Abstract
Abiotic and biotic stresses lead to massive reprogramming of different life processes and are the major limiting factors hampering crop productivity. Omics-based research platforms allow for a holistic and comprehensive survey on crop stress responses and hence may bring forth better crop improvement strategies. Since high-throughput approaches generate considerable amounts of data, bioinformatics tools will play an essential role in storing, retrieving, sharing, processing, and analyzing them. Genomic and functional genomic studies in crops still lag far behind similar studies in humans and other animals. In this review, we summarize some useful genomics and bioinformatics resources available to crop scientists. In addition, we also discuss the major challenges and advancements in the “-omics” studies, with an emphasis on their possible impacts on crop stress research and crop improvement.
Keywords: bioinformatics, crops, genomics, stresses
1. Introduction
According to the Food and Agricultural Organization of the United Nations (FAO), food production must be increased by 70% in the next 40 years to meet the increasing global demand [1]. Abiotic and biotic stresses are major limiting factors hampering crop productivity. Therefore, understanding the stress responses of crops using genomic information is important in bringing forth more effective crop improvement strategies.
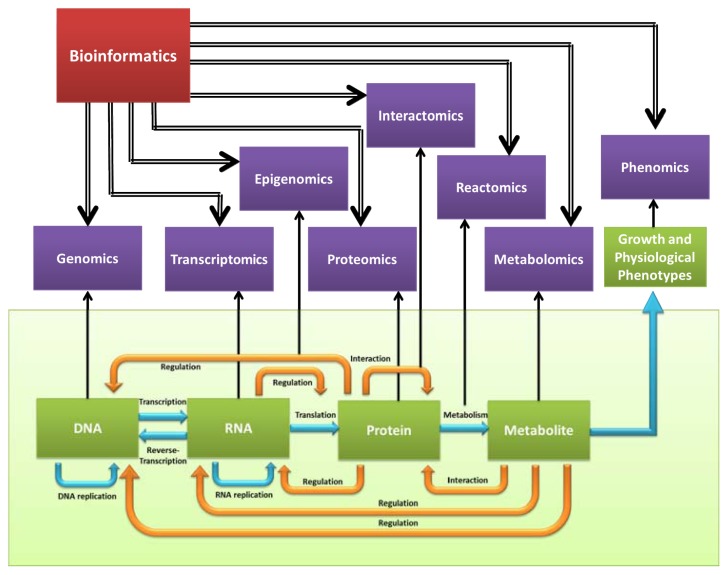
The publishing of the Arabidopsis thaliana genome in 2000 is a cornerstone of the plant genomics era [2]. Taking advantage of the high-throughput data acquisition platforms of the next generation sequencing technology, additional crop genomes have been subsequently decoded. So far, the draft genomes of more than 40 plants have been completed, including those processed in the 1000 Plant and Animal Project [3]. Other “-omics” technologies such as transcriptomics, proteomics, metabolomics, and phenomics (Figure 1) have also undergone rapid development in recent years. Together, there is a large volume of accumulated data, and hence data management and data mining have become a bottleneck for “-omics” researches.
Figure 1.
Infusion of biological “-omics” with bioinformatics.
To convert the great amount of data into manageable information, it is essential to establish standard formats and methods for storing, retrieving, and sharing data. Algorithms based on mathematical and statistical models are needed to handle biological data. This review aims to provide a systematic summary of the currently available databases and bioinformatics resources and highlight some challenges and advancements in the study of genomics and other “-omics”, with emphasis on their implications on crop stress research.
2. General Bioinformatics Resources
2.1. Databases
Various databases have been developed to accommodate the comprehensive -omics data and some of them also provide onsite analytical tools (Table 1). The three commonly used sequence databases are GenBank in USA, European Nucleotide Archive (ENA) in Europe, and DNA Data Bank of Japan (DDBJ). They are collaboratively accommodated by the International Nucleotide Sequence Databases (INSD), and the deposited data are frequently synchronized. There are also repositories designated specifically for plants, such as Phytozome that holds the genomic information of more than 40 plant species, including all the sequenced crops. Besides basic genomic information, databases such as Legume Information System (LIS) facilitate synteny analyses and comparative genomic studies between closely related crop plants.
Table 1.
Example of some commonly used databases.
| Database name | URL | Reference |
|---|---|---|
| GenBank | http://www.ncbi.nlm.nih.gov/genbank/ | [4] |
| ENA | http://www.ebi.ac.uk/ena/ | [5] |
| DDBJ | http://www.ddbj.nig.ac.jp/ | [6] |
| Phytozome | http://www.phytozome.net/ | [7] |
| Gramene | http://www.gramene.org/ | [8] |
| KEGG | http://www.genome.jp/kegg/ | [9] |
| PlantGDB | http://www.plantgdb.org/ | [10] |
| EnsemblPlants | http://plants.ensembl.org/index.html | [11] |
| VISTA | http://genome.lbl.gov/vista/index.shtml | [12] |
| PLAZA | http://bioinformatics.psb.ugent.be/plaza/ | [13] |
| GigaDB | http://gigadb.org/ | [14] |
| SGN | http://solgenomics.net/ | [15] |
| GrainGenes | http://wheat.pw.usda.gov | [16] |
| LIS | http://www.comparative-legumes.org/ | [17] |
Online resources for individual crops, together with massive datasets, have been developed (Table 2) where systematically integrated information including: genetic resources (genetic maps, molecular markers, and quantitative trait loci (QTL)); genomic resources (DNA sequences, gene models, and regulatory elements); gene expression data (ESTs, cDNA sequences, and transcriptomes); and functional units (proteomic and metabolomic data), is provided. Crops of higher economic values are usually accompanied with a more comprehensive database. The genomic sequences of some economically less important crops, such as foxtail millet, sorghum, and barley, have been released recently [18–20] and their corresponding integrated databases are still under development.
Table 2.
Data repositories for crop plants.
| Crop | Database name | URL of related database | Ref |
|---|---|---|---|
| Rice | RAP-DB | http://rapdb.dna.affrc.go.jp/ | [23] |
| Maize | MaizeGDB | http://www.maizegdb.org/ | [24] |
| Medicago |
Medicago truncatula SEQUENCING RESOURCE |
http://www.medicago.org/genome/index_old.php | - |
| Wheat | GrainGenes | http://wheat.pw.usda.gov/ | [16] |
| Potato | Solanaceae Genomics Resource | http://solanaceae.plantbiology.msu.edu/index.shtml | - |
| Soybean | SoyBase | http://soybase.org | [25] |
| Tomato | TOMATO FUNCTIONAL GENOMICS DATABASE | http://ted.bti.cornell.edu/ | [26] |
Some data repositories also provide information related to abiotic and biotic stress responses. For example, in MaizeGDB, there are well documented records for tropical maize exhibiting tolerance to drought stress [21]. In SoyBase, genetic markers associated with salt tolerance, drought tolerance, and cyst nematode resistance are incorporated with genomic and expression information. Databases for individual crops could also facilitate the unveiling of the genetic basis of specific traits. For example, the tomato genome sequence helped identify the R-genes which were then incorporated in the Plant Resistance Genes database [22].
2.2. Biological Ontologies Related to Crop Stress Research
The standardization of ontology is important for the structuring of huge datasets, interconnection between databases, merging resources, and curation of information. Each ontology term has its own name, identifier/ID/accession number and definition. The identifier/ID/accession number is usually made up of a prefix and a number. For example, the Gene Ontology term “lipid binding” has the accession number GO:0008289. The definition of “lipid binding” is a gene product that can interact selectively and non-covalently with a lipid.
The Gene Ontology (GO) project provides a well-established and controlled vocabulary database for describing the function of a gene and its gene product. The ontology covers three aspects, including cellular component, molecular function, and biological process. GO is used in genome annotation to provide information on gene products. An evidence code (by Evidence Code ontology) is used to describe the evidence that links the GO annotation with the gene product. The Evidence Ontology (EO) suggests whether an annotation has been made manually by a curator or by automated electronic annotation. For example, EXP refers to: “inferred from experiment”; IBA refers to: “inferred from biological aspect of ancestor”; and IEA refers to: “inferred from electronic annotation”. All this information can be found in the Gene Ontology website [27].
Plant Trait Ontology (TO) is a controlled vocabulary for describing the plant trait and phenotype. In addition to anatomical and morphological traits, TO also includes a subset of controlled vocabularies for abiotic and biotic stress traits. For example, the yellow dwarf disease resistance (TO:0000292) is the child term of resistance to disease by mycophasma-like organism (TO:0000013) under the lineage of stress trait (TO:0000164).
There are many other biological ontology projects for different research fields. Ontologies listed in Table 3 contain the information related to crop stress responses.
Table 3.
List of ontologies containing information related to crop stress responses.
| Domain | Prefix | Description | Reference/website |
|---|---|---|---|
| Plant Environmental Conditions | EO | Controlled vocabulary for the representation of plant environmental conditions | http://www.gramene.org/db/ontology/search?id=EO:0007359 |
| Gene Ontology | GO | Controlled vocabulary for genes and gene products | [28] |
| Taxonomy Ontology | GR_tax | Representation of the taxonomic tree of plants in the ontology format | http://www.gramene.org/db/ontology/search?id=GR_tax:090165 |
| The Plant-Associated Microbe Gene Ontology | PAMGO | Controlled vocabulary for the interaction of microbes with their hosts | [29] |
| Plant Ontology | PO | Controlled vocabulary for anatomy, morphology and stages of development for all plants | [30] |
| Sequence Ontology | SO | Controlled vocabulary for sequence annotations, for the exchange of annotation data and for the description of sequence objects in databases | [31] |
| Plant Trait Ontology | TO | Controlled vocabulary for phenotypic traits in plants | http://www.gramene.org/db/ontology/search?id=TO:0000387 |
3. Recent Advances and Challenges in Crop Genomics
3.1. Polyploidy as a Major Challenge in Crop Genome Assembly
Polyploidy is a major hindrance in crop genome assembly. One of the ways to tackle the highly polyploid genomes is to make references to the closely related, putative progenitor diploid genomes if they are available. The Catalogue of Life [32] and the Integrated Taxonomic Information System [33] may help to identify such related species. For example, the fiber-producing cotton (Gossypium hirsutum) is tetraploid, comprising an A-genome and a D-genome. To assist the assembly of the tetraploid genome, the diploid D-genome of G. raimondii was first sequenced and assembled [34]. A second example is strawberry (Fragaria × ananassa), with an estimated genome size of about 600 Mb. Although this is much smaller than other crop genomes, it is an octaploid (AAA′A′BBB′B′) [35]. Therefore, the genome sequence of the woodland strawberry (Fragaria vesca), a potential progenitor of Fragaria × ananassa, was completed in 2012 to provide the first diploid model for the genomes of F. spp. [35,36]. Wheat is another example of polyploid crop genomes. The hexaploid bread wheat (Triticum aestivum) contains the A, B and D genomes, which probably originated from Triticum urartu (A genome), Aegilops tauschii (D genome), and an unknown species related to Aegilops speltoides (B genome). The genomic sequence information of T. aestivum, T. monococcum (a community standard line related to the A-genome donor), and Ae. Tauschii, as well as the cDNA sequence information of T. aestivum and Ae. Speltodies, were obtained [37]. With reference to the respective diploid genome information, over 90% of the wheat genes were successfully assembled into the A, B, or D genome with over 70% precision [37]. The drafted de novo genomes of T. urartu and Ae. tauschii were recently published, representing 94.3% and 97.0% of the predicted genome sizes respectively [38,39]. Although the lack of a good reference for the B genome is still an obstacle in building the T. aestivum genome, these pieces of work have built a good framework for the further whole genome assembly of bread wheat, and established a model for the study of other polyploid genomes.
3.2. Reduced Genetic Diversity of Modern Crops
Modern crops originated from a small number of plants. Bottleneck effects during domestication and prolonged human selection together have significantly reduced the genetic diversity of modern crops. Such a reduction in genetic diversity has been confirmed by several genomic studies (Supplementary Table S1). For example, whole-genome resequencing of 14 cultivated and 17 wild soybean genomes revealed that the wild soybeans have higher numbers of SNPs and genetic diversity compared to those of the cultivated ones [40]. The domesticated rice cultivars (Oryza sativa indica and Oryza sativa japonica) also show a lower genetic diversity than their wild relatives (O. rufipogon and O. nivara) in a study on 50 accessions of cultivated and wild rice [41]. More interestingly, even though both indica rice and japonica rice are cultivated, the japonica rice shows significantly lower genetic diversity than the indica rice, suggesting that the japonica rice has suffered from a stronger bottleneck effect under domestication [41]. On the other hand, although maize landraces and improved lines have retained a higher nucleotide diversity from their wild progenitor, as compared to other self-fertilizing crop species, a weak bottleneck effect can still be observed [42]. Reduced genomic diversity of major staple crops limits their adaptability to the changing environment and reduces the room for crop improvement. Therefore, crop improvement programs should turn their focus to the genetically compatible wild species, which have higher biodiversity and can serve as natural genetic reservoirs.
3.3. Sequence and Structural Variations in Genomes Providing Clues for Stress Studies
Sequence differences and structural variations in genomes are usually identified by comparing the genomes of wild species to their related landraces and modern cultivars, and also to other model plants. These differences can, on the one hand, provide information about genome evolution, and, on the other hand, serve as molecular markers for genetic mapping. Sequence differences and structural variations that affect gene structure, gene expression, and gene copy number are major determinants shaping the diversity among different varieties of the same species. For instance, wild soybeans and some rice accessions possess some present/absent variations or unmapped contigs that contain bona fide genes annotated to be involved in abiotic and biotic stresses [40,41,43,44]. One specific example relating to biotic stresses is the enrichment and over-representation of LRR (leucine-rich repeat) and NB-ARC (nucleotide-binding adaptor shared by APAF-1, certain R gene products and CED-4) domain-containing genes in some crop genomes [19,45]. In plant genomes, disease resistance (R) genes are responsible for defense responses [46]. LRR and NB-ARC are two important domains found on the R proteins [46]. The LRR domain-containing proteins play important roles in pathogen-host interactions and the activation of defense responses [47,48]. On the other hand, the NB-ARC domain is responsible for the mulitmerization and autoactivation of the R proteins upon stimulus [49]. The LRR and NB-ARC-containing genes exhibit higher ratios of nonsynonymous-to-synonymous SNPs than the genome average in crops such as soybean [40], rice [41,50], and sorghum [51]. In maize, 101 out of 3490 large-effect SNPs detected are located on 49 LRR domain-containing genes [44]. LRR and NB-ARC domain-containing genes are important components in the plant defense response system [46,49,52] while the high nonsynonymous-to-synonymous SNP ratio of LRR or NB-ARC domain-containing genes suggests a dynamic evolution of these genes to combat pathogens.
In addition to disease resistance genes, some transcription factors are found to be over-retained after the whole-genome duplication in Musa α/β (banana) [53]. Some of these transcription factors such as Myb, AP2/ERF, and WRKY are known to be important regulators in abiotic stress responses [54]. On the other hand, compared to rice, sorghum, and maize, there are more genes encoding for cytochrome P450, CCAT-binding factor transcription factors, late-embryogenesis-abundant proteins, and osmoprotectant biosynthesis proteins in the Ae. tauschii genome (progenitor B genome of wheat) [38]. These genes are important for the adaptation to cold and physiological drought. Moreover, a significantly higher number of transmembrane ATPase subunits, which are probably involved in Na+ exclusion and mineral uptake, have been detected in Ae. tauschii than in wheat [37,38]. The extra genes in Ae. tauschii may be good candidates for wheat improvement.
3.4. Advances in Ultra-High-Density Genetic Mapping Using SNPs
Genetic mapping using genetic populations is one classical strategy to identify genes related to stress responses. Members in the mapping population can either be related (e.g., QTL mapping using bi-parental populations) or unrelated (e.g., genome-wide association study (GWAS) using germplasm collections) (for population structure, data characteristics and methods, see reviews [55,56]). There are some successful cases in identifying stress tolerance causal genes through mapping [57–59]. For example, a salt tolerance-conferring sodium transporter from rice was identified through QTL mapping [58]. The SKC1 locus corresponding to shoot K+ content was mapped with a BC2F2 population generated from a cross between a salt-tolerant indica variety and a susceptible japonica variety [58]. The SKC1 locus was further confined to a 7.4-kb stretch by the BC3F4 progeny testing of fixed recombinant plants. The locus contains only a single open reading frame, which encodes for a HKT-type transporter. SKC1 near-isogenic lines accumulated less Na+ under salt treatment compared to the susceptible parent. Voltage-clamp also supports the notion that the SKC1 protein functions as a Na+-selective transporter that probably regulates K+/Na+ homeostasis under salt stress [58].
