Abstract
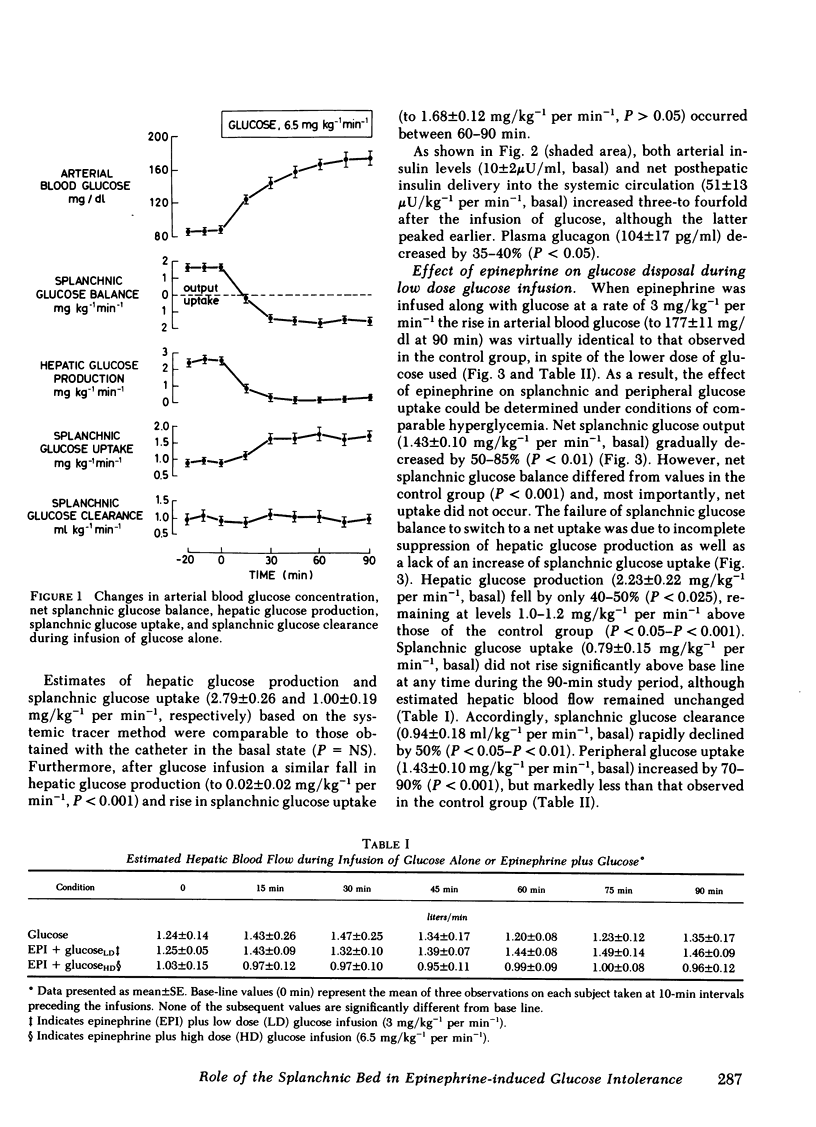
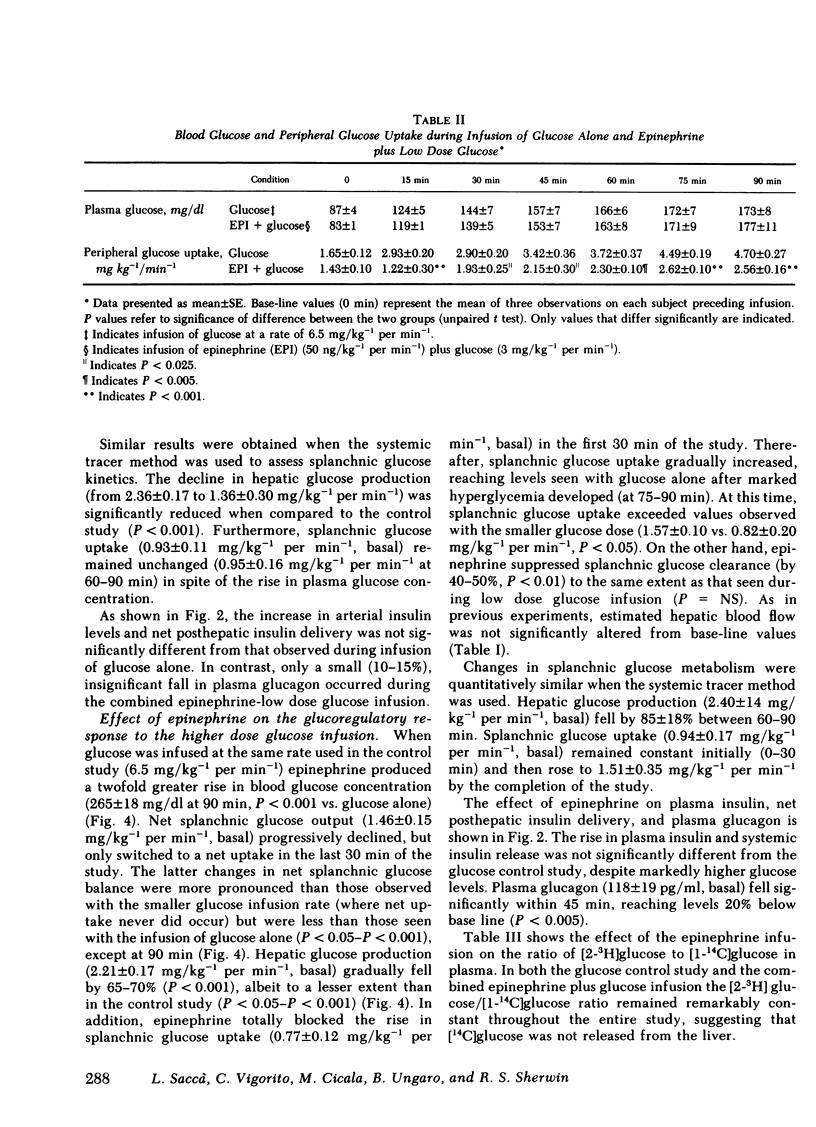
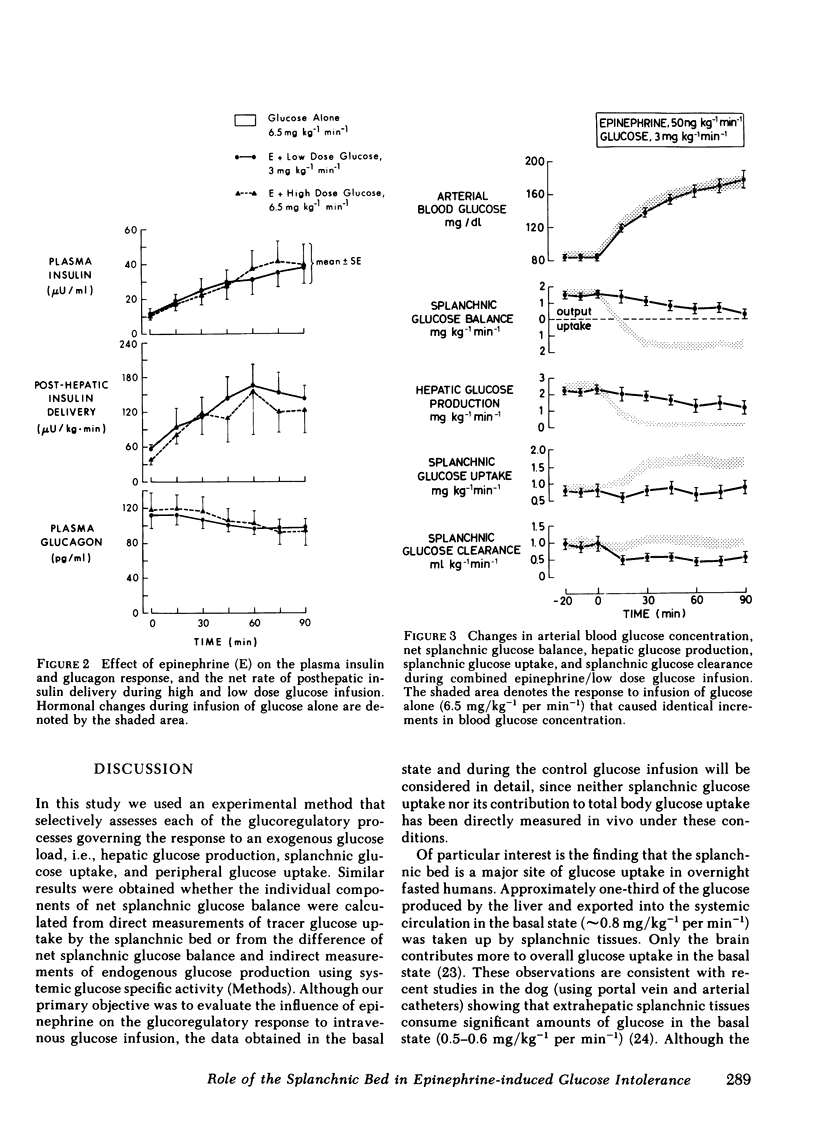
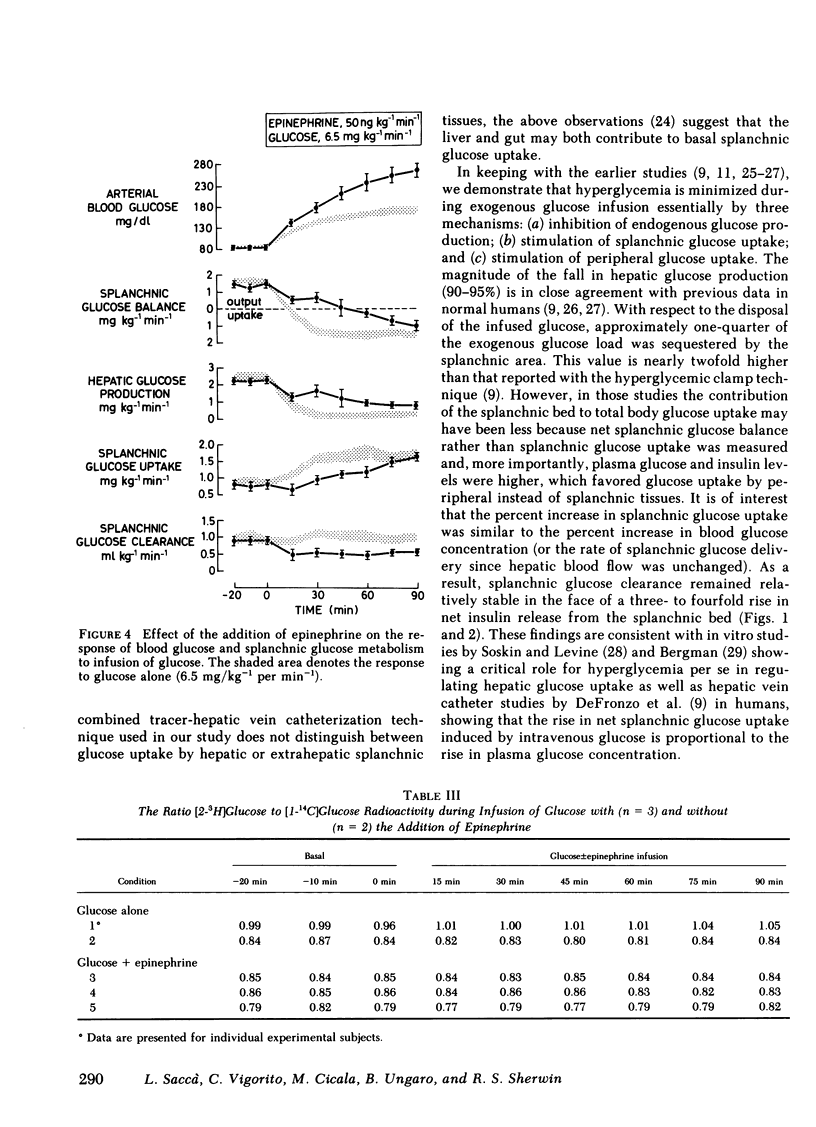
To evaluate the role of the splanchnic bed in epinephrine-induced glucose intolerance, we selectively assessed the components of net splanchnic glucose balance, i.e., splanchnic glucose uptake and hepatic glucose production, and peripheral glucose uptake by combining infusion of [3-3H]glucose with hepatic vein catheterization. Normal humans received a 90-min infusion of either glucose alone (6.5 mg/kg−1 per min−1) or epinephrine plus glucose at two dose levels: (a) in amounts that simulated the hyperglycemia seen with glucose alone (3.0 mg/kg−1 per min−1); and (b) in amounts identical to the control study. During infusion of glucose alone, blood glucose rose twofold, insulin levels and net posthepatic insulin release increased three- to fourfold, and net splanchnic glucose output switched from a net output (1.65±0.12 mg/kg−1 per min−1) to a net uptake (1.56±0.18). This was due to a 90-95% fall (P < 0.001) in hepatic glucose production and a 100% rise (P < 0.001) in splanchnic glucose uptake (from 0.86±0.14 to 1.71±0.12 mg/kg−1 per min−1), which in the basal state amounted to 30-35% of total glucose uptake. Peripheral glucose uptake rose by 170-185% (P < 0.001). When epinephrine was combined with the lower glucose dose, blood glucose, insulin release, and hepatic blood flow were no different from values observed with glucose alone. However, hepatic glucose production fell only 40-45% (P < 0.05 vs. glucose alone) and, most importantly, the rise