Abstract
Penile carcinoma (PeCa) represents an important public health problem in poor and developing countries. Despite its unpredictable behavior and aggressive treatment, there have only been a few reports regarding its molecular data, especially epigenetic mechanisms. The functional diversity in different cell types is acquired by chromatin modifications, which are established by epigenetic regulatory mechanisms involving DNA methylation, histone acetylation, and miRNAs. Recent evidence indicates that the dysregulation in these processes can result in the development of several diseases, including cancer. Epigenetic alterations, such as the methylation of CpGs islands, may reveal candidates for the development of specific markers for cancer detection, diagnosis and prognosis. There are a few reports on the epigenetic alterations in PeCa, and most of these studies have only focused on alterations in specific genes in a limited number of cases. This review aims to provide an overview of the current knowledge of the epigenetic alterations in PeCa and the promising results in this field. The identification of epigenetically altered genes in PeCa is an important step in understanding the mechanisms involved in this unexplored disease.
Keywords: penile cancer, epigenetic, DNA methylation, molecular markers
1. Introduction
Penile carcinoma (PeCa), although relatively rare in developed countries of the world, is associated with high morbidity and mortality rates in poor and developing countries. The incidence of PeCa varies among populations, with an incidence rate of 0.58 and 0.84 per 100,000 males in United States and in the Western world, respectively [1,2]. In contrast, this rate is higher in developing countries, ranging from 2.3 to 8 cases per 100,000 men [3]. In Brazil, the incidence rate of penile cancer varies between 2.9 and 6.8 per 100,000 men and represents 2.1% of all cancer cases in men [4,5]. The mean age at presentation of penile cancer is 60 years [6].
The involvement of regional lymph nodes is the best indicator of long-term survival in patients with invasive penile carcinomas [7]. In addition to lymph node metastasis, other pathological factors, including grade, histological type, lymphovascular embolization, and stage and perineural invasion, have been described to be of prognostic value in PeCa [8–10]. However, none of them can effectively predict outcome.
There is a lack of information regarding the molecular genetic and epigenetic alterations and PeCa. Several studies in other cancer types have focused on epigenetic alterations, and there are promising data from clinical trials regarding the targeting of genes that regulate epigenetic events [11,12]. However, the published studies on PeCa are limited to the evaluation of CpG islands in specific genes.
2. Risk Factors: Human Papillomavirus as One of the Main Actors
Several risk factors have been associated with the development of malignant penile lesions. The most important risk factors are the presence of phimosis [13], poor genital hygiene [14], tobacco usage [15], and human papillomavirus (HPV) infection [16].
A history of phimosis is found in approximately 25% of penile cancer patients [2,17] and is strongly associated with invasive PeCa [17,18]. While circumcision shortly after birth is a protective factor, it does not have the same protective potential when carried out later in life [19]. The lack of circumcision, together with poor genital hygiene, contributes to the accumulation of smegma (which forms from desquamated epithelial cells) and consequently to the risk of developing PeCa [14]. The treatment of psoriasis patients with psoralen and ultraviolet A photo chemotherapy has also been identified as a risk factor for PeCa [20].
Human papillomavirus infection has been reported to have an important role in the development of a subset of PeCa, and its presence is thought to be related to the histological type [16]. Basaloid and warty penile cancers are very frequently HPV-positive, ranging from about 80% to 100%. Conversely, viral DNA is only found in a small fraction of verrucous penile carcinomas [20]. High-risk HPVs exert their oncogenic effect by expressing the oncoproteins, E6 and E7, which bind to and inactivate the tumor suppressor proteins, p53 and Rb, respectively [21].
It has been hypothesized that penile squamous cell carcinomas (PeSCC) arise by two distinct etiologic pathways. One is mediated by HPV infection and most likely involves sexual contact. The second occurs via a non-viral pathway and is related to other risk factors, such as poor genital hygiene and the presence of phimosis [22,23]. The incidence of HPV DNA found in penile carcinoma tissue ranges from 15% to 78% and varies according to the population studied, the method of specimen collection, and the protocol used for HPV detection [24].
In general, HPV infection, mainly by the HPV-16 and HPV-18 genotypes, is found in approximately 50% of all PeSCC [16,25] and is associated with multiple sexual partners [26]. Reports have indicated a positive association between the incidence of HPV infection in male sexual partners of women who had been diagnosed with cervical neoplasia [27]. Men vaccinated with the quadrivalent HPV vaccine that protects against HPV 6/11/16/18 have also been shown to have significantly less HPV-associated anogenital infection and PeCa [28].
Epstein-Barr virus (EBV) is another sexually transmitted virus that could be a cofactor in cancer development. The oncogenic potential of EBV is well known in lymphoproliferative disorders, such as Hodgkin’s lymphoma, Burkitt’s lymphoma, and lymphoma in immune compromised individuals [29]. The association between EBV and HPV has also been suggested in cervical cancer [30]. EBV is one of the most efficient cellular growth-transforming viruses known, and it has been found frequently in the genital mucosa, urethral discharges, and genital ulcers. In penile cancer, Epstein-Barr virus positivity was found in 46.7% of cases, and more than 23% of the men were co-infected with both HPV and EBV [31].
In addition, a number of other penile conditions have been associated with an increased risk of PeCa, including balanitis xerotica obliterans, Bowen’s disease, erythroplasia of Queyrat, bowenoid papulosis, and giant condyloma [32]. Bowen’s disease, erythroplasia of Queyrat, and bowenoid papulosis are uncommon pre-malignant disorders of the anogenital skin that may be confused with a variety of other lesions. Bowen’s disease has been reported to degenerate into invasive carcinoma in 5% to 10% of cases [33], while erythroplasia of Queyrat has been associated with invasive carcinoma in up to 30% of cases [34]. Penile lichen sclerosus, also known as balanitis xerotica obliterans, is a chronic inflammatory disorder that occurs in men of all ages [35], and which is associated with PeSCC in 4% to 6% of patients [36].
Overall, PeCa seems to be a multifactorial disease with several risk factors, including HPV and EBV infection, phimosis, poor hygiene habits, and tobacco usage. Several penile diseases have also been associated with a higher risk of developing PeCa.
3. Histopathological Data: Involvement of Lymph Nodes as the Major Predictive Factor of Poor Prognosis in Penile Cancer
Penile cancer usually originates in the epithelium of the inner prepuce (21% of cases), and the glans (48% of cases) [37]. Most penile malignancies (95%) are squamous cell carcinomas (SCCs). However, PeSCC represents a heterogeneous group of histopathological entities that differ in terms of morphology, pathogenesis, and prognosis [38]. The most common PeSSC type is the usual, followed by verrucous, papillary, basaloid, sarcomatoid, warty, cuniculatum, pseudohyperplastic, adenosquamous, and acantholytic [39]. The histological subtypes carry different risks of developing metastasis to lymph nodes, with usual SCC having a risk of 56.7% and sarcomatoid carcinoma having a risk of 89% [40].
