Abstract
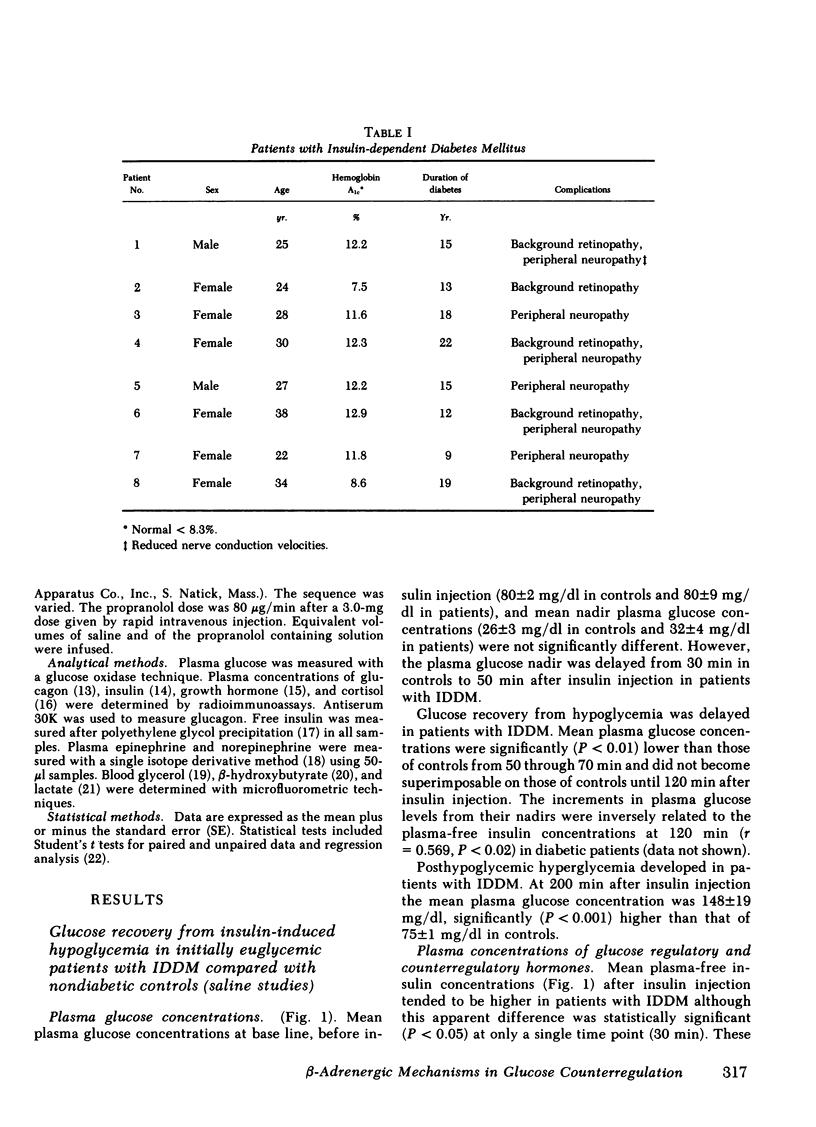
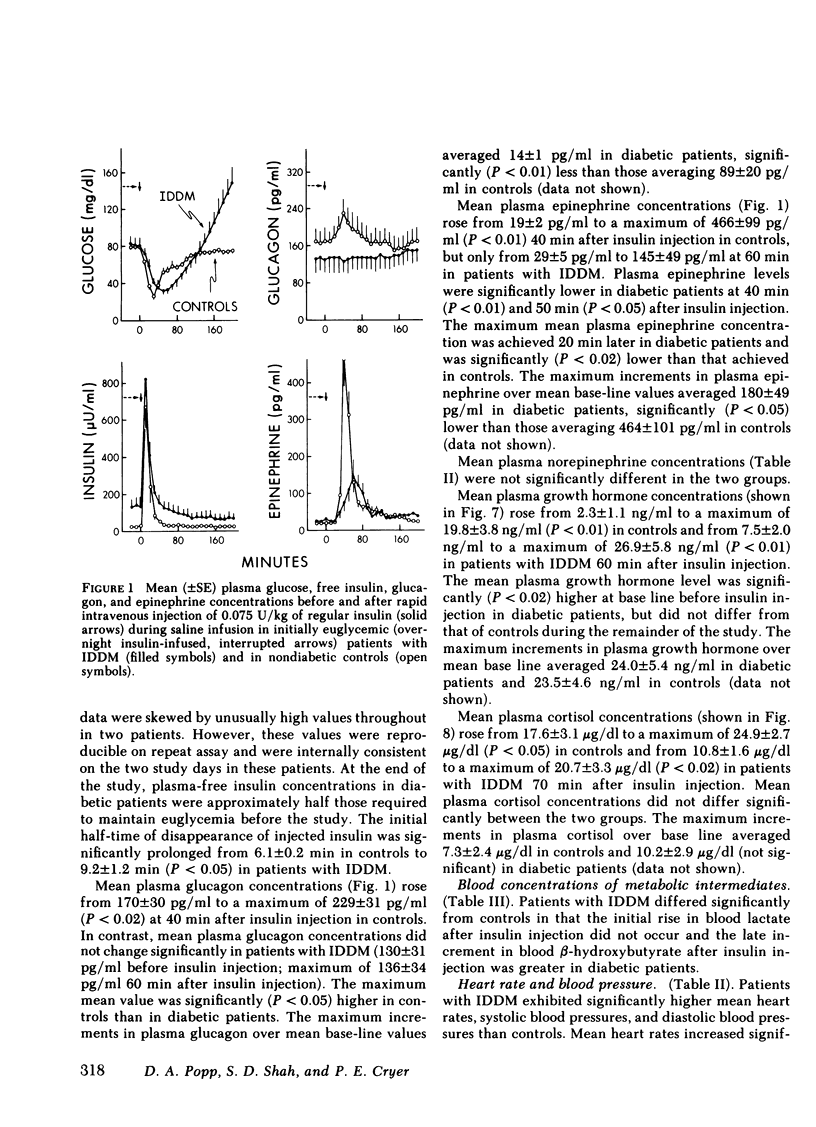
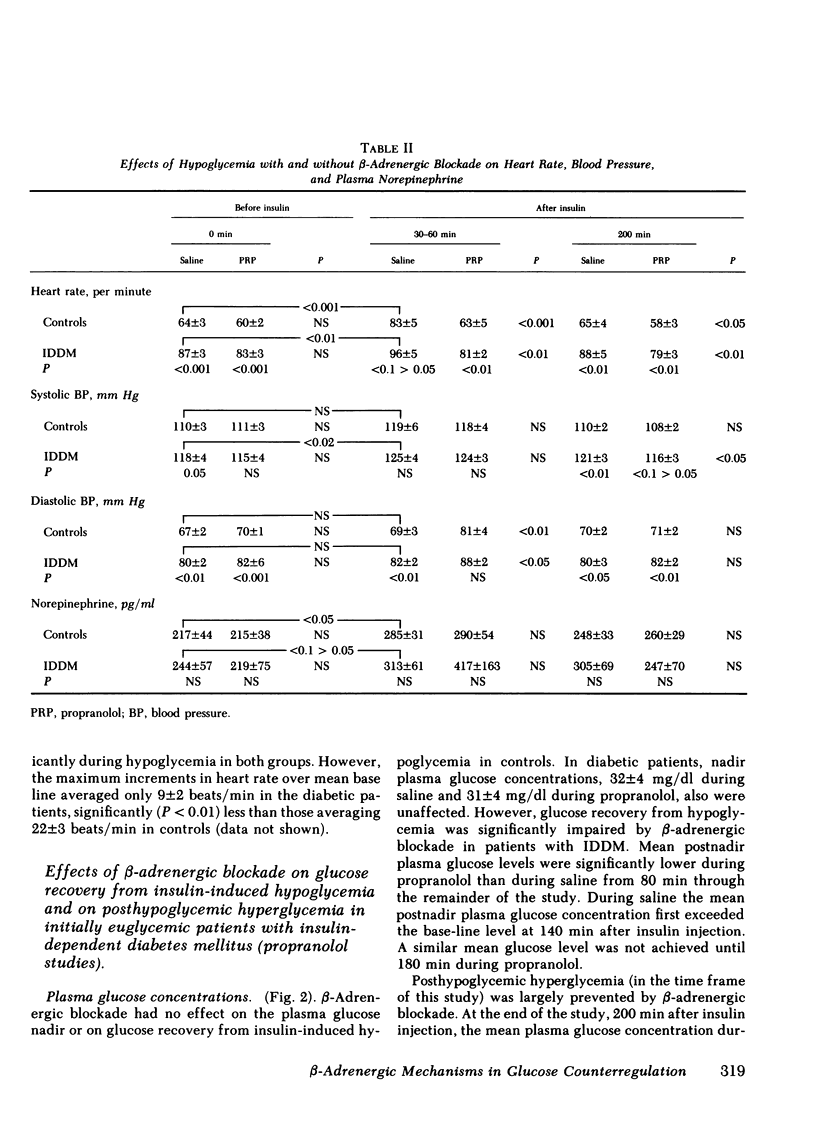
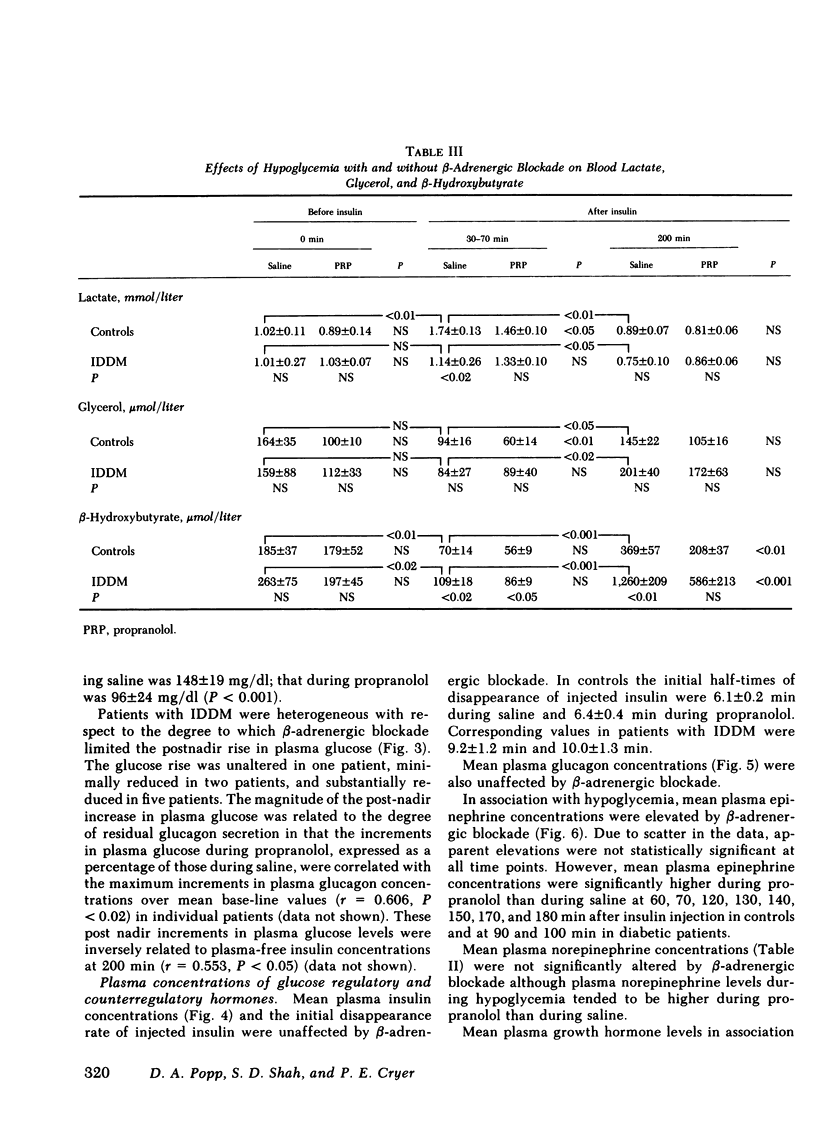
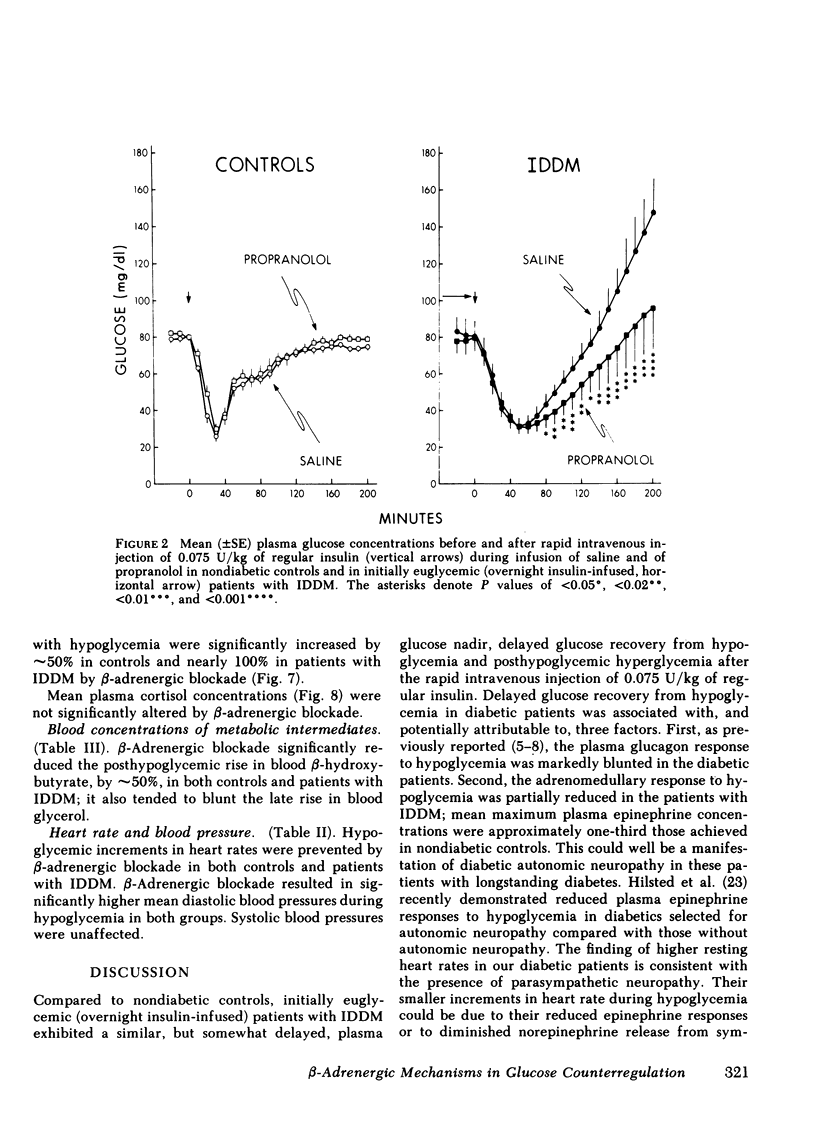
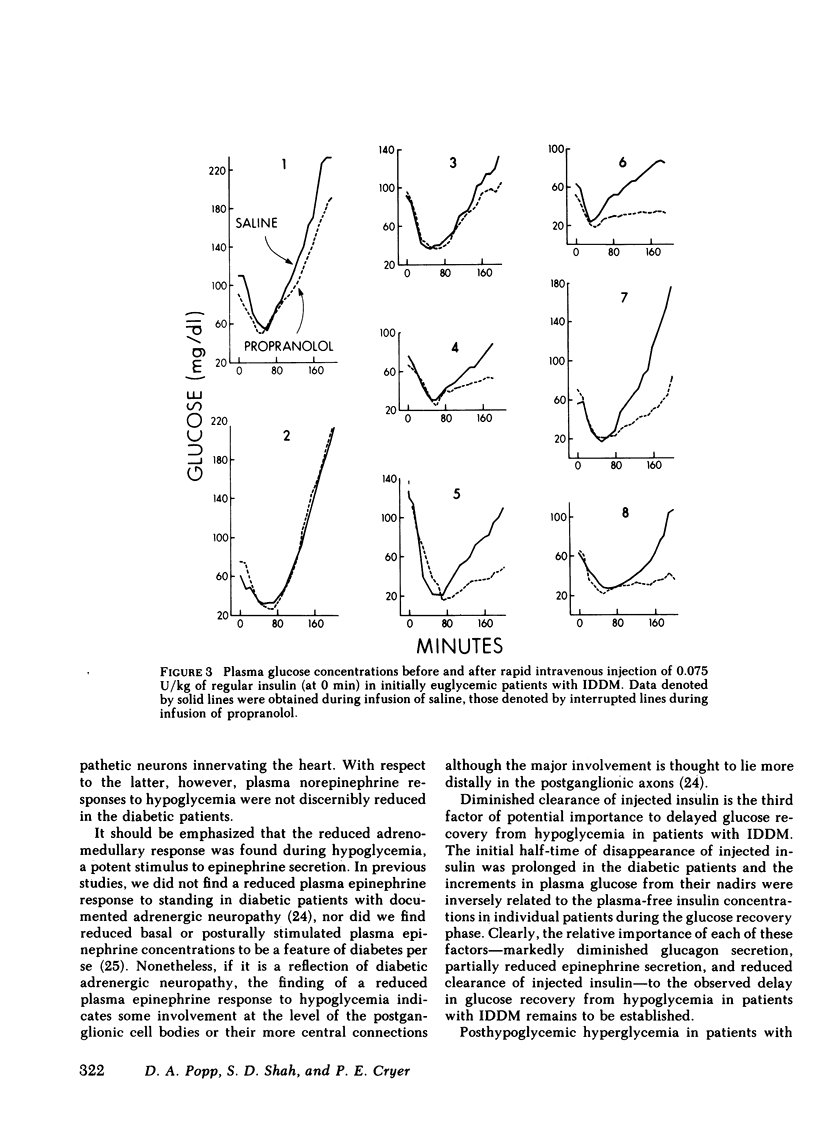
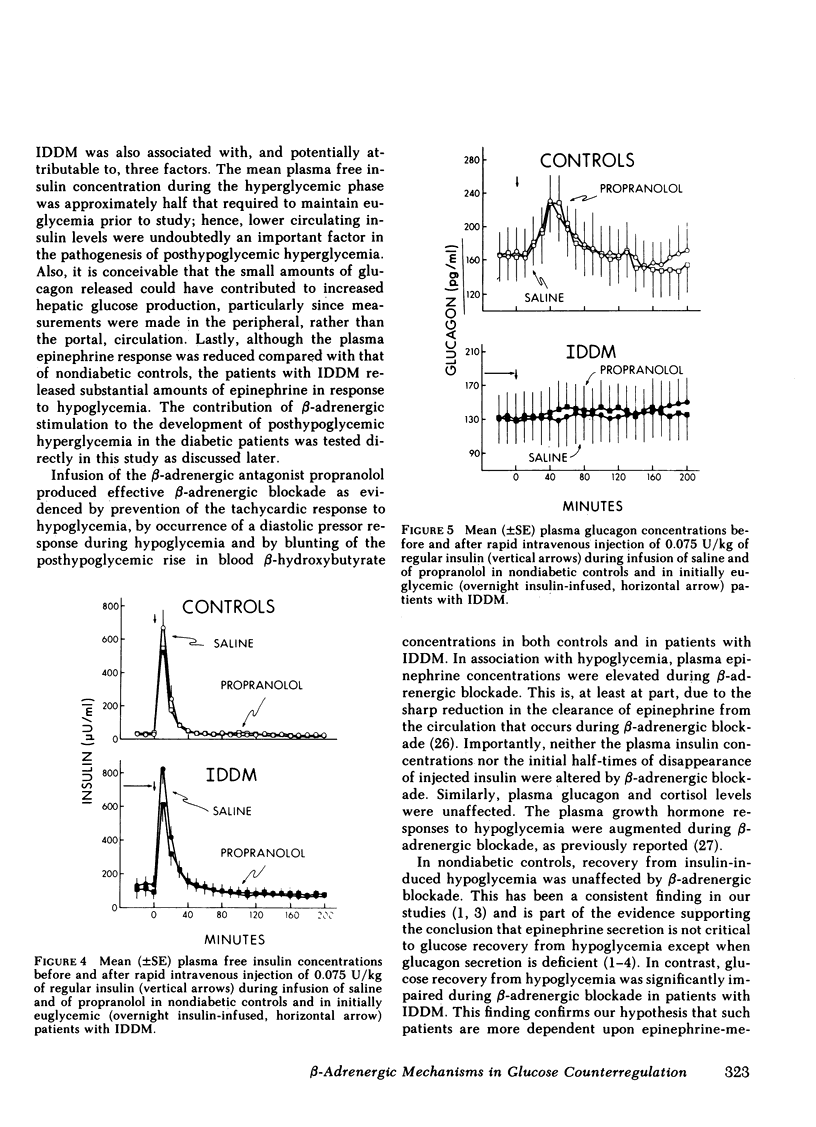
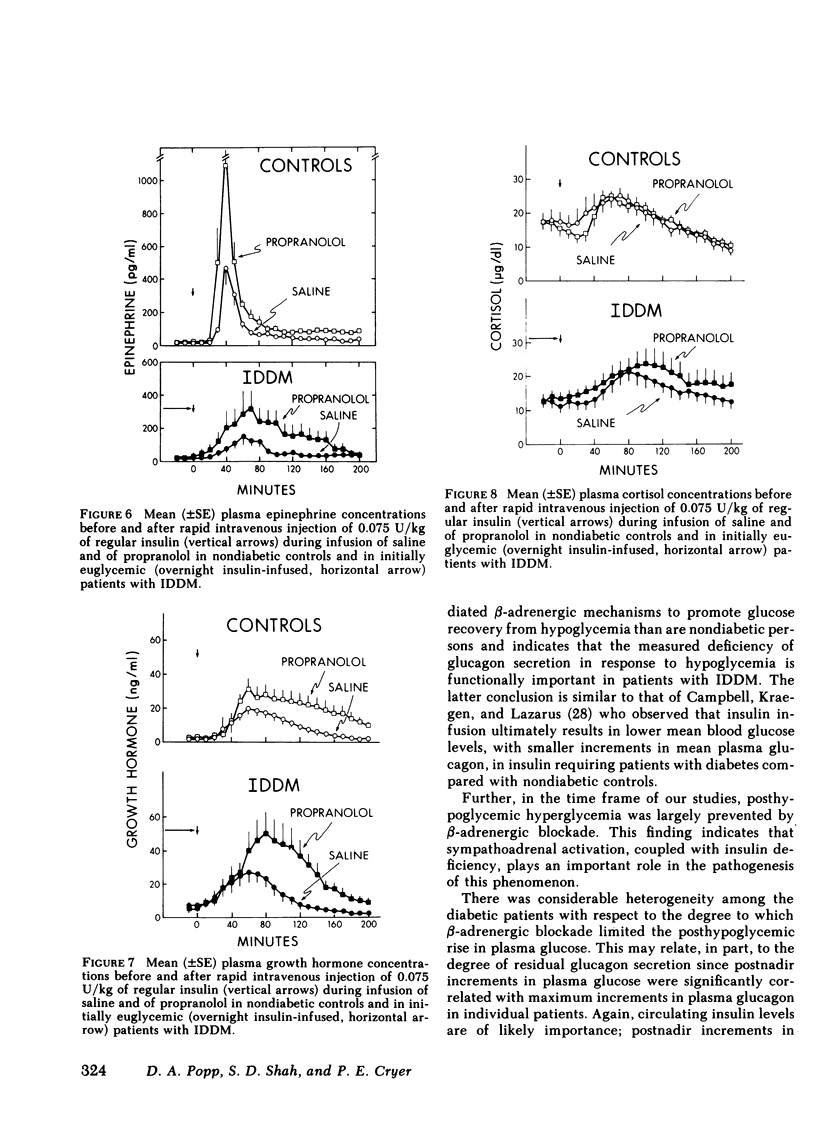
Initially euglycemic (overnight insulin-infused) patients with insulin-dependent diabetes mellitus (IDDM), compared with nondiabetic controls, exhibit similar, but somewhat delayed plasma glucose nadirs, delayed glucose recovery from hypoglycemia, and posthypoglycemic hyperglycemia after the rapid intravenous injection of 0.075 U/kg of regular insulin. These abnormalities are associated with and potentially attributable to markedly diminished glucagon secretory responses, partially reduced epinephrine secretory responses and delayed clearance of injected insulin in the diabetic patients. Because glucagon normally plays a primary role in hypoglycemic glucose