in splanchnic glucose uptake was totally blocked. As a result, splanchnic glucose clearance fell by 50% (P < 0.05), and net splanchnic glucose uptake did not occur. The rise in peripheral glucose uptake was also reduced by 50-60% (P < 0.001). When epinephrine was added to the same dose of glucose used in the control study, blood glucose rose twofold higher (P < 0.001). The initial rise in splanchnic glucose uptake was totally prevented; however, beyond 30 min, splanchnic glucose uptake increased, reaching levels seen in the control study when severe hyperglycemia occurred. Splanchnic glucose clearance, nevertheless, remained suppressed throughout the entire study (40%-50%, P < 0.01).
It is concluded that (a) the splanchnic bed accounts for one-third of total body glucose uptake in the basal state in normal humans; (b) epinephrine markedly inhibits the rise in splanchnic glucose uptake induced by infusion of glucose; and (c) this effect does not require a fall in insulin and is modulated by the level of hyperglycemia. Our data indicate that the splanchnic bed is an important site of glucose uptake in post-absorptive humans and that epinephrine impairs glucose tolerance by suppressing glucose uptake by both splanchnic and peripheral tissues, as well as by its well known stimulatory effect on endogenous glucose production.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP J. S., STEELE R., ALTSZULER N., DUNN A., BJERKNES C., DEBODO R. C. EFFECTS OF INSULIN ON LIVER GLYCOGEN SYNTHESIS AND BREAKDOWN IN THE DOG. Am J Physiol. 1965 Feb;208:307–316. doi: 10.1152/ajplegacy.1965.208.2.307. [DOI] [PubMed] [Google Scholar]