PeSCC can also be divided into five categories according to its way of growth as follows: superficially spreading, vertical growth, verrucous, multicentric, and mixed [41]. Superficially spreading squamous cell cancers occur most frequently, and lymph node metastasis are present in 42% of cases. Lesions with a deeper vertical growth present with positive lymph nodes in 82% of cases. Multicentric lesions have positive lymph nodes in 33% of cases, whereas verrucous lesions rarely present with metastasis to the lymph nodes [42]. The pattern of growth in PeSCC and the depth of invasion are important prognostic determinants [10]. The histopathological grading is based on the Broder’s system that classifies the tumors in well-differentiated tumors (grade I) to undifferentiated and invasive tumors (grade IV) [43]. Two staging systems are used in penile carcinoma: the Jackson classification [44], and the TNM classification [45].
The presence of metastasis in regional lymph nodes is the main factor predicting an unfavorable prognosis for patients with PeSCC [9]. Palpable inguinal lymphadenopathy is present at diagnosis in 58% of patients (range from 20% to 96%), and metastatic carcinoma in 45% of these patients [37]. In the early stages of the disease, radical inguinal lymphadenectomy has been demonstrated to convey survival benefits [7,46]. However, these surgical techniques are limited by their high rates of morbidity and mortality [47]. Recent studies have shown new surgical techniques that have improved patient survival [48,49].
The prognosis of patients with lymph node metastasis varies according to the number of positive lymph nodes, the presence of uni- or bilateral inguinal extension, pelvic node involvement, and the presence of lymph node capsular involvement [9].
Kattan et al. [50] and Ficarra et al. [51] developed nomograms to predict inguinal lymph node involvement and the five-year cancer-specific survival of PeCa patients. These predictive models of patient outcome integrated the information about inguinal lymph node stage, pathologic tumor thickness, growth pattern, histologic grade, lymphatic and venous embolization, corpora cavernosa infiltration, corpus spongiosum, and urethral infiltration. Although nomograms allow improvements in prognostic accuracy compared with the use of each single variable, their use in clinical practice is potentially limited by the lack of external validation [9].
The studies evaluating the impact of HPV infection on the prognosis of patients with PeCa are controversial. Some studies have found an association between HPV positive infection and poor prognosis [52,53], while others have suggested that HPV status does not influence prognosis in invasive penile carcinoma [54–56]. HPV infection has also been related to favorable prognosis, as reported by Lont et al. [57], who showed a five-year cancer-specific survival rate of 92% for HPV-positive and 78% for HPV-negative patients. In the same study, the presence of positive lymph nodes was detected in 71% of HPV-negative cases, compared to 29% of HPV-positive patients.
4. Epigenetic Alterations and Cancer: Emerging Potential Markers of Diagnosis, Prognosis, and Therapy
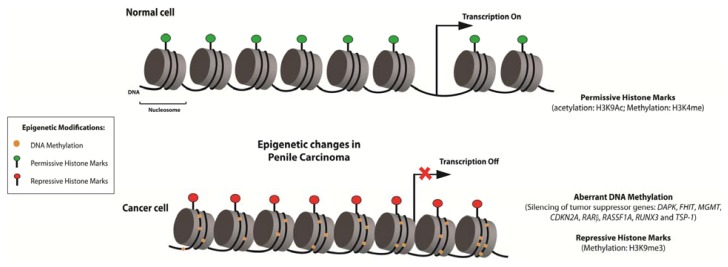
Epigenetic modifications are potentially reversible alterations in DNA methylation or chromatin that are not associated with changes in the DNA sequence. These modifications specify functional outputs from the DNA template and are often heritable through cell division [58–61]. The epigenetic regulatory mechanisms are comprised of DNA methylation, histone modifications, and transcriptional alterations induced by noncoding RNAs. Aberrant epigenetic regulation can lead to alterations in global gene expression and genomic instability, which have been shown to have clear implications in the development of cancer [62].
DNA methylation changes include locus-targeted hypermethylation and global hypomethylation [63,64]. DNA methylation is catalyzed by a family of enzymes called DNA methyltransferases (DNMTs). These enzymes transfer a methyl group, donated by S-adenosylmethionine (SAM), to the fifth position carbon of cytosine. Three catalytically active DNMTs, DNMT1, DNMT3A, and DNMT3B are described in the mammals genome [65].
In mammals, DNA methylation primarily occurs by the covalent modification of cytosine residues in CpG dinucleotides. CpG dinucleotides are not evenly distributed across the human genome, but they are instead concentrated in short CpG-rich DNA stretches called “CpG islands” and in regions of large repetitive sequences [66–68].
CpG islands are preferentially located at the 5′ end of genes and occupy approximately 60% of human gene promoters [69]. While most of the CpG sites in the genome are methylated, the majority of CpG islands usually remain unmethylated during development and in differentiated tissues. However, some CpG island promoters become methylated during cancer development and progression. In contrast, the repetitive genomic sequences, retrotransposons, introns, and gene deserts, which are scattered throughout the human genome, become unmethylated during tumorigenesis. The global hypomethylation of these DNA regions during cancer development leads to increased genomic instability and results in chromosomal rearrangement [67,70].
The investigation of aberrant CpG island methylation has primarily been carried out using a candidate gene approach [71]. Several methods can be used to determine methylation patterns [67], including methylation-specific polymerase chain reaction (MSP) [72], MethyLight [73], combined bisulfite restriction analysis (COBRA) [74], methylation-specific single-nucleotide primer extension (MS-SNuPE) [75], methylation sensitive high resolution melting (MS-HRM) [76], and quantitative bisulfite pyrosequencing [77]. The gold standard technique to detect DNA methylation at a specific locus is quantitative bisulfite pyrosequencing, which analyzes bisulfite-modified and PCR-amplified DNA and provides information on the methylation status of individual CpG sites [77].
A large number of techniques are available for studying global DNA methylation. Genome-wide approaches can be broadly grouped into three strategies according to how DNA is modified before it is interrogated using microarrays or next generation sequencing platforms. These modifications include bisulfite converted DNA, affinity assays that precipitate methylated DNA (MeDIP and MCIP), and restriction enzyme methods that recognize methylated and unmethylated sequences (CHARM, LUMA, HELP) [78–83]. After the initial DNA enrichment or chemical modification, genome-wide analyses can be performed by array hybridization systems, such as the Illumina Infinium and GoldenGate systems (Illumina, Inc., San Diego, CA, USA), or oligonucleotide tiling arrays, such as the Nimblegen (Roche NimbleGen, Madison, WI, USA) and Agilent CpG Islands plus Promoters arrays (Agilent, Santa Clara, CA, USA), pyrosequencers, and next generation sequencing platforms such as Illumina/Solexa, ABI/SOLiD, Roche 454 and Helicos/Single molecule sequencing [84,85]. Although some of these techniques present biases or limitations, they are still useful for interrogating epigenetic marks, especially DNA methylation profiles.
Identifying changes in the methylation profile in tumors allows the identification of molecular markers for diagnosis and prognosis in cancer that could also be translated into therapeutic targets [77]. Wei et al. [86] reported that global hypomethylation was associated with worse prognosis or recurrence after treatment in ovarian cancer patients. In addition, methylation profiles have been demonstrated as important tools for the diagnosis of disease and the prediction of disease progression [86–89].