Classical molecular markers for mapping such as AFLP, RFLP, and SSR markers are sparsely distributed in the genome, and hence limit the mapping resolution and pose difficulties in pinpointing the phenotype-causal genes. With the availability of genomic sequence data, SNP markers become more accessible for use in mapping, to help achieve a much better resolution. However, conventional PCR-based methods are laborious and time-consuming while the resolution of array-based methods is limited by the number of probes on the array.
High-resolution genotyping by whole-genome resequencing has been established [60,61], making the ultra-high-density genetic mapping more attainable. In principle, this method can achieve the highest resolution, provided that there are enough resources to capture all the SNPs in a population. In reality, polymorphic SNPs are usually captured by low-coverage sequencing (~1X for unrelated populations [58] and <0.1X for recombinant populations [60,62,63]).
In a QTL study of recombinant inbred populations originating from indica and japonica rice, SNPs between the parental reference genomes were first identified using DiffSeq in the EMBOSS package and cleaned by SSAHASNP in the ssaha2 package. Low-depth sequencing reads of recombinant inbred lines (RILs) were mapped to the parents’ pseudomolecules by using the SSAHA2 software [64] to determine the genotype of each RIL. SNPs were analyzed by a sliding window approach to determine the recombinant break points within the genome of every single line in the population to form a bin map [60]. This sliding window strategy can accommodate the high error rate of next generation sequencing and allow missing data resulting from low-coverage sequencing [60]. Each “bin” will serve as a “marker” in the subsequent linkage map construction using MAPMAKER/EXP and in QTL mapping using QTL Cartographer. In this study, using 150 rice RILs, the sequencing-based method increased the resolution by 35-fold and greatly reduced the time needed for genotyping, compared to the map generated from 287 PCR-based markers [57]. The power of this method was further illustrated in a study using 210 rice RILs to map the GS3 and GW5/qSW5 loci related to the grain length and grain width, respectively [62].
Since missing genotypes in low-depth sequencing would reduce the effectiveness of GWAS, after SNPs have been identified by mapping the sequencing reads, the k-nearest neighbor method (KNN) that uses in-house algorithms for data-imputation can be adopted in addition to increasing the sequencing depth, in order to reduce the missing genotypes [61]. GWAS has been conducted in mapping 14 agronomic traits, including drought tolerance, using 373 indica rice lines. One to seven loci have been mapped for each trait, and some of them overlap with the previously known loci/genes identified through bi-parental QTL mapping or mutant studies [61]. With the great reduction of sequencing cost (<US$0.1 per raw megabase in 2012) [65], we anticipate that mapping by sequencing will become a popular method to obtain high resolution maps for stress-related loci/genes.
3.5. Genomic Selections
Genomic selection (GS) is introduced to evaluate the overall effects of all contributing loci genome-wide [66]. During the process of GS, a training population will be used for computational model training to obtain the genomic estimated breeding values (GEBVs) [67]. Complex traits such as drought tolerance are usually determined by multiple small-effect QTLs. GEBV associates markers and QTLs by regarding all the markers as variables contributing to the trait and the effect of each marker allele towards the complex QTLs is quantified (it can be zero). GEBV determines the sum of the marker effects and thus indicates the breeding value of an individual; favorable individuals with high GEBVs from breeding populations will be selected for field application. Genotypic and phenotypic information of the breeding population can be used to further improve the computational model to form a training-breeding cycle [66]. Unlike GWAS and QTL studies, which are designed to reduce the breeding time by selecting plants with desired molecular markers at early growth stages instead of evaluating the actual phenotypes at a later stage, GEBVs serve only as selection criteria but do not lead to target markers or causal genes.
As high-throughput genotyping and phenotyping have accelerated GS studies by increasing marker density and selection capacity, one of the major challenges of GS is selection accuracy. Evaluations of GS accuracy have been performed in maize [68], wheat [69,70], barley [71], and cassava [72]. Several statistical models for GEBV calculations, including best linear unbiased prediction (BLUP) [67], Bayesian shrinkage regression (BayesA, BayesB, etc.) [73], and mixed models have been employed. There is no agreement on which model is the most efficient, because many factors such as population size and genetic background may affect statistical power [71]. It is believed that GS is a valuable approach for plant breeding [74], however, it will take some time for this concept to develop into a practical tool [75]. A GS-based breeding scheme has already been proposed and is considered to be an important tool for developing durable stem rust-resistant wheat [76].
3.6. Identification of Stress-Related Gene Families
When properly annotated genomes are available, the genome-wide identification of all members of a gene family will become feasible. Since genome duplication (polyploidy or paleopolyploidy) and single gene duplication are common in crops [77], genes usually exist in multiple copies and/or in gene families. Identifying all members of a gene family may give a more comprehensive view on the possible functions of a group of evolutionarily related genes. Bioinformatics tools such as Fgenesh [78], GAZE [79], and JIGSAW [80] have been adopted for searching gene families in crops.
Two typical ways to identify members of gene families from within a genome are keyword search and pattern/homology search. Keyword search usually requires precise keywords including gene names and controlled vocabularies. The most commonly used controlled vocabularies are Gene Ontology, as mentioned in section 2.2, and the functional classification by Pfam, InterPro and KEGG [81–83].
A genome-wide pattern search usually begins with searching sequence databases using programs like BLASTP or TBLASTN [84]. Databases can either be online resources (Table 1) or in-house databases. The occurrence of the desired functional domains in the potential sequences can then be verified using the Pfam protein families database [81], SMART database [85], or HMMER [86]. When the BLAST results are associated with unannotated sequences, these will require further analyses to determine the putative gene structures. One example of applying the above strategy to identify stress-related genes is the analysis of AP2/EREBPs in the rice genome [87]. “AP2/EREBP” was used as the keyword in searching databases, including DRTF, MSU NCBI, and KOMBE. Any non-redundant sequences obtained were then used as query terms in the TBLAST and BLASTP searches of the MSU and NCBI databases. Four genes with an incomplete AP2 domain were excluded after Pfam and SMART analyses because of their very small AP2/ERF domain. A total of 163 genes were identified using this method, in contrast to the 139 genes as suggested previously [87]. Expression studies revealed that a number of the members are responsive to abiotic or biotic stresses. A few of them can even be induced by multiple stresses, suggesting their possible involvement in stress responses [87].
Supplementary Table S2 summarizes the strategies and tools used in recent literature on genome-wide analyses of gene families related to stress responses in major crops.
4. Functional Genomics
4.1. Transcriptome
There are two major technologies for obtaining the overall transcription map of specific plant tissues: hybridization-based microarray technology [88] and next generation RNA sequencing technology (RNA-seq) [89]. RNA-seq technology, in conjunction with efficient bioinformatics tools, is now more widely used to support predicted gene models, extract differentially expressed genes, and find novel transcripts in de novo assemblies. Public repositories such as ArrayExpress [90] are designed for the storage of expression data. Standard data formats including Minimum Information about Microarray Experiments (MIAME) or Minimum Information about Sequencing Experiments (MINSEQE) are unified to facilitate transcriptome data submission/downloading. Bioinformatics tools dealing with transcriptome alignment, splicing event prediction, and de novo assembly are also available (Table 4).
Table 4.
Widely used bioinformatics tools for the analysis of transcriptome data.
| Software | Description | Download URL | Reference |
|---|---|---|---|
| ABMapper | RNA-seq data alignment | http://hkbic.cuhk.edu.hk/software/abmapper | [91] |
| Bowtie | RNA-seq data alignment | http://bowtie-io.sourceforge.net/bowtie2/index.shtml | [92] |
| Cufflinks | Transcript assembly | http://cufflinks.cbcb.umd.edu/ | [93] |
| DEGseq | Differential gene expression detection | http://www.bioconductor.org/packages/2.11/bioc/html/DEGseq.html | [94] |
| Infernal | RNA-seq data alignment | http://infernal.janelia.org/ | [95] |
| Oases | De novo assembly | www.ebi.ac.uk/~zerbino/oases/ | [96] |
| Tophat | RNA-seq data alignment & Alternative splicing detection | http://tophat.cbcb.umd.edu/ | [97] |
| Trans-AByss | De novo assembly | http://www.bcgsc.ca/platform/bioinfo/software/ | [98] |
| Trinity | De novo assembly | http://trinityrnaseq.sourceforge.net/ | [99] |
Crops such as maize [100] and soybean [101] have their own transcriptome atlases, compiled from sub-transcriptomes from multiple tissues and different developmental stages. For the transcriptome atlas of soybean, plant ontology (PO) was used to describe the developmental stage of each experimental tissue, providing a common ground for readers and users to discuss and perform further analyses. The cDNA short reads generated by Illumina Genome Analyzer were aligned to the soybean reference genome sequence assembly using GSNAP, released in 2005. The digital expression counts were determined using the R programming language and normalized using a variation of RPKM methods [101]. The global inventory of expressed transcripts of crops under stress is dynamic, both temporally and spatially. Time series sampling is a typical experimental design to trace the trajectory of such differentially expressed transcripts of crops under stress conditions. A typical example was the study of the soybean transcriptome under alkaline stress. Soybean plants were treated with NaHCO3 and transcriptomes were analyzed using microarray [102]. GO terms were successfully assigned to the 1380 significantly changed probe sets that are related to metabolism, signal transduction, energy, transcription, secondary metabolism, transporter, as well as disease and defense. A time series study revealed the interplay of signal transduction and metabolism during the progression of the treatment. MapMan tools were used to visualize these changes [102]. Other time series studies include the studies of rice root under low potassium [103], cassava under cold stress [104], and soybean subjected to Pseudomonas syringae infection [105]. The other widely reported experimental design is the comparative transcriptome study performed among crop accessions with different degrees of stress tolerance, such as the study of soybean accessions exhibiting differential tolerance toward low potassium [106], rice cultivars with contrasting abilities to withstand drought [107] and chilling [108], wheat with differential drought tolerance [109], and Medicago [110] and foxtail millet [111] cultivars with differential salt tolerance.
Another strategy to associate transcript abundance to genomic variations is the expression QTL (eQTL), which use differentially expressed transcripts as the quantitative traits [112]. The eQTL maps of maize root [113] and rice shoots [114] have identified thousands of cis and trans regulation factors by population transcriptome screening. The eQTLs co-localizing with traditional QTL regions could give supportive evidence explaining the genetic basis of the targeted phenotypic characters. One successful example is the eQTL study of the partial resistance toward Puccinia hordei in barley [115], in which some eQTLs were reported to co-localize with previously known rust resistance QTL regions.
4.2. Proteome
Due to the alternative splicing of RNA transcripts and post-translational modifications of the proteins themselves, the proteome within a cell can be much more complicated than the corresponding genome. The gel-based proteomics technology will soon be obsolete due to its limited sensitivity and semi-quantitative nature [116]. The rise of the next generation proteomics systems such as Orbitrap and QStar, together with the application of isotopic tag-based quantitative proteomics (ICATs [117], SILAC [118], isobaric tag-based quantitative proteomics (ITRAQ [119]), and label-free quantitative proteomics (MaxQuant [120], Serac [121], SIEVE (Thermo Scientific, San Jose CA, USA)) have expedited the development of high-throughput proteomic studies. Nevertheless, the pace of adopting these platforms in plant stress studies is far behind studies in humans.
Despite the advancement in the proteomics platforms, the application of de novo peptide sequencing is still limited. Protein identifications still largely rely on database searches in which experimental peptide mass spectra are compared with theoretical peptide mass spectra generated from existing sequence databases. Some commonly used databases and useful algorithms are summarized in Table 5. Since the genomes of many crops have not been completely sequenced, and some others are still unknown, proteins of species without a genome database are frequently identified by referring to cross-species databases. In these cases, it is not uncommon that molecular weights and isoelectric points (pI) of the identified proteins may deviate from the actual spot position on the 2D gel, despite the high protein scores.
Table 5.
Bioinformatics resources commonly used in crop proteomic studies.
| Program | URL |
|---|---|
| Mascot | http://www.matrixscience.com |
| SEQUEST | http://fields.scripps.edu/sequest/index.html |
| X!Tandem | http://www.thegpm.org/TANDEM/index.html |
|
| |
| Database | URL |
|
| |
| Expasy | http://www.uniprot.org/help/uniprotkb |
| UniprotKB/SwissProt and UniProtKB/TrEMBL database | http://www.uniprot.org/help/uniprotkb |
| Protein Information Resource (PIR) | http://pir.georgetown.edu/ |
| RCSB Protein Data Bank (RCSB PDB) | http://www.rcsb.org/pdb/download/download.do |
| EMBL-EBI’s Protein Data Bank in Europe (PDBe) | http://www.ebi.ac.uk/pdbe/ |
| SWISS-2DPAGE | http://world-2dpage.expasy.org/swiss-2dpage/ |
| The Plant Proteome Database (PPDB) | http://ppdb.tc.cornell.edu/ |
| Plant Protein Phosphorylation DataBase (P3DB) | http://www.p3db.org/ |
| RIKEN Plant Phosphoproteome Database (RIPP) | https://database.riken.jp/sw/links/en/ria102i/ |
| Secretom—The Tomato Fruit Glycoproteome | http://solgenomics.net/secretom/detail/glycoproteome |
| Plant Secretome KnowledgeBase (PlantSecKB) | http://proteomics.ysu.edu/secretomes/plant.php |
Comprehensive reviews summarizing plant proteomic studies from 2006 to 2008 are available [122,123]. We have also summarized the plant proteomic studies in 2012 (Supplementary Table S3). Recently, plant proteomic investigations have been subdivided into several areas, including subcellular proteomics and proteomics-related post-translational modifications. For example, 21 differentially expressed proteins were identified from salt-treated wheat chloroplasts [124], and 13 and 11 differentially expressed microsomal proteins, respectively, were identified from two distinct cadmium-accumulating soybean cultivars [125].
Stress-induced posttranslational modifications of proteins are common. They are either the results of deleterious damage from the stress, or beneficial modifications to regulate the functions of the proteins in order to cope with the stress. To study posttranslational modifications of proteins, special techniques within proteomics are used. Redox proteomics requires special labeling methods, including the reduction and subsequent labeling of the oxidized thiol groups with 5-iodoacetamidofluorescein (IAF) [126]. Twenty-two highly oxidized proteins involved in a wide range of biological processes were identified in ozone-treated rice using this method [127]. Phosphoproteome [128], glycoproteome, and secretome [129–132] are sub-categories of proteomics that require special staining and enrichment techniques. Post-translational modifications involved in gene expression regulations will be discussed in the Epigenomics section below.
4.3. Interactome
Protein-protein interactions determine the contextual functions of a protein and hence play a crucial role in regulation and signal transduction [133]. There are several commonly used experimental systems to identify protein-protein interactions, including: (1) yeast two hybrid (Y2H) (reviewed in [134]); (2) biomolecular fluorescence complementation (BiFC) (reviewed in [134]); (3) affinity pull-down coupled with mass spectrometry (AP-MS) (reviewed in [134]); (4) blue native PAGE [135]; and (5) structural analysis of protein crystals [136,137]. In addition, literature curation involving tedious literature searches can be used to supplement the experimental efforts [138] and in silico prediction can be done by searching for orthologous pairs which interact in other systems, to identify possible interologues [134,139]. Multiple systems are generally adopted to authenticate the interactions.
The concept of the plant interactome was initiated years ago, and was based mainly on the information collected through literature curation [140]. Subsequently, an experimentally constructed interactome map of A. thaliana was established via intensive screening, recording a total of 6200 high-confidence interactions among 2700 proteins through the screening of proteins encoded by 8000 open reading frames in the Arabidopsis genome [141]. It is estimated that this screening only captured around 2% of the binary protein-protein interactome in A. thaliana [141]. Using the in silico interolog prediction method, more than 37,000 interactions among 4567 rice proteins were predicted, 168 of which have been experimentally confirmed [139]. In this piece of work, the INPARANOID 3.0 program was used to predict high-confidence protein orthologues in 12 species including rice. With the assumption that protein-protein interactions are retained in evolutionarily conserved orthologous proteins, rice protein-protein interactions were compiled using the predicted orthologous proteins and the known interactions in interactome databases [139]. Only a few studies directly related to crop stress interactomes have been published (Table 6). In the search for rice stress-related interactomes, 4 stress proteins related to disease (XA21 and NH1) and flooding (SUB1A and SUB1C) were used as baits for the initial interactome screens by Y2H [142]. Preys identified from the initial screens were then used as baits for subsequent screens. Together with the information from literature curation, an interactome network consisting of 100 proteins were constructed. The interactomes of the two kinds of stresses were linked by proteins such as SNRK1A, which has been shown to be related to ABA, a positive regulator of abiotic stress responses and a negative regulator of biotic stress responses [142].
Table 6.