- Bergman R. N. Integrated control of hepatic glucose metabolism. Fed Proc. 1977 Feb;36(2):265–270. [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Harris M. S. Relationship between the plasma glucose level and glucose uptake in the conscious dog. Metabolism. 1978 Jul;27(7):787–791. doi: 10.1016/0026-0495(78)90213-5. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibert D. C., DeFronzo R. A. Epinephrine-induced insulin resistance in man. J Clin Invest. 1980 Mar;65(3):717–721. doi: 10.1172/JCI109718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975 May;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hamburg S., Hendler R., Sherwin R. S. Influence of small increments of epinephrine on glucose tolerance in normal humans. Ann Intern Med. 1980 Oct;93(4):566–568. doi: 10.7326/0003-4819-93-4-566. [DOI] [PubMed] [Google Scholar]
- Holst J. J., Madsen O. G., Knop J., Schmidt A. The effect of intraportal and peripheral infusions of glucagon on insulin and glucose concentrations and glucose tolerance in normal man. Diabetologia. 1977 Sep;13(5):487–490. doi: 10.1007/BF01234501. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- LEEVY C. M., MENDENHALL C. L., LESKO W., HOWARD M. M. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962 May;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Rabin D. Lack of a role for glucagon in the disposal of an oral glucose load in normal man. J Clin Endocrinol Metab. 1979 Dec;49(6):937–939. doi: 10.1210/jcem-49-6-937. [DOI] [PubMed] [Google Scholar]
