Abstract
Are there common pathways underlying the broad spectrum of tissue pathologies that develop upon injuries and from subsequent tissue remodeling? Here, we explain the pathophysiological impact of a set of evolutionary conserved danger control programs for tissue pathology. These programs date back to the survival benefits of the first multicellular organisms upon traumatic injuries by launching a series of danger control responses, i.e., 1. Haemostasis, or clotting to control bleeding; 2. Host defense, to control pathogen entry and spreading; 3. Re-epithelialisation, to recover barrier functions; and 4. Mesenchymal, to repair to regain tissue stability. Taking kidney pathology as an example, we discuss how clotting, inflammation, epithelial healing, and fibrosis/sclerosis determine the spectrum of kidney pathology, especially when they are insufficiently activated or present in an overshooting and deregulated manner. Understanding the evolutionary benefits of these response programs may refine the search for novel therapeutic targets to limit organ dysfunction in acute injuries and in progressive chronic tissue remodeling.
Keywords: regeneration, fibrosis, coagulation, stem cells, inflammation, acute kidney injury, chronic kidney disease, healing, repair
1. Introduction
Most acute and chronic disorders involve a combination of direct and indirect tissue injuries, i.e., the damage caused by the injurious trigger, and that caused by a series of different danger response programs, i.e., clotting, inflammation, epithelial regeneration, and mesenchymal repair. These processes predominate in a serial manner during acute disorders, with some overlap. However, in chronic non-communicable diseases this overlap turns into a concomitant persistence of some of these programs, e.g., inflammation and healing, which often leads to significant parenchymal atrophy and fibrosis. In this review we use kidney pathology as an example for the spectrum of pathological manifestations that derive from these danger response programs and explain why evolution’s risk-benefit assessment conserved them across species over time. Better understanding of the origin of tissue pathologies should be instrumental to better define target pathways for therapeutic interventions.
2. Injuries Trigger a Series of Host Response Programs
Wounding requires immediate actions to control the dangers that come with the injury followed either by regeneration or repair, a concept that applies to plants and animals [1,2]. Focal wounding requires local danger control, e.g., of pathogen entry, to prevent systemic consequences such as fatal sepsis. Hence, such survival benefits from local danger control, outweighs any risk of focal collateral injury or tissue remodeling that comes with these danger responses. What remains problematic are systemic triggers of danger responses, such as toxic (drugs), hemodynamic (shock or arterial hypertension), or metabolic alterations (diabetes, hyperlipidemia) because these induce responses that affect multiple organ systems and organ compartments, which implies that any collateral damage threatens entire organs, if not the whole body. In the following paragraphs we will introduce the four major danger response programs along wound healing after acute skin injury [3–5].
2.1. Clotting Addresses the Risk of Potentially Fatal Bleeding
Skin injury causes bleeding, which implies the risk of dying from hemorrhagic shock. Clotting addresses this type of danger within minutes by haemolymph aggregation in arthropods, and by a more sophisticated interplay of injured endothelial cells, coagulation factors, and platelets in vertebrates [6–9]. Overshooting clotting causes tissue ischemia via intravascular coagulation or thromboembolism.
2.2. Inflammation Addresses the Risk of Fatal Sepsis
The balance between microbe virulence and host defense has gained considerable complexity along the evolution of monocellular organisms [10–13]. Outer surface wounding allows pathogen entry and may lead to fatal sepsis, if not immediately addressed by local inflammation to control pathogen spreading [14,15]. Extrinsic pathogen-associated molecular patterns (PAMPs) and intrinsic damage-associated molecular patterns (DAMPs) act as alarmins that activate the same innate immunity pattern recognition receptors in infectious or sterile inflammations [16–21]. Inflammation is already activated by clotting, a process referred to as immunothrombosis [6,22–24], as platelet aggregates release chemokines that trigger the recruitment of neutrophils [6,25,26]. Overshooting local inflammation causes unnecessary immunopathology and loss of parenchyma, e.g., in pyoderma gangraenosum [27,28]. Overshooting systemic inflammation contributes to the early phase of sepsis, while insufficient systemic inflammation accounts for lethality in the late phase of sepsis [14,29].
2.3. Epithelial Regeneration Restores Barrier Functions
Non-sterile barrier defects require rapid regeneration of the barrier to limit pathogen entry [5,30]. Epithelial barriers have also other important functions, such as nutrient absorbtion (gut), gas transfer (lungs), or solute secretion/re-absorption (kidney), which require rapid restoration upon injury, e.g., by signals that trigger re-epitheliasation from wound borders [2,5,31]. Components of the coagulation cascade are the first mediators inside a wound that elicit mitogenic effects on the surviving epithelial cells [2,7,25,31,32]. Inflammatory mediators with mitogenic properties, such as epithelial growth factors, hepatocyte growth factor, IL-6, IL-17, fractalkine, CXCL10, and IL-22, as well as certain miRNAs, stimulate epithelial repair [31,33–40]. Local progenitor cells that are committed to the specific epithelial lineage phenotype contribute to re-epithelialisation [30,40–42]. Insufficient re-epitheliasation creates chronic wounds, which implies a risk of infections, while overshooting or uncoordinated re-epitheliasation, can also cause, problematically, hyperplastic lesions [2,43].
2.4. Mesenchymal Repair Restores Tissue Stability
Insufficient re-epithelialisation, loss of tissue parenchyma, or injury to mesenchymal tissues activates the wound healing program of mesenchymal repair. This process is needed to stabilize the organ’s shape and structure. Insufficient epithelial repair directly stimulates mesenchymal healing, as epithelial-mesenchymal transition (EMT) of epithelial cells, and their arrest in the G2/M phase of the cell cycle induce the secretion of the pro-fibrotic cytokine TGF-β [44]. The associated accumulation of collagen-producing cells [45,46] relates to an influx of bone marrow-derived fibrocytes [47,48], a mesenchymal transition of also pericytes and endothelial cells [48,49], as well as the proliferation of resident fibroblasts that transform to myofibroblasts [49]. The accumulation of extracellular matrix (referred to as fibrosis) stiffens the tissue (referred to as sclerosis). Insufficient mesenchymal healing destabilizes tissues while overshooting mesenchymal healing produces fibrotic lesions such as keloid, and diffuse fibrotic disorders such as scleroderma.
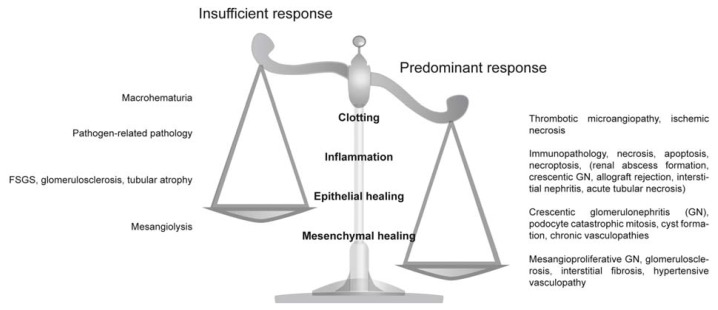
In the following we discuss how these ancient danger control programs account for the spectrum of organ abnormalities known from pathology textbooks. Based on our own experience we focus to kidney pathology. We describe in more detail how common histomorphological abnormalities and disease entities develop, either from insufficient, or overshooting danger control programs (Figure 1).
Figure 1.
Insufficient and predominant danger response programs determine kidney pathology. Distinct entities of clinical syndromes or kidney injury patterns are consequences of insufficient or overshooting danger control programs.
3. Clotting
3.1. Overshooting Clotting in the Kidney
Thrombotic microangiopathies that affect the kidney result from an abnormal activation of microvessel endothelial cells, and subsequent activation of platelets and plasmatic coagulation (Figure 2) [50,51]. Microvascular clotting causes tissue ischemia and necrosis. In crescentic glomerulonephritis, vascular necrosis leads to perforations, ruptures in the glomerular basement membrane (GBM), plasma leakage, and bleeding [52]. Glomerular bleeding manifests clinically as hematuria, a process that should activate clotting inside the glomerulus. In fact, fibrin deposition is usually seen in areas of loop necrosis in crescentic glomerulonephritis, in turn, used as a diagnostic marker to identify necrotizing glomerulonephritis [53,54]. In Alport nephropathy, a genetic GBM abnormality not associated with intense inflammation, progressive intrinsic degradation and disintegration of the GBM, generates similar GBM perforations and ruptures that are associated with hematuria, fibrin deposits, and plasma leakage [43]. As part of the clot formation, activated platelets release pro-inflammatory and mitogenic factors, which activate subsequent inflammation as well as and epithelial regeneration [7,25,26,31,55]. The link of clotting and inflammation, referred to as immmunothrombosis, is well defined in the field of immunology and currently understood as a mechanism to limit pathogen spreading from the entry site [6,56–59]. This evolutionary conserved process certainly contributes to atherosclerosis and -thrombosis, and it is reasonable to assume that this link is as important in other non-communicable disorders.
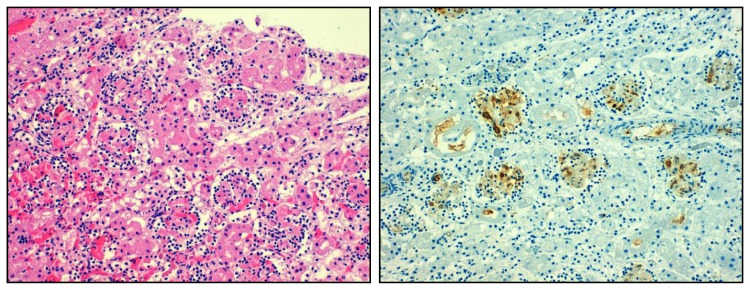
Figure 2.
Overshooting clotting in thrombotic microangiopathy. Local activation microvascular endothelial cells and lack of distinct inhibitory factors can lead to thrombotic microangiopathy which is characterized by microthrombi obstructing arteriolar and glomerular vessels, a lesion associated with significant inflammatory cell infiltrates (left). Fibrin immunostaining displayes clot formation within glomeruli (right). Original magnification 200×.
3.2. Insufficient Clotting in the Kidney
IgA nephropathy, and several other renal disorders, may present with intermittent macrohematuric episodes, implicating that vascular sealing by clotting is insufficient [60]. Urokinase expression further down the urinary tract elicits fibrinolytic activity that may maintain the bleeding [61].
4. Inflammation
4.1. Overshooting Inflammation in the Kidney
Resident and infiltrating mononuclear phagocytes express the entire spectrum of innate pattern recognition receptors that translate danger recognition into a rapid inflammatory response [62,63]. In contrast, renal parenchymal cells, i.e., mesangial cells, endothelial cells, podocytes, tubular cells, and fibroblasts express only a limited spectrum of such receptors [64,65]. For example, they lack some of the endosomal nucleic acid-specific Toll-like receptors, and do not produce IL-1beta upon activation of the NLRP3 inflammasome, even though they easily get activated by bacterial endotoxin or other PAMPs and DAMPs [66,67]. This mechanism can explain how systemic or extrarenal infections can cause flares of pre-existing and smoldering forms of renal inflammation, e.g., chronic glomerulonephritis or renal vasculitis. We had addressed this concept by transiently injecting agonists to several pattern recognition receptors in mice with experimental immune complex glomerulonephritis, which then triggered the intrarenal production of cytokines, type I interferons, which increases inflammation and tissue damage [65,68–77]. PAMP-mediated renal inflammation induces the loss of renal parenchymal cells, especially podocytes [78,79], because these cannot be easily regenerated [80]. For example, mice with Alport nephropathy transiently exposed to bacterial CpG-DNA, display a transient activation of resident dendritic cells and infiltrating Ly6Chigh+ macrophages that produce pro-inflammatory mediators such as TNF, which triggered podocyte loss and thereby accelerated proteinuria and glomerular scarring [78].