Recent large-scale stress interactome studies in crop plants.
Online resources such as PRIN [143] can help to predict rice interactomes, while BioGRID [144], DIP [145], PlaPID [146], and InAct [147] can be queried for some pre-determined interactomes in certain plant species. Recent large-scale stress interactome studies in crop plants are shown in Table 6.
4.4. Epigenome
In addition to the genetic information encoded by DNA, epigenetic modifications of DNA and histones provide another dimension of regulation to influence gene expressions. Chromatin-associated proteins, including DNA methylase, histones, and histone-modifying enzymes, are cataloged in the ChromDB [151]. Technological platforms for epigenomic research can be considered as an extension of genomic and proteomic studies with modifications in analysis protocols.
For example, cytosine DNA methylation, one of the epigenetic modifications, plays an important role in gene silencing and genomic imprinting [152,153]. The transcriptional levels of endogenous genes are highly correlated with the methylation status within their promoter or transcribed regions [154,155]. One way to detect DNA methylation is to capture and enrich the methylated DNA fragments by immunoprecipitation [156]. Bisulfite treatment is another way to distinguish between methylated and unmethylated DNA. The bisulfite treatment converts unmethylated (but not methylated) cytosines to uracils [157]. Both immunoprecipitation-enriched and bisulfite-treated DNA can be analyzed by microarray- or sequencing-based methods to the single-base level of resolution [157–159]. A number of bioinformatics tools are designed to handle the bisulfite sequencing data (Table 7).
Table 7.
Bioinformatics tools for the analysis of bisulfite sequencing data.
| Tools | Descriptions | Reference |
|---|---|---|
| BiQ Analyzer HT | Quantitative study of locus-specific DNA methylation patterns from bisulfite sequencing data. | [166] |
| Bismark | Mapping of bisulfite-sequencing reads and methylation calling. | [167] |
| BRAT-BW | Genome-wide single base-resolution methylation data analysis | [168] |
| BSmooth | Providing estimate of methylation profiles with low-coverage whole-genome bisulfite-sequencing data. | [169] |
| BS seeker | Mapping of bisulfite-sequencing reads. | [170] |
| BSMAP | Bisulfite reads mapping algorithm. | [171] |
| CpG_MPs | Analysis of biosulfite-sequencing read and identification of genome-wide methylation pattern | [172] |
| GBSA | Both gene-centric or gene-independent analyses of whole-genome bisulfite sequencing data | [173] |
| Kismeth | Analysis of plant bisulfite sequencing results, with a tool for designing bisulfite sequencing primers. | [174] |
| QUMA | Quantification tool for methylation analysis | [175] |
| RRBSMAP | Derivative of BSMAP—a specific tool for reduced-representation bisulfite sequencing | [176] |
Both biotic and abiotic stresses will lead to massive changes in the DNA methylation status [160–162]. Some stress-induced DNA methylations can be inherited by the next generation. The mechanism for trans-generation DNA methylation may be partially mediated by small RNAs [163]. This trans-generation DNA methylation has been observed in some crops in response to stress [164,165], as a way of pre-acquiring immunity toward the upcoming stresses via designed parental priming [164].
Histone proteins are responsible for the packing of DNA. The epigenetic modifications of core histones affecting the tightness of DNA packing are called histone codes that can relay important information to affect gene expressions [177]. The Histone Sequence Database provides a comprehensive collection of histone sequences and structural information [178].
The addition of acetyl groups to histones neutralizes the positive charges and hence loosens the condensed DNA, leading to transcriptional activation [179], while the methylation of histones results in gene deactivation or repression [180]. The phosphorylation of histones causes the relaxation of chromatin and modulates histone acetylation and methylation [181].
Individual types of histone modifications on specific amino acid residues can be detected using specific antibodies or various mass spectrometries while genome-wide histone-DNA associations can be captured by chromatin immunoprecipitation (ChIP) and subsequently analyzed using either microarray (ChIP-Chip) [182] or sequencing (ChIP-seq) [183].
Some histone-modifying enzymes are induced in crops under stress. For example, a trithorax-like H3K4 methyltransferase was found to be induced by drought in drought-tolerant barley cultivars [184] while a histone deacetylase was found to be induced by compatible infections and repressed by incompatible infections [185]. The methylation statuses of four transcription factors were affected by salt stress. The expression of three of these transcription factors were also found to be correlated with their H3 methylation and acetylation statuses [186]. A genome-wide study in rice identified 4837 genes that harbor differential H3K4me3 modification under drought stress, in which the expression of 609 genes were significantly correlated with the H3K4me3 modification [187].
4.5. Phenome
Every observable biological characteristic beyond the genotype can be regarded as the phenotype. Phenotypes can be observed at the molecular, cellular, organismal, and population levels. Phenotypes also vary throughout the organism’s lifecycle, spanning different growth stages, and during different periods of stress. The environment can also exert significant influences on the phenotype. The total sum of phenotypes of an organism or a population constitutes the phenome.
As mentioned in section 2.2, to make phenotypic data in public databases more searchable and accessible to users of bioinformatics tools, ontologies are used to describe the setup of the experiment and the phenotypic data. For example, one may study salt tolerance (TO:0006001) at the whole-plant flowering stage (PO:0007016) and the days to flower (TO:0000344) of Oryza sativa (GR_tax:013681), in a greenhouse study (EO:0007248) under a sodium chloride regimen (EO:0007048). These ontologies provide a common language to describe an experiment and render it understandable by both researchers and computational algorithms. For instance, some people may record certain phenotypes during the flowering stage. However, what does it mean by “flowering stage”? Some people refer to “flowering stage” as the time when the first flower opens. Others may refer to it as having half of the individual plants with flowers opened. In this case, the flowering time is well defined in plant ontology. PO:0007026, PO:0007034, PO:0007053 and PO:0007052 refer to the stage at which the first flower, 1/4 of the flowers, 1/2 of the flowers, and 3/4 of the flowers, open, respectively. PO:0007024 marks the end of the flowering stage. The application of these ontologies can thus reduce the discrepancies in annotating the phenotypes and treatment conditions.
High-quality phenotypic information is essential for mapping, association studies, gene identifications, gene functional studies and genomic selections. To design experiments to collect phenotypic information, some critical parameters have to be considered, such as the sample/population sizes, experimental conditions, phenotypes to be assessed, and the data acquisition methods.
The size of a population can vary from a few plants for functional studies, several hundred lines for mapping and GS, to as many as a thousand germplasms for GWAS. Some public collections of germplasms or populations are available for public requests. The United States Department of Agriculture National Plant Germplasm System has a collection of over 500,000 germplasm accessions from 10,000 plant species including rice, soybean, tomato and many other staple crops. Table 8 summarized some publicly available mutant and germplasm collections, some of which also provide phenotypic descriptions and photos of the mutant.
Table 8.
Databases for mutant and germplasm resources.
| Species | Databases | URL |
|---|---|---|
| Barley | Barley DB | http://www.shigen.nig.ac.jp/barley/ |
| Barley | NordGen Plant Collection | http://www.nordgen.org |
| Maize | RescueMu Maize Mutant Phenotype Database | http://maizegdb.org/rescuemu-phenotype.php |
| Rice | Oryza Tag Line | http://oryzatagline.cirad.fr/ |
| Rice | Rice Tos17 Insertion Mutant Database | http://pfg101.nias.affrc.go.jp/ |
| Soybean | SoyBase—Fast Neutron Mutants | http://www.soybase.org/mutants/index.php |
| Tomato | Genes that Make Tomatoes | http://zamir.sgn.cornell.edu/mutants/index.html |
| Tomato | LycoTILL | http://www.agrobios.it/tilling/index.html |
| Tomato | TOMATOMA | http://tomatoma.nbrp.jp/index.jsp |
| Tomato/Pea | URGV TILLING Database | http://urgv.evry.inra.fr/UTILLdb |
| Tomato/Potato | SOL genomics network | http://solgenomics.net/ |
| Wheat | The Scottish Wheat Variety Database | http://wheat.agricrops.org/menu.php |
Since the phenome is the overall outcome of the interactions between the genotype and the environment, whether the phenotypic data are collected in a controlled environment or not can greatly affect the final interpretation of results. Field experiments can better mimic the actual conditions of crop production, but the consistency of the phenotype greatly depends on the location of the field, the soil composition, weather conditions, season, and so on. The interpretation of results can thus be complicated. For example, a change in the transpiration rate in some of the plants may not solely be the result of the stress treatment, but also the result of localized changes in light intensity and/or temperature in the field [188]. In general, a larger number of replications are required to compensate for the effects due to environmental variations. A controlled environment such as that in a greenhouse or a growth chamber can minimize the effects of environmental fluctuations and hence will emphasize the contribution of the genotype. However, data from controlled experiments are usually limited in scale and may overlook the fast-changing environment in the real production field.
Choosing the appropriate phenotypes to be assessed is also important. For example, stomatal conductance and pathogen titer are good indicators of osmotic stress tolerance and disease resistance, respectively. However, they are not quite applicable in large-scale experiments due to the limitation of the machine and the laborious procedures. On the other hand, fresh weight and biomass can truly reflect the productivity of the crops, but taking these measurements is destructive to the plant. For morphological and physiological phenotyping of crops under stress, a conversion of stress symptoms to parameters that can be captured and digitized is needed for high-throughput automation. Commonly used methods include: 2D or 3D visible light imaging [189,190], infrared thermography [188], near-infrared imaging, spectral reflectance [191], fluorescence analysis [191,192], stable isotope analysis [193] and X-ray imaging [194]. For example, a study of wheat salt stress response suggested that the shoot area calculated from 3 digital images (2 side and 1 top images) showed a strong positive correlation with manually measured leaf area and shoot fresh weight which commonly serve as the indicators of salt tolerance in crops [195]. As a non-destructive method, the imaging system could continuously monitor the growth of the plant and distinguish its bi-phasic (osmotic stress phase and ionic stress phase) growth under salinity stress [195]. Another example is related to osmotic stresses (salinity and drought) that reduce stomatal conductance. Since the reduction in stomatal conductance will halt the cooling effect of transpiration, infrared thermal imaging can be used to monitor the degree of salinity and drought stress [188,196]. In the case of lesions on leaf surfaces caused by plant diseases, instead of measuring the lesion area on each leaf, determining the reduction in chlorophyll fluorescence is a possible alternative [197].
In addition to the physiological phenotypes, metabolite profiles in crops are also altered by both biotic and abiotic stresses [198,199]. Deleterious metabolites such as reactive oxygen species might be generated through the disruption of normal cellular processes while beneficial metabolites such as signaling molecules and osmoprotectants may be generated to alleviate the stress [200,201].
Current platforms for metabolomic analyses include various forms of liquid chromatography-coupled mass spectrometry (LC-MS), gas chromatography-coupled MS (GC-MS), capillary electrophoresis-coupled MS (CE-MS), fourier transform MS (FT-MS), fourier transform infrared spectrometry (FT-IR), and one- or two-dimension nuclear magnetic resonance (NMR). Raw spectra generated by mass spectrometry or NMR can be analyzed by making references to either in-house or online databases (Table 9). Biological multivariate data generated from metabolomic studies are commonly analyzed using principal component analysis (PCA) [202], partial least square (PLS) [203], and orthogonal projections to latent structures (O-PLS) [203,204].
Table 9.
Mass spectra databases and other bioinformatics resources for metabolomic studies.
| Database | URL | Reference |
|---|---|---|
| AOCS Lipid Library | http://lipidlibrary.aocs.org/index.html | - |
| Golm Metabolome Database | http://gmd.mpimp-golm.mpg.de/ | [205] |
| Lipidomics Gateway | http://www.lipidmaps.org/ | [206] |
| Madison-Qingdao Metabolomics Consortium Database | http://mmcd.nmrfam.wisc.edu/ | [207] |
| Manchester Metabolomics Database | http://dbkgroup.org/MMD/ | [208] |
| MassBank | http://www.massbank.jp/index.html | [209] |
| Metabolome Express | https://www.metabolome-express.org/ | [210] |
| METLIN | http://metlin.scripps.edu/ | [211] |
| NIST Chemistry WebBook | http://webbook.nist.gov/chemistry/#Notes | - |
|
| ||
| Software/Tools | URL | Reference |
|
| ||
| AMDIS | http://www.amdis.net/ | - |
| COLMAR | http://spinportal.magnet.fsu.edu/ | [212] |
| MetDat | http://smbl.nus.edu.sg/METDAT2/ | [213] |
| MetaboSearch | http://omics.georgetown.edu/MetaboSearch.html | [214] |
Numerous metabolomic studies have been done on crops under stress, including: salinity [215–217], drought [218–221], flooding [222], ozone treatment [223], fungal infections [224–226], bacterial infections [217,227], other infections [228], and multiple stresses [229,230].
There are two major research strategies in metabolomic studies: metabolic fingerprinting and metabolic profiling. Metabolic fingerprinting uses the mass-to-charge ratio of mass spectrometry, the peak height and/or retention time of chromatography and the strength of NMR signal as the metabolomic signature in specific samples, such that the identity of each metabolite is not necessary [231]. This helps to classify different samples into categories. For example, metabolic fingerprints have been made to differentiate between disease-resistant and susceptible varieties [217,227,228] or between salt-tolerant and sensitive varieties [232]. In one study, fourier transform infrared (FT-IR) spectroscopy was used for the metabolic fingerprinting of salt-treated tomatoes [233]. A total of 882 FT-IR spectra variables were collected between the wave number 4000 to 600 cm−1 for each sample [233]. Through discriminant function analysis (DFA) of the spectra variables, without knowing the identity and the quantity of each metabolite, salt-treated and control samples can be discriminated. Furthermore, key regions within the spectrum distinguishing the treated from the untreated samples were identified through genetic algorithms, and the major components were found to be amino radicals and nitrile-containing compounds [233]. Thus, disease resistance and stress tolerance of novel crop varieties can be assessed by comparing their metabolic fingerprints with those of well characterized varieties, facilitating the screening process.
On the other hand, metabolic profiling compares the metabolic compositions between samples and hence the quantitation and identification of the metabolites are required. Signal patterns must be matched to known standards or depositions in the databases in order to identify the actual compounds. For example, the accumulation of compatible solutes, such as proline, glycine-betaine, and their precursors, is usually observed in osmotically stressed crops, especially in tolerant varieties [216,220,221]. A specific example is the mitochondrial metabolic profile of flood-stressed soybean; metabolites were extracted from the roots and hypocotyls of soybean seedlings with or without submergence stress [226], and were then analyzed using capillary electrophoresis mass spectrometry. Eighty-one mitochondria-related metabolites were identified and quantified with reference to the commercially available standards [226]. There was an accumulation of TCA cycle-related metabolites, including citrate, succinate, and aconitate, but a reduction in ATP in flood-stressed plants, which can be explained by the arrest of aerobic respiration due to anoxia [222]. Following a similar logic, the accumulation of antimicrobial compounds, such as caffeic acid, phytoalexins, glycoalkaloids, and other polyphenolic compounds, are common in pathogen-infected crops compared to their uninfected counterparts [224–226,228]. Glucose oxidase secreted by a fungal pathogen, Botrytis cinerea, can also lead to the accumulation of gluconic acid in Vitis vinifera cv. Chardonnay berries [226].
5. Future Perspectives
Sequencing throughput is no longer the major limiting factor in genomics and transcriptomics studies. The next generation sequencing platforms can actually generate enough depth for genome assembly in one or several runs [234]. However, sequence assembly and annotation for complex genomes remain challenging. The data acquisition platforms for other “-omics”, on the other hand, are under rapid development to catch up with the pace of genomic research. While the data source is no longer a rate-determining step, data integration and interpretation have become the bottleneck in the research pipeline. One obstacle hindering the cross-platform analyses of different datasets is the variations in experimental designs, treatment conditions, and data formats. Drawing meaningful conclusions may sometimes be difficult when there are discrepancies between two germplasms. For example, the transcriptomic data of one germplasm may not be used effectively to explain the proteomic data of another germplasm. Researchers should therefore strategically design experiments to generate interrelated -omics data using carefully selected germplasms. The standardization of data acquisition and storage formats using strictly controlled vocabulary is also important.
With the advance of computer technology and high-throughput analysis platforms, life processes can now be captured, digitized, and stored in the hard disk of a computer. Yet, no matter how perfectly a genome is sequenced and assembled, biological data from experiments are still essential to connect the genotypes and the phenotypes. A few softwares/platforms have been developed to integrate the interactions of cellular components into networks [235,236]. For example, the VirtualPlant has been developed as a software platform for the integration and analysis of different levels of data [237]. It provides large datasets of Arabidopsis gene annotation, gene functional categories, microarray data, biochemical pathways, interaction information, and microRNA:mRNA interaction information. Users can also upload their own gene lists and microarray data for analysis, and identify coexpressed genes, interacting proteins, and metabolites associated with their genes of interest. Building a similar platform for crop plants could be extremely useful but it requires a well-coordinated effort among different research centers and groups.