counterregulation and enhanced epinephrine secretion largely compensates for glucagon deficiency, we hypothesized that patients with IDDM, who exhibit diminished glucagon secretory responses to hypoglycemia, would be more dependent upon epinephrine to promote glucose recovery from hypoglycemia than are nondiabetic persons. To test this hypothesis, glucose counterregulation during β-adrenergic blockade with propranolol was compared with that during saline infusion in both nondiabetic controls and in patients with IDDM. Glucose counterregulation was unaffected by β-adrenergic blockade in controls. In contrast, glucose recovery from hypoglycemia was significantly impaired during β-adrenergic blockade in diabetic patients. This finding confirms the hypothesis that such patients are more dependent upon epinephrine-mediated β-adrenergic mechanisms to promote glucose recovery from hypoglycemia and indicates that the measured deficiency of glucagon secretion is functionally important in patients with IDDM. Further, in the time frame of these studies, posthypoglycemic hyperglycemia was prevented by β-adrenergic blockade in these patients. There was considerable heterogeneity among the diabetic patients with respect to the degree to which β-adrenergic blockade limited the posthypoglycemic rise in plasma glucose. This rise was directly related to the degree of residual glucagon secretion and inversely related to plasma-free insulin concentrations.
Thus, we conclude: (a) that patients with IDDM are, to varying degrees, dependent upon epinephrine-mediated β-adrenergic mechanisms to promote glucose recovery from hypoglycemia and that the degree of this dependence upon epinephrine is an inverse function of the residual capacity to secrete glucagon in response to hypoglycemia in individual patients; (b) that sympathoadrenal activation, coupled with the inability to secrete insulin, plays an important role in the pathogenesis of posthypoglycemic hyperglycemia in patients with