In lupus nephritis, endogenous nucleic acids drive immunity in a similar manner [81,82]. Nuclear particles that contain immunostimulatory endogenous RNA and/or DNA activate antigen-presenting dendritic cells, macrophages, and B cells, to mature and to release numerous pro-inflammatory mediators including type I interferon [82–84]. The latter sets off a coordinated antiviral immune response explaining the similarities between the clinical manifestations of viral infections and systemic lupus [85]. This process also occurs inside the kidney, as documented by the antiviral gene expression signature in renal biopsies [86,87]. For example, mesangial cells and glomerular endothelial cells use their cytosolic nucleic acid sensors to translate nucleic acid recognition into the release of type I interferons, which contribute to renal inflammation and tissue damage [88–93].
Tissue necrosis is associated with a release of endogenous ligands to pattern recognition receptors [64,94,95]. For example, postischemic tubular cell necrosis releases HMGB1 and histones that ligate TLR2 and TLR4, which trigger an acute intrarenal inflammatory response that determines the extent of AKI [66,95–99]. In addition, Tamm-Horsfall protein/uromodulin, a kidney-specific protein exclusively expressed within the distal tubule, acts as a TLR4 and NLRP3 agonists when tubular injury allows its leakage into the renal interstitium [100,101]. TLR signaling occurs in renal parenchymal cells [64] and is tightly regulated in the intrarenal network of dendritic cells by the constitutive and induced expression of several inhibitory molecules that are absent or dysfunctional in tubular epithelial cells [102–108]. In contrast, the NLRP3 inflammasome enhances tubulointerstitial but not to glomerular inflammation [19,109–111], because activated glomerular cells do not induce pro-IL-1β [110].
Triggered by the local release of chemokines, various subsets of leukocytes sequentially recruit into the kidney [112–120]. Macrophages, T cells, and B cells polarize into functionally distinct subsets that differently affect renal pathology [62,114,121–125]. PAMPs and DAMPs turn non-activated intrarenal and circulating mononuclear phagocytes into cells that promote immunopathology [126–128]. Blocking the CC-chemokine CCL2, or its receptor CCR2, prevents the recruitment and expansion of such classically-activated macrophages, and thereby reduces renal immunopathology in glomeruli and the tubulointerstitium, but it does not affect alternatively-activated macrophages [129–137]. Together, the kidney is mostly affected by renal inflammation that is triggered by extrarenal infections that release immunostimulatory PAMPs into the circulation, or by intrarenal release of DAMPs that promote a sterile inflammatory response [138,139]. These immunostimulatory molecules promote unnecessary (collateral) damage to renal cells (Figure 3).
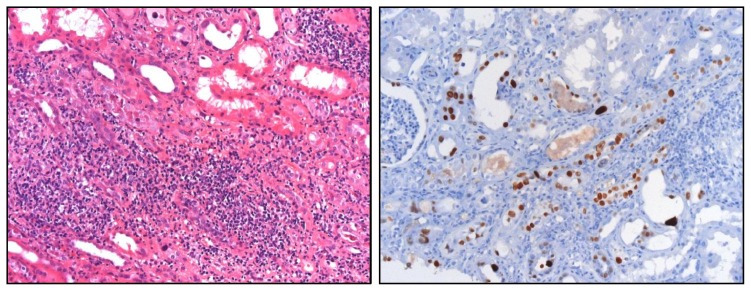
Figure 3.
Interstitial nephritis in polyoma (BK) virus nephropathy. Local activation of inflammation can destroy renal parenchyma and cause acute kidney injury. In this example of BK virus reactivation in a kidney allograft was proven by immunostaining for BK viral protein with positivity in affected tubular epithelial cells (right). Viral replication activates antiviral immunity and immunopathology as evidenced by the dense leukocyte infiltrates in the renal interstitium (left). Original magnification 200×.
Thus, suppressing renal inflammation appears as an important strategy to preserve renal tissue, especially those epithelial cells that cannot be easily regenerated. Anti-inflammatory drugs that do not elicit systemic immunosuppressive effects hold new promise for that [140].
4.2. Insufficient Inflammation in the Kidney
While TLR-mediated renal inflammation contributes to inappropriate renal immunopathology in sterile nephropathies, it remains an important element of pathogen control in renal infections [66,141]. For example, some degree of innate immune activation is needed to keep BK virus in check to avoid viral replication and BK virus infection of the allograft [142,143]. The enigmatic importance of renal inflammation as part of the local host defense becomes evident also during bacterial pyelonephritis [144]. Intrarenal host defense is needed to limit pathogen growth and spreading to systemic infections, which happens in TLR4-mutant mice inoculated with uropathogenic E. coli. While these mice are protected from renal abscess formation [145], this occurs at the price of insufficient pathogen control at the entry site and could cause fatal gram negative sepsis [14]. In addition, TLR2 is required for the recognition of leptospiral outer membrane proteins in proximal tubular epithelial cells [146].
5. Epithelial Regeneration
Epithelial cells determine most of the kidney’s functions, in the glomerular compartment (filtration barrier) as well as the tubular compartment of the kidney (reabsorbtion and secretion). Transient and short-term injuries to the tubules are usually followed by sufficient epithelial regeneration that rapidly restores renal function [147,148]. Numerous growth factors such as HGF, PDFG, EGF, and BMP-7 drive the repair of the epithelial monolayers after injury [7,25,31,149]. In addition, the cell cycle regulator murine double minute (MDM)-2 assures cell cycle entry of surviving tubular cells by inhibiting p53-dependent cell cycle arrest [150], in addition to its role in NF-κB signaling [150,151] Epithelial regeneration becomes effective only after the resolution of inflammation [2,5,152]. Switching the phenotype of macrophages from a pro-inflammatory (M1) toward an anti-inflammatory (M2) is important in this process [122,125,153–156]. This process is associated with the release of additional growth factors that drive epithelial recovery in the kidney, including CSF-1 that enhances M2 macrophage accumulation [137,152,155–159]. However, epithelial regeneration needs to be tightly balanced to avoid renal pathology [80].
5.1. Overshooting Epithelial Regeneration in the Kidney
When epithelial (progenitor) cells get heavily activated in the absence of the necessary signals for differentiation, overshooting and maladaptive epithelial repair results in additional renal pathology [80,160,161]. In rapid progressive glomerulonephritis glomerular vascular necrosis, and subsequent activation of the coagulation cascade, drive intense intraglomerular inflammation [162–164]. Both, epithelial injury and inflammation induce the proliferation of parietal epithelial cells without their differentiation into podocytes, which would be required for podocyte regeneration [43,161–163]. The resulting parietal epithelial cell hyperplasia generates the initial step in glomerular crescent formation and nephron loss (Figure 4). This overshooting epithelial hyperplasia does not necessarily require inflammation as a trigger. In Col4A3-deficient mice disruption of glomerular capillaries was sufficient to trigger parietal epithelial cell hyperplasia [43]. Plasma leakage seemed to be the mitogenic stimulus for these epithelial cells that normally reside, devoid of serum contact, in the urinary space [165,166].
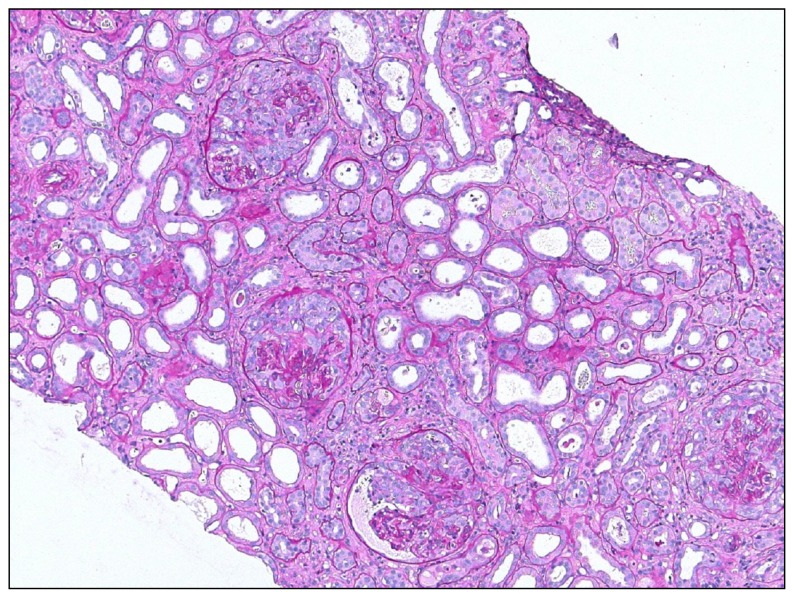
Figure 4.
Overshooting epithelial regeneration in crescentic glomerulonephritis. Massive and uncoordinated proliferation of parietal epithelial cells leads to crescent formation in Bowman’s space, e.g., in necrotizing renal vasculitis. Original magnification 100×.
Epithelial hyperplasia in the tubular compartment is less obvious. The tubular progenitor cells are located at the junction of glomeruli and proximal tubules, while single progenitor cells are scattered in the proximal and distal tubules of the cortex [167,168]. Chevalier et al., have recently demonstrated that the phenomenon of atubular glomeruli originates from an obstruction of the tubular lumen by epithelial cells [169,170], a process that contributes to tip lesions in focal-segmental glomerulosclerosis and diabetic nephropathy [171,172].