Supplementary Information
Acknowledgments
This work is supported by the Hong Kong RGC Collaborative Research Fund (CUHK3/CRF/11G), the Hong Kong RGC General Research Fund (468610), and funding from the Lo Kwee-Seong Biomedical Research Fund and Lee Hysan Foundation. Jee Yan Chu copy-edited this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.FAO. How to Feed the World in 2050. FAO; Rome, Italy: 2009. p. 35. [Google Scholar]
- 2.Arabidopsis Genome Initiative. The Arabidopsis genome initiative analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 3.BGI Library of Digital Life: Plants. [(accessed on 5 January 2013)]. Available online: http://ldl.genomics.org.cn/page/pa-plant.jsp.
- 4.Sayers E.W., Barrett T., Benson D.A., Bolton E., Bryant S.H., Canese K., Chetvernin V., Church D.M., DiCuccio M., Federhen S., et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2012;40:D13–D25. doi: 10.1093/nar/gkr1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leinonen R., Akhtar R., Birney E., Bower L., Cerdeno-Tárraga A., Cheng Y., Cleland I., Faruque N., Goodgame N., Gibson R., et al. The European nucleotide archive. Nucleic Acids Res. 2011;39:D28–D31. doi: 10.1093/nar/gkq967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyazaki S., Sugawara H., Ikeo K., Gojobori T., Tateno Y. DDBJ in the stream of various biological data. Nucleic Acids Res. 2004;32:D31–D34. doi: 10.1093/nar/gkh127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodstein D.M., Shu S., Howson R., Neupane R., Hayes R.D., Fazo J., Mitros T., Dirks W., Hellsten U., Putnam N., et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang C., Jaiswal P., Hebbard C., Avraham S., Buckler E.S., Casstevens T., Hurwitz B., McCouch S., Ni J., Pujar A., et al. Gramene: A growing plant comparative genomics resource. Nucleic Acids Res. 2008;36:D947–D953. doi: 10.1093/nar/gkm968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., Katayama T., Kawashima S., Okuda S., Tokimatsu T., et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvick J., Fu A., Muppirala U., Sabharwal M., Wilkerson M.D., Lawrence C.J., Lushbough C., Brendel V. PlantGDB: A resource for comparative plant genomics. Nucleic Acids Res. 2008;36:D959–D965. doi: 10.1093/nar/gkm1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersey P.J., Staines D.M., Lawson D., Kulesha E., Derwent P., Humphrey J.C., Hughes D.S.T., Keenan S., Kerhornou A., Koscielny G., et al. Ensembl Genomes: An integrative resource for genome-scale data from non-vertebrate species. Nucleic Acids Res. 2012;40:D91–D97. doi: 10.1093/nar/gkr895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazer K.A., Pachter L., Poliakov A., Rubin E.M., Dubchak I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proost S., van Bel M., Sterck L., Billiau K., van Parys T., van de Peer Y., Vandepoele K. PLAZA: A comparative genomics resource to study gene and genome evolution in plants. Plant Cell. 2009;21:3718–3731. doi: 10.1105/tpc.109.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sneddon T.P., Li P., Edmunds S.C. GigaDB: Announcing the GigaScience database. GigaScience. 2012;1:1–2. doi: 10.1186/2047-217X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bombarely A., Menda N., Tecle I.Y., Buels R.M., Strickler S., Fischer-York T., Pujar A., Leto J., Gosselin J., Mueller L.A. The Sol Genomics Network (solgenomics.net): Growing tomatoes using Perl. Nucleic Acids Res. 2011;39:D1149–D1155. doi: 10.1093/nar/gkq866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carollo V., Matthews D.E., Lazo G.R., Blake T.K., Hummel D.D., Lui N., Hane D.L., Anderson O.D. GrainGenes 2.0. An improved resource for the small-grains community. Plant Physiol. 2005;139:643–651. doi: 10.1104/pp.105.064485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzales M.D., Archuleta E., Farmer A., Gajendran K., Grant D., Shoemaker R., Beavis W.D., Waugh M.E. The Legume Information System (LIS): An integrated information resource for comparative legume biology. Nucleic Acids Res. 2005;33:D660–D665. doi: 10.1093/nar/gki128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paterson A.H., Bowers J.E., Bruggmann R., Dubchak I., Grimwood J., Gundlach H., Haberer G., Hellsten U., Mitros T., Poliakov A., et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 19.The International Barley Genome Sequencing Consortium. A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012;491:711–716. doi: 10.1038/nature11543. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G., Liu X., Quan Z., Cheng S., Xu X., Pan S., Xie M., Zeng P., Yue Z., Wang W., et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotech. 2012;30:549–554. doi: 10.1038/nbt.2195. [DOI] [PubMed] [Google Scholar]
- 21.Messmer R., Fracheboud Y., Bänziger M., Vargas M., Stamp P., Ribaut J.-M. Drought stress and tropical maize: QTL-by-environment interactions and stability of QTLs across environments for yield components and secondary traits. Theor. Appl. Genet. 2009;119:913–930. doi: 10.1007/s00122-009-1099-x. [DOI] [PubMed] [Google Scholar]
- 22.Sanseverino W., Hermoso A., D’Alessandro R., Vlasova A., Andolfo G., Frusciante L., Lowy E., Roma G., Ercolano M.R. PRGdb 2.0: Towards a community-based database model for the analysis of R-genes in plants. Nucleic Acids Res. 2013;41:D1167–D1171. doi: 10.1093/nar/gks1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai H., Lee S.S., Tanaka T., Numa H., Kim J., Kawahara Y., Wakimoto H., Yang C.-C., Iwamoto M., Abe T., et al. Rice annotation project database (RAP-DB): An integrative and interactive database for rice genomics. Plant Cell Physiol. 2013;54:e6. doi: 10.1093/pcp/pcs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaeffer M.L., Harper L.C., Gardiner J.M., Andorf C.M., Campbell D.A., Cannon E.K.S., Sen T.Z., Lawrence C.J. MaizeGDB: Curation and outreach go hand-in-hand. Database. 2011;2011:bar022. doi: 10.1093/database/bar022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant D., Nelson R.T., Cannon S.B., Shoemaker R.C. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 2010;38:D843–D846. doi: 10.1093/nar/gkp798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fei Z., Joung J.-G., Tang X., Zheng Y., Huang M., Lee J.M., McQuinn R., Tieman D.M., Alba R., Klee H.J., et al. Tomato functional genomics database: A comprehensive resource and analysis package for tomato functional genomics. Nucleic Acids Res. 2011;39:D1156–D1163. doi: 10.1093/nar/gkq991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gene Ontology Consortium. The Gene Ontology Website. [(accessed on 10 January 2013)]. Available online: http://www.geneontology.org/
- 28.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torto-Alalibo T., Collmer C., Gwinn-Giglio M. The Plant-Associated Microbe Gene Ontology (PAMGO) Consortium: Community development of new Gene Ontology terms describing biological processes involved in microbe-host interactions. BMC Microbiol. 2009;9:1–5. doi: 10.1186/1471-2180-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avraham S., Tung C.W., Ilic K., Jaiswal P., Kellogg E.A., McCouch S., Pujar A., Reiser L., Rhee S.Y., Sachs M.M., et al. The Plant Ontology Database: A community resource for plant structure and developmental stages controlled vocabulary and annotations. Nucleic Acids Res. 2008;36:D449–D454. doi: 10.1093/nar/gkm908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eilbeck K., Lewis S.E., Mungall C.J., Yandell M., Stein L., Durbin R., Ashburner M. The Sequence Ontology: A tool for the unification of genome annotations. Genome Biol. 2005;6:R44. doi: 10.1186/gb-2005-6-5-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Catalogue of Life. [(accessed on 8 January 2013)]. Available online: http://www.catalogueoflife.org/
- 33.Integrated Taxonomic Information System. [(accessed on 8 January 2013)]. Available online: http://www.itis.gov/
- 34.Wang K., Wang Z., Li F., Ye W., Wang J., Song G., Yue Z., Cong L., Shang H., Zhu S., et al. The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet. 2012;44:1098–1103. doi: 10.1038/ng.2371. [DOI] [PubMed] [Google Scholar]
- 35.Shulaev V., Korban S.S., Sosinski B., Abbott A.G., Aldwinckle H.S., Folta K.M., Iezzoni A., Main D., Arús P., Dandekar A.M., et al. Multiple models for rosaceae genomics. Plant Physiol. 2008;147:985–1003. doi: 10.1104/pp.107.115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulaev V., Sargent D.J., Crowhurst R.N., Mockler T.C., Folkerts O., Delcher A.L., Jaiswal P., Mockaitis K., Liston A., Mane S.P., et al. The genome of woodland strawberry (Fragaria vesca) Nat. Genet. 2011;43:109–116. doi: 10.1038/ng.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenchley R., Spannagl M., Pfeifer M., Barker G.L.A., D’Amore R., Allen A.M., McKenzie N., Kramer M., Kerhornou A., Bolser D., et al. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature. 2012;491:705–710. doi: 10.1038/nature11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia J., Zhao S., Kong X., Li Y., Zhao G., He W., Appels R., Pfeifer M., Tao Y., Zhang X., et al. Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature. 2013;496:91–95. doi: 10.1038/nature12028. [DOI] [PubMed] [Google Scholar]
- 39.Ling H.-Q., Zhao S., Liu D., Wang J., Sun H., Zhang C., Fan H., Li D., Dong L., Tao Y., et al. Draft genome of the wheat A-genome progenitor Triticum urartu. Nature. 2013;496:87–90. doi: 10.1038/nature11997. [DOI] [PubMed] [Google Scholar]
- 40.Lam H.M., Xu X., Liu X., Chen W.B., Yang G.H., Wong F.L., Li M.W., He W.M., Qin N., Wang B., et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010;42:1053–1059. doi: 10.1038/ng.715. [DOI] [PubMed] [Google Scholar]
- 41.Xu X., Liu X., Ge S., Jensen J.D., Hu F., Li X., Dong Y., Gutenkunst R.N., Fang L., Huang L., et al. Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat. Biotechnol. 2012;30:105–111. doi: 10.1038/nbt.2050. [DOI] [PubMed] [Google Scholar]
- 42.Hufford M.B., Xu X., van Heerwaarden J., Pyhajarvi T., Chia J.-M., Cartwright R.A., Elshire R.J., Glaubitz J.C., Guill K.E., Kaeppler S.M., et al. Comparative population genomics of maize domestication and improvement. Nat. Genet. 2012;44:808–811. doi: 10.1038/ng.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M.Y., Lee S., Van K., Kim T.-H., Jeong S.-C., Choi I.-Y., Kim D.-S., Lee Y.-S., Park D., Ma J., et al. Whole-genome sequencing and intensive analysis of the undomesticated soybean (Glycine soja Sieb. and Zucc.) genome. Proc. Natl. Acad. Sci. USA. 2010;107:22032–22037. doi: 10.1073/pnas.1009526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai J., Li R., Xu X., Jin W., Xu M., Zhao H., Xiang Z., Song W., Ying K., Zhang M., et al. Genome-wide patterns of genetic variation among elite maize inbred lines. Nat. Genet. 2010;42:1027–1030. doi: 10.1038/ng.684. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Mas J., Benjak A., Sanseverino W., Bourgeois M., Mir G., Gonzalez V.M., Henaff E., Camara F., Cozzuto L., Lowy E., et al. The genome of melon (Cucumis melo L.) Proc. Natl. Acad. Sci. USA. 2012;109:11872–11877. doi: 10.1073/pnas.1205415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dangl J.L., Jones J.D.G. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 47.Shanmugam V. Role of extracytoplasmic leucine rich repeat proteins in plant defence mechanisms. Microbiol. Res. 2005;160:83–94. doi: 10.1016/j.micres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Torii K.U. Leucine-rich repeat receptor kinases in plants: Structure, function, and signal transduction pathways. Int. Rev. Cytol. 2004;234:1–46. doi: 10.1016/S0074-7696(04)34001-5. [DOI] [PubMed] [Google Scholar]
- 49.Van Ooijen G., Mayr G., Kasiem M.M.A., Albrecht M., Cornelissen B.J.C., Takken F.L.W. Structure—Function analysis of the NB-ARC domain of plant disease resistance proteins. J. Exp. Bot. 2008;59:1383–1397. doi: 10.1093/jxb/ern045. [DOI] [PubMed] [Google Scholar]
- 50.McNally K.L., Childs K.L., Bohnert R., Davidson R.M., Zhao K., Ulat V.J., Zeller G., Clark R.M., Hoen D.R., Bureau T.E., et al. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc. Natl. Acad. Sci. USA. 2009;106:12273–12278. doi: 10.1073/pnas.0900992106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng L.-Y., Guo X.-S., He B., Sun L.-J., Peng Y., Dong S.-S., Liu T.-F., Jiang S., Ramachandran S., Liu C.-M., et al. Genome-wide patterns of genetic variation in sweet and grain sorghum (Sorghum bicolor) Genome Biol. 2011;12:R114. doi: 10.1186/gb-2011-12-11-r114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodds P.N., Rathjen J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 53.D’Hont A., Denoeud F., Aury J.-M., Baurens F.-C., Carreel F., Garsmeur O., Noel B., Bocs S., Droc G., Rouard M., et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature. 2012;488:213–217. doi: 10.1038/nature11241. [DOI] [PubMed] [Google Scholar]
- 54.Singh K.B., Foley R.C., Oñate-Sánchez L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002;5:430–436. doi: 10.1016/s1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 55.Collard B.C.Y., Jahufer M.Z.Z., Brouwer J.B., Pang E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica. 2005;142:169–196. [Google Scholar]
- 56.Rafalski J.A. Association genetics in crop improvement. Curr. Opin. Plant Biol. 2010;13:174–180. doi: 10.1016/j.pbi.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Huang S., Spielmeyer W., Lagudah E.S., James R.A., Platten J.D., Dennis E.S., Munns R. A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol. 2006;142:1718–1727. doi: 10.1104/pp.106.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren Z.-H., Gao J.-P., Li L.-G., Cai X.-L., Huang W., Chao D.-Y., Zhu M.-Z., Wang Z.-Y., Luan S., Lin H.-X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 59.Sutton T., Baumann U., Hayes J., Collins N.C., Shi B.-J., Schnurbusch T., Hay A., Mayo G., Pallotta M., Tester M., et al. Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science. 2007;318:1446–1449. doi: 10.1126/science.1146853. [DOI] [PubMed] [Google Scholar]
- 60.Huang X., Feng Q., Qian Q., Zhao Q., Wang L., Wang A., Guan J., Fan D., Weng Q., Huang T., et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 2009;19:1068–1076. doi: 10.1101/gr.089516.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang X., Wei X., Sang T., Zhao Q., Feng Q., Zhao Y., Li C., Zhu C., Lu T., Zhang Z., et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010;42:U961–U976. doi: 10.1038/ng.695. [DOI] [PubMed] [Google Scholar]
- 62.Yu H., Xie W., Wang J., Xing Y., Xu C., Li X., Xiao J., Zhang Q. Gains in QTL detection using an ultra-high density SNP map based on population sequencing relative to traditional RFLP/SSR markers. PLoS One. 2011;6:e17595. doi: 10.1371/journal.pone.0017595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou G., Zhai G., Feng Q., Yan S., Wang A., Zhao Q., Shao J., Zhang Z., Zou J., Han B., et al. Identification of QTLs for eight agronomically important traits using an ultra-high-density map based on SNPs generated from high-throughput sequencing in sorghum under contrasting photoperiods. J. Exp. Bot. 2012;63:5451–5462. doi: 10.1093/jxb/ers205. [DOI] [PubMed] [Google Scholar]
- 64.Li H., Durbin R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wetterstrand K. DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program (GSP) [(accessed on 3 January 2013)]. Available online: http://www.genome.gov/sequencingcosts.