The cytosine methylation in CpG islands at promoter regions provides a stable gene silencing mechanism that plays an important role in regulating gene expression and chromatin architecture. Methylation often occurs in association with histone modifications and other chromatin-associated proteins. Histone proteins, which comprise the nucleosome core, contain a globular C-terminal domain and an unstructured N-terminal tail [90]. Post-translational modifications of histone tails determine which regions of the genome are in a transcriptionally active conformation or in a transcriptionally inactive form. The modifications of histone tails include acetylation, methylation, ubiquitylation, phosphorylation, sumoylation, and ribosylation. Each of these modifications regulate key cellular processes, such as transcription, replication, and repair [91–93].
Histone modifications can lead to either the activation or the repression of target genes, depending on the specific residues modified and the type of modifications present. Several active and repressive histone modifications have been identified, and these constitute a complex gene regulatory network in cells, which is known as the “histone code” [94]. The importance of epigenetic regulation is highlighted by the disruption of multiple epigenetic marks in various disease states. This is commonly associated with the deregulation of miRNA expression.
miRNAs are small, noncoding RNAs that regulate gene expression at the posttranscriptional level and are critical in many biological processes and cellular pathways [95,96]. miRNA expression profiles of human cancers have been described in several tumors, and the main causes of the aberrant miRNA expression patterns are DNA copy number alterations, the failure of miRNA post-transcriptional regulation, and genetic mutation or transcriptional silencing associated with the hypermethylation of CpG island promoters [96–101]. Recent studies have identified a number of miRNAs as potential biomarkers of diagnosis and prognosis, as well as targets for cancer therapy [102].
In recent years, remarkable progress has been made in target identification, drug discovery, and clinical validation for epigenetic therapeutics [103]. Inhibitors of two classes of epigenetic enzymes, i.e., DNA methyltransferases (DNMTs) and histone deacetylases (HDACs), have already demonstrated utility as molecularly targeted chemotherapeutic agents for specific cancers and have received approval for these indications [11]. Furthermore, a database called HEMD (Human Epigenetic Enzyme & Modulator Database), which integrates human epigenetic enzymes and their modulators, has been developed to facilitate the investigation of epigenetic mechanisms and to provide subsidies for novel drug design [104].
Epigenetic alterations are present in all steps of cancer development and progression. With the improvement of techniques in the epigenetic field, especially those identifying global profiles, potential markers for diagnosis, prognosis and therapy have emerged for a series of tumors. Large-scale studies were also important in improving the knowledge about the mechanisms involved in several cancers. However, there is a lack of information regarding both genetic and epigenetic factors that are involved in PeCa.
5. Epigenetics Studies in PeCa
To the best of our knowledge, there are eight studies in the literature describing epigenetic alterations in PeCa [105–112], most of which evaluated the methylation pattern of CpG islands in specific genes (Table 1). Six of these studies investigated the CpG island status of CDKN2A. The CDKN2A locus encodes two tumor suppressor proteins, p16INK4A and p14ARF, which control cell growth through the Rb-CDK4 and p53 pathways, respectively [113]. The tumor suppressor gene CDKN2A blocks the cyclin-dependent kinases 4 and 6, which are involved in the activation of the cell cycle and the inhibition of CDK-mediated phosphorylation of the RB gene. Furthermore, the epigenetically mediated loss of CDKN2A is one of the most common and earliest events in human cancers [114].
Table 1.
Summary of the epigenetics studies in penile carcinomas described in the literature.
| References | Number of samples | Method | Gene studied | % methylation | HPV infection | HPV 16 |
|---|---|---|---|---|---|---|
| Ferreux et al. [105] | 53 | Methylation-specific PCR | CDKN2A | 9 (17%) | 20 (38%) | 15 (28%) |
| Poetsch et al. [112] | 52 | Methylation-specific PCR | CDKN2A | 22 (42%) | 20 (38%) | 18 (35%) |
| Soufir et al. [106] | 3 | Methylation-specific PCR | CDKN2A | 0 (0%) | 2 (66.3%) | 2 (100%) |
| Guerreto et al. [108] | 24 | Methylation-specific PCR |
CDKN2A RASSF1A TSP-1 |
9 (38%) 10 (42%) 11 (46%) |
11 (46%) | 10 (42%) |
| Yanagawa et al. [109] | 26 | Methylation-specific PCR |
DAPK FHIT MGMT CDKN2A (p16INK4A) CDKN2A (p14ARF) RARβ RASSF1A RUNX3 |
7 (26.9%) 23 (88.4%) 5 (19.2%) 1 (3.8%) 6 (23.1%) 6 (23.1%) 3 (11.5%) 11 (42.3%) |
3 (11.5%) | 3 (11.5%) |
| Yanagawa et al. [110] | 25 | Methylation-specific PCR |
DAPK FHIT MGMT CDKN2A (p16INK4A) CDKN2A (p14ARF) RARβ RASSF1A RUNX3 |
7 (28%) 23 (92%) 5(20%) 1 (4%) 6 (24%) 6 (24%) 3 (12%) 11 (44%) |
3 (12%) | 3 (12%) |
| Kalantari et al. [107] | 24 | DNA sequencing | L1 HPV16 LCR HPV16 |
58% 22% |
24 (100%) | 19 (79%) |
| Rogenhofer et al. [111] | 65 | Immunohistochemical | H3K4 H3K9 H3K27 |
H3K4me1 Decrease H3K9me1 Decrease H3K9me2 Decrease H3K27me2 Decrease H3K27me3 Decrease H3K9me3 Increase |
Considering all studies of methylation in PeCa, the promoter of CDKN2A has been globally investigated in 183 cases, and its methylation levels vary from 0% to 42% (Table 1). Reports with a larger number of individuals also presented a higher frequency of CDKN2A methylation [105,108,112]. Three studies evaluated the same CpG island and an amplicon with 150 bp [106,108,112] (Figure 1). Two of these studies found similar frequencies of methylation [108,112] (Table 1); however, Soufir et al. [106] reported 0% of methylation level. The low frequency detected by these authors may be related to the small number of invasive carcinomas studied (3 samples) or to HPV infection, which was found in two out of three PeCa samples. The CDKN2A primer sequences were not available in other studies [105,109,110].
Figure 1.
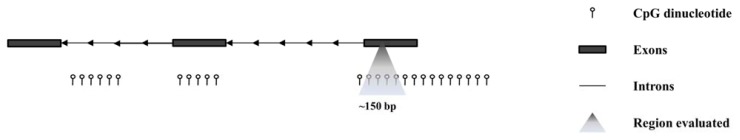
CpG islands described on CDKN2A gene. Three studies evaluated the same CpG island in an amplicon with 150 bases pair [106,108,112].
Ferreux et al. [105] suggested at least three plausible mechanisms that could be involved in the disruption of the p16INK4A/cyclinD/Rb pathway during penile carcinogenesis, specifically, high-risk HPV infection, CDKN2A promoter methylation and BMI-1 overexpression, which is an alternative mechanism that down-regulates p16INK4A [115]. A significant overexpression of BMI-1 was detected in tumors without methylation of the CDKN2A gene promoter. The data revealed that strong p16INK4A immunostaining was significantly associated with carcinomas positive for high-risk HPV. In addition, the frequency of CDKN2A promoter methylation was higher in HPV-negative tumors than in positive cases. According to Guerrero et al. [108], the hypermethylation of CDKN2A was correlated with negative and weak expression of the p16 protein, and all of the HPV-negative cases had weak or no p16 expression. The difference in p16 expression and the methylation patterns of CDKN2A between HPV positive and negative cases reinforce the hypothesis that PeCa is etiologically heterogeneous and may develop by distinct pathways. Furthermore, in other squamous cell carcinomas, there is evidence that differences in HPV status can lead to different tumor behavior and patient prognosis [116,117].