- Radziuk J., McDonald T. J., Rubenstein D., Dupre J. Initial splanchnic extraction of ingested glucose in normal man. Metabolism. 1978 Jun;27(6):657–669. doi: 10.1016/0026-0495(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R., Haymond M., Cryer P., Gerich J. Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol. 1979 Oct;237(4):E356–E362. doi: 10.1152/ajpendo.1979.237.4.E356. [DOI] [PubMed] [Google Scholar]
- SOMOGYI M. Studies of arteriovenous differences in blood sugar. V. Effect of epinephrine on the rate of glucose assimilation. J Biol Chem. 1950 Oct;186(2):513–526. [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Saccà L., Cicala M., Corso G., Ungaro B., Sherwin R. S. Effect of counterregulatory hormones on kinetic response to ingested glucose in dogs. Am J Physiol. 1981 May;240(5):E465–E473. doi: 10.1152/ajpendo.1981.240.5.E465. [DOI] [PubMed] [Google Scholar]
- Saccà L., Eigler N., Cryer P. E., Sherwin R. S. Insulin antagonistic effects of epinephrine and glucagon in the dog. Am J Physiol. 1979 Dec;237(6):E487–E492. doi: 10.1152/ajpendo.1979.237.6.E487. [DOI] [PubMed] [Google Scholar]
- Saccà L., Morrone G., Cicala M., Corso G., Ungaro B. Influence of epinephrine, norepinephrine, and isoproterenol on glucose homeostasis in normal man. J Clin Endocrinol Metab. 1980 Apr;50(4):680–684. doi: 10.1210/jcem-50-4-680. [DOI] [PubMed] [Google Scholar]
- Saccà L., Perez G. Influence of prostaglandins on plasma glucagon levels in the rat. Metabolism. 1976 Feb;25(2):127–130. doi: 10.1016/0026-0495(76)90041-x. [DOI] [PubMed] [Google Scholar]
- Saccà L., Trimarco B., Perez G., Rengo F. Studies on the mechanism underlying the influence of alanine infusion on glucose dynamics in the dog. Diabetes. 1977 Apr;26(4):262–270. doi: 10.2337/diab.26.4.262. [DOI] [PubMed] [Google Scholar]