- 66.Heffner E.L., Sorrells M.E., Jannink J.-L. Genomic selection for crop improvement. Crop Sci. 2009;49:1–12. [Google Scholar]
- 67.Meuwissen T.H.E., Hayes B.J., Goddard M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001;157:1819–1829. doi: 10.1093/genetics/157.4.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y., Gowda M., Liu W., Würschum T., Maurer H.P., Longin F.H., Ranc N., Reif J.C. Accuracy of genomic selection in European maize elite breeding populations. Theor. Appl. Genet. 2012;124:769–776. doi: 10.1007/s00122-011-1745-y. [DOI] [PubMed] [Google Scholar]
- 69.Heffner E.L., Jannink J.-L., Iwata H., Souza E., Sorrells M.E. Genomic selection accuracy for grain quality traits in biparental wheat populations. Crop Sci. 2011;51:2597–2606. [Google Scholar]
- 70.Heffner E.L., Jannink J.-L., Sorrells M.E. Genomic selection accuracy using multifamily prediction models in a wheat breeding program. Plant Gen. 2011;4:65–75. [Google Scholar]
- 71.Zhong S.Q., Dekkers J.C.M., Fernando R.L., Jannink J.L. Factors affecting accuracy from genomic selection in populations derived from multiple inbred lines: A barley case study. Genetics. 2009;182:355–364. doi: 10.1534/genetics.108.098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliveira E., Resende M., Silva Santos V., Ferreira C., Oliveira G., Silva M., Oliveira L., Aguilar-Vildoso C. Genome-wide selection in cassava. Euphytica. 2012;187:263–276. [Google Scholar]
- 73.Xu S. Estimating polygenic effects using markers of the entire genome. Genetics. 2003;163:789–801. doi: 10.1093/genetics/163.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakaya A., Isobe S.N. Will genomic selection be a practical method for plant breeding? Ann. Bot. 2012;110:1303–1316. doi: 10.1093/aob/mcs109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jannink J.-L., Lorenz A.J., Iwata H. Genomic selection in plant breeding: From theory to practice. Brief. Funct. Genomics. 2010;9:166–177. doi: 10.1093/bfgp/elq001. [DOI] [PubMed] [Google Scholar]
- 76.Rutkoski J., Heffner E., Sorrells M. Genomic selection for durable stem rust resistance in wheat. Euphytica. 2011;179:161–173. [Google Scholar]
- 77.Salse J. In silico archeogenomics unveils modern plant genome organisation, regulation and evolution. Curr. Opin. Plant Biol. 2012;15:122–130. doi: 10.1016/j.pbi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Salamov A.A., Solovyev V.V. Ab initio gene finding in drosophila genomic DNA. Genome Res. 2000;10:516–522. doi: 10.1101/gr.10.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Howe K.L., Chothia T., Durbin R. GAZE: A generic framework for the integration of gene-prediction data by dynamic programming. Genome Res. 2002;12:1418–1427. doi: 10.1101/gr.149502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen J.E., Salzberg S.L. JIGSAW: Integration of multiple sources of evidence for gene prediction. Bioinformatics. 2005;21:3596–3603. doi: 10.1093/bioinformatics/bti609. [DOI] [PubMed] [Google Scholar]
- 81.Finn R.D., Mistry J., Tate J., Coggill P., Heger A., Pollington J.E., Gavin O.L., Gunasekaran P., Ceric G., Forslund K., et al. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hunter S., Jones P., Mitchell A., Apweiler R., Attwood T.K., Bateman A., Bernard T., Binns D., Bork P., Burge S., et al. InterPro in 2011: New developments in the family and domain prediction database. Nucleic Acids Res. 2012;40:D306–D312. doi: 10.1093/nar/gkr948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanehisa M., Goto S., Sato Y., Furumichi M., Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 85.Letunic I., Doerks T., Bork P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012;40:D302–D305. doi: 10.1093/nar/gkr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finn R.D., Clements J., Eddy S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharoni A.M., Nuruzzaman M., Satoh K., Shimizu T., Kondoh H., Sasaya T., Choi I.-R., Omura T., Kikuchi S. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol. 2011;52:344–360. doi: 10.1093/pcp/pcq196. [DOI] [PubMed] [Google Scholar]
- 88.Schena M., Shalon D., Davis R.W., Brown P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 89.Ozsolak F., Milos P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rustici G., Kolesnikov N., Brandizi M., Burdett T., Dylag M., Emam I., Farne A., Hastings E., Ison J., Keays M., et al. ArrayExpress update—Trends in database growth and links to data analysis tools. Nucleic Acids Res. 2013;41:D987–D990. doi: 10.1093/nar/gks1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lou S.-K., Ni B., Lo L.-Y., Tsui S.K.-W., Chan T.-F., Leung K.-S. ABMapper: A suffix array-based tool for multi-location searching and splice-junction mapping. Bioinformatics. 2011;27:421–422. doi: 10.1093/bioinformatics/btq656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Meth. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L., Feng Z., Wang X., Wang X., Zhang X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 95.Nawrocki E.P., Kolbe D.L., Eddy S.R. Infernal 1.0: Inference of RNA alignments. Bioinformatics. 2009;25:1335–1337. doi: 10.1093/bioinformatics/btp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schulz M.H., Zerbino D.R., Vingron M., Birney E. Oases: Robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28:1086–1092. doi: 10.1093/bioinformatics/bts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trapnell C., Pachter L., Salzberg S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robertson G., Schein J., Chiu R., Corbett R., Field M., Jackman S.D., Mungall K., Lee S., Okada H.M., Qian J.Q., et al. De novo assembly and analysis of RNA-seq data. Nat. Meth. 2010;7:909–912. doi: 10.1038/nmeth.1517. [DOI] [PubMed] [Google Scholar]
- 99.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sekhon R.S., Lin H., Childs K.L., Hansey C.N., Buell C.R., de Leon N., Kaeppler S.M. Genome-wide atlas of transcription during maize development. Plant J. 2011;66:553–563. doi: 10.1111/j.1365-313X.2011.04527.x. [DOI] [PubMed] [Google Scholar]
- 101.Severin A., Woody J., Bolon Y.-T., Joseph B., Diers B., Farmer A., Muehlbauer G., Nelson R., Grant D., Specht J., et al. RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010;10:160. doi: 10.1186/1471-2229-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ge Y., Li Y., Zhu Y.-M., Bai X., Lv D.-K., Guo D., Ji W., Cai H. Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol. 2010;10:153. doi: 10.1186/1471-2229-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma T.-L., Wu W.-H., Wang Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 2012;12:161. doi: 10.1186/1471-2229-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.An D., Yang J., Zhang P. Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genomics. 2012;13:64. doi: 10.1186/1471-2164-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zabala G., Zou J., Tuteja J., Gonzalez D., Clough S., Vodkin L. Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol. 2006;6:26. doi: 10.1186/1471-2229-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang C., Chen H., Hao Q., Sha A., Shan Z., Chen L., Zhou R., Zhi H., Zhou X. Transcript profile of the response of two soybean genotypes to potassium deficiency. PLoS One. 2012;7:e39856. doi: 10.1371/journal.pone.0039856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lenka S.K., Katiyar A., Chinnusamy V., Bansal K.C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011;9:315–327. doi: 10.1111/j.1467-7652.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- 108.Zhang T., Zhao X., Wang W., Pan Y., Huang L., Liu X., Zong Y., Zhu L., Yang D., Fu B. Comparative transcriptome profiling of chilling stress responsiveness in two contrasting rice genotypes. PLoS One. 2012;7:e43274. doi: 10.1371/journal.pone.0043274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y.C., Meng F.R., Zhang C.Y., Zhang N., Sun M.S., Ren J.P., Niu H.B., Wang X., Yin J. Comparative analysis of water stress-responsive transcriptomes in drought-susceptible and -tolerant wheat (Triticum aestivum L.) J. Plant Biol. 2012;55:349–360. [Google Scholar]
- 110.Zahaf O., Blanchet S., de Zelicourt A., Alunni B., Plet J., Laffont C., de Lorenzo L., Imbeaud S., Ichante J.-L., Diet A., et al. Comparative transcriptomic analysis of salt adaptation in roots of contrasting Medicago truncatula genotypes. Mol. Plant. 2012;5:1068–1081. doi: 10.1093/mp/sss009. [DOI] [PubMed] [Google Scholar]
- 111.Puranik S., Jha S., Srivastava P.S., Sreenivasulu N., Prasad M. Comparative transcriptome analysis of contrasting foxtail millet cultivars in response to short-term salinity stress. J. Plant Physiol. 2011;168:280–287. doi: 10.1016/j.jplph.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Delker C., Quint M. Expression level polymorphisms: Heritable traits shaping natural variation. Trends Plant Sci. 2011;16:481–488. doi: 10.1016/j.tplants.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 113.Holloway B., Luck S., Beatty M., Rafalski J.-A., Li B. Genome-wide expression quantitative trait loci (eQTL) analysis in maize. BMC Genomics. 2011;12:336. doi: 10.1186/1471-2164-12-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang J., Yu H., Xie W., Xing Y., Yu S., Xu C., Li X., Xiao J., Zhang Q. A global analysis of QTLs for expression variations in rice shoots at the early seedling stage. Plant J. 2010;63:1063–1074. doi: 10.1111/j.1365-313X.2010.04303.x. [DOI] [PubMed] [Google Scholar]
- 115.Chen X., Hackett C.A., Niks R.E., Hedley P.E., Booth C., Druka A., Marcel T.C., Vels A., Bayer M., Milne I., et al. An eQTL Analysis of partial resistance to Puccinia hordei in barley. PLoS One. 2010;5:e8598. doi: 10.1371/journal.pone.0008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mann M. Can proteomics retire the western blot? J. Proteome Res. 2008;7:3065–3065. doi: 10.1021/pr800463v. [DOI] [PubMed] [Google Scholar]
- 117.Gygi S.P., Rist B., Gerber S.A., Turecek F., Gelb M.H., Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 118.Ong S.E., Blagoev B., Kratchmarova I., Kristensen D.B., Steen H., Pandey A., Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 119.Wiese S., Reidegeld K.A., Meyer H.E., Warscheid B. Protein labeling by iTRAQ: A new tool for quantitative mass spectrometry in proteome research. Proteomics. 2007;7:340–350. doi: 10.1002/pmic.200600422. [DOI] [PubMed] [Google Scholar]
- 120.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 121.Old W.M., Meyer-Arendt K., Aveline-Wolf L., Pierce K.G., Mendoza A., Sevinsky J.R., Resing K.A., Ahn N.G. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol. Cell. Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 122.Jorrín J.V., Maldonado A.M., Castillejo M.A. Plant proteome analysis: A 2006 update. Proteomics. 2007;7:2947–2962. doi: 10.1002/pmic.200700135. [DOI] [PubMed] [Google Scholar]
- 123.Jorrin-Novo J.V., Maldonado A.M., Echevarria-Zomeno S., Valledor L., Castillejo M.A., Curto M., Valero J., Sghaier B., Donoso G., Redondo I. Plant proteomics update (2007–2008): Second-generation proteomic techniques, an appropriate experimental design, and data analysis to fulfill MIAPE standards, increase plant proteome coverage and expand biological knowledge. J. Proteomics. 2009;72:285–314. doi: 10.1016/j.jprot.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 124.Kamal A.H.M., Cho K., Kim D.-E., Uozumi N., Chung K.-Y., Lee S.Y., Choi J.-S., Cho S.-W., Shin C.-S., Woo S.H. Changes in physiology and protein abundance in salt-stressed wheat chloroplasts. Mol. Biol. Rep. 2012;39:9059–9074. doi: 10.1007/s11033-012-1777-7. [DOI] [PubMed] [Google Scholar]
- 125.Ahsan N., Nakamura T., Komatsu S. Differential responses of microsomal proteins and metabolites in two contrasting cadmium (Cd)-accumulating soybean cultivars under Cd stress. Amino Acids. 2012;42:317–327. doi: 10.1007/s00726-010-0809-7. [DOI] [PubMed] [Google Scholar]
- 126.Wang H., Wang S., Xia Y. Identification and verification of redox-sensitive proteins in Arabidopsis thaliana. Methods Mol. Biol. 2012;876:83–94. doi: 10.1007/978-1-61779-809-2_6. [DOI] [PubMed] [Google Scholar]
- 127.Galant A., Koester R.P., Ainsworth E.A., Hicks L.M., Jez J.M. From climate change to molecular response: Redox proteomics of ozone-induced responses in soybean. New Phytol. 2012;194:220–229. doi: 10.1111/j.1469-8137.2011.04037.x. [DOI] [PubMed] [Google Scholar]
- 128.Nakagami H., Sugiyama N., Mochida K., Daudi A., Yoshida Y., Toyoda T., Tomita M., Ishihama Y., Shirasu K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol. 2010;153:1161–1174. doi: 10.1104/pp.110.157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Agrawal G.K., Jwa N.-S., Lebrun M.-H., Job D., Rakwal R. Plant secretome: Unlocking secrets of the secreted proteins. Proteomics. 2010;10:799–827. doi: 10.1002/pmic.200900514. [DOI] [PubMed] [Google Scholar]
- 130.Alexandersson E., Ashfaq A., Resjö S., Andreasson E. Plant secretome proteomics. Front. Plant Sci. 2013;4:9. doi: 10.3389/fpls.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Catalá C., Howe K.J., Hucko S., Rose J.K.C., Thannhauser T.W. Towards characterization of the glycoproteome of tomato (Solanum lycopersicum) fruit using Concanavalin A lectin affinity chromatography and LC-MALDI-MS/MS analysis. Proteomics. 2011;11:1530–1544. doi: 10.1002/pmic.201000424. [DOI] [PubMed] [Google Scholar]
- 132.Ruiz-May E., Kim S.J., Brandizzi F., Rose J.K.C. The secreted plant n-glycoproteome and associated secretory pathways. Front. Plant Sci. 2012;3:117. doi: 10.3389/fpls.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pawson T., Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000;14:1027–1047. [PubMed] [Google Scholar]
- 134.Zhang Y., Gao P., Yuan J.S. Plant protein-protein interaction network and interactome. Curr. Genomics. 2010;11:40–46. doi: 10.2174/138920210790218016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wittig I., Braun H.-P., Schagger H. Blue native PAGE. Nat. Protoc. 2006;1:418–428. doi: 10.1038/nprot.2006.62. [DOI] [PubMed] [Google Scholar]
- 136.Hue M., Riffle M., Vert J.-P., Noble W. Large-scale prediction of protein-protein interactions from structures. BMC Bioinforma. 2010;11:144. doi: 10.1186/1471-2105-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moal I.H., Agius R., Bates P.A. Protein-protein binding affinity prediction on a diverse set of structures. Bioinformatics. 2011;27:3002–3009. doi: 10.1093/bioinformatics/btr513. [DOI] [PubMed] [Google Scholar]
- 138.Cusick M.E., Yu H., Smolyar A., Venkatesan K., Carvunis A.-R., Simonis N., Rual J.-F., Borick H., Braun P., Dreze M., et al. Literature-curated protein interaction datasets. Nat. Meth. 2009;6:39–46. doi: 10.1038/nmeth.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ho C.-L., Wu Y., Shen H.-B., Provart N., Geisler M. A predicted protein interactome for rice. Rice. 2012;5:15. doi: 10.1186/1939-8433-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cui J., Li P., Li G., Xu F., Zhao C., Li Y.H., Yang Z.N., Wang G., Yu Q.B., Li Y.X., et al. AtPID: Arabidopsis thaliana protein interactome database—An integrative platform for plant systems biology. Nucleic Acids Res. 2008;36:D999–D1008. doi: 10.1093/nar/gkm844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arabidopsis interactome mapping consortium. Evidence for network evolution in an Arabidopsis interactome map. Science. 2011;333:601–607. doi: 10.1126/science.1203877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seo Y.-S., Chern M., Bartley L.E., Han M., Jung K.-H., Lee I., Walia H., Richter T., Xu X., Cao P., et al. Towards establishment of a rice stress response interactome. PLoS Genet. 2011;7:e1002020. doi: 10.1371/journal.pgen.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gu H., Zhu P., Jiao Y., Meng Y., Chen M. PRIN: A predicted rice interactome network. BMC Bioinforma. 2011;12:161. doi: 10.1186/1471-2105-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chatr-aryamontri A., Breitkreutz B.-J., Heinicke S., Boucher L., Winter A., Stark C., Nixon J., Ramage L., Kolas N., O’Donnell L., et al. The BioGRID interaction database: 2013 update. Nucleic Acids Res. 2013;41:D816–D823. doi: 10.1093/nar/gks1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xenarios I., Salwínski L., Duan X.J., Higney P., Kim S.-M., Eisenberg D. DIP, the Database of Interacting Proteins: A research tool for studying cellular networks of protein interactions. Nucleic Acids Res. 2002;30:303–305. doi: 10.1093/nar/30.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mingwei M., Haoyang C., Wen Z., Zhirui Y., Xiao L., Xinjian F., Quansheng F. PlaPID: A Database of Protein-Protein Interactions in Plants. Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE); Chengdu, China. 18–20 June 2010; pp. 1–4. [Google Scholar]
