated with three-fraction radiotherapy.
REFERENCES
- 1.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–98. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 2.Yang HC, Kano H, Lunsford LD, et al. What factors predict the response of larger brain metastases to radiosurgery. Neurosurgery. 2011;68:682–90. doi: 10.1227/NEU.0b013e318207a58b. [DOI] [PubMed] [Google Scholar]
- 3.Andrews DW. Current neurosurgical management of brain metastases. Semin Oncol. 2008;35:100–7. doi: 10.1053/j.seminoncol.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 5.Inoue HK, Hayashi S, Ishihara J, et al. Fractionated Gamma Knife radiosurgery for malignant gliomas: neurobiological effects and FDG-PET studies. Stereotact Funct Neurosurg. 1995;64:249–57. doi: 10.1159/000098786. [DOI] [PubMed] [Google Scholar]
- 6.Adler JR, Jr, Gibbs IC, Puataweepong P, et al. Visual field preservation after multisession CyberKnife radiosurgery for perioptic lesions. Neurosurgery. 2006;59:244–54. doi: 10.1227/01.NEU.0000223512.09115.3E. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215–22. doi: 10.1016/j.semradonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Inoue HK. Characteristics of radiosurgery. In: Inoue HK, editor. Textbook of Radiosurgery. Osaka: MedicaPress; 2012. pp. 14–15. (Jpn) [Google Scholar]
- 9.Pan HC, Cheng FC, Sun MH, et al. Prediction of volumetric data errors in patients treated with Gamma Knife radiosurgery. Stereotact Funct Neurosurg. 2007;85:184–91. doi: 10.1159/000101297. [DOI] [PubMed] [Google Scholar]
- 10.Barker FG, Butler WE, Lyons S, et al. Dose-volume prediction of radiation-related complications after proton beam radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 2003;99:254–63. doi: 10.3171/jns.2003.99.2.0254. [DOI] [PubMed] [Google Scholar]
- 11.Sanghavi SN, Miranpuri SS, Chappell R, et al. Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol Biol Phys. 2001;51:426–34. doi: 10.1016/s0360-3016(01)01622-4. [DOI] [PubMed] [Google Scholar]
- 12.Hatiboglu MA, Chang EL, Suki D, et al. Outcomes and prognostic factors for patients with brainstem metastases undergoing stereotactic radiosurgery. Neurosurgery. 2011;69:796–806. doi: 10.1227/NEU.0b013e31821d31de. [DOI] [PubMed] [Google Scholar]
- 13.Sharma MS, Kondziolka D, Khan A, et al. Radiation tolerance limits of the brainstem. Neurosurgery. 2008;63:728–33. doi: 10.1227/01.NEU.0000325726.72815.22. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi Y, Serizawa T, Nagano O, et al. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009;74:1543–8. doi: 10.1016/j.ijrobp.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Nishizaki T, Saito K, Jimi Y, et al. The role of CyberKnife radiosurgery/radiotherapy for brain metastases of multiple or large-size tumors. Minim Invasive Neurosurg. 2006;49:203–9. doi: 10.1055/s-2006-947998. [DOI] [PubMed] [Google Scholar]
- 16.Fahrig A, Ganslandt O, Lambrecht U, et al. Hypofractionated stereotactic radiotherapy for brain metastases–results from three different dose concepts. Strahlenther Onkol. 2007;183:625–30. doi: 10.1007/s00066-007-1714-1. [DOI] [PubMed] [Google Scholar]
- 17.Wiggenraad R, Verbeek-de Kanter A, Mast M, et al. Local progression and pseudo progression after single fraction or fractionated stereotactic radiotherapy for large brain metastases. A single centre study. Strahlenther Onkol. 2012;188:696–701. doi: 10.1007/s00066-012-0122-3. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh J, Saito Y, Kazumoto T, et al. Therapeutic effect of linac-based stereotactic radiotherapy with a micro-multileaf collimator for the treatment of patients with brain metastases from lung cancer. Jpn J Clin Oncol. 2010;40:119–24. doi: 10.1093/jjco/hyp128. [DOI] [PubMed] [Google Scholar]
- 19.Inoue HK. Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation. J Neurosurg. 2005;102(Suppl):111–13. [PubMed] [Google Scholar]
- 20.Friedman WA, Bova FJ, Bollampally S, et al. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296–307. doi: 10.1227/01.neu.0000043692.51385.91. [DOI] [PubMed] [Google Scholar]
- 21.Inoue HK. Long-term results of Gamma Knife surgery for arteriovenous malformations: 10- to 15-year follow up in patients treated with lower doses. J Neurosurg. 2006;105(Suppl):64–8. doi: 10.3171/sup.2006.105.7.64. [DOI] [PubMed] [Google Scholar]
- 22.Pan DH, Guo WY, Chang YC, et al. The effectiveness and factors related to treatment results of gamma knife radiosurgery for meningiomas. Stereotact Funct Neurosurg. 1998;70(Suppl 1):19–32. doi: 10.1159/000056403. [DOI] [PubMed] [Google Scholar]
- 23.Inoue H, Negishi M, Zama A, et al. Fractionated Gamma Knife radiotherapy: Experimental studies and follow-up studies of clinical trials. Kitakanto Med J. 1997;47:217–22. [Google Scholar]
- 24.Inoue HK, Hirato M, Zama A, et al. Long-term results of fractionated radiotherapy using a Gamma Unit: A study of the biologically effective dose compared with radiosurgery. Stereotactic Radiotherapy. 1999;3:75–9. (Jpn, Eng. Abst.) [Google Scholar]
- 25.Otsuka S, Shibamoto Y, Iwata H, et al. Compatibility of the linear-quadratic formalism and biologically effective dose concept to high-dose-per-fraction irradiation in a murine tumor. Int J Radiat Oncol Biol Phys. 2011;81:1538–43. doi: 10.1016/j.ijrobp.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 26.Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94:899–904. doi: 10.3171/jns.2001.94.6.0899. [DOI] [PubMed] [Google Scholar]
- 27.Inoue H, Seto K, Nozaki A, et al. Optimal multisession radiosurgery for large brain metastases in critical areas. Stereotact Funct Neurosurg. 2012;90(Suppl 1):106. [Google Scholar]