5.2. Insufficient Epithelial Regeneration in the Kidney
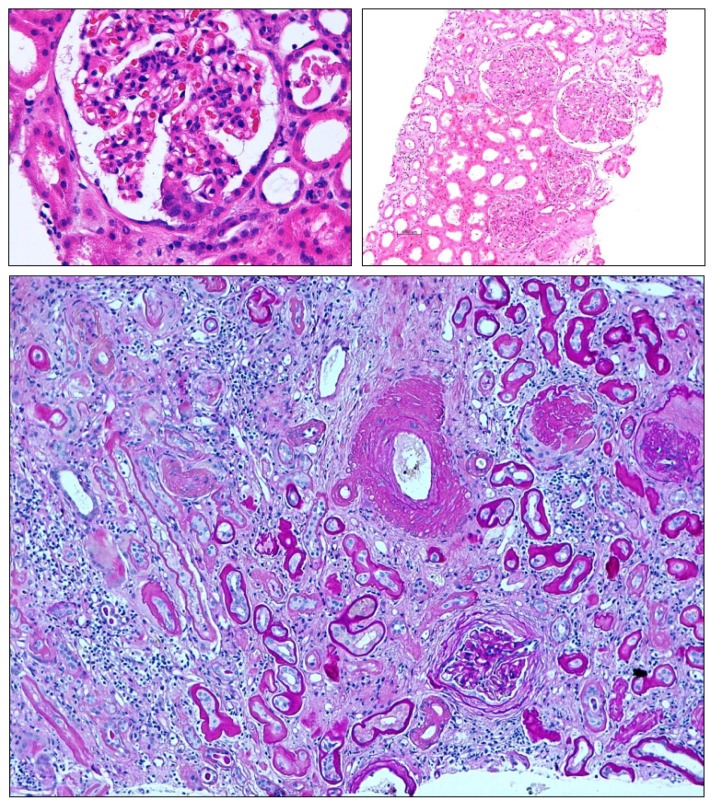
Insufficient glomerular epithelial (podocyte) regeneration is the predominant cause for chronic kidney disease (CKD) and for progression to end stage renal disease (ESRD) (Figure 5). The particular interaction of differentiated podocytes with each other, and with the GBM that maintains the glomerular filtration barrier remains a major obstacle for rapid repair [173–176]. This is because podocytes involve all their cytoskeleton to maintain the secondary foot processes and the slit diaphragm, which is not compatible with reorganizing the cytoskeleton to form the mitotic spindle during mitosis [177]. Thus, when podocytes enter the S phase of the cell cycle to undergo hypertrophy, cell cycle arrest at the G1 and G2/M restriction points are needed to prevent the podocyte to undergo mitosis or, otherwise, podocytes will subsequently detach and die, i.e., mitotic catastrophe [177–180]. There has been a controversial debate whether bone marrow-derived progenitors are able to replace lost podocytes [181–183], but meanwhile it has been demonstrated that podocytes originate from local epithelial progenitors at the urinary pole of the glomerulus that can migrate to the vascular pole and differentiate into terminally differentiated podocytes on the glomerular tuft [178,184,185]. This process contributes to renal development and podocyte expansion during kidney growth in early childhood [101] but its capacity to replace injured podocytes seems to be limited in adults [184,186]. The signaling pathways that regulate podocyte renewal from parietal epithelial cells remain to be clarified. Notch and Wnt signaling, EGF and SDF-1/CXCL12 seem to have a role [164,178,187,188]. Specific epigenetic imprinting at histone H3K9, H3K23 (acetylation), H3K4 (dimethylation), and H3K4 phosphorylation at serine 10, which alters gene expression and cell growth, are associated with incomplete podocyte recovery [189,190].
Figure 5.
Insufficient epithelial repair results in mesenchymal repair. Loss of glomerular epithelial cells (podocytes) cannot be easily repaired in adults leading to focal-segmental glomerulosclerosis (upper left). Global glomerulosclerosis (upper right) results from the progression of focal-segmental lesions or as a consequence of diffuse and persistent disease mechanisms such as diabetes, hypertension, or immune complex glomerulonephritis. Here also, mesangial cells contribute to the sclerosis by excess production of mesangial matrix. In chronic kidney disease insufficient tubular regeneration results in tubular atrophy, which is usually associated concomitant tubulointerstitial fibrosis. These complex lesions make it difficult to appreciate the individual contributing danger response programs. Magnification 100×–400×.
The persistence of classically-activated mononuclear phagocytes or repetitive/persistent triggers of kidney injury also impair epithelial repair in the tubulointerstitial compartment. CSF-1 is a mediator of this process [159]. In addition, severe kidney injury may eradicate the tubular progenitor cells that have a higher capacity to survive stress [191]. Bone marrow stem cells do not directly replace tubular cells by differentiation, but provide paracrine support to the regeneratory capacity of local progenitor cells and other surviving epithelial cells [154,168,192–194]. An insufficient regeneration of injured tubular epithelial cells will lead to tubular atrophy and nephron loss, a typical characteristic of progressive CKD (Figure 5).
Together, a coordinated epithelial regeneration is needed upon injury, which first requires vascular sealing and the resolution of inflammation. Insufficient epithelial regeneration leads to atrophy and mesenchymal repair, i.e., tubular atrophy and glomerulosclerosis. Overshooting epithelial repair, i.e., hyperplasia, is another form of renal pathology, e.g., the glomerular crescent. Finding ways to enhance a coordinated proliferation and differentiation of surviving epithelial cells remains a challenge for the future.
6. Mesenchymal Repair
Tissue healing is not limited to epithelia but also includes recovery of vasculature, tendons, fasciae, bones, and muscles, all being mesenchymal structures that contribute to function, not only of the musculoskeletal system but also to that of solid organs. For example, mesenchymal repair contributes to mechanical stabilization of organs like the lung, the heart, and the kidney, which is why epithelial growth factors are secreted together with growth factors that stimulate mesenchymal repair, e.g., by increasing the secretion of extracellular matrix components [31]. In skin wounds, dermal fibroblasts get activated to transform into myofibroblasts that produce large amounts of type I collagen, which promotes wound contraction as a way to reduce wound size for more rapid re-epithelisation [2]. Irreversible tissue losses such as in ruptured ligaments or burned skin get filled by fibrous tissue to regain tissue stability [2]. These benefits of mesenchymal repair explain why this response program was positively selected and maintained along evolution. Solid organs like the kidney, however, often suffer from global scarring and progressive fibrosis because of the diffuse nature of the metabolic, hemodynamic, and toxic injuries that commonly affect the kidney (Figure 5). [49]. That is why it is not at all clear whether diffuse renal fibrosis is an overshooting, or simply a diffuse response. For example, interstitial fibrosis in focal-segmental glomerulosclerosis (FSGS) starts in a focal manner around those single nephrons that succumb to scarring [173]. The diffuse appearance of interstitial fibrosis only develops once many nephrons undergo the same process. The mesenchymal “repair” of each dying nephron then leads to confluent fibrotic lesions that give the impression that it is the fibrosis that accounts for renal dysfunction [46,49,173,195]. The extracellular matrix mainly replaces lost renal epithelia. As such, reducing interstitial fibrosis may result in even smaller kidneys, if not being accompanied by sufficient generation of new nephrons, a process which fish can do, but mammals cannot [196]. For the sake of didactic clarity, we will continue to use the term “overshooting” mesenchymal repair for the discussion of fibrosis as a danger response program that accounts for renal pathology.
6.1. Insufficient Mesenchymal Repair in the Kidney
Insufficient mesangial repair is a hardly known phenomenon in kidney pathology, or at least poorly defined. Mesangiolysis can be considered as a lesion of incomplete mesenchymal repair, but mesangiolysis is rarely described in renal biopsies, as it is mostly a transient phenomenon followed by rapid, (and often overshooting) mesangial cell recovery [197,198]. Mesangiolysis often results from massive renal complement activation, as in C3 glomerulopathies, atypical hemolytic uremic syndrome, or immune complex glomerulonephritis [199].
6.2. Overshooting Mesenchymal Repair in the Kidney
Mesangial repair after inflammatory injury can occur from three sources: surviving mesangial cells [198], from the extraglomerular mesangium [200], and from the bone marrow [201]. Mesangial injury rarely occurs in an isolated manner, but complicates diseases that are associated with extensive complement activation such as hemolytic uremic syndrome. The rat anti-Thy1.1 model is frequently used to study the mechanisms of mesangial repair upon mesangiolysis. Hugo et al., first reported that the hyperproliferative response upon mesangiolysis originates from surviving mesangial cells or local progenitor cells that reside in the extraglomerular mesangium [200]. Mesangial hyperproliferation also contributes to a histopathological lesion named “membrano-proliferative glomerulonephritis” where hereditary, or acquired forms, of extensive complement activation within the mesangium lead to a persistent expansion of mesangial cells that even extend into the space between the GBM and the endothelial cells [202]. The mesangial matrix produced along these mesangial cell extensions stains positive with silver, which then gives glomerular capillaries a splitted GBM appearance on light microscopy [202].
In the glomerulus this causal relationship becomes clear, as podocyte renewal from local progenitors is mostly insufficient, especially in proteinuric disorders of the adult [80,203]. In FSGS, parietal epithelial cells rather produce extracellular matrix, and generate segmental sclerotic lesions, than regenerating lost podocytes [204]. This focal synechia has still the potential to stabilize the focal loss of podocytes as in some forms of secondary FSGS [171]. However, beyond a certain amount of lost podocytes, the scarring process acquires its own dynamic and progresses to global glomerulosclerosis [175], because hyperfiltration of the remaining glomerulus adds more hemodynamic stress on the surviving podocytes [173,205]. The mesenchymal transition of parietal epithelial cells further contributes to fibrocellular crescent formation, implying an irreversible loss of the entire nephron [206]. In this way, parietal epithelial cells directly contribute, not only to epithelial, but also to mesenchymal repair [166].
The process of epithelial-mesenchymal transition of surviving epithelial cells that cannot rapidly regenerate epithelial injuries has attracted a lot of attention [46,207–209]. It is based on the observation that epithelial cells of mesenchymal origin, like the renal epithelia, re-express mesenchymal markers upon injury in vitro and in vivo [46]. This has led to the assumption that such cells could leave tubular compartment and migrate into the renal interstitium where they may fuel into the heterogeneous pool of fibroblasts and contribute extracellular matrix production and fibrotic lesions [46,49]. The significance of this phenomenon for human kidney disease remains under debate because clear evidence for tubular cells leaving the tubular compartment in vivo is still lacking [46,207–209]. In the glomerulus, however, parietal epithelial cells that do not adequately differentiate into podocytes clearly undergo this mesenchymal transition and cause scarring, as there is no need for them to leave their home compartment (Figure 5) [43,206].
The concept that renal interstitial fibrosis accounts for renal dysfunction originates from the close association of the extent of renal fibrosis with poor outcomes of primary glomerular disorders [210], but functional studies do not always support this causal relationship [49,196]. It is of note that the driving factor of interstitial fibrosis seems to be epithelial injury and insufficient epithelial repair [211], e.g., when proliferating epithelial cells get arrested in the G2/M phase and start to produce tumor growth factor-beta [44]. This process is also triggered by aristocholic acid [44,212], the nephrotoxic element of Chinese herb nephropathy [213]. Bone marrow progenitor cells and leukocytes enhance the process of renal fibrosis, as evidenced by experimental interventions that block leukocyte recruitment and can prevent interstitial fibrosis either as a direct or an indirect effect [112,134,214–219]. For example, inhibition, genetic deletion, or depletion of alternatively-activated (M2) macrophages protects from renal fibrosis [122,155,156,220–227]. Fibrocytes are a particular type of Ly6G+ collagen-producing cell that originate from myeloid precursors in the bone marrow and that recruit to sites of chronic kidney injury [228,229]. Ly6G+ fibrocytes specifically recruit via CCL21-CCR7 and not via CCL2-CCR2 like pro-inflammatory macrophages, but once they reach the kidney they contribute to local collagen deposition and interstitial fibrosis [230,231].