- 147.Kerrien S., Aranda B., Breuza L., Bridge A., Broackes-Carter F., Chen C., Duesbury M., Dumousseau M., Feuermann M., Hinz U., et al. The IntAct molecular interaction database in 2012. Nucleic Acids Res. 2012;40:D841–D846. doi: 10.1093/nar/gkr1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cooper B., Clarke J.D., Budworth P., Kreps J., Hutchison D., Park S., Guimil S., Dunn M., Luginbühl P., Ellero C., et al. A network of rice genes associated with stress response and seed development. Proc. Natl. Acad. Sci. USA. 2003;100:4945–4950. doi: 10.1073/pnas.0737574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tardif G., Kane N., Adam H., Labrie L., Major G., Gulick P., Sarhan F., Laliberté J.-F. Interaction network of proteins associated with abiotic stress response and development in wheat. Plant Mol. Biol. 2007;63:703–718. doi: 10.1007/s11103-006-9119-6. [DOI] [PubMed] [Google Scholar]
- 150.Afzal A.J., Natarajan A., Saini N., Iqbal M.J., Geisler M., El Shemy H.A., Mungur R., Willmitzer L., Lightfoot D.A. The nematode resistance allele at the rhg1 locus alters the proteome and primary metabolism of soybean roots. Plant Physiol. 2009;151:1264–1280. doi: 10.1104/pp.109.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gendler K., Paulsen T., Napoli C. ChromDB: The chromatin database. Nucleic Acids Res. 2008;36:D298–D302. doi: 10.1093/nar/gkm768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morison I.M., Ramsay J.P., Spencer H.G. A census of mammalian imprinting. Trends Genet. 2005;21:457–465. doi: 10.1016/j.tig.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 153.Tsukahara S., Kobayashi A., Kawabe A., Mathieu O., Miura A., Kakutani T. Bursts of retrotransposition reproduced in Arabidopsis. Nature. 2009;461:423–426. doi: 10.1038/nature08351. [DOI] [PubMed] [Google Scholar]
- 154.Zhang X.Y., Yazaki J., Sundaresan A., Cokus S., Chan S.W.L., Chen H.M., Henderson I.R., Shinn P., Pellegrini M., Jacobsen S.E., et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell. 2006;126:1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 155.Zilberman D., Gehring M., Tran R.K., Ballinger T., Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 2007;39:61–69. doi: 10.1038/ng1929. [DOI] [PubMed] [Google Scholar]
- 156.Seifert M., Cortijo S., Colome-Tatche M., Johannes F., Roudier F., Colot V. MeDIP-HMM: Genome-wide identification of distinct DNA methylation states from high-density tiling arrays. Bioinformatics. 2012;28:2930–2939. doi: 10.1093/bioinformatics/bts562. [DOI] [PubMed] [Google Scholar]
- 157.Cokus S.J., Feng S.H., Zhang X.Y., Chen Z.G., Merriman B., Haudenschild C.D., Pradhan S., Nelson S.F., Pellegrini M., Jacobsen S.E. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bibikova M., Barnes B., Tsan C., Ho V., Klotzle B., Le J.M., Delano D., Zhang L., Schroth G.P., Gunderson K.L., et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. doi: 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 159.Meissner A., Gnirke A., Bell G.W., Ramsahoye B., Lander E.S., Jaenisch R. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Res. 2005;33:5868–5877. doi: 10.1093/nar/gki901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dowen R.H., Pelizzola M., Schmitz R.J., Lister R., Dowen J.M., Nery J.R., Dixon J.E., Ecker J.R. Widespread dynamic DNA methylation in response to biotic stress. Proc. Natl. Acad. Sci. USA. 2012;109:E2183–E2191. doi: 10.1073/pnas.1209329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang W.S., Pan Y.J., Zhao X.Q., Dwivedi D., Zhu L.H., Ali J., Fu B.Y., Li Z.K. Drought-induced site-specific DNA methylation and its association with drought tolerance in rice (Oryza sativa L.) J. Exp. Bot. 2011;62:1951–1960. doi: 10.1093/jxb/erq391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhong L., Xu Y.H., Wang J.B. DNA-methylation changes induced by salt stress in wheat Triticum aestivum. Afr. J. Biotechnol. 2009;8:6201–6207. [Google Scholar]
- 163.Calarco J.P., Borges F., Donoghue M.T., van Ex F., Jullien P.E., Lopes T., Gardner R., Berger F., Feijo J.A., Becker J.D., et al. Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell. 2012;151:194–205. doi: 10.1016/j.cell.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Holeski L.M., Jander G., Agrawal A.A. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 2012;27:618–626. doi: 10.1016/j.tree.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 165.Kou H.P., Li Y., Song X.X., Ou X.F., Xing S.C., Ma J., von Wettstein D., Liu B. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice (Oryza sativa L.) J. Plant Physiol. 2011;168:1685–1693. doi: 10.1016/j.jplph.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 166.Lutsik P., Feuerbach L., Arand J., Lengauer T., Walter J., Bock C. BiQ Analyzer HT: Locus-specific analysis of DNA methylation by high-throughput bisulfite sequencing. Nucleic Acids Res. 2011;39:W551–W556. doi: 10.1093/nar/gkr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Krueger F., Andrews S.R. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harris E.Y., Ponts N., Le Roch K.G., Lonardi S. BRAT-BW: Efficient and accurate mapping of bisulfite-treated reads. Bioinformatics. 2012;28:1795–1796. doi: 10.1093/bioinformatics/bts264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hansen K.D., Langmead B., Irizarry R.A. BSmooth: From whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol. 2012;13:R83. doi: 10.1186/gb-2012-13-10-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen P.Y., Cokus S.J., Pellegrini M. BS Seeker: Precise mapping for bisulfite sequencing. BMC Bioinforma. 2010;11:203. doi: 10.1186/1471-2105-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xi Y., Li W. BSMAP: Whole genome bisulfite sequence MAPping program. BMC Bioinforma. 2009;10:232. doi: 10.1186/1471-2105-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Su J.Z., Yan H.D., Wei Y.J., Liu H.B., Liu H., Wang F., Lv J., Wu Q., Zhang Y. CpG_MPs: Identification of CpG methylation patterns of genomic regions from high-throughput bisulfite sequencing data. Nucleic Acids Res. 2013;41:e4. doi: 10.1093/nar/gks829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Benoukraf T., Wongphayak S., Hadi L.H., Wu M., Soong R. GBSA: A comprehensive software for analysing whole genome bisulfite sequencing data. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gruntman E., Qi Y.J., Slotkin R.K., Roeder T., Martienssen R.A., Sachidanandam R. Kismeth: Analyzer of plant methylation states through bisulfite sequencing. BMC Bioinforma. 2008;9:371. doi: 10.1186/1471-2105-9-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kumaki Y., Oda M., Okano M. QUMA: Quantification tool for methylation analysis. Nucleic Acids Res. 2008;36:W170–W175. doi: 10.1093/nar/gkn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xi Y.X., Bock C., Muller F., Sun D.Q., Meissner A., Li W. RRBSMAP: A fast, accurate and user-friendly alignment tool for reduced representation bisulfite sequencing. Bioinformatics. 2012;28:430–432. doi: 10.1093/bioinformatics/btr668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strahl B.D., Allis C.D. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 178.Marino-Ramirez L., Levine K.M., Morales M., Zhang S.Y., Moreland R.T., Baxevanis A.D., Landsman D. The histone database: An integrated resource for histones and histone fold-containing proteins. Database-Oxford. 2011 doi: 10.1093/database/bar048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee K.K., Workman J.L. Histone acetyltransferase complexes: One size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 180.Barski A., Cuddapah S., Cui K.R., Roh T.Y., Schones D.E., Wang Z.B., Wei G., Chepelev I., Zhao K.J. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 181.Oki M., Aihara H., Ito T. Role Of Histone Phosphorylation In Chromatin Dynamics And Its Implications in Diseases. In: Kundu T., Bittman R., Dasgupta D., Engelhardt H., Flohe L., Herrmann H., Holzenburg A., Nasheuer H.P., Rottem S., Wyss M., Zwickl P., editors. Chromatin and Disease. Vol. 41. Springer; London, UK: 2007. pp. 323–340. [PubMed] [Google Scholar]
- 182.Shivaswamy S., Iyer V.R. Genome-wide analysis of chromatin status using tiling microarrays. Methods. 2007;41:304–311. doi: 10.1016/j.ymeth.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Johnson D.S., Mortazavi A., Myers R.M., Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 184.Papaefthimiou D., Tsaftaris A.S. Characterization of a drought inducible trithorax-like H3K4 methyltransferase from barley. Biol. Plant. 2012;56:683–692. [Google Scholar]
- 185.Ding B., Bellizzi M.D.R., Ning Y., Meyers B.C., Wang G.-L. HDT701, a Histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice. Plant Cell. 2012;24:3783–3794. doi: 10.1105/tpc.112.101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Song Y.G., Ji D.D., Li S., Wang P., Li Q., Xiang F.N. The dynamic changes of DNA methylation and histone modifications of salt responsive transcription factor genes in soybean. PLoS One. 2012;7:e41274. doi: 10.1371/journal.pone.0041274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zong W., Zhong X., You J., Xiong L. Genome-wide profiling of histone H3K4-tri-methylation and gene expression in rice under drought stress. Plant Mol. Biol. 2013;81:175–188. doi: 10.1007/s11103-012-9990-2. [DOI] [PubMed] [Google Scholar]
- 188.Munns R., James R.A., Sirault X.R.R., Furbank R.T., Jones H.G. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 2010;61:3499–3507. doi: 10.1093/jxb/erq199. [DOI] [PubMed] [Google Scholar]
- 189.Golzarian M., Frick R., Rajendran K., Berger B., Roy S., Tester M., Lun D. Accurate inference of shoot biomass from high-throughput images of cereal plants. Plant Methods. 2011;7:2. doi: 10.1186/1746-4811-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Paproki A., Sirault X., Berry S., Furbank R., Fripp J. A novel mesh processing based technique for 3D plant analysis. BMC Plant Biol. 2012;12:63. doi: 10.1186/1471-2229-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Spielbauer G., Armstrong P., Baier J.W., Allen W.B., Richardson K., Shen B., Settles A.M. High-throughput near-infrared reflectance spectroscopy for predicting quantitative and qualitative composition phenotypes of individual maize kernels. Cereal Chem. 2009;86:556–564. [Google Scholar]
- 192.Baker N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 193.Condon A.G., Richards R.A., Rebetzke G.J., Farquhar G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- 194.Hargreaves C., Gregory P., Bengough A.G. Measuring root traits in barley (Hordeum vulgare ssp. vulgare and ssp. spontaneum) seedlings using gel chambers, soil sacs and X-ray microtomography. Plant Soil. 2009;316:285–297. [Google Scholar]
- 195.Rajendran K., Tester M., Roy S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009;32:237–249. doi: 10.1111/j.1365-3040.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 196.Jones H.G., Serraj R., Loveys B.R., Xiong L., Wheaton A., Price A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009;36:978–989. doi: 10.1071/FP09123. [DOI] [PubMed] [Google Scholar]
- 197.Moshou D., Bravo C., Oberti R., West J., Bodria L., McCartney A., Ramon H. Plant disease detection based on data fusion of hyper-spectral and multi-spectral fluorescence imaging using Kohonen maps. Real-Time Imaging. 2005;11:75–83. [Google Scholar]
- 198.Genga A., Mattana M., Coraggio I., Locatelli F., Piffanelli P., Consonni R. Plant Metabolomics: A Characterisation of Plant Responses to Abiotic Stresses. In: Shanker A., Venkateswarlu B., editors. Abiotic Stress in Plants—Mechanisms and Adaptations. InTech; Rijeka, Croatia: 2011. [Google Scholar]
- 199.Kooke R., Keurentjes J.J.B. Multi-dimensional regulation of metabolic networks shaping plant development and performance. J. Exp. Bot. 2011 doi: 10.1093/jxb/err373. [DOI] [PubMed] [Google Scholar]
- 200.Cramer G.R., Urano K., Delrot S., Pezzotti M., Shinozaki K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011;11:163. doi: 10.1186/1471-2229-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang G.-T., Ma S.-L., Bai L.-P., Zhang L., Ma H., Jia P., Liu J., Zhong M., Guo Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012;39:969–987. doi: 10.1007/s11033-011-0823-1. [DOI] [PubMed] [Google Scholar]
- 202.Liland K.H. Multivariate methods in metabolomics—From pre-processing to dimension reduction and statistical analysis. Trends Anal. Chem. 2011;30:827–841. [Google Scholar]
- 203.Stenlund H., Gorzsas A., Persson P., Sundberg B., Trygg J. Orthogonal projections to latent structures discriminant analysis modeling on in situ FT-IR spectral imaging of liver tissue for identifying sources of variability. Anal. Chem. 2008;80:6898–6906. doi: 10.1021/ac8005318. [DOI] [PubMed] [Google Scholar]
- 204.Trygg J., Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemom. 2002;16:119–128. [Google Scholar]
- 205.Kopka J., Schauer N., Krueger S., Birkemeyer C., Usadel B., Bergmüller E., Dörmann P., Weckwerth W., Gibon Y., Stitt M., et al. GMD@CSB.DB: The golm metabolome database. Bioinformatics. 2005;21:1635–1638. doi: 10.1093/bioinformatics/bti236. [DOI] [PubMed] [Google Scholar]
- 206.Fahy E., Sud M., Cotter D., Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35:W606–W612. doi: 10.1093/nar/gkm324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cui Q., Lewis I.A., Hegeman A.D., Anderson M.E., Li J., Schulte C.F., Westler W.M., Eghbalnia H.R., Sussman M.R., Markley J.L. Metabolite identification via the Madison Metabolomics Consortium Database. Nat. Biotechnol. 2008;26:162–164. doi: 10.1038/nbt0208-162. [DOI] [PubMed] [Google Scholar]
- 208.Brown M., Dunn W.B., Dobson P., Patel Y., Winder C.L., Francis-McIntyre S., Begley P., Carroll K., Broadhurst D., Tseng A., et al. Mass spectrometry tools and metabolite-specific databases for molecular identification in metabolomics. Analyst. 2009;134:1322–1332. doi: 10.1039/b901179j. [DOI] [PubMed] [Google Scholar]
- 209.Horai H., Arita M., Kanaya S., Nihei Y., Ikeda T., Suwa K., Ojima Y., Tanaka K., Tanaka S., Aoshima K., et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010;45:703–714. doi: 10.1002/jms.1777. [DOI] [PubMed] [Google Scholar]
- 210.Carroll A., Badger M., Harvey Millar A. The MetabolomeExpress Project: Enabling web-based processing, analysis and transparent dissemination of GC/MS metabolomics datasets. BMC Bioinforma. 2010;11:376. doi: 10.1186/1471-2105-11-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tautenhahn R., Cho K., Uritboonthai W., Zhu Z., Patti G.J., Siuzdak G. An accelerated workflow for untargeted metabolomics using the METLIN database. Nat. Biotechnol. 2012;30:826–828. doi: 10.1038/nbt.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang F., Robinette S.L., Bruschweiler-Li L., Brüschweiler R. Web server suite for complex mixture analysis by covariance NMR. Magn. Reson. Chem. 2009;47:S118–S122. doi: 10.1002/mrc.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Biswas A., Mynampati K.C., Umashankar S., Reuben S., Parab G., Rao R., Kannan V.S., Swarup S. MetDAT: A modular and workflow-based free online pipeline for mass spectrometry data processing, analysis and interpretation. Bioinformatics. 2010;26:2639–2640. doi: 10.1093/bioinformatics/btq436. [DOI] [PubMed] [Google Scholar]