Poetsch et al. [112] investigated the effect of loss of heterozygosity (LOH), immunohistochemistry, point mutations and promoter methylation of CDKN2A. Fifty percent of primary PeCa showed p16INK4A overexpression, and cases that were negative for p16INK4A expression showed LOH near the CDKN2A locus and/or hypermethylation of the gene promoter. The absence of p16INK4A protein expression, LOH and promoter hypermethylation was significantly associated with the occurrence of lymph node metastasis. While p16 overexpression is almost always associated with the presence of HPV DNA in cervical carcinomas, Poetsch et al. [112] showed that the overexpression of p16INK4A, which is a frequent event in penile carcinomas, occurs in both HPV-positive and HPV-negative cases. The authors suggested that other pathways leading to the coactivation of p53 and p16INK4A that are independent of HPV must be considered because they found the expression of p16INK4A and p53 without the presence of HPV-DNA.
The reported methylation frequencies for RASSF1A varied from 11.5% to 45% (Table 1) [109,110]. Guerrero et al. [108] also investigated the expression of Thrombospondin-1 (TSP-1) and the methylation status of its promoter region. TSP-1 is a cell adhesion glycoprotein secreted by several types of normal cells and by tumor cells [118]. The hypermethylation of the TSP1 gene was associated with unfavorable histological grade, vascular and tumor invasion, weak expression of TSP-1 protein and shorter overall survival. The association of the hypermethylation of TSP1 with poor prognosis makes it a potential marker that could be used to detect more aggressive penile tumors. The methylation pattern of eight genes, i.e., DAPK, FHIT, MGMT, CDKN2A (region p16INK4A and p14ARF), RARβ, RASSF1A, and RUNX3, revealed that at least one of them was methylated in each case [109]. In particular, the tumor suppressor gene, FHIT, and the gene, RUNX3, were methylated in 88% and 42% of the cases, respectively. Subsequently, the authors evaluated the same genes in 25 PeCa cases and included the FHIT protein expression by immunohistochemical staining [110]. Hypermethylation was detected in 92% of the cases, and decreased expression levels of FHIT protein were shown in 88% of cases. Twenty out of the 22 cases negative for FHIT protein expression showed FHIT methylation. Five genes, DAPK, MGMT, CDKN2A, RARβ, and RUNX3, were methylated in more than 20% of the cases. According to the authors, because the methylation of the FHIT gene was more common than the presence of HPV infection, which occurred in less than 5% of patients, this gene might play an important role in the pathogenesis of penile squamous cell carcinoma.
Recently, Rogenhofer et al. [111] evaluated the global methylation levels of the histones, H3K4, H3K9 and H3K27, on a tissue microarray platform containing 65 penile carcinomas, six metastatic lesions, and 30 normal skin samples by immunohistochemistry. A variation in the overall level of histone methylation was detected between normal and tumor samples. The overall levels of H3K4me1, H3K9me1, H3K9me2, H3K27me2, and H3K27me3 were decreased, whereas H3K9me3 levels were increased in PeCa. Hierarchical clustering analysis demonstrated that cancer and normal tissues were differentiated based on the histone methylation pattern of H3K9 and H3K27. A trend towards increased global histone methylation levels was detected in metastasis, and high H3K9me2 levels could be related to poor outcomes in PeCa patients. The epigenetic alterations described in PeCa are summarized in Figure 2.
Figure 2.
Epigenetic alterations were described in eight studies of penile carcinoma (PeCa). Aberrant DNA methylation pattern of DAPK, FHIT, MGMT, CDKN2A, RARβ, RASSF1A, TSP-1, and RUNX3 and alteration in the expression levels of histones were events related with this disease. In special, CDKN2A gene was evaluated in six studies.
At present, only a single study has investigated the pattern of methylation of genes in the HPV virus of men with PeCa. According to Kalantari et al. [107] the mechanisms involved in penile carcinogenesis related to HPV infection are similar to those involved in cervical carcinoma. The authors investigated three properties of the HPV genomes in penile carcinomas patients, specifically, the methylation of HPV DNA, the junctions between HPV and cellular DNA, and the genomic variation. The authors found that the HPV16 and 18 L1 genes showed similar patterns of hypermethylation in penile and cervical carcinomas, and as such, the methylation of HPV16 and 18 L1 DNA can serve as a biomarker of integration between HPV and cellular DNA in PeCa.
In summary, a substantial variability of methylation has been described for CDKN2A and RASSF1A in PeCa. Additionally, gene silencing through CpG island hypermethylation and FHIT downregulation have been suggested as potential markers in PeCa. Although several genes have been described to be epigenetically regulated in PeCa, the available data are limited, and only a few reports have confirmed the analysis using gene or protein expression.
6. Future Perspectives and Direction
It has been established that epigenetic changes are critical for the development and progression of several tumors. The majority of studies regarding epigenetic alterations in PeCa have only evaluated the patterns of specific genes. However, the assessment of relevant markers for this disease requires methods that detect alterations in methylation on a genome-wide level. Large-scale studies in PeCa are needed to better comprehend tumor behavior and to determine the molecular markers involved in this disease. In a recent review in penile cancer, Sonpavde et al. [119] emphasized the importance of a better understanding of the basic biology of PeCa to guide the design of clinical trials. In our opinion, given the significant number of PeCa cases that are positive for HPV and EBV infections, it is a necessity to investigate epigenetic alterations based on these patterns using more robust, genome-wide methods. These studies may identify new molecular markers that could be useful for designing effective therapeutic strategies against this clinically and psychologically aggressive disease.