Vascular reconstruction is another element of mesenchymal healing [232]. Pericytes stabilize microvessels not only during homeostasis, but also during microvessel recovery, a process mediated by TIMP3 and ADAMTS1 [233]. Their capacity to produce collagen adds pericytes to the list of cells that contribute to renal interstitial fibrosis and sclerosis [234].
Together, mesenchymal repair is needed to stabilize and rebuild tissues after injury, especially after loss of parenchymal tissue. Insufficient scarring is rarely a problem in the kidney. In contrast, scarring within the glomerulus, usually upon dysregulated epithelial repair like in podocyte loss, or crescent formation, is the greatest concern as FSGS and fibrocellular crescents both eventually lead to loss of the entire nephron (Figure 5). Within the interstitial compartment fibroblast- and pericyte-derived extracellular matrix fills the gaps left by dying nephrons and, this way, stabilizes the remaining nephrons. This process, however, further contributes to vascular rarefication and renal ischemia and is thought to further promote the progression of kidney disease. Hence, an otherwise beneficial wound healing response turns into a maladaptive process that promotes organ failure, mainly because of the diffuse nature of most kidney diseases.
7. Summary
Clotting, inflammation, epithelial, and mesenchymal healing represent ancient danger response mechanisms that were positively selected throughout evolution for their benefits on host survival upon focal injury. In focal injuries the associated collateral damages may be acceptable. In contrast, in diffuse injuries, as they usually affect the kidney, these danger response programs often turn into maladaptive pathomechanisms that account for organ failure. Research efforts can benefit from dissecting these individual danger response programs, and from studying their regulatory interactions. From a therapeutic perspective, inhibiting the unnecessary inflammatory response in renal sterile inflammation and stimulating a coordinated epithelial repair should be the most promising strategies to avoid kidney pathology and disease progression.
Acknowledgments
This work was funded by the Deutsche Forschungsgemeinschaft (AN372/12-2, 14-1) and the Else Kröner-Fresenius Stiftung (2011_A95).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Schilmiller A.L., Howe G.A. Systemic signaling in the wound response. Curr. Opin. Plant Biol. 2005;8:369–377. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 3.Singer A.J., Clark R.A. Cutaneous wound healing. N. Engl. J. Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 4.Clark R.A. Cutaneous tissue repair: Basic biologic considerations. I. J. Am. Acad. Dermatol. 1985;13:701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- 5.Martin P. Wound healing—Aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 6.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 7.Nurden A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011;105:S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 8.Furie B., Furie B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 9.Aird W.C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 11.Messier-Solek C., Buckley K.M., Rast J.P. Highly diversified innate receptor systems and new forms of animal immunity. Semin. Immunol. 2010;22:39–47. doi: 10.1016/j.smim.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier M.E., Du Pasquier L., Degnan B.M. The genome of the sponge Amphimedon queenslandica provides new perspectives into the origin of Toll-like and interleukin 1 receptor pathways. Evol. Dev. 2010;12:519–533. doi: 10.1111/j.1525-142X.2010.00436.x. [DOI] [PubMed] [Google Scholar]
- 13.Wiens M., Korzhev M., Perovic-Ottstadt S., Luthringer B., Brandt D., Klein S., Muller W.E. Toll-like receptors are part of the innate immune defense system of sponges (demospongiae: Porifera) Mol. Biol. Evol. 2007;24:792–804. doi: 10.1093/molbev/msl208. [DOI] [PubMed] [Google Scholar]
- 14.Stearns-Kurosawa D.J., Osuchowski M.F., Valentine C., Kurosawa S., Remick D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickey M.J., Kubes P. Intravascular immunity: The host-pathogen encounter in blood vessels. Nat. Rev. Immunol. 2009;9:364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 16.Chan J.K., Roth J., Oppenheim J.J., Tracey K.J., Vogl T., Feldmann M., Horwood N., Nanchahal J. Alarmins: awaiting a clinical response. J. Clin. Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rock K.L., Latz E., Ontiveros F., Kono H. The sterile inflammatory response. Annu. Rev. Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders H.J. Toll-like receptors and danger signaling in kidney injury. J. Am. Soc. Nephrol. 2010;21:1270–1274. doi: 10.1681/ASN.2010030233. [DOI] [PubMed] [Google Scholar]
- 19.Mulay S.R., Kulkarni O.P., Rupanagudi K.V., Migliorini A., Darisipudi M.N., Vilaysane A., Muruve D., Shi Y., Munro F., Liapis H., et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Invest. 2013;123:236–246. doi: 10.1172/JCI63679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannemeier C., Shibamiya A., Nakazawa F., Trusheim H., Ruppert C., Markart P., Song Y., Tzima E., Kennerknecht E., Niepmann M., et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc. Natl. Acad. Sci. USA. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semeraro F., Ammollo C.T., Morrissey J.H., Dale G.L., Friese P., Esmon N.L., Esmon C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delvaeye M., Conway E.M. Coagulation and innate immune responses: Can we view them separately? Blood. 2009;114:2367–2374. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 23.Niessen F., Schaffner F., Furlan-Freguia C., Pawlinski R., Bhattacharjee G., Chun J., Derian C.K., Andrade-Gordon P., Rosen H., Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 24.Van der Poll T., de Boer J.D., Levi M. The effect of inflammation on coagulation and vice versa. Curr. Opin. Infect. Dis. 2011;24:273–278. doi: 10.1097/QCO.0b013e328344c078. [DOI] [PubMed] [Google Scholar]
- 25.Semple J.W., Italiano J.E., Jr, Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 26.Palabrica T., Lobb R., Furie B.C., Aronovitz M., Benjamin C., Hsu Y.M., Sajer S.A., Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 27.Ahronowitz I., Harp J., Shinkai K. Etiology and management of pyoderma gangrenosum: A comprehensive review. Am. J. Clin. Dermatol. 2012;13:191–211. doi: 10.2165/11595240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Bonventre J.V., Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss R.S., Coopersmith C.M., McDunn J.E., Ferguson T.A. The sepsis seesaw: Tilting toward immunosuppression. Nat. Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romagnani P. From proteus to prometheus: Learning from fish to modulate regeneration. J. Am. Soc. Nephrol. 2010;21:726–728. doi: 10.1681/ASN.2010020228. [DOI] [PubMed] [Google Scholar]
- 31.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 32.Sopova K., Tatsidou P., Stellos K. Platelets and platelet interaction with progenitor cells in vascular homeostasis and inflammation. Curr. Vasc. Pharmacol. 2012;10:555–562. doi: 10.2174/157016112801784486. [DOI] [PubMed] [Google Scholar]
- 33.Braun R.K., Ferrick C., Neubauer P., Sjoding M., Sterner-Kock A., Kock M., Putney L., Ferrick D.A., Hyde D.M., Love R.B. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–179. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 34.Jiang G.X., Zhong X.Y., Cui Y.F., Liu W., Tai S., Wang Z.D., Shi Y.G., Zhao S.Y., Li C.L. IL-6/STAT3/TFF3 signaling regulates human biliary epithelial cell migration and wound healing in vitro. Mol. Biol. Rep. 2010;37:3813–3818. doi: 10.1007/s11033-010-0036-z. [DOI] [PubMed] [Google Scholar]
- 35.Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm. Bowel. Dis. 2012;18:1777–1784. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida T., Nakamura M., Mishima H., Otori T. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J. Cell. Physiol. 1992;153:1–5. doi: 10.1002/jcp.1041530102. [DOI] [PubMed] [Google Scholar]
- 37.Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H.A., Hirth S., Weigmann B., Wirtz S., et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugawara T., Gallucci R.M., Simeonova P.P., Luster M.I. Regulation and role of interleukin 6 in wounded human epithelial keratinocytes. Cytokine. 2001;15:328–336. doi: 10.1006/cyto.2001.0946. [DOI] [PubMed] [Google Scholar]
- 39.Zenewicz L.A., Flavell R.A. Recent advances in IL-22 biology. Int. Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 40.Sallustio F., Costantino V., Cox S.N., Loverre A., Divella C., Rizzi M., Schena F.P. Human renal stem/progenitor cells repair tubular epithelial cell injury through TLR2-driven inhibin-A and microvesicle-shuttled decorin. Kidney Int. 2013;83:392–403. doi: 10.1038/ki.2012.413. [DOI] [PubMed] [Google Scholar]
- 41.Brockes J.P. Amphibian limb regeneration: Rebuilding a complex structure. Science. 1997;276:81–87. doi: 10.1126/science.276.5309.81. [DOI] [PubMed] [Google Scholar]
- 42.Sipos F., Valcz G., Molnar B. Physiological and pathological role of local and immigrating colonic stem cells. World J. Gastroenterol. 2012;18:295–301. doi: 10.3748/wjg.v18.i4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryu M., Migliorini A., Miosge N., Gross O., Shankland S., Brinkkoetter P.T., Hagmann H., Romagnani P., Liapis H., Anders H.J. Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury. J. Pathol. 2012;228:482–494. doi: 10.1002/path.4046. [DOI] [PubMed] [Google Scholar]
- 44.Yang L., Besschetnova T.Y., Brooks C.R., Shah J.V., Bonventre J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niedermeier M., Reich B., Rodriguez Gomez M., Denzel A., Schmidbauer K., Gobel N., Talke Y., Schweda F., Mack M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc. Natl. Acad. Sci. USA. 2009;106:17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V., Valerius M.T., McMahon A.P., Duffield J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeisberg M., Neilson E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 50.Chapman K., Seldon M., Richards R. Thrombotic microangiopathies, thrombotic thrombocytopenic purpura, and ADAMTS-13. Semin. Thromb. Hemost. 2012;38:47–54. doi: 10.1055/s-0031-1300951. [DOI] [PubMed] [Google Scholar]
- 51.Amengual O., Atsumi T., Koike T. Pathophysiology of thrombosis and potential targeted therapies in antiphospholipid syndrome. Curr. Vasc. Pharmacol. 2011;9:606–618. doi: 10.2174/157016111796642715. [DOI] [PubMed] [Google Scholar]