- Saccà L., Vitale D., Cicala M., Trimarco B., Ungaro B. The glucoregulatory response to intravenous glucose infusion in normal man: roles of insulin and glucose. Metabolism. 1981 May;30(5):457–461. doi: 10.1016/0026-0495(81)90180-3. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Hendler R., Sherwin R. S. Altered responsiveness to cortisol, epinephrine, and glucagon in insulin-infused juvenile-onset diabetics. A mechanism for diabetic instability. Diabetes. 1980 Apr;29(4):284–291. doi: 10.2337/diab.29.4.284. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Fisher M., Hendler R., Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976 Feb 26;294(9):455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Liljenquist J. E., Williams P. E., Lacy W. W., Cherrington A. D. Glucose disposal during insulinopenia in somatostatin-treated dogs. The roles of glucose and glucagon. J Clin Invest. 1978 Aug;62(2):487–491. doi: 10.1172/JCI109150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skikama H., Ui M. Metabolic background for glucose tolerance: mechanism for epinephrine-induced impairment. Am J Physiol. 1975 Oct;229(4):955–961. doi: 10.1152/ajplegacy.1975.229.4.955. [DOI] [PubMed] [Google Scholar]
- Steele R., Rostami H., Altszuler N. A two-compartment calculator for the dog glucose pool in the nonsteady state. Fed Proc. 1974 Jul;33(7):1869–1876. [PubMed] [Google Scholar]
- Wolfe R. R., Allsop J. R., Burke J. F. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979 Mar;28(3):210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]