Acknowledgments
The authors would like to thank the National Council of Technological and Scientific Development (CNPq), Coordination for the Improvement of Higher Level Personnel (CAPES) and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP: 2010/51601-6 and 2009/52088-3). Authors would like to thank Cláudia A. Rainho for her critical review and support with the figures.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Parkin D., Whelan S., Ferlay J., Teppo L., Thomas D. Cancer Incidence in Five Continents. VIII IARC Scientific Publications; Lyon, France: 2002. [Google Scholar]
- 2.Barnholtz-Sloan J.S., Maldonado J.L., Pow-sang J., Giuliano A.R., Guiliano A.R. Incidence trends in primary malignant penile cancer. Urol. Oncol. 2007;25:361–367. doi: 10.1016/j.urolonc.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Misra S., Chaturvedi A., Misra N.C. Penile carcinoma: A challenge for the developing world. Lancet Oncol. 2004;5:240–247. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- 4.Solsona E., Algaba F., Horenblas S., Pizzocaro G., Windahl T., Urology E.A.O. Eau guidelines on penile cancer. Eur. Urol. 2004;46:1–8. doi: 10.1016/j.eururo.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Favorito L.A., Nardi A.C., Ronalsa M., Zequi S.C., Sampaio F.J., Glina S. Epidemiologic study on penile cancer in Brazil. Int. Braz. J. Urol. 2008;34:587–591. doi: 10.1590/s1677-55382008000500007. [DOI] [PubMed] [Google Scholar]
- 6.Daling J.R., Madeleine M.M., Johnson L.G., Schwartz S.M., Shera K.A., Wurscher M.A., Carter J.J., Porter P.L., Galloway D.A., McDougall J.K., et al. Penile cancer: Importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int. J. Cancer. 2005;116:606–616. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 7.Ornellas A.A., Kinchin E.W., Nóbrega B.L., Wisnescky A., Koifman N., Quirino R. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian national cancer institute long-term experience. J. Surg. Oncol. 2008;97:487–495. doi: 10.1002/jso.20980. [DOI] [PubMed] [Google Scholar]
- 8.Calmon M.F., Tasso Mota M., Vassallo J., Rahal P. Penile carcinoma: Risk factors and molecular alterations. ScientificWorldJournal. 2011;11:269–282. doi: 10.1100/tsw.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ficarra V., Akduman B., Bouchot O., Palou J., Tobias-Machado M. Prognostic factors in penile cancer. Urology. 2010;76:S66–S73. doi: 10.1016/j.urology.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Lopes A., Hidalgo G.S., Kowalski L.P., Torloni H., Rossi B.M., Fonseca F.P. Prognostic factors in carcinoma of the penis: Multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J. Urol. 1996;156:1637–1642. doi: 10.1016/s0022-5347(01)65471-5. [DOI] [PubMed] [Google Scholar]
- 11.Copeland R.A., Olhava E.J., Scott M.P. Targeting epigenetic enzymes for drug discovery. Curr. Opin. Chem. Biol. 2010;14:505–510. doi: 10.1016/j.cbpa.2010.06.174. [DOI] [PubMed] [Google Scholar]
- 12.Nebbioso A., Carafa V., Benedetti R., Altucci L. Trials with “epigenetic” drugs: An update. Mol. Oncol. 2012;6:657–682. doi: 10.1016/j.molonc.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillner J., von Krogh G., Horenblas S., Meijer C.J. Etiology of squamous cell carcinoma of the penis. Scand. J. Urol. Nephrol. Suppl. 2000;205:189–193. doi: 10.1080/00365590050509913. [DOI] [PubMed] [Google Scholar]
- 14.Kochen M., McCurdy S. Circumcision and the risk of cancer of the penis. A life-table analysis. Am. J. Dis. Child. 1980;134:484–486. doi: 10.1001/archpedi.1980.02130170034012. [DOI] [PubMed] [Google Scholar]
- 15.Harish K., Ravi R. The role of tobacco in penile carcinoma. Br. J. Urol. 1995;75:375–377. doi: 10.1111/j.1464-410x.1995.tb07352.x. [DOI] [PubMed] [Google Scholar]
- 16.Miralles-Guri C., Bruni L., Cubilla A.L., Castellsagué X., Bosch F.X., de Sanjosé S. Human papillomavirus prevalence and type distribution in penile carcinoma. J. Clin. Pathol. 2009;62:870–878. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- 17.Tsen H.F., Morgenstern H., Mack T., Peters R.K. Risk factors for penile cancer: Results of a population-based case-control study in los angeles county (united states) Cancer Causes Control. 2001;12:267–277. doi: 10.1023/a:1011266405062. [DOI] [PubMed] [Google Scholar]
- 18.Madsen B.S., van den Brule A.J., Jensen H.L., Wohlfahrt J., Frisch M. Risk factors for squamous cell carcinoma of the penis—Population-based case-control study in Denmark. Cancer Epidemiol. Biomark. Prev. 2008;17:2683–2691. doi: 10.1158/1055-9965.EPI-08-0456. [DOI] [PubMed] [Google Scholar]
- 19.Schoen E.J., Oehrli M., Colby C., Machin G. The highly protective effect of newborn circumcision against invasive penile cancer. Pediatrics. 2000;105:E36. doi: 10.1542/peds.105.3.e36. [DOI] [PubMed] [Google Scholar]
- 20.Gross G., Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med. Microbiol. Immunol. 2004;193:35–44. doi: 10.1007/s00430-003-0181-2. [DOI] [PubMed] [Google Scholar]
- 21.Zur Hausen H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 22.Rubin M.A., Kleter B., Zhou M., Ayala G., Cubilla A.L., Quint W.G., Pirog E.C. Detection and typing of human papillomavirus dna in penile carcinoma: Evidence for multiple independent pathways of penile carcinogenesis. Am. J. Pathol. 2001;159:1211–1218. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaux A., Cubilla A.L. The role of human papillomavirus infection in the pathogenesis of penile squamous cell carcinomas. Semin. Diagn. Pathol. 2012;29:67–71. doi: 10.1053/j.semdp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Pascual A., Pariente M., Godínez J.M., Sánchez-Prieto R., Atienzar M., Segura M., Poblet E. High prevalence of human papillomavirus 16 in penile carcinoma. Histol. Histopathol. 2007;22:177–183. doi: 10.14670/HH-22.177. [DOI] [PubMed] [Google Scholar]
- 25.Backes D.M., Kurman R.J., Pimenta J.M., Smith J.S. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449–457. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- 26.Schottenfeld D., Beebe-Dimmer J. Alleviating the burden of cancer: A perspective on advances, challenges, and future directions. Cancer Epidemiol. Biomarkers Prev. 2006;15:2049–2055. doi: 10.1158/1055-9965.EPI-06-0603. [DOI] [PubMed] [Google Scholar]
- 27.Rombaldi R.L., Serafini E.P., Villa L.L., Vanni A.C., Baréa F., Frassini R., Xavier M., Paesi S. Infection with human papillomaviruses of sexual partners of women having cervical intraepithelial neoplasia. Braz. J. Med. Biol. Res. 2006;39:177–187. doi: 10.1590/s0100-879x2006000200003. [DOI] [PubMed] [Google Scholar]
- 28.Gillison M.L., Chaturvedi A.K., Lowy D.R. Hpv prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Daraji W.I., Smith J.H. Infection and cervical neoplasia: Facts and fiction. Int. J. Clin. Exp. Pathol. 2009;2:48–64. [PMC free article] [PubMed] [Google Scholar]