- 52.Bonsib S.M. Glomerular basement membrane discontinuities. Scanning electron microscopic study of acellular glomeruli. Am. J. Pathol. 1985;119:357–360. [PMC free article] [PubMed] [Google Scholar]
- 53.Sorensen I., Susnik N., Inhester T., Degen J.L., Melk A., Haller H., Schmitt R. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011;80:1035–1044. doi: 10.1038/ki.2011.214. [DOI] [PubMed] [Google Scholar]
- 54.Drew A.F., Tucker H.L., Liu H., Witte D.P., Degen J.L., Tipping P.G. Crescentic glomerulonephritis is diminished in fibrinogen-deficient mice. Am. J. Physiol. Renal. Physiol. 2001;281:F1157–F1163. doi: 10.1152/ajprenal.2001.281.6.F1157. [DOI] [PubMed] [Google Scholar]
- 55.Downing L.J., Wakefield T.W., Strieter R.M., Prince M.R., Londy F.J., Fowlkes J.B., Hulin M.S., Kadell A.M., Wilke C.A., Brown S.L., et al. Anti-P-selectin antibody decreases inflammation and thrombus formation in venous thrombosis. J. Vasc. Surg. 1997;25:816–827. doi: 10.1016/s0741-5214(97)70211-8. ; discussion 828. [DOI] [PubMed] [Google Scholar]
- 56.Esmon C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 57.Loof T.G., Morgelin M., Johansson L., Oehmcke S., Olin A.I., Dickneite G., Norrby-Teglund A., Theopold U., Herwald H. Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood. 2011;118:2589–2598. doi: 10.1182/blood-2011-02-337568. [DOI] [PubMed] [Google Scholar]
- 58.Loof T.G., Schmidt O., Herwald H., Theopold U. Coagulation systems of invertebrates and vertebrates and their roles in innate immunity: The same side of two coins? J. Innate Immun. 2011;3:34–40. doi: 10.1159/000321641. [DOI] [PubMed] [Google Scholar]
- 59.Rivers R.P., Hathaway W.E., Weston W.L. The endotoxin-induced coagulant activity of human monocytes. Br. J. Haematol. 1975;30:311–316. doi: 10.1111/j.1365-2141.1975.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 60.Cleary C.M., Moreno J.A., Fernández B., Ortiz A., Parra E.G., Gracia C., Blanco-Colio L.M., Barat A., Egido J. Glomerular haematuria, renal interstitial haemorrhage and acute kidney injury. Nephrol. Dial. Transpl. 2010;25:4103–4106. doi: 10.1093/ndt/gfq493. [DOI] [PubMed] [Google Scholar]
- 61.Degen J.L., Bugge T.H., Goguen J.D. Fibrin and fibrinolysis in infection and host defense. J. Thromb. Haemost. 2007;5:24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 62.Nelson P.J., Rees A.J., Griffin M.D., Hughes J., Kurts C., Duffield J. The renal mononuclear phagocytic system. J. Am. Soc. Nephrol. 2012;23:194–203. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lech M., Avila-Ferrufino A., Skuginna V., Susanti H.E., Anders H.J. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int. Immunol. 2010;22:717–728. doi: 10.1093/intimm/dxq058. [DOI] [PubMed] [Google Scholar]
- 64.Anders H.J., Banas B., Schlondorff D. Signaling danger: Toll-Like receptors and their potential roles in kidney disease. J. Am. Soc. Nephrol. 2004;15:854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 65.Patole P.S., Pawar R.D., Lech M., Zecher D., Schmidt H., Segerer S., Ellwart A., Henger A., Kretzler M., Anders H.J. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol. Dial. Transplant. 2006;21:3062–3073. doi: 10.1093/ndt/gfl336. [DOI] [PubMed] [Google Scholar]
- 66.Anders H.J., Schlondorff D. Toll-Like receptors: Emerging concepts in kidney disease. Curr. Opin. Nephrol. Hypertens. 2007;16:177–183. doi: 10.1097/MNH.0b013e32803fb767. [DOI] [PubMed] [Google Scholar]
- 67.Anders H.J., Muruve D.A. The inflammasomes in kidney disease. J. Am. Soc. Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 68.Pawar R.D., Castrezana-Lopez L., Allam R., Kulkarni O.P., Segerer S., Radomska E., Meyer T.N., Schwesinger C.M., Akis N., Grone H.J., et al. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology. 2009;128:e206–e221. doi: 10.1111/j.1365-2567.2008.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patole P.S., Grone H.J., Segerer S., Ciubar R., Belemezova E., Henger A., Kretzler M., Schlondorff D., Anders H.J. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J. Am. Soc. Nephrol. 2005;16:1326–1338. doi: 10.1681/ASN.2004100820. [DOI] [PubMed] [Google Scholar]
- 70.Anders H.J., Banas B., Linde Y., Weller L., Cohen C.D., Kretzler M., Martin S., Vielhauer V., Schlondorff D., Grone H.J. Bacterial CpG-DNA aggravates immune complex glomerulonephritis: Role of TLR9-mediated expression of chemokines and chemokine receptors. J. Am. Soc. Nephrol. 2003;14:317–326. doi: 10.1097/01.asn.0000042169.23931.73. [DOI] [PubMed] [Google Scholar]
- 71.Anders H.J., Vielhauer V., Eis V., Linde Y., Kretzler M., Perez de Lema G., Strutz F., Bauer S., Rutz M., Wagner H., et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J. 2004;18:534–536. doi: 10.1096/fj.03-0646fje. [DOI] [PubMed] [Google Scholar]
- 72.Allam R., Pawar R.D., Kulkarni O.P., Hornung V., Hartmann G., Segerer S., Akira S., Endres S., Anders H.J. Viral 5′-triphosphate RNA and non-CpG DNA aggravate autoimmunity and lupus nephritis via distinct TLR-independent immune responses. Eur. J. Immunol. 2008;38:3487–3498. doi: 10.1002/eji.200838604. [DOI] [PubMed] [Google Scholar]
- 73.Brown H.J., Lock H.R., Sacks S.H., Robson M.G. TLR2 stimulation of intrinsic renal cells in the induction of immune-mediated glomerulonephritis. J. Immunol. 2006;177:1925–1931. doi: 10.4049/jimmunol.177.3.1925. [DOI] [PubMed] [Google Scholar]
- 74.Brown H.J., Lock H.R., Wolfs T.G., Buurman W.A., Sacks S.H., Robson M.G. Toll-like receptor 4 ligation on intrinsic renal cells contributes to the induction of antibody-mediated glomerulonephritis via CXCL1 and CXCL2. J. Am. Soc. Nephrol. 2007;18:1732–1739. doi: 10.1681/ASN.2006060634. [DOI] [PubMed] [Google Scholar]
- 75.Brown H.J., Sacks S.H., Robson M.G. Toll-like receptor 2 agonists exacerbate accelerated nephrotoxic nephritis. J. Am. Soc. Nephrol. 2006;17:1931–1939. doi: 10.1681/ASN.2005111167. [DOI] [PubMed] [Google Scholar]
- 76.Wornle M., Schmid H., Banas B., Merkle M., Henger A., Roeder M., Blattner S., Bock E., Kretzler M., Grone H.J., et al. Novel role of toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am. J. Pathol. 2006;168:370–385. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lichtnekert J., Vielhauer V., Zecher D., Kulkarni O.P., Clauss S., Segerer S., Hornung V., Mayadas T.N., Beutler B., Akira S., et al. Trif is not required for immune complex glomerulonephritis: Dying cells activate mesangial cells via Tlr2/Myd88 rather than Tlr3/Trif. Am. J. Physiol. Renal. Physiol. 2009;296:F867–F874. doi: 10.1152/ajprenal.90213.2008. [DOI] [PubMed] [Google Scholar]
- 78.Ryu M., Kulkarni O.P., Radomska E., Miosge N., Gross O., Anders H.J. Bacterial CpG-DNA accelerates Alport glomerulosclerosis by inducing an M1 macrophage phenotype and tumor necrosis factor-alpha-mediated podocyte loss. Kidney Int. 2011;79:189–198. doi: 10.1038/ki.2010.373. [DOI] [PubMed] [Google Scholar]
- 79.Brahler S., Ising C., Hagmann H., Rasmus M., Hoehne M., Kurschat C., Kisner T., Goebel H., Shankland S., Addicks K., et al. Intrinsic proinflammatory signaling in podocytes contributes to podocyte damage and prolonged proteinuria. Am. J. Physiol. Renal. Physiol. 2012;303:F1473–F1485. doi: 10.1152/ajprenal.00031.2012. [DOI] [PubMed] [Google Scholar]
- 80.Lasagni L., Romagnani P. Glomerular epithelial stem cells: The good, the bad, and the ugly. J. Am. Soc. Nephrol. 2010;21:1612–1619. doi: 10.1681/ASN.2010010048. [DOI] [PubMed] [Google Scholar]
- 81.Pawar R.D., Patole P.S., Wornle M., Anders H.J. Microbial nucleic acids pay a Toll in kidney disease. Am. J. Physiol. Renal. Physiol. 2006;291:F509–F516. doi: 10.1152/ajprenal.00453.2005. [DOI] [PubMed] [Google Scholar]
- 82.Marshak-Rothstein A., Rifkin I.R. Immunologically active autoantigens: The role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 83.Leadbetter E.A., Rifkin I.R., Hohlbaum A.M., Beaudette B.C., Shlomchik M.J., Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 84.Steinman R.M., Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 85.Migliorini A., Anders H.J. A novel pathogenetic concept-antiviral immunity in lupus nephritis. Nat. Rev. Nephrol. 2012;8:183–189. doi: 10.1038/nrneph.2011.197. [DOI] [PubMed] [Google Scholar]
- 86.Anders H.J. Pseudoviral immunity—A novel concept for lupus. Trends Mol. Med. 2009;15:553–561. doi: 10.1016/j.molmed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Anders H.J., Lichtnekert J., Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010;77:848–854. doi: 10.1038/ki.2010.71. [DOI] [PubMed] [Google Scholar]
- 88.Flur K., Allam R., Zecher D., Kulkarni O.P., Lichtnekert J., Schwarz M., Beutler B., Vielhauer V., Anders H.J. Viral RNA induces type I interferon-dependent cytokine release and cell death in mesangial cells via melanoma-differentiation-associated gene-5: Implications for viral infection-associated glomerulonephritis. Am. J. Pathol. 2009;175:2014–2022. doi: 10.2353/ajpath.2009.080585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hagele H., Allam R., Pawar R.D., Anders H.J. Double-stranded RNA activates type I interferon secretion in glomerular endothelial cells via retinoic acid-inducible gene (RIG)-1. Nephrol. Dial. Transplant. 2009;24:3312–3318. doi: 10.1093/ndt/gfp339. [DOI] [PubMed] [Google Scholar]
- 90.Hagele H., Allam R., Pawar R.D., Reichel C.A., Krombach F., Anders H.J. Double-stranded DNA activates glomerular endothelial cells and enhances albumin permeability via a toll-like receptor-independent cytosolic DNA recognition pathway. Am. J. Pathol. 2009;175:1896–1904. doi: 10.2353/ajpath.2009.090182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allam R., Lichtnekert J., Moll A., Taubitz A., Vielhauer V., Anders H.J. Viral RNA and DNA sense common antiviral responses including type I interferons in mesangial cells. J. Am. Soc. Nephrol. 2009;20:1986–1996. doi: 10.1681/ASN.2008101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fairhurst A.M., Mathian A., Connolly J.E., Wang A., Gray H.F., George T.A., Boudreaux C.D., Zhou X.J., Li Q.Z., Koutouzov S., et al. Systemic IFN-alpha drives kidney nephritis in B6.Sle123 mice. Eur. J. Immunol. 2008;38:1948–1960. doi: 10.1002/eji.200837925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fairhurst A.M., Xie C., Fu Y., Wang A., Boudreaux C., Zhou X.J., Cibotti R., Coyle A., Connolly J.E., Wakeland E.K., et al. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J. Immunol. 2009;183:6831–6838. doi: 10.4049/jimmunol.0900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pawar R.D., Ramanjaneyulu A., Kulkarni O.P., Lech M., Segerer S., Anders H.J. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 2007;18:1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 95.Wu H., Ma J., Wang P., Corpuz T.M., Panchapakesan U., Wyburn K.R., Chadban S.J. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shigeoka A.A., Holscher T.D., King A.J., Hall F.W., Kiosses W.B., Tobias P.S., Mackman N., McKay D.B. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J. Immunol. 2007;178:6252–6258. doi: 10.4049/jimmunol.178.10.6252. [DOI] [PubMed] [Google Scholar]