- 214.Zhou B., Wang J., Ressom H.W. MetaboSearch: Tool for mass-based metabolite identification using multiple databases. PLoS One. 2012;7:e40096. doi: 10.1371/journal.pone.0040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gavaghan C.L., Li J.V., Hadfield S.T., Hole S., Nicholson J.K., Wilson I.D., Howe P.W.A., Stanley P.D., Holmes E. Application of NMR-based Metabolomics to the Investigation of Salt Stress in Maize (Zea mays) Phytochem. Anal. 2011;22:214–224. doi: 10.1002/pca.1268. [DOI] [PubMed] [Google Scholar]
- 216.Widodo Patterson J.H., Newbigin E., Tester M., Bacic A., Roessner U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009;60:4089–4103. doi: 10.1093/jxb/erp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wu J., Yu H., Dai H., Mei W., Huang X., Zhu S., Peng M. Metabolite profiles of rice cultivars containing bacterial blight-resistant genes are distinctive from susceptible rice. Acta Biochim. Biophys. Sin. 2012;44:650–659. doi: 10.1093/abbs/gms043. [DOI] [PubMed] [Google Scholar]
- 218.Levi A., Paterson A.H., Cakmak I., Saranga Y. Metabolite and mineral analyses of cotton near-isogenic lines introgressed with QTLs for productivity and drought-related traits. Physiol. Plant. 2011;141:265–275. doi: 10.1111/j.1399-3054.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 219.Semel Y., Schauer N., Roessner U., Zamir D., Fernie A.R. Metabolite analysis for the comparison of irrigated and non-irrigated field grown tomato of varying genotype. Metabolomics. 2007;3:289–295. [Google Scholar]
- 220.Silvente S., Sobolev A.P., Lara M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS One. 2012;7:e38554. doi: 10.1371/journal.pone.0038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Witt S., Galicia L., Lisec J., Cairns J., Tiessen A., Luis Araus J., Palacios-Rojas N., Fernie A.R. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol. Plant. 2012;5:401–417. doi: 10.1093/mp/ssr102. [DOI] [PubMed] [Google Scholar]
- 222.Komatsu S., Yamamoto A., Nakamura T., Nouri M.-Z., Nanjo Y., Nishizawa K., Furukawa K. Comprehensive analysis of mitochondria in roots and hypocotyls of soybean under flooding stress using proteomics and metabolomics techniques. J. Proteome Res. 2011;10:3993–4004. doi: 10.1021/pr2001918. [DOI] [PubMed] [Google Scholar]
- 223.Cho K., Shibato J., Agrawal G.K., Jung Y.-H., Kubo A., Jwa N.-S., Tamogami S., Satoh K., Kikuchi S., Higashi T., et al. Integrated transcriptomics, proteomics, and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J. Proteome Res. 2008;7:2980–2998. doi: 10.1021/pr800128q. [DOI] [PubMed] [Google Scholar]
- 224.Aliferis K.A., Jabaji S. FT-ICR/MS and GC-EI/MS metabolomics networking unravels global potato sprout’s responses to Rhizoctonia solani infection. PLoS One. 2012;7:e42576. doi: 10.1371/journal.pone.0042576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Figueiredo A., Fortes A.M., Ferreira S., Sebastiana M., Choi Y.H., Sousa L., Acioli-Santos B., Pessoa F., Verpoorte R., Pais M.S. Transcriptional and metabolic profiling of grape (Vitis vinifera L.) leaves unravel possible innate resistance against pathogenic fungi. J. Exp. Bot. 2008;59:3371–3381. doi: 10.1093/jxb/ern187. [DOI] [PubMed] [Google Scholar]
- 226.Hong Y.-S., Martinez A., Liger-Belair G., Jeandet P., Nuzillard J.-M., Cilindre C. Metabolomics reveals simultaneous influences of plant defence system and fungal growth in Botrytis cinerea-infected Vitis vinifera cv. Chardonnay berries. J. Exp. Bot. 2012;63:5773–5785. doi: 10.1093/jxb/ers228. [DOI] [PubMed] [Google Scholar]
- 227.Cevallos-Cevallos J.M., Futch D.B., Shilts T., Folimonova S.Y., Reyes-De-Corcuera J.I. GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol. Biochem. 2012;53:69–76. doi: 10.1016/j.plaphy.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 228.Ali K., Maltese F., Figueiredo A., Rex M., Fortes A.M., Zyprian E., Pais M.S., Verpoorte R., Choi Y.H. Alterations in grapevine leaf metabolism upon inoculation with Plasmopara viticola in different time-points. Plant Sci. 2012;191:100–107. doi: 10.1016/j.plantsci.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 229.Fumagalli E., Baldoni E., Abbruscato P., Piffanelli P., Genga A., Lamanna R., Consonni R. NMR techniques coupled with multivariate statistical analysis: Tools to analyse Oryza sativa metabolic content under stress conditions. J. Agron. Crop Sci. 2009;195:77–88. [Google Scholar]
- 230.Rose M.T., Rose T.J., Pariasca-Tanaka J., Yoshihashi T., Neuweger H., Goesmann A., Frei M., Wissuwa M. Root metabolic response of rice (Oryza sativa L.) genotypes with contrasting tolerance to zinc deficiency and bicarbonate excess. Planta. 2012;236:959–973. doi: 10.1007/s00425-012-1648-4. [DOI] [PubMed] [Google Scholar]
- 231.Shulaev V. Metabolomics technology and bioinformatics. Brief. Bioinforma. 2006;7:128–139. doi: 10.1093/bib/bbl012. [DOI] [PubMed] [Google Scholar]
- 232.Wu W., Zhang Q., Zhu Y., Lam H.-M., Cai Z., Guo D. Comparative metabolic profiling reveals secondary metabolites correlated with soybean salt tolerance. J. Agric. Food Chem. 2008;56:11132–11138. doi: 10.1021/jf8024024. [DOI] [PubMed] [Google Scholar]
- 233.Johnson H.E., Broadhurst D., Goodacre R., Smith A.R. Metabolic fingerprinting of salt-stressed tomatoes. Phytochemistry. 2003;62:919–928. doi: 10.1016/s0031-9422(02)00722-7. [DOI] [PubMed] [Google Scholar]
- 234.Liu L., Li Y.H., Li S.L., Hu N., He Y.M., Pong R., Lin D.N., Lu L.H., Law M. Comparison of next-generation sequencing systems. J. Biomed. Biotechnol. 2012 doi: 10.1155/2012/251364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kao H.-L., Gunsalus K.C. Current Protocols in Bioinformatics. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2002. Browsing Multidimensional Molecular Networks with the Generic Network Browser (N-Browse) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Smoot M.E., Ono K., Ruscheinski J., Wang P.-L., Ideker T. Cytoscape 2.8: New features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Katari M.S., Nowicki S.D., Aceituno F.F., Nero D., Kelfer J., Thompson L.P., Cabello J.M., Davidson R.S., Goldberg A.P., Shasha D.E., et al. VirtualPlant: A software platform to support systems biology research. Plant Physiol. 2010;152:500–515. doi: 10.1104/pp.109.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jami S.K., Clark G.B., Ayele B.T., Ashe P., Kirti P.B. Genome-wide comparative analysis of annexin superfamily in plants. PLoS One. 2012;7:e47801. doi: 10.1371/journal.pone.0047801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wan H., Yuan W., Bo K., Shen J., Pang X., Chen J. Genome-wide analysis of NBS-encoding disease resistance genes in Cucumis sativus and phylogenetic study of NBS-encoding genes in Cucurbitaceae crops. BMC Genomics. 2013;14:109. doi: 10.1186/1471-2164-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Hu L., Liu S. Genome-wide identification and phylogenetic analysis of the ERF gene family in cucumbers. Genet. Mol. Biol. 2011;34:624–633. doi: 10.1590/S1415-47572011005000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li Q., Zhang C., Li J., Wang L., Ren Z. Genome-wide identification and characterization of R2R3MYB family in Cucumis sativus. PLoS One. 2012;7:e47576. doi: 10.1371/journal.pone.0047576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ling J., Jiang W., Zhang Y., Yu H., Mao Z., Gu X., Huang S., Xie B. Genome-wide analysis of WRKY gene family in Cucumis sativus. BMC Genomics. 2011;12:471. doi: 10.1186/1471-2164-12-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kang Y., Kim K., Shim S., Yoon M., Sun S., Kim M., Van K., Lee S.-H. Genome-wide mapping of NBS-LRR genes and their association with disease resistance in soybean. BMC Plant Biol. 2012;12:139. doi: 10.1186/1471-2229-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Du H., Yang S.-S., Liang Z., Feng B.-R., Liu L., Huang Y.-B., Tang Y.-X. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biol. 2012;12:106. doi: 10.1186/1471-2229-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dung Tien L., Nishiyama R., Watanabe Y., Mochida K., Yamaguchi-Shinozaki K., Shinozaki K., Lam-Son Phan T. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011;18:263–276. doi: 10.1093/dnares/dsr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Osorio M.B., Buecker-Neto L., Castilhos G., Turchetto-Zolet A.C., Wiebke-Strohm B., Bodanese-Zanettini M.H., Margis-Pinheiro M. Identification and in silico characterization of soybean trihelix-GT and bHLH transcription factors involved in stress responses. Genet. Mol. Biol. 2012;35:233–246. doi: 10.1590/S1415-47572012000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tran L.-S.P., Quach T.N., Guttikonda S.K., Aldrich D.L., Kumar R., Neelakandan A., Valliyodan B., Nguyen H.T. Molecular characterization of stress-inducible GmNAC genes in soybean. Mol. Genet. Genomics. 2009;281:647–664. doi: 10.1007/s00438-009-0436-8. [DOI] [PubMed] [Google Scholar]
- 248.Zhou Q.-Y., Tian A.-G., Zou H.-F., Xie Z.-M., Lei G., Huang J., Wang C.-M., Wang H.-W., Zhang J.-S., Chen S.-Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008;6:486–503. doi: 10.1111/j.1467-7652.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 249.Liang D., Xia H., Wu S., Ma F. Genome-wide identification and expression profiling of dehydrin gene family in Malus domestica. Mol. Biol. Rep. 2012;39:10759–10768. doi: 10.1007/s11033-012-1968-2. [DOI] [PubMed] [Google Scholar]
- 250.Zhao T., Liang D., Wang P., Liu J., Ma F. Genome-wide analysis and expression profiling of the DREB transcription factor gene family in Malus under abiotic stress. Mol. Genet. Genomics. 2012;287:423–436. doi: 10.1007/s00438-012-0687-7. [DOI] [PubMed] [Google Scholar]
- 251.Agalou A., Purwantomo S., Oevernaes E., Johannesson H., Zhu X., Estiati A., de Kam R.J., Engstroem P., Slamet-Loedin I.H., Zhu Z., et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol. Biol. 2008;66:87–103. doi: 10.1007/s11103-007-9255-7. [DOI] [PubMed] [Google Scholar]
- 252.Agarwal P., Arora R., Ray S., Singh A.K., Singh V.P., Takatsuji H., Kapoor S., Tyagi A.K. Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol. Biol. 2007;65:467–485. doi: 10.1007/s11103-007-9199-y. [DOI] [PubMed] [Google Scholar]
- 253.Amrutha R.N., Sekhar P.N., Varshney R.K., Kishor P.B.K. Genome-wide analysis and identification of genes related to potassium transporter families in rice (Oryza sativa L.) Plant Sci. 2007;172:708–721. [Google Scholar]
- 254.Chen R., Jiang Y., Dong J., Zhang X., Xiao H., Xu Z., Gao X. Genome-wide analysis and environmental response profiling of SOT family genes in rice (Oryza sativa) Genes Genomics. 2012;34:549–560. [Google Scholar]
- 255.Ding X., Hou X., Xie K., Xiong L. Genome-wide identification of BURP domain-containing genes in rice reveals a gene family with diverse structures and responses to abiotic stresses. Planta. 2009;230:149–163. doi: 10.1007/s00425-009-0929-z. [DOI] [PubMed] [Google Scholar]
- 256.Gollan P.J., Bhave M. Genome-wide analysis of genes encoding FK506-binding proteins in rice. Plant Mol. Biol. 2010;72:1–16. doi: 10.1007/s11103-009-9547-1. [DOI] [PubMed] [Google Scholar]
- 257.Huang J., Zhao X., Yu H., Ouyang Y., Wang L., Zhang Q. The ankyrin repeat gene family in rice: Genome-wide identification, classification and expression profiling. Plant Mol. Biol. 2009;71:207–226. doi: 10.1007/s11103-009-9518-6. [DOI] [PubMed] [Google Scholar]
- 258.Jain M., Tyagi A.K., Khurana J.P. Genome-wide identification, classification, evolutionary expansion and expression analyses of homeobox genes in rice. FEBS J. 2008;275:2845–2861. doi: 10.1111/j.1742-4658.2008.06424.x. [DOI] [PubMed] [Google Scholar]
- 259.Jiang S.-Y., Ramamoorthy R., Bhalla R., Luan H.-F., Venkatesh P.N., Cai M., Ramachandran S. Genome-wide survey of the RIP domain family in Oryza sativa and their expression profiles under various abiotic and biotic stresses. Plant Mol. Biol. 2008;67:603–614. doi: 10.1007/s11103-008-9342-4. [DOI] [PubMed] [Google Scholar]
- 260.Nuruzzaman M., Manimekalai R., Sharoni A.M., Satoh K., Kondoh H., Ooka H., Kikuchi S. Genome-wide analysis of NAC transcription factor family in rice. Gene. 2010;465:30–44. doi: 10.1016/j.gene.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 261.Nuruzzaman M., Sharoni A.M., Satoh K., Al-Shammari T., Shimizu T., Sasaya T., Omura T., Kikuchi S. The thioredoxin gene family in rice: Genome-wide identification and expression profiling under different biotic and abiotic treatments. Biochem. Biophys. Res. Commun. 2012;423:417–423. doi: 10.1016/j.bbrc.2012.05.142. [DOI] [PubMed] [Google Scholar]
- 262.Ouyang Y., Chen J., Xie W., Wang L., Zhang Q. Comprehensive sequence and expression profile analysis of Hsp20 gene family in rice. Plant. Mol. Biol. 2009;70:341–357. doi: 10.1007/s11103-009-9477-y. [DOI] [PubMed] [Google Scholar]
- 263.Vij S., Giri J., Dansana P.K., Kapoor S., Tyagi A.K. The receptor-like cytoplasmic kinase (OsRLCK) gene family in rice: Organization, phylogenetic relationship, and expression during development and stress. Mol. Plant. 2008;1:732–750. doi: 10.1093/mp/ssn047. [DOI] [PubMed] [Google Scholar]
- 264.Wang D., Guo Y., Wu C., Yang G., Li Y., Zheng C. Genome-wide analysis of CCCH zinc finger family in Arabidopsis and rice. BMC Genomics. 2008;9:44. doi: 10.1186/1471-2164-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhao H., Ma H., Yu L., Wang X., Zhao J. Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.) PLoS One. 2012;7:e49210. doi: 10.1371/journal.pone.0049210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wu J., Peng Z., Liu S., He Y., Cheng L., Kong F., Wang J., Lu G. Genome-wide analysis of Aux/IAA gene family in Solanaceae species using tomato as a model. Mol. Genet. Genomics. 2012;287:295–311. doi: 10.1007/s00438-012-0675-y. [DOI] [PubMed] [Google Scholar]
- 267.Bai M., Yang G.-S., Chen W.-T., Mao Z.-C., Kang H.-X., Chen G.-H., Yang Y.-H., Xie B.-Y. Genome-wide identification of Dicer-like, Argonaute and RNA-dependent RNA polymerase gene families and their expression analyses in response to viral infection and abiotic stresses in Solanum lycopersicum. Gene. 2012;501:52–62. doi: 10.1016/j.gene.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 268.Huang S., Gao Y., Liu J., Peng X., Niu X., Fei Z., Cao S., Liu Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genomics. 2012;287:495–513. doi: 10.1007/s00438-012-0696-6. [DOI] [PubMed] [Google Scholar]
- 269.Kong F., Wang J., Cheng L., Liu S., Wu J., Peng Z., Lu G. Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum. Gene. 2012;499:108–120. doi: 10.1016/j.gene.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 270.Gan D., Jiang H., Zhang J., Zhao Y., Zhu S., Cheng B. Genome-wide analysis of BURP domain-containing genes in Maize and Sorghum. Mol. Biol. Rep. 2011;38:4553–4563. doi: 10.1007/s11033-010-0587-z. [DOI] [PubMed] [Google Scholar]
- 271.Vannozzi A., Dry I.B., Fasoli M., Zenoni S., Lucchin M. Genome-wide analysis of the grapevine stilbene synthase multigenic family: Genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 2012;12:130. doi: 10.1186/1471-2229-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhuang J., Peng R.-H., Cheng Z.-M., Zhang J., Cai B., Zhang Z., Gao F., Zhu B., Fu X.-Y., Jin X.-F., et al. Genome-wide analysis of the putative AP2/ERF family genes in Vitis vinifera. Sci. Hortic. 2009;123:73–81. [Google Scholar]