IDDM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. W., Jr, Johnson D. G., Palmer J. P., Werner P. L., Ensinck J. W. Glucagon and catecholamine secretion during hypoglycemia in normal and diabetic man. J Clin Endocrinol Metab. 1977 Mar;44(3):459–464. doi: 10.1210/jcem-44-3-459. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Heidingsfelder S. A. Adrenergic receptor control mechanism for growth hormone secretion. J Clin Invest. 1968 Jun;47(6):1407–1414. doi: 10.1172/JCI105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. V., Kraegen E. W., Lazarus L. Defective blood glucose counter-regulation in diabetics is a selective form of autonomic neuropathy. Br Med J. 1977 Dec 10;2(6101):1527–1529. doi: 10.1136/bmj.2.6101.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke W. L., Santiago J. V., Thomas L., Ben-Galim E., Haymond M. W., Cryer P. E. Adrenergic mechanisms in recovery from hypoglycemia in man: adrenergic blockade. Am J Physiol. 1979 Feb;236(2):E147–E152. doi: 10.1152/ajpendo.1979.236.2.E147. [DOI] [PubMed] [Google Scholar]
- Cryer P. E. Glucose counterregulation in man. Diabetes. 1981 Mar;30(3):261–264. doi: 10.2337/diab.30.3.261. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Rizza R. A., Haymond M. W., Gerich J. E. Epinephrine and norepinephrine are cleared through beta-adrenergic, but not alpha-adrenergic, mechanisms in man. Metabolism. 1980 Nov;29(11 Suppl 1):1114–1118. doi: 10.1016/0026-0495(80)90019-0. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Santiago J. V., Shah S. Measurement of norepinephrine and epinephrine in small volumes of human plasma by a single isotope derivative method: response to the upright posture. J Clin Endocrinol Metab. 1974 Dec;39(6):1025–1029. doi: 10.1210/jcem-39-6-1025. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Silverberg A. B., Santiago J. V., Shah S. D. Plasma catecholamines in diabetes. The syndromes of hypoadrenergic and hyperadrenergic postural hypotension. Am J Med. 1978 Mar;64(3):407–416. doi: 10.1016/0002-9343(78)90220-6. [DOI] [PubMed] [Google Scholar]