- 97.Leemans J.C., Stokman G., Claessen N., Rouschop K.M., Teske G.J., Kirschning C.J., Akira S., van der Poll T., Weening J.J., Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J. Clin. Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu H., Chen G., Wyburn K.R., Yin J., Bertolino P., Eris J.M., Alexander S.I., Sharland A.F., Chadban S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allam R., Scherbaum C.R., Darisipudi M.N., Mulay S.R., Hagele H., Lichtnekert J., Hagemann J.H., Rupanagudi K.V., Ryu M., Schwarzenberger C., et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J. Am. Soc. Nephrol. 2012;23:1375–1388. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saemann M.D., Weichhart T., Zeyda M., Staffler G., Schunn M., Stuhlmeier K.M., Sobanov Y., Stulnig T.M., Akira S., von Gabain A., et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J. Clin. Invest. 2005;115:468–475. doi: 10.1172/JCI22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Darisipudi M.N., Thomasova D., Mulay S.R., Brech D., Noessner E., Liapis H., Anders H.J. Uromodulin triggers IL-1beta-dependent innate immunity via the NLRP3 inflammasome. J. Am. Soc. Nephrol. 2012;23:1783–1789. doi: 10.1681/ASN.2012040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lech M., Garlanda C., Mantovani A., Kirschning C.J., Schlondorff D., Anders H.J. Different roles of TiR8/Sigirr on toll-like receptor signaling in intrarenal antigen-presenting cells and tubular epithelial cells. Kidney Int. 2007;72:182–192. doi: 10.1038/sj.ki.5002293. [DOI] [PubMed] [Google Scholar]
- 103.Lassen S., Lech M., Rommele C., Mittruecker H.W., Mak T.W., Anders H.J. Ischemia reperfusion induces IFN regulatory factor 4 in renal dendritic cells, which suppresses postischemic inflammation and prevents acute renal failure. J. Immunol. 2010;185:1976–1983. doi: 10.4049/jimmunol.0904207. [DOI] [PubMed] [Google Scholar]
- 104.Lech M., Avila-Ferrufino A., Allam R., Segerer S., Khandoga A., Krombach F., Garlanda C., Mantovani A., Anders H.J. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J. Immunol. 2009;183:4109–4118. doi: 10.4049/jimmunol.0900118. [DOI] [PubMed] [Google Scholar]
- 105.Gong J., Wei T., Stark R.W., Jamitzky F., Heckl W.M., Anders H.J., Lech M., Rossle S.C. Inhibition of Toll-like receptors TLR4 and 7 signaling pathways by SIGIRR: A computational approach. J. Struct. Biol. 2010;169:323–330. doi: 10.1016/j.jsb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 106.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 107.John R., Nelson P.J. Dendritic cells in the kidney. J. Am. Soc. Nephrol. 2007;18:2628–2635. doi: 10.1681/ASN.2007030273. [DOI] [PubMed] [Google Scholar]
- 108.Kruger T., Benke D., Eitner F., Lang A., Wirtz M., Hamilton-Williams E.E., Engel D., Giese B., Muller-Newen G., Floege J., et al. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J. Am. Soc. Nephrol. 2004;15:613–621. doi: 10.1097/01.asn.0000114553.36258.91. [DOI] [PubMed] [Google Scholar]
- 109.Vilaysane A., Chun J., Seamone M.E., Wang W., Chin R., Hirota S., Li Y., Clark S.A., Tschopp J., Trpkov K., et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lichtnekert J., Kulkarni O.P., Mulay S.R., Rupanagudi K.V., Ryu M., Allam R., Vielhauer V., Muruve D., Lindenmeyer M.T., Cohen C.D., et al. Anti-GBM glomerulonephritis involves IL-1 but is independent of NLRP3/ASC inflammasome-mediated activation of caspase-1. PLoS One. 2011;6:e26778. doi: 10.1371/journal.pone.0026778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iyer S.S., Pulskens W.P., Sadler J.J., Butter L.M., Teske G.J., Ulland T.K., Eisenbarth S.C., Florquin S., Flavell R.A., Leemans J.C., et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anders H.J., Vielhauer V., Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- 113.Vielhauer V., Kulkarni O., Reichel C.A., Anders H.J. Targeting the recruitment of monocytes and macrophages in renal disease. Semin. Nephrol. 2010;30:318–333. doi: 10.1016/j.semnephrol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 114.Heller F., Lindenmeyer M.T., Cohen C.D., Brandt U., Draganovici D., Fischereder M., Kretzler M., Anders H.J., Sitter T., Mosberger I., et al. The contribution of B cells to renal interstitial inflammation. Am. J. Pathol. 2007;170:457–468. doi: 10.2353/ajpath.2007.060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steinmetz O.M., Stahl R.A., Panzer U. Chemokines and B cells in renal inflammation and allograft rejection. Front. Biosci. (Schol Ed.) 2009;1:13–22. doi: 10.2741/S2. [DOI] [PubMed] [Google Scholar]
- 116.Swaminathan S., Griffin M.D. First responders: Understanding monocyte-lineage traffic in the acutely injured kidney. Kidney Int. 2008;74:1509–1511. doi: 10.1038/ki.2008.555. [DOI] [PubMed] [Google Scholar]
- 117.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 118.Stroo I., Stokman G., Teske G.J., Raven A., Butter L.M., Florquin S., Leemans J.C. Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int. Immunol. 2010;22:433–442. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Segerer S., Nelson P.J. Chemokines in renal diseases. ScientificWorldJournal. 2005;5:835–844. doi: 10.1100/tsw.2005.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ishida Y., Gao J.L., Murphy P.M. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J. Immunol. 2008;180:569–579. doi: 10.4049/jimmunol.180.1.569. [DOI] [PubMed] [Google Scholar]
- 121.Panzer U., Kurts C. T cell cross-talk with kidney dendritic cells in glomerulonephritis. J. Mol. Med. (Berl. ) 2010;88:19–26. doi: 10.1007/s00109-009-0541-5. [DOI] [PubMed] [Google Scholar]
- 122.Anders H.J., Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 123.Lech M., Anders H.J. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Biophys. Acta. 2012;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 124.Lech M., Grobmayr R., Weidenbusch M., Anders H.J. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: The immunoregulatory role of changing tissue environments. Mediators Inflamm. 2012;2012:951390. doi: 10.1155/2012/951390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duffield J.S. Macrophages and immunologic inflammation of the kidney. Semin. Nephrol. 2010;30:234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anders H.J., Frink M., Linde Y., Banas B., Wornle M., Cohen C.D., Vielhauer V., Nelson P.J., Grone H.J., Schlondorff D. CC chemokine ligand 5/RANTES chemokine antagonists aggravate glomerulonephritis despite reduction of glomerular leukocyte infiltration. J. Immunol. 2003;170:5658–5666. doi: 10.4049/jimmunol.170.11.5658. [DOI] [PubMed] [Google Scholar]
- 127.Pawar R.D., Patole P.S., Ellwart A., Lech M., Segerer S., Schlondorff D., Anders H.J. Ligands to nucleic acid-specific toll-like receptors and the onset of lupus nephritis. J. Am. Soc. Nephrol. 2006;17:3365–3373. doi: 10.1681/ASN.2006030263. [DOI] [PubMed] [Google Scholar]
- 128.Anders H.J., Zecher D., Pawar R.D., Patole P.S. Molecular mechanisms of autoimmunity triggered by microbial infection. Arthritis Res. Ther. 2005;7:215–224. doi: 10.1186/ar1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ble A., Mosca M., Di Loreto G., Guglielmotti A., Biondi G., Bombardieri S., Remuzzi G., Ruggenenti P. Antiproteinuric effect of chemokine C-C motif ligand 2 inhibition in subjects with acute proliferative lupus nephritis. Am. J. Nephrol. 2011;34:367–372. doi: 10.1159/000330685. [DOI] [PubMed] [Google Scholar]
- 130.Kulkarni O., Pawar R.D., Purschke W., Eulberg D., Selve N., Buchner K., Ninichuk V., Segerer S., Vielhauer V., Klussmann S., et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 2007;18:2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- 131.Ninichuk V., Clauss S., Kulkarni O., Schmid H., Segerer S., Radomska E., Eulberg D., Buchner K., Selve N., Klussmann S., et al. Late onset of Ccl2 blockade with the Spiegelmer mNOX-E36–3′PEG prevents glomerulosclerosis and improves glomerular filtration rate in db/db mice. Am. J. Pathol. 2008;172:628–637. doi: 10.2353/ajpath.2008.070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kulkarni O., Eulberg D., Selve N., Zollner S., Allam R., Pawar R.D., Pfeiffer S., Segerer S., Klussmann S., Anders H.J. Anti-Ccl2 Spiegelmer permits 75% dose reduction of cyclophosphamide to control diffuse proliferative lupus nephritis and pneumonitis in MRL-Fas(lpr) mice. J. Pharmacol. Exp. Ther. 2009;328:371–377. doi: 10.1124/jpet.108.142711. [DOI] [PubMed] [Google Scholar]
- 133.Sayyed S.G., Ryu M., Kulkarni O.P., Schmid H., Lichtnekert J., Gruner S., Green L., Mattei P., Hartmann G., Anders H.J. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011;80:68–78. doi: 10.1038/ki.2011.102. [DOI] [PubMed] [Google Scholar]
- 134.Clauss S., Gross O., Kulkarni O., Avila-Ferrufino A., Radomska E., Segerer S., Eulberg D., Klussmann S., Anders H.J. Ccl2/Mcp-1 blockade reduces glomerular and interstitial macrophages but does not ameliorate renal pathology in collagen4A3-deficient mice with autosomal recessive Alport nephropathy. J. Pathol. 2009;218:40–47. doi: 10.1002/path.2505. [DOI] [PubMed] [Google Scholar]
- 135.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R.K., Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 137.Lucas T., Waisman A., Ranjan R., Roes J., Krieg T., Muller W., Roers A., Eming S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 138.Xu J., Zhang X., Monestier M., Esmon N.L., Esmon C.T. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu J., Zhang X., Pelayo R., Monestier M., Ammollo C.T., Semeraro F., Taylor F.B., Esmon N.L., Lupu F., Esmon C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kulkarni O.P., Ryu M., Kantner C., Sardy M., Naylor D., Lambert D., Brown R., Anders H.J. Recombinant chaperonin 10 suppresses cutaneous lupus and lupus nephritis in MRL-(Fas)lpr mice. Nephrol. Dial. Transplant. 2011;27:1358–1367. doi: 10.1093/ndt/gfr544. [DOI] [PubMed] [Google Scholar]
- 141.Vandewalle A. Toll-like receptors and renal bacterial infections. Chang. Gung Med. J. 2008;31:525–537. [PubMed] [Google Scholar]