- 30.Sasagawa T., Shimakage M., Nakamura M., Sakaike J., Ishikawa H., Inoue M. Epstein-barr virus (ebv) genes expression in cervical intraepithelial neoplasia and invasive cervical cancer: A comparative study with human papillomavirus (hpv) infection. Hum. Pathol. 2000;31:318–326. doi: 10.1016/s0046-8177(00)80245-2. [DOI] [PubMed] [Google Scholar]
- 31.Afonso L.A., Moyses N., Alves G., Ornellas A.A., Passos M.R., Oliveira L.O.H., Cavalcanti S.M. Prevalence of human papillomavirus and epstein-barr virus dna in penile cancer cases from brazil. Mem. Inst. Oswaldo. Cruz. 2012;107:18–23. doi: 10.1590/s0074-02762012000100003. [DOI] [PubMed] [Google Scholar]
- 32.Buechner S.A. Common skin disorders of the penis. BJU Int. 2002;90:498–506. doi: 10.1046/j.1464-410x.2002.02962.x. [DOI] [PubMed] [Google Scholar]
- 33.Schellhammer P.F., Jordan G.H., Robey E.L., Spaulding J.T. Premalignant lesions and nonsquamous malignancy of the penis and carcinoma of the scrotum. Urol. Clin. N. Am. 1992;19:131–142. [PubMed] [Google Scholar]
- 34.Wieland U., Jurk S., Weissenborn S., Krieg T., Pfister H., Ritzkowsky A. Erythroplasia of queyrat: Coinfection with cutaneous carcinogenic human papillomavirus type 8 and genital papillomaviruses in a carcinoma in situ. J. Invest. Dermatol. 2000;115:396–401. doi: 10.1046/j.1523-1747.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 35.Val I., Almeida G. An overview of lichen sclerosus. Clin. Obstet. Gynecol. 2005;48:808–817. doi: 10.1097/01.grf.0000179635.64663.3d. [DOI] [PubMed] [Google Scholar]
- 36.Tasker G.L., Wojnarowska F. Lichen sclerosus. Clin. Exp. Dermatol. 2003;28:128–133. doi: 10.1046/j.1365-2230.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- 37.Sufrin G., Huber R. Benign and Malignant Lesions of the Penis. J.Y. Gillenwater; St. Louis, MO, USA: 1991. pp. 1997–2042. [Google Scholar]
- 38.Mosconi A.M., Roila F., Gatta G., Theodore C. Cancer of the penis. Crit. Rev. Oncol. Hematol. 2005;53:165–177. doi: 10.1016/j.critrevonc.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Chaux A., Velazquez E.F., Algaba F., Ayala G., Cubilla A.L. Developments in the pathology of penile squamous cell carcinomas. Urology. 2010;76:S7–S14. doi: 10.1016/j.urology.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 40.Pizzocaro G., Algaba F., Horenblas S., Solsona E., Tana S., van der Poel H., Watkin N.A. Eau penile cancer guidelines 2009. Eur. Urol. 2010;57:1002–1012. doi: 10.1016/j.eururo.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 41.Cubilla A.L., Velazquez E.F., Young R.H. Epithelial lesions associated with invasive penile squamous cell carcinoma: A pathologic study of 288 cases. Int. J. Surg. Pathol. 2004;12:351–364. doi: 10.1177/106689690401200408. [DOI] [PubMed] [Google Scholar]
- 42.Cubilla A.L., Barreto J., Caballero C., Ayala G., Riveros M. Pathologic features of epidermoid carcinoma of the penis. A prospective study of 66 cases. Am. J. Surg. Pathol. 1993;17:753–763. doi: 10.1097/00000478-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Lucia M.S., Miller G.J. Histopathology of malignant lesions of the penis. Urol. Clin. N. Am. 1992;19:227–246. [PubMed] [Google Scholar]
- 44.Jackson S.M. The treatment of carcinoma of the penis. Br. J. Surg. 1966;53:33–35. doi: 10.1002/bjs.1800530108. [DOI] [PubMed] [Google Scholar]
- 45.Sobin L., Wittekind C. TNM Classification of Malignant Tumours. 6th ed. Wiley-Liss; New York, NY, USA: 2002. [Google Scholar]
- 46.Kroon B.K., Horenblas S., Lont A.P., Tanis P.J., Gallee M.P., Nieweg O.E. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J. Urol. 2005;173:816–819. doi: 10.1097/01.ju.0000154565.37397.4d. [DOI] [PubMed] [Google Scholar]
- 47.Ravi R. Morbidity following groin dissection for penile carcinoma. Br. J. Urol. 1993;72:941–945. doi: 10.1111/j.1464-410x.1993.tb16304.x. [DOI] [PubMed] [Google Scholar]
- 48.Britto C.A., Rebouças R.B., Lopes T.R., Silva da Costa T., Leite R.E.C., de Carvalho P.S. Video-assisted left inguinal lymphadenectomy for penile cancer. Int. Braz. J. Urol. 2012;38:289. doi: 10.1590/s1677-55382012000200020. [DOI] [PubMed] [Google Scholar]
- 49.Canter D.J., Dobbs R.W., Jafri S.M., Herrel L.A., Ogan K., Delman K.A., Master V.A. Functional, oncologic, and technical outcomes after endoscopic groin dissection for penile carcinoma. Can. J. Urol. 2012;19:6395–6400. [PubMed] [Google Scholar]
- 50.Kattan M.W., Ficarra V., Artibani W., Cunico S.C., Fandella A., Martignoni G., Novara G., Galetti T.P., Zattoni F., Members G.P.C.P. Nomogram predictive of cancer specific survival in patients undergoing partial or total amputation for squamous cell carcinoma of the penis. J. Urol. 2006;175:2103–2108. doi: 10.1016/S0022-5347(06)00313-2. [DOI] [PubMed] [Google Scholar]
- 51.Ficarra V., Zattoni F., Artibani W., Fandella A., Martignoni G., Novara G., Galetti T.P., Zambolin T., Kattan M.W. Penile Cancer Project Members. Nomogram predictive of pathological inguinal lymph node involvement in patients with squamous cell carcinoma of the penis. J. Urol. 2006;175:1700–1704. doi: 10.1016/S0022-5347(05)01003-7. [DOI] [PubMed] [Google Scholar]
- 52.Gregoire L., Cubilla A.L., Reuter V.E., Haas G.P., Lancaster W.D. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J. Natl. Cancer Inst. 1995;87:1705–1709. doi: 10.1093/jnci/87.22.1705. [DOI] [PubMed] [Google Scholar]
- 53.Burger R.A., Monk B.J., Kurosaki T., Anton-Culver H., Vasilev S.A., Berman M.L., Wilczynski S.P. Human papillomavirus type 18: Association with poor prognosis in early stage cervical cancer. J. Natl. Cancer Inst. 1996;88:1361–1368. doi: 10.1093/jnci/88.19.1361. [DOI] [PubMed] [Google Scholar]
- 54.Bezerra A.L., Lopes A., Santiago G.H., Ribeiro K.C., Latorre M.R., Villa L.L. Human papillomavirus as a prognostic factor in carcinoma of the penis: Analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer. 2001;91:2315–2321. [PubMed] [Google Scholar]
- 55.Kirrander P., Kolaric A., Helenius G., Windahl T., Andrén O., Stark J.R., Lillsunde-Larsson G., Elgh F., Karlsson M. Human papillomavirus prevalence, distribution and correlation to histopathological parameters in a large swedish cohort of men with penile carcinoma. BJU Int. 2011;108:355–359. doi: 10.1111/j.1464-410X.2010.09770.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhai J.P., Li M., Wang Q.Y., Wei D., Xu K.X. Correlation of cd82 and htert expressions and hpv infection with penile cancer. Zhonghua Nan Ke Xue. 2011;17:817–822. [PubMed] [Google Scholar]