- Farmer R. W., Pierce C. E. Plasma cortisol determination: radioimmunoassay and competitive protein binding compared. Clin Chem. 1974 Apr;20(4):411–414. [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Gerich J., Davis J., Lorenzi M., Rizza R., Bohannon N., Karam J., Lewis S., Kaplan R., Schultz T., Cryer P. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979 Apr;236(4):E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsted J., Madsbad S., Krarup T., Sestoft L., Christensen N. J., Tronier B., Galbo H. Hormonal, metabolic, and cardiovascular responses to hypoglycemia in diabetic autonomic neuropathy. Diabetes. 1981 Aug;30(8):626–633. doi: 10.2337/diab.30.8.626. [DOI] [PubMed] [Google Scholar]
- Kuzuya H., Blix P. M., Horwitz D. L., Steiner D. F., Rubenstein A. H. Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes. 1977 Jan;26(1):22–29. doi: 10.2337/diab.26.1.22. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Lager I., Blohmé G., Smith U. Effect of cardioselective and non-selective beta-blockade on the hypoglycaemic response in insulin-dependent diabetics. Lancet. 1979 Mar 3;1(8114):458–462. doi: 10.1016/s0140-6736(79)90821-3. [DOI] [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Leveston S. A., Shah S. D., Cryer P. E. Cholinergic stimulation of norepinephrine release in man. Evidence of a sympathetic postganglionic axonal lesion in diabetic adrenergic neuropathy. J Clin Invest. 1979 Aug;64(2):374–380. doi: 10.1172/JCI109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher T. D., Tanenberg R. J., Greenberg B. Z., Hoffman J. E., Doe R. P., Goetz F. C. Lack of glucagon response to hypoglycemia in diabetic autonomic neuropathy. Diabetes. 1977 Mar;26(3):196–200. doi: 10.2337/diab.26.3.196. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALCH D. S., PARKER M. L. A SENSITIVE DOUBLE ANTIBODY IMMUNOASSAY FOR HUMAN GROWTH HORMONE IN PLASMA. Nature. 1964 Sep 12;203:1141–1142. doi: 10.1038/2031141a0. [DOI] [PubMed] [Google Scholar]
- Santiago J. V., Clarke W. L., Shah S. D., Cryer P. E. Epinephrine, norepinephrine, glucagon, and growth hormone release in association with physiological decrements in the plasma glucose concentration in normal and diabetic man. J Clin Endocrinol Metab. 1980 Oct;51(4):877–883. doi: 10.1210/jcem-51-4-877. [DOI] [PubMed] [Google Scholar]
- Viberti G. C., Keen H., Bloom S. R. Beta blockade and diabetes mellitus: effect of oxprenolol and metoprolol on the metabolic, cardiovascular, and hormonal response to insulin-induced hypoglycemia in insulin-dependent diabetics. Metabolism. 1980 Sep;29(9):873–879. doi: 10.1016/0026-0495(80)90127-4. [DOI] [PubMed] [Google Scholar]
- Viberti G. C., Keen H., Bloom S. R. Beta blockade and diabetes mellitus: effect of oxprenolol and metoprolol on the metabolic, cardiovascular, and hormonal response to insulin-induced hypoglycemia in normal subjects. Metabolism. 1980 Sep;29(9):866–872. doi: 10.1016/0026-0495(80)90126-2. [DOI] [PubMed] [Google Scholar]