- 142.Ribeiro A., Wornle M., Motamedi N., Anders H.J., Grone E.F., Nitschko H., Kurktschiev P., Debiec H., Kretzler M., Cohen C.D., et al. Activation of innate immune defense mechanisms contributes to polyomavirus BK-associated nephropathy. Kidney Int. 2012;81:100–111. doi: 10.1038/ki.2011.311. [DOI] [PubMed] [Google Scholar]
- 143.Babel N., Volk H.D., Reinke P. BK polyomavirus infection and nephropathy: the virus-immune system interplay. Nat. Rev. Nephrol. 2011;7:399–406. doi: 10.1038/nrneph.2011.59. [DOI] [PubMed] [Google Scholar]
- 144.Anders H.J., Patole P.S. Toll-like receptors recognize uropathogenic Escherichia coli and trigger inflammation in the urinary tract. Nephrol. Dial. Transplant. 2005;20:1529–1532. doi: 10.1093/ndt/gfh922. [DOI] [PubMed] [Google Scholar]
- 145.Patole P.S., Schubert S., Hildinger K., Khandoga S., Khandoga A., Segerer S., Henger A., Kretzler M., Werner M., Krombach F., et al. Toll-like receptor-4: Renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int. 2005;68:2582–2587. doi: 10.1111/j.1523-1755.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- 146.Yang C.W., Hung C.C., Wu M.S., Tian Y.C., Chang C.T., Pan M.J., Vandewalle A. Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney Int. 2006;69:815–822. doi: 10.1038/sj.ki.5000119. [DOI] [PubMed] [Google Scholar]
- 147.Bonventre J.V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003;14:S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 148.Abuelo J.G. Normotensive ischemic acute renal failure. N. Engl. J. Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 149.Sugimoto H., Lebleu V.S., Bosukonda D., Keck P., Taduri G., Bechtel W., Okada H., Carlson W., Bey P., Rusckowski M., et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat. Med. 2012;18:396–404. doi: 10.1038/nm.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mulay S.R., Thomasova D., Ryu M., Anders H.J. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int. 2012;81:1199–1211. doi: 10.1038/ki.2011.482. [DOI] [PubMed] [Google Scholar]
- 151.Thomasova D., Mulay S.R., Bruns H., Anders H.J. p53-Independent Roles of MDM2 in NF-kappaB signaling: Implications for cancer therapy, wound healing, and autoimmune diseases. Neoplasia. 2012;14:1097–1101. doi: 10.1593/neo.121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weidenbusch M., Anders H.J. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J. Innate Immun. 2012;4:463–477. doi: 10.1159/000336717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ricardo S.D., van Goor H., Eddy A.A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duffield J.S., Park K.M., Hsiao L.L., Kelley V.R., Scadden D.T., Ichimura T., Bonventre J.V. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J. Clin. Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee S., Huen S., Nishio H., Nishio S., Lee H.K., Choi B.S., Ruhrberg C., Cantley L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang M.Z., Yao B., Yang S., Jiang L., Wang S., Fan X., Yin H., Wong K., Miyazawa T., Chen J., et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Invest. 2012;122:4519–4532. doi: 10.1172/JCI60363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duffield J.S., Forbes S.J., Constandinou C.M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee S.B., Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int. 2010;78:S22–S26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Iwata Y., Bostrom E.A., Menke J., Rabacal W.A., Morel L., Wada T., Kelley V.R. Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J. Immunol. 2012;188:4568–4580. doi: 10.4049/jimmunol.1102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Smeets B., Angelotti M.L., Rizzo P., Dijkman H., Lazzeri E., Mooren F., Ballerini L., Parente E., Sagrinati C., Mazzinghi B., et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2009;20:2593–2603. doi: 10.1681/ASN.2009020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Smeets B., Uhlig S., Fuss A., Mooren F., Wetzels J.F., Floege J., Moeller M.J. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J. Am. Soc. Nephrol. 2009;20:2604–2615. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Atkins R.C., Nikolic-Paterson D.J., Song Q., Lan H.Y. Modulators of crescentic glomerulonephritis. J. Am. Soc. Nephrol. 1996;7:2271–2278. doi: 10.1681/ASN.V7112271. [DOI] [PubMed] [Google Scholar]
- 163.Tipping P.G., Kitching P.R., Holdsworth S.R. The Formation of the Glomerular Crescent. In: Neilson E.G., Couser W.G., editors. Immunologic Renal Diseases. 2nd ed. Lippincott Williams & Wilkins Publishers; Philadelphia, PA, USA: 2001. [Google Scholar]
- 164.Bollee G., Flamant M., Schordan S., Fligny C., Rumpel E., Milon M., Schordan E., Sabaa N., Vandermeersch S., Galaup A., et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat. Med. 2011;17:1242–1250. doi: 10.1038/nm.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ohse T., Pippin J.W., Chang A.M., Krofft R.D., Miner J.H., Vaughan M.R., Shankland S.J. The enigmatic parietal epithelial cell is finally getting noticed: A review. Kidney Int. 2009;76:1225–1238. doi: 10.1038/ki.2009.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shankland S.J., Anders H.J., Romagnani P. Glomerular parietal epithelial cells in kidney physiology, pathology, and repair. Curr. Opin. Nephrol. Hypertens. 2013;22:302–309. doi: 10.1097/MNH.0b013e32835fefd4. [DOI] [PubMed] [Google Scholar]
- 167.Lindgren D., Bostrom A.K., Nilsson K., Hansson J., Sjolund J., Moller C., Jirstrom K., Nilsson E., Landberg G., Axelson H., et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am. J. Pathol. 2011;178:828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Romagnani P. Family portrait: Renal progenitor of Bowman’s capsule and its tubular brothers. Am. J. Pathol. 2011;178:490–493. doi: 10.1016/j.ajpath.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Forbes M.S., Thornhill B.A., Chevalier R.L. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am. J. Physiol. Renal. Physiol. 2011;301:F110–F117. doi: 10.1152/ajprenal.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chevalier R.L., Forbes M.S. Generation and evolution of atubular glomeruli in the progression of renal disorders. J. Am. Soc. Nephrol. 2008;19:197–206. doi: 10.1681/ASN.2007080862. [DOI] [PubMed] [Google Scholar]
- 171.D’Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N. Engl. J. Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 172.Romagnani P., Remuzzi G. Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol. Metab. 2013;24:13–20. doi: 10.1016/j.tem.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 173.Kriz W., Lemley K.V. The role of the podocyte in glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 1999;8:489–497. doi: 10.1097/00041552-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 174.de Teixeira V.P., Blattner S.M., Li M., Anders H.J., Cohen C.D., Edenhofer I., Calvaresi N., Merkle M., Rastaldi M.P., Kretzler M. Functional consequences of integrin-linked kinase activation in podocyte damage. Kidney Int. 2005;67:514–523. doi: 10.1111/j.1523-1755.2005.67108.x. [DOI] [PubMed] [Google Scholar]
- 175.Wharram B.L., Goyal M., Wiggins J.E., Sanden S.K., Hussain S., Filipiak W.E., Saunders T.L., Dysko R.C., Kohno K., Holzman L.B., et al. Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 176.Sachs N., Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol. 2013;9:200–210. doi: 10.1038/nrneph.2012.291. [DOI] [PubMed] [Google Scholar]
- 177.Lasagni L., Lazzeri E., Shankland S.J., Anders H.J., Romagnani P. Podocyte mitosis—A catastrophe. Curr. Mol. Med. 2013;13:13–23. doi: 10.2174/1566524011307010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lasagni L., Ballerini L., Angelotti M.L., Parente E., Sagrinati C., Mazzinghi B., Peired A., Ronconi E., Becherucci F., Bani D., et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010;28:1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pippin J.W., Durvasula R., Petermann A., Hiromura K., Couser W.G., Shankland S.J. DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J. Clin. Invest. 2003;111:877–885. doi: 10.1172/JCI15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mulay S.R., Thomasova D., Ryu M., Kulkarni O.P., Migliorini A., Bruns H., Gröbmayr R., Lazzeri E., Lasagni L., Liapis H., Romagnani P., Anders H.-J. Podocyte loss involves MDM2-driven mitotic catastrophe of podocytes. J. Pathol. 2013 doi: 10.1002/path.4193. in press. [DOI] [PubMed] [Google Scholar]
- 181.Sugimoto H., Mundel T.M., Sund M., Xie L., Cosgrove D., Kalluri R. Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc. Natl. Acad. Sci. USA. 2006;103:7321–7326. doi: 10.1073/pnas.0601436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.LeBleu V., Sugimoto H., Mundel T.M., Gerami-Naini B., Finan E., Miller C.A., Gattone V.H., II, Lu L., Shield C.F., III, Folkman J., et al. Stem cell therapies benefit Alport syndrome. J. Am. Soc. Nephrol. 2009;20:2359–2370. doi: 10.1681/ASN.2009010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gross O., Borza D.B., Anders H.J., Licht C., Weber M., Segerer S., Torra R., Gubler M.C., Heidet L., Harvey S., et al. Stem cell therapy for Alport syndrome: The hope beyond the hype. Nephrol. Dial. Transplant. 2009;24:731–734. doi: 10.1093/ndt/gfn722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lazzeri E., Crescioli C., Ronconi E., Mazzinghi B., Sagrinati C., Netti G.S., Angelotti M.L., Parente E., Ballerini L., Cosmi L., et al. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J. Am. Soc. Nephrol. 2007;18:3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- 185.Ronconi E., Sagrinati C., Angelotti M.L., Lazzeri E., Mazzinghi B., Ballerini L., Parente E., Becherucci F., Gacci M., Carini M., et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Appel D., Kershaw D.B., Smeets B., Yuan G., Fuss A., Frye B., Elger M., Kriz W., Floege J., Moeller M.J. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sayyed S.G., Hagele H., Kulkarni O.P., Endlich K., Segerer S., Eulberg D., Klussmann S., Anders H.J. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia. 2009;52:2445–2454. doi: 10.1007/s00125-009-1493-6. [DOI] [PubMed] [Google Scholar]
- 188.Darisipudi M.N., Kulkarni O.P., Sayyed S.G., Ryu M., Migliorini A., Sagrinati C., Parente E., Vater A., Eulberg D., Klussmann S., et al. Dual blockade of the homeostatic chemokine CXCL12 and the proinflammatory chemokine CCL2 has additive protective effects on diabetic kidney disease. Am. J. Pathol. 2011;179:116–124. doi: 10.1016/j.ajpath.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gaikwad A.B., Sayyed S.G., Lichtnekert J., Tikoo K., Anders H.J. Renal failure increases cardiac histone h3 acetylation, dimethylation, and phosphorylation and the induction of cardiomyopathy-related genes in type 2 diabetes. Am. J. Pathol. 2010;176:1079–1083. doi: 10.2353/ajpath.2010.090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sayyed S.G., Gaikwad A.B., Lichtnekert J., Kulkarni O., Eulberg D., Klussmann S., Tikoo K., Anders H.J. Progressive glomerulosclerosis in type 2 diabetes is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation and phosphorylation at serine 10. Nephrol. Dial. Transplant. 2010;25:1811–1817. doi: 10.1093/ndt/gfp730. [DOI] [PubMed] [Google Scholar]