- 273.Cheng Y., Li X., Jiang H., Ma W., Miao W., Yamada T., Zhang M. Systematic analysis and comparison of nucleotide-binding site disease resistance genes in maize. FEBS J. 2012;279:2431–2443. doi: 10.1111/j.1742-4658.2012.08621.x. [DOI] [PubMed] [Google Scholar]
- 274.Gomez-Anduro G., Adriana Ceniceros-Ojeda E., Edith Casados-Vazquez L., Bencivenni C., Sierra-Beltran A., Murillo-Amador B., Tiessen A. Genome-wide analysis of the beta-glucosidase gene family in maize (Zea mays L. var B73) Plant Mol. Biol. 2011;77:159–183. doi: 10.1007/s11103-011-9800-2. [DOI] [PubMed] [Google Scholar]
- 275.Lin Y.-X., Jiang H.-Y., Chu Z.-X., Tang X.-L., Zhu S.-W., Cheng B.-J. Genome-wide identification, classification and analysis of heat shock transcription factor family in maize. BMC Genomics. 2011;12:76. doi: 10.1186/1471-2164-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Peng X., Zhao Y., Cao J., Zhang W., Jiang H., Li X., Ma Q., Zhu S., Cheng B. CCCH-type zinc finger family in Maize: Genome-wide identification, classification and expression profiling under abscisic acid and drought treatments. PLoS One. 2012;7:e40120. doi: 10.1371/journal.pone.0040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang W.W., Ma Q., Xiang Y., Zhu S.W., Cheng B.J. Genome-wide analysis of immunophilin FKBP genes and expression patterns in Zea mays. Genet. Mol. Res. 2012;11:1690–1700. doi: 10.4238/2012.June.25.2. [DOI] [PubMed] [Google Scholar]
- 278.Wei K.-F., Chen J., Chen Y.-F., Wu L.-J., Xie D.-X. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in Maize. DNA Res. 2012;19:153–164. doi: 10.1093/dnares/dsr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhang Z., Zhang J., Chen Y., Li R., Wang H., Wei J. Genome-wide analysis and identification of HAK potassium transporter gene family in maize (Zea mays L.) Mol. Biol. Rep. 2012;39:8465–8473. doi: 10.1007/s11033-012-1700-2. [DOI] [PubMed] [Google Scholar]
- 280.Zhou M.-L., Zhang Q., Zhou M., Sun Z.-M., Zhu X.-M., Shao J.-R., Tang Y.-X., Wu Y.-M. Genome-wide identification of genes involved in raffinose metabolism in Maize. Glycobiology. 2012;22:1775–1785. doi: 10.1093/glycob/cws121. [DOI] [PubMed] [Google Scholar]
- 281.Wendelboe-Nelson C., Morris P.C. Proteins linked to drought tolerance revealed by DIGE analysis of drought resistant and susceptible barley varieties. Proteomics. 2012;12:3374–3385. doi: 10.1002/pmic.201200154. [DOI] [PubMed] [Google Scholar]
- 282.Fatehi F., Hosseinzadeh A., Alizadeh H., Brimavandi T., Struik P.C. The proteome response of salt-resistant and salt-sensitive barley genotypes to long-term salinity stress. Mol. Biol. Rep. 2012;39:6387–6397. doi: 10.1007/s11033-012-1460-z. [DOI] [PubMed] [Google Scholar]
- 283.Cheng Z.Y., Woody O.Z., McConkey B.J., Glick B.R. Combined effects of the plant growth-promoting bacterium Pseudomonas putida UW4 and salinity stress on the Brassica napus proteome. Appl. Soil Ecol. 2012;61:255–263. [Google Scholar]
- 284.Louarn S., Nawrocki A., Edelenbos M., Jensen D.F., Jensen O.N., Collinge D.B., Jensen B. The influence of the fungal pathogen Mycocentrospora acerina on the proteome and polyacetylenes and 6-methoxymellein in organic and conventionally cultivated carrots (Daucus carota) during post harvest storage. J. Proteomics. 2012;75:962–977. doi: 10.1016/j.jprot.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 285.Deeba F., Pandey A.K., Ranjan S., Mishra A., Singh R., Sharma Y.K., Shirke P.A., Pandey V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochnol. 2012;53:6–18. doi: 10.1016/j.plaphy.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 286.Zheng M., Wang Y.H., Liu K., Shu H.M., Zhou Z.G. Protein expression changes during cotton fiber elongation in response to low temperature stress. J. Plant Physiol. 2012;169:399–409. doi: 10.1016/j.jplph.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 287.Wang Y.H., Zheng M., Gao X.B., Zhou Z.G. Protein differential expression in the elongating cotton (Gossypium hirsutum L.) fiber under nitrogen stress. Sci. China Life Sci. 2012;55:984–992. doi: 10.1007/s11427-012-4390-z. [DOI] [PubMed] [Google Scholar]
- 288.Li J., Sun J., Yang Y.J., Guo S.R., Glick B.R. Identification of hypoxic-responsive proteins in cucumber roots using a proteomic approach. Plant Physiol. Biochnol. 2012;51:74–80. doi: 10.1016/j.plaphy.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 289.Palmieri M.C., Perazzolli M., Matafora V., Moretto M., Bachi A., Pertot I. Proteomic analysis of grapevine resistance induced by Trichoderma harzianum T39 reveals specific defence pathways activated against downy mildew. J. Exp. Bot. 2012;63:6237–6251. doi: 10.1093/jxb/ers279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang Z., Zhao F.X., Zhao X., Ge H., Chai L.J., Chen S.W., Perl A., Ma H.Q. Proteomic analysis of berry-sizing effect of GA3 on seedless Vitis vinifera L. Proteomics. 2012;12:86–94. doi: 10.1002/pmic.201000668. [DOI] [PubMed] [Google Scholar]
- 291.Minas I.S., Tanou G., Belghazi M., Job D., Manganaris G.A., Molassiotis A., Vasilakakis M. Physiological and proteomic approaches to address the active role of ozone in kiwifruit post-harvest ripening. J. Exp. Bot. 2012;63:2449–2464. doi: 10.1093/jxb/err418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Huang H., Moller I.M., Song S.Q. Proteomics of desiccation tolerance during development and germination of maize embryos. J. Proteomics. 2012;75:1247–1262. doi: 10.1016/j.jprot.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 293.Benesova M., Hola D., Fischer L., Jedelsky P.L., Hnilicka F., Wilhelmova N., Rothova O., Kocova M., Prochazkova D., Honnerova J., et al. The physiology and proteomics of drought tolerance in Maize: Early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS One. 2012;7:e38017. doi: 10.1371/journal.pone.0038017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Fristedt R., Wasilewska W., Romanowska E., Vener A.V. Differential phosphorylation of thylakoid proteins in mesophyll and bundle sheath chloroplasts from maize plants grown under low or high light. Proteomics. 2012;12:2852–2861. doi: 10.1002/pmic.201200196. [DOI] [PubMed] [Google Scholar]
- 295.Muneer S., Kim T.H., Qureshi M.I. Fe modulates Cd-induced oxidative stress and the expression of stress responsive proteins in the nodules of Vigna radiata. Plant Growth Regul. 2012;68:421–433. [Google Scholar]
- 296.Rodrigues S.P., Ventura J.A., Aguilar C., Nakayasu E.S., Choi H., Sobreira T.J.P., Nohara L.L., Wermelinger L.S., Almeida I.C., Zingali R.B., et al. Label-free quantitative proteomics reveals differentially regulated proteins in the latex of sticky diseased Carica papaya L. plants. J. Proteomics. 2012;75:3191–3198. doi: 10.1016/j.jprot.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mohammadi P.P., Moieni A., Komatsu S. Comparative proteome analysis of drought-sensitive and drought-tolerant rapeseed roots and their hybrid F1 line under drought stress. Amino Acids. 2012;43:2137–2152. doi: 10.1007/s00726-012-1299-6. [DOI] [PubMed] [Google Scholar]
- 298.Zhu M.M., Dai S.J., Zhu N., Booy A., Simons B., Yi S., Chen S.X. Methyl jasmonate responsive proteins in Brassica napus guard cells revealed by iTRAQ-based quantitative proteomics. J. Proteome Res. 2012;11:3728–3742. doi: 10.1021/pr300213k. [DOI] [PubMed] [Google Scholar]
- 299.Chen J.H., Tian L., Xu H.F., Tian D.G., Luo Y.M., Ren C.M., Yang L.M., Shi J.S. Cold-induced changes of protein and phosphoprotein expression patterns from rice roots as revealed by multiplex proteomic analysis. Plant Omics. 2012;5:194–199. [Google Scholar]
- 300.Ji K.X., Wang Y.Y., Sun W.N., Lou Q.J., Mei H.W., Shen S.H., Chen H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012;169:336–344. doi: 10.1016/j.jplph.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 301.Mirzaei M., Soltani N., Sarhadi E., Pascovici D., Keighley T., Salekdeh G.H., Haynes P.A., Atwell B.J. Shotgun proteomic analysis of long-distance drought signaling in rice roots. J. Proteome Res. 2012;11:348–358. doi: 10.1021/pr2008779. [DOI] [PubMed] [Google Scholar]
- 302.Koga H., Dohi K., Nishiuchi T., Kato T., Takahara H., Mori M., Komatsu S. Proteomic analysis of susceptible rice plants expressing the whole plant-specific resistance against Magnaporthe oryzae: Involvement of a thaumatin-like protein. Physiol. Mol. Plant P. 2012;77:60–66. [Google Scholar]
- 303.Li Y.F., Zhang Z.H., Nie Y.F., Zhang L.H., Wang Z.Z. Proteomic analysis of salicylic acid-induced resistance to Magnaporthe oryzae in susceptible and resistant rice. Proteomics. 2012;12:2340–2354. doi: 10.1002/pmic.201200054. [DOI] [PubMed] [Google Scholar]
- 304.Hakeem K.R., Chandna R., Ahmad A., Qureshi M.I., Iqbal M. Proteomic analysis for low and high nitrogen-responsive proteins in the leaves of rice genotypes grown at three nitrogen levels. Appl. Biochem. Biotechnol. 2012;168:834–850. doi: 10.1007/s12010-012-9823-4. [DOI] [PubMed] [Google Scholar]
- 305.Sawada H., Komatsu S., Nanjo Y., Khan N.A., Kohno Y. Proteomic analysis of rice response involved in reduction of grain yield under elevated ozone stress. Environ. Exp. Bot. 2012;77:108–116. [Google Scholar]
- 306.Wang Y.D., Wang X., Wong Y.S. Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J. Proteomics. 2012;75:1849–1866. doi: 10.1016/j.jprot.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 307.Li D.X., Wang L.J., Teng S.L., Zhang G.G., Guo L.J., Mao Q., Wang W., Li M., Chen L. Proteomics analysis of rice proteins up-regulated in response to bacterial leaf streak disease. J. Plant Biol. 2012;55:316–324. [Google Scholar]
- 308.Ngara R., Ndimba R., Borch-Jensen J., Jensen O.N., Ndimba B. Identification and profiling of salinity stress-responsive proteins in Sorghum bicolor seedlings. J. Proteomics. 2012;75:4139–4150. doi: 10.1016/j.jprot.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 309.Hossain Z., Hajika M., Komatsu S. Comparative proteome analysis of high and low cadmium accumulating soybeans under cadmium stress. Amino Acids. 2012;43:2393–2416. doi: 10.1007/s00726-012-1319-6. [DOI] [PubMed] [Google Scholar]
- 310.Mohammadi P.P., Moieni A., Hiraga S., Komatsu S. Organ-specific proteomic analysis of drought-stressed soybean seedlings. J. Proteomics. 2012;75:1906–1923. doi: 10.1016/j.jprot.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 311.Salavati A., Khatoon A., Nanjo Y., Komatsu S. Analysis of proteomic changes in roots of soybean seedlings during recovery after flooding. J. Proteomics. 2012;75:878–893. doi: 10.1016/j.jprot.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 312.Yanagawa Y., Komatsu S. Ubiquitin/proteasome-mediated proteolysis is involved in the response to flooding stress in soybean roots, independent of oxygen limitation. Plant Sci. 2012;185:250–258. doi: 10.1016/j.plantsci.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 313.Khatoon A., Rehman S., Salavati A., Komatsu S. A comparative proteomics analysis in roots of soybean to compatible symbiotic bacteria under flooding stress. Amino Acids. 2012;43:2513–2525. doi: 10.1007/s00726-012-1333-8. [DOI] [PubMed] [Google Scholar]
- 314.Wang L.Q., Ma H., Song L.R., Shu Y.J., Gu W.H. Comparative proteomics analysis reveals the mechanism of pre-harvest seed deterioration of soybean under high temperature and humidity stress. J. Proteomics. 2012;75:2109–2127. doi: 10.1016/j.jprot.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 315.Wang Y., Yuan X.Z., Hu H., Liu Y., Sun W.H., Shan Z.H., Zhou X.A. Proteomic analysis of differentially expressed proteins in resistant soybean leaves after Phakopsora pachyrhizi infection. J. Phytopathol. 2012;160:554–560. [Google Scholar]
- 316.Ma H., Song L., Shu Y., Wang S., Niu J., Wang Z., Yu T., Gu W., Ma H. Comparative proteomic analysis of seedling leaves of different salt tolerant soybean genotypes. J. Proteomics. 2012;75:1529–1546. doi: 10.1016/j.jprot.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 317.Khatoon A., Rehman S., Hiraga S., Makino T., Komatsu S. Organ-specific proteomics analysis for identification of response mechanism in soybean seedlings under flooding stress. J. Proteomics. 2012;75:5706–5723. doi: 10.1016/j.jprot.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 318.Nanjo Y., Skultety L., Uvackova L., Kubicova K., Hajduch M., Komatsu S. Mass spectrometry-based analysis of proteomic changes in the root tips of flooded soybean seedlings. J. Proteome Res. 2012;11:372–385. doi: 10.1021/pr200701y. [DOI] [PubMed] [Google Scholar]
- 319.Khatoon A., Rehman S., Oh M.W., Woo S.H., Komatsu S. Analysis of response mechanism in soybean under low oxygen and flooding stresses using gel-base proteomics technique. Mol. Biol. Rep. 2012;39:10581–10594. doi: 10.1007/s11033-012-1946-8. [DOI] [PubMed] [Google Scholar]
- 320.Koehler G., Wilson R.C., Goodpaster J.V., Sonsteby A., Lai X., Witzmann F.A., You J.S., Rohloff J., Randall S.K., Alsheikh M. Proteomic study of low-temperature responses in strawberry cultivars (Fragaria x ananassa) that differ in cold tolerance. Plant Physiol. 2012;159:1787–1805. doi: 10.1104/pp.112.198267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fang X.P., Chen W.Y., Xin Y., Zhang H.M., Yan C.Q., Yu H., Liu H., Xiao W.F., Wang S.Z., Zheng G.Z., et al. Proteomic analysis of strawberry leaves infected with Colletotrichum fragariae. J. Proteomics. 2012;75:4074–4090. doi: 10.1016/j.jprot.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 322.Zhou G., Yang L.T., Li Y.R., Zou C.L., Huang L.P., Qiu L.H., Huang X., Srivastava M.K. Proteomic analysis of osmotic stress-responsive proteins in sugarcane leaves. Plant Mol. Biol. Rep. 2012;30:349–359. [Google Scholar]
- 323.Shah P., Powell A.L.T., Orlando R., Bergmann C., Gutierrez-Sanchez G. Proteomic analysis of ripening tomato fruit infected by Botrytis cinerea. J. Proteome Res. 2012;11:2178–2192. doi: 10.1021/pr200965c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ge P., Ma C., Wang S., Gao L., Li X., Guo G., Ma W., Yan Y. Comparative proteomic analysis of grain development in two spring wheat varieties under drought stress. Anal. Bioanal. Chem. 2012;402:1297–1313. doi: 10.1007/s00216-011-5532-z. [DOI] [PubMed] [Google Scholar]
- 325.Kang G.Z., Li G.Z., Xu W., Peng X.Q., Han Q.X., Zhu Y.J., Guo T.C. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J. Proteome Res. 2012;11:6066–6079. doi: 10.1021/pr300728y. [DOI] [PubMed] [Google Scholar]
- 326.Vitamvas P., Prasil I.T., Kosova K., Planchon S., Renaut J. Analysis of proteome and frost tolerance in chromosome 5A and 5B reciprocal substitution lines between two winter wheats during long-term cold acclimation. Proteomics. 2012;12:68–85. doi: 10.1002/pmic.201000779. [DOI] [PubMed] [Google Scholar]
- 327.Gunnaiah R., Kushalappa A.C., Duggavathi R., Fox S., Somers D.J. Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL (Fhb1) contributes to resistance against Fusarium graminearum. PLoS One. 2012;7:e40695. doi: 10.1371/journal.pone.0040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ravalason H., Grisel S., Chevret D., Favel A., Berrin J.G., Sigoillot J.C., Herpoel-Gimbert I. Fusarium verticillioides secretome as a source of auxiliary enzymes to enhance saccharification of wheat straw. Bioresour. Technol. 2012;114:589–596. doi: 10.1016/j.biortech.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 329.Kang G.Z., Li G.Z., Zheng B.B., Han Q.X., Wang C.Y., Zhu Y.J., Guo T.C. Proteomic analysis on salicylic acid-induced salt tolerance in common wheat seedlings (Triticum aestivum L.) Biochim. Biophys. Acta. 2012;1824:1324–1333. doi: 10.1016/j.bbapap.2012.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.