- 57.Lont A.P., Kroon B.K., Horenblas S., Gallee M.P., Berkhof J., Meijer C.J., Snijders P.J. Presence of high-risk human papillomavirus dna in penile carcinoma predicts favorable outcome in survival. Int. J. Cancer. 2006;119:1078–1081. doi: 10.1002/ijc.21961. [DOI] [PubMed] [Google Scholar]
- 58.Herceg Z., Hernandez-Vargas H. New concepts of old epigenetic phenomena and their implications for selecting specific cell populations for epigenomic research. Epigenomics. 2011;3:383–386. doi: 10.2217/epi.11.64. [DOI] [PubMed] [Google Scholar]
- 59.Feinberg A.P., Ohlsson R., Henikoff S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 60.Jones P.A., Baylin S.B. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feinberg A.P. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 62.Willard S.S., Koochekpour S. Regulators of gene expression as biomarkers for prostate cancer. Am. J. Cancer Res. 2012;2:620–657. [PMC free article] [PubMed] [Google Scholar]
- 63.Jovanovic J., Rønneberg J.A., Tost J., Kristensen V. The epigenetics of breast cancer. Mol. Oncol. 2010;4:242–254. doi: 10.1016/j.molonc.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandoval J., Esteller M. Cancer epigenomics: Beyond genomics. Curr. Opin. Genet. Dev. 2012;22:50–55. doi: 10.1016/j.gde.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Bestor T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 66.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 67.Takai D., Jones P.A. Comprehensive analysis of CPG islands in human chromosomes 21 and 22. Proc. Natl. Acad. Sci. USA. 2002;99:3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki M.M., Bird A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y., Leung F.C. An evaluation of new criteria for CPG islands in the human genome as gene markers. Bioinformatics. 2004;20:1170–1177. doi: 10.1093/bioinformatics/bth059. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez J., Frigola J., Vendrell E., Risques R.A., Fraga M.F., Morales C., Moreno V., Esteller M., Capellà G., Ribas M., et al. Chromosomal instability correlates with genome-wide dna demethylation in human primary colorectal cancers. Cancer Res. 2006;66:8462–9468. doi: 10.1158/0008-5472.CAN-06-0293. [DOI] [PubMed] [Google Scholar]
- 71.Dahl C., Guldberg P. DNA methylation analysis techniques. Biogerontology. 2003;4:233–250. doi: 10.1023/a:1025103319328. [DOI] [PubMed] [Google Scholar]
- 72.Herman J.G., Graff J.R., Myöhänen S., Nelkin B.D., Baylin S.B. Methylation-specific PCR: A novel PCR assay for methylation status of CPG islands. Proc. Natl. Acad. Sci. USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eads C.A., Danenberg K.D., Kawakami K., Saltz L.B., Blake C., Shibata D., Danenberg P.V., Laird P.W. Methylight: A high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiong Z., Laird P.W. Cobra: A sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 1997;25:2532–2534. doi: 10.1093/nar/25.12.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalgo M.L., Jones P.A. Rapid quantitation of methylation differences at specific sites using methylation-sensitive single nucleotide primer extension (ms-snupe) Nucleic Acids Res. 1997;25:2529–2531. doi: 10.1093/nar/25.12.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wojdacz T.K., Dobrovic A. Methylation-sensitive high resolution melting (ms-hrm): A new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007;35:e41. doi: 10.1093/nar/gkm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tost J., Gut I.G. DNA methylation analysis by pyrosequencing. Nat. Protoc. 2007;2:2265–2275. doi: 10.1038/nprot.2007.314. [DOI] [PubMed] [Google Scholar]
- 78.Mohn F., Weber M., Schübeler D., Roloff T.C. Methylated DNA immunoprecipitation (medip) Methods Mol. Biol. 2009;507:55–64. doi: 10.1007/978-1-59745-522-0_5. [DOI] [PubMed] [Google Scholar]
- 79.Irizarry R.A., Ladd-Acosta C., Carvalho B., Wu H., Brandenburg S.A., Jeddeloh J.A., Wen B., Feinberg A.P. Comprehensive high-throughput arrays for relative methylation (charm) Genome Res. 2008;18:780–790. doi: 10.1101/gr.7301508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serre D., Lee B.H., Ting A.H. Mbd-isolated genome sequencing provides a high-throughput and comprehensive survey of DNA methylation in the human genome. Nucleic Acids Res. 2010;38:391–399. doi: 10.1093/nar/gkp992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karimi M., Johansson S., Ekström T.J. Using luma: A luminometric-based assay for global DNA-methylation. Epigenetics. 2006;1:45–48. doi: 10.4161/epi.1.1.2587. [DOI] [PubMed] [Google Scholar]
- 82.Oda M., Greally J.M. The help assay. Methods Mol. Biol. 2009;507:77–87. doi: 10.1007/978-1-59745-522-0_7. [DOI] [PubMed] [Google Scholar]
- 83.Sonnet M., Baer C., Rehli M., Weichenhan D., Plass C. Enrichment of methylated DNA by methyl-CPG immunoprecipitation. Methods Mol. Biol. 2013;971:201–212. doi: 10.1007/978-1-62703-269-8_11. [DOI] [PubMed] [Google Scholar]
- 84.Ammerpohl O., Martín-Subero J.I., Richter J., Vater I., Siebert R. Hunting for the 5th base: Techniques for analyzing DNA methylation. Biochim. Biophys. Acta. 2009;1790:847–862. doi: 10.1016/j.bbagen.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Brebi-Mieville P., Ili-Gangas C., Leal-Rojas P., Noordhuis M.G., Soudry E., Perez J., Roa J.C., Sidransky D., Guerrero-Preston R. Clinical and public health research using methylated dna immunoprecipitation (medip): A comparison of commercially available kits to examine differential DNA methylation across the genome. Epigenetics. 2012;7:106–112. doi: 10.4161/epi.7.1.18647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei S.H., Chen C.M., Strathdee G., Harnsomburana J., Shyu C.R., Rahmatpanah F., Shi H., Ng S.W., Yan P.S., Nephew K.P., et al. Methylation microarray analysis of late-stage ovarian carcinomas distinguishes progression-free survival in patients and identifies candidate epigenetic markers. Clin. Cancer Res. 2002;8:2246–2252. [PubMed] [Google Scholar]
- 87.Vincent A., Omura N., Hong S.M., Jaffe A., Eshleman J., Goggins M. Genome-wide analysis of promoter methylation associated with gene expression profile in pancreatic adenocarcinoma. Clin. Cancer Res. 2011;17:4341–4354. doi: 10.1158/1078-0432.CCR-10-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Capoa A., Musolino A., Della Rosa S., Caiafa P., Mariani L., del Nonno F., Vocaturo A., Donnorso R.P., Niveleau A., Grappelli C. DNA demethylation is directly related to tumour progression: Evidence in normal, pre-malignant and malignant cells from uterine cervix samples. Oncol. Rep. 2003;10:545–549. [PubMed] [Google Scholar]