- 191.Angelotti M.L., Ronconi E., Ballerini L., Peired A., Mazzinghi B., Sagrinati C., Parente E., Gacci M., Carini M., Rotondi M., et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 192.Humphreys B.D., Czerniak S., DiRocco D.P., Hasnain W., Cheema R., Bonventre J.V. Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl. Acad. Sci. USA. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Humphreys B.D., Valerius M.T., Kobayashi A., Mugford J.W., Soeung S., Duffield J.S., McMahon A.P., Bonventre J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 194.Togel F.E., Westenfelder C. Mesenchymal stem cells: A new therapeutic tool for AKI. Nat. Rev. Nephrol. 2010;6:179–183. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- 195.Higgins D.F., Lappin D.W., Kieran N.E., Anders H.J., Watson R.W., Strutz F., Schlondorff D., Haase V.H., Fitzpatrick J.M., Godson C., et al. DNA oligonucleotide microarray technology identifies fisp-12 among other potential fibrogenic genes following murine unilateral ureteral obstruction (UUO): Modulation during epithelial-mesenchymal transition. Kidney Int. 2003;64:2079–2091. doi: 10.1046/j.1523-1755.2003.00306.x. [DOI] [PubMed] [Google Scholar]
- 196.Ninichuk V., Gross O., Segerer S., Hoffmann R., Radomska E., Buchstaller A., Huss R., Akis N., Schlondorff D., Anders H.J. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 197.Migliorini A., Ebid R., Scherbaum C.R., Anders H.J. The danger control concept in kidney disease: mesangial cells. J. Nephrol. 2013;26:437–449. doi: 10.5301/jn.5000247. [DOI] [PubMed] [Google Scholar]
- 198.Johnson R.J., Raines E.W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J. Exp. Med. 1992;175:1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bomback A.S., Appel G.B. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat. Rev. Nephrol. 2012;8:634–642. doi: 10.1038/nrneph.2012.213. [DOI] [PubMed] [Google Scholar]
- 200.Hugo C., Shankland S.J., Bowen-Pope D.F., Couser W.G., Johnson R.J. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J. Clin. Invest. 1997;100:786–794. doi: 10.1172/JCI119592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Imasawa T., Utsunomiya Y., Kawamura T., Zhong Y., Nagasawa R., Okabe M., Maruyama N., Hosoya T., Ohno T. The potential of bone marrow-derived cells to differentiate to glomerular mesangial cells. J. Am. Soc. Nephrol. 2001;12:1401–1409. doi: 10.1681/ASN.V1271401. [DOI] [PubMed] [Google Scholar]
- 202.Sethi S., Fervenza F.C. Membranoproliferative glomerulonephritis—A new look at an old entity. N. Engl. J. Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 203.Remuzzi G., Benigni A., Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Smeets B., Kuppe C., Sicking E.M., Fuss A., Jirak P., van Kuppevelt T.H., Endlich K., Wetzels J.F., Grone H.J., Floege J., et al. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 2011;22:1262–1274. doi: 10.1681/ASN.2010090970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Helal I., Fick-Brosnahan G.M., Reed-Gitomer B., Schrier R.W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012;8:293–300. doi: 10.1038/nrneph.2012.19. [DOI] [PubMed] [Google Scholar]
- 206.Bariety J., Hill G.S., Mandet C., Irinopoulou T., Jacquot C., Meyrier A., Bruneval P. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol. Dial. Transplant. 2003;18:1777–1784. doi: 10.1093/ndt/gfg231. [DOI] [PubMed] [Google Scholar]
- 207.Duffield J.S. Epithelial to mesenchymal transition in injury of solid organs: Fact or artifact? Gastroenterology. 2010;139 doi: 10.1053/j.gastro.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 208.Zeisberg M., Duffield J.S. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 209.Kriz W., Kaissling B., Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: Fact or fantasy? J. Clin. Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bohle A., Wehrmann M., Bogenschutz O., Batz C., Vogl W., Schmitt H., Muller C.A., Muller G.A. The long-term prognosis of the primary glomerulonephritides. A morphological and clinical analysis of 1747 cases. Pathol. Res. Pract. 1992;188:908–924. doi: 10.1016/s0344-0338(11)80252-9. [DOI] [PubMed] [Google Scholar]
- 211.Famulski K.S., Reeve J., de Freitas D.G., Kreepala C., Chang J., Halloran P.F. Kidney transplants with progressing chronic diseases express high levels of acute kidney injury transcripts. Am. J. Transplant. 2013;13:634–644. doi: 10.1111/ajt.12080. [DOI] [PubMed] [Google Scholar]
- 212.Li Y., Liu Z., Guo X., Shu J., Chen Z., Li L. Aristolochic acid I-induced DNA damage and cell cycle arrest in renal tubular epithelial cells in vitro. Arch. Toxicol. 2006;80:524–532. doi: 10.1007/s00204-006-0090-4. [DOI] [PubMed] [Google Scholar]
- 213.Debelle F.D., Vanherweghem J.L., Nortier J.L. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 214.Ninichuk V., Anders H.J. Bone marrow-derived progenitor cells and renal fibrosis. Front. Biosci. 2008;13:5163–5173. doi: 10.2741/3072. [DOI] [PubMed] [Google Scholar]
- 215.Vielhauer V., Anders H.J., Mack M., Cihak J., Strutz F., Stangassinger M., Luckow B., Grone H.J., Schlondorff D. Obstructive nephropathy in the mouse: Progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J. Am. Soc. Nephrol. 2001;12:1173–1187. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- 216.Anders H.J., Vielhauer V., Kretzler M., Cohen C.D., Segerer S., Luckow B., Weller L., Grone H.J., Schlondorff D. Chemokine and chemokine receptor expression during initiation and resolution of immune complex glomerulonephritis. J. Am. Soc. Nephrol. 2001;12:919–931. doi: 10.1681/ASN.V125919. [DOI] [PubMed] [Google Scholar]
- 217.Mayer V., Hudkins K.L., Heller F., Schmid H., Kretzler M., Brandt U., Anders H.J., Regele H., Nelson P.J., Alpers C.E., et al. Expression of the chemokine receptor CCR1 in human renal allografts. Nephrol. Dial. Transplant. 2007;22:1720–1729. doi: 10.1093/ndt/gfm007. [DOI] [PubMed] [Google Scholar]
- 218.Vielhauer V., Anders H.J. Chemokines and chemokine receptors as therapeutic targets in chronic kidney disease. Front. Biosci. (Schol Ed. ) 2009;1:1–12. doi: 10.2741/s1. [DOI] [PubMed] [Google Scholar]
- 219.Jedlicka J., Soleiman A., Draganovici D., Mandelbaum J., Ziegler U., Regele H., Wuthrich R.P., Gross O., Anders H.J., Segerer S. Interstitial inflammation in Alport syndrome. Hum. Pathol. 2010;41:582–593. doi: 10.1016/j.humpath.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 220.Anders H.J., Ninichuk V., Schlondorff D. Progression of kidney disease: Blocking leukocyte recruitment with chemokine receptor CCR1 antagonists. Kidney Int. 2006;69:29–32. doi: 10.1038/sj.ki.5000053. [DOI] [PubMed] [Google Scholar]
- 221.Eis V., Vielhauer V., Anders H.J. Targeting the chemokine network in renal inflammation. Arch. Immunol. Ther. Exp. (Warsz) 2004;52:164–172. [PubMed] [Google Scholar]
- 222.Eis V., Luckow B., Vielhauer V., Siveke J.T., Linde Y., Segerer S., Perez De Lema G., Cohen C.D., Kretzler M., Mack M., et al. Chemokine receptor CCR1 but not CCR5 mediates leukocyte recruitment and subsequent renal fibrosis after unilateral ureteral obstruction. J. Am. Soc. Nephrol. 2004;15:337–347. doi: 10.1097/01.asn.0000111246.87175.32. [DOI] [PubMed] [Google Scholar]
- 223.Anders H.J., Vielhauer V., Frink M., Linde Y., Cohen C.D., Blattner S.M., Kretzler M., Strutz F., Mack M., Grone H.J., et al. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J. Clin. Invest. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Anders H.J., Belemezova E., Eis V., Segerer S., Vielhauer V., Perez de Lema G., Kretzler M., Cohen C.D., Frink M., Horuk R., et al. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J. Am. Soc. Nephrol. 2004;15:1504–1513. doi: 10.1097/01.asn.0000130082.67775.60. [DOI] [PubMed] [Google Scholar]
- 225.Vielhauer V., Berning E., Eis V., Kretzler M., Segerer S., Strutz F., Horuk R., Grone H.J., Schlondorff D., Anders H.J. CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int. 2004;66:2264–2278. doi: 10.1111/j.1523-1755.2004.66038.x. [DOI] [PubMed] [Google Scholar]
- 226.Ninichuk V., Gross O., Reichel C., Khandoga A., Pawar R.D., Ciubar R., Segerer S., Belemezova E., Radomska E., Luckow B., et al. Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J. Am. Soc. Nephrol. 2005;16:977–985. doi: 10.1681/ASN.2004100871. [DOI] [PubMed] [Google Scholar]
- 227.Ninichuk V., Khandoga A.G., Segerer S., Loetscher P., Schlapbach A., Revesz L., Feifel R., Khandoga A., Krombach F., Nelson P.J., et al. The role of interstitial macrophages in nephropathy of type 2 diabetic db/db mice. Am. J. Pathol. 2007;170:1267–1276. doi: 10.2353/ajpath.2007.060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sakai N., Furuichi K., Shinozaki Y., Yamauchi H., Toyama T., Kitajima S., Okumura T., Kokubo S., Kobayashi M., Takasawa K., et al. Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Hum. Pathol. 2010;41:672–678. doi: 10.1016/j.humpath.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 229.Wada T., Sakai N., Matsushima K., Kaneko S. Fibrocytes: A new insight into kidney fibrosis. Kidney Int. 2007;72:269–273. doi: 10.1038/sj.ki.5002325. [DOI] [PubMed] [Google Scholar]
- 230.Reich B., Schmidbauer K., Rodriguez Gomez M., Johannes Hermann F., Gobel N., Bruhl H., Ketelsen I., Talke Y., Mack M. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 2013 doi: 10.1038/ki.2013.84. [DOI] [PubMed] [Google Scholar]
- 231.Sakai N., Wada T., Yokoyama H., Lipp M., Ueha S., Matsushima K., Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc. Natl. Acad. Sci. USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Duffield J.S., Lupher M., Thannickal V.J., Wynn T.A. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schrimpf C., Xin C., Campanholle G., Gill S.E., Stallcup W., Lin S.L., Davis G.E., Gharib S.A., Humphreys B.D., Duffield J.S. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J. Am. Soc. Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fligny C., Duffield J.S. Activation of pericytes: Recent insights into kidney fibrosis and microvascular rarefaction. Curr. Opin. Rheumatol. 2013;25:78–86. doi: 10.1097/BOR.0b013e32835b656b. [DOI] [PubMed] [Google Scholar]