- 89.Hernandez-Vargas H., Lambert M.P., le Calvez-Kelm F., Gouysse G., McKay-Chopin S., Tavtigian S.V., Scoazec J.Y., Herceg Z. Hepatocellular carcinoma displays distinct DNA methylation signatures with potential as clinical predictors. PLoS One. 2010;5:e9749. doi: 10.1371/journal.pone.0009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luger K., Mäder A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 a resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 91.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 92.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 93.Huang Y., Nayak S., Jankowitz R., Davidson N.E., Oesterreich S. Epigenetics in breast cancer: What’s new? Breast Cancer Res. 2011;13:225. doi: 10.1186/bcr2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Linggi B.E., Brandt S.J., Sun Z.W., Hiebert S.W. Translating the histone code into leukemia. J. Cell Biochem. 2005;96:938–950. doi: 10.1002/jcb.20604. [DOI] [PubMed] [Google Scholar]
- 95.Krol J., Loedige I., Filipowicz W. The widespread regulation of microrna biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 96.Lopez-Serra P., Esteller M. DNA methylation-associated silencing of tumor-suppressor micrornas in cancer. Oncogene. 2012;31:1609–1622. doi: 10.1038/onc.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Calin G.A., Croce C.M. Microrna-cancer connection: The beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 98.Thomson J.M., Newman M., Parker J.S., Morin-Kensicki E.M., Wright T., Hammond S.M. Extensive post-transcriptional regulation of micrornas and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang T.C., Yu D., Lee Y.S., Wentzel E.A., Arking D.E., West K.M., Dang C.V., Thomas-Tikhonenko A., Mendell J.T. Widespread microrna repression by MYC contributes to tumorigenesis. Nat. Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Melo S.A., Kalluri R. Molecular pathways: Micrornas as cancer therapeutics. Clin. Cancer Res. 2012;18:4234–4239. doi: 10.1158/1078-0432.CCR-11-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamamoto H., Adachi Y., Taniguchi H., Kunimoto H., Nosho K., Suzuki H., Shinomura Y. Interrelationship between microsatellite instability and microrna in gastrointestinal cancer. World J. Gastroenterol. 2012;18:2745–2755. doi: 10.3748/wjg.v18.i22.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho W.C. Great potential of mirnas as predictive and prognostic markers for cancer. Expert Rev. Mol. Diagn. 2012;12:315–318. doi: 10.1586/erm.12.21. [DOI] [PubMed] [Google Scholar]
- 103.Kelly T.K., de Carvalho D.D., Jones P.A. Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 2010;28:1069–1078. doi: 10.1038/nbt.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Z., Jiang H., Liu X., Chen Y., Wong J., Wang Q., Huang W., Shi T., Zhang J. Hemd: An integrated tool of human epigenetic enzymes and chemical modulators for therapeutics. PLoS One. 2012;7:e39917. doi: 10.1371/journal.pone.0039917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferreux E., Lont A.P., Horenblas S., Gallee M.P., Raaphorst F.M., von Knebel Doeberitz M., Meijer C.J., Snijders P.J. Evidence for at least three alternative mechanisms targeting the p16ink4a/cyclin d/rb pathway in penile carcinoma, one of which is mediated by high-risk human papillomavirus. J. Pathol. 2003;201:109–118. doi: 10.1002/path.1394. [DOI] [PubMed] [Google Scholar]
- 106.Soufir N., Queille S., Liboutet M., Thibaudeau O., Bachelier F., Delestaing G., Balloy B.C., Breuer J., Janin A., Dubertret L., et al. Inactivation of the cdkn2a and the p53 tumour suppressor genes in external genital carcinomas and their precursors. Br. J. Dermatol. 2007;156:448–453. doi: 10.1111/j.1365-2133.2006.07604.x. [DOI] [PubMed] [Google Scholar]
- 107.Kalantari M., Villa L.L., Calleja-Macias I.E., Bernard H.U. Human papillomavirus-16 and -18 in penile carcinomas: DNA methylation, chromosomal recombination and genomic variation. Int. J. Cancer. 2008;123:1832–1840. doi: 10.1002/ijc.23707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guerrero D., Guarch R., Ojer A., Casas J.M., Ropero S., Mancha A., Pesce C., Lloveras B., Garcia-Bragado F., Puras A. Hypermethylation of the thrombospondin-1 gene is associated with poor prognosis in penile squamous cell carcinoma. BJU Int. 2008;102:747–755. doi: 10.1111/j.1464-410X.2008.07603.x. [DOI] [PubMed] [Google Scholar]
- 109.Yanagawa N., Osakabe M., Hayashi M., Tamura G., Motoyama T. Detection of HPV-DNA, p53 alterations, and methylation in penile squamous cell carcinoma in japanese men. Pathol. Int. 2008;58:477–482. doi: 10.1111/j.1440-1827.2008.02259.x. [DOI] [PubMed] [Google Scholar]
- 110.Yanagawa N., Osakabe M., Hayashi M., Tamura G., Motoyama T. Frequent epigenetic silencing of the FHIT gene in penile squamous cell carcinomas. Virchows. Arch. 2008;452:377–382. doi: 10.1007/s00428-008-0597-6. [DOI] [PubMed] [Google Scholar]
- 111.Rogenhofer S., Miersch H., Göke F., Kahl P., Wieland W.F., Hofstädter F., Kristiansen G., von Ruecker A., Müller S.C., Ellinger J. Histone methylation defines an epigenetic entity in penile squamous cell carcinoma. J. Urol. 2012;189:1117–1122. doi: 10.1016/j.juro.2012.08.221. [DOI] [PubMed] [Google Scholar]
- 112.Poetsch M., Hemmerich M., Kakies C., Kleist B., Wolf E., vom Dorp F., Hakenberg O.W., Protzel C. Alterations in the tumor suppressor gene p16(ink4a) are associated with aggressive behavior of penile carcinomas. Virchows. Arch. 2011;458:221–229. doi: 10.1007/s00428-010-1007-4. [DOI] [PubMed] [Google Scholar]
- 113.Larsen C.J. Contribution of the dual coding capacity of the p16ink4a/mts1/cdkn2 locus to human malignancies. Prog. Cell Cycle Res. 1997;3:109–124. doi: 10.1007/978-1-4615-5371-7_9. [DOI] [PubMed] [Google Scholar]
- 114.Kim W.Y., Sharpless N.E. The regulation of ink4/arf in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 115.Jacobs J.J., Kieboom K., Marino S., DePinho R.A., van Lohuizen M. The oncogene and polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 116.Deng Z., Hasegawa M., Yamashita Y., Matayoshi S., Kiyuna A., Agena S., Uehara T., Maeda H., Suzuki M. Prognostic value of human papillomavirus and squamous cell carcinoma antigen in head and neck squamous cell carcinoma. Cancer Sci. 2012;103:2127–2134. doi: 10.1111/cas.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang H., Zhang B., Chen W., Zhou S.M., Zhang Y.X., Gao L., Xu Z.G., Qiao Y.L., Tang P.Z. Human papillomavirus infection and prognostic predictors in patients with oropharyngeal squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2012;13:891–896. doi: 10.7314/apjcp.2012.13.3.891. [DOI] [PubMed] [Google Scholar]
- 118.Bornstein P. Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sonpavde G., Pagliaro L.C., Buonerba C., Dorff T.B., Lee R.J., di Lorenzo G. Penile cancer: Current therapy and future directions. Ann. Oncol. 2013;24:1179–1189. doi: 10.1093/annonc/mds635. [DOI] [PMC free article] [PubMed] [Google Scholar]