Abstract
Cold acclimation of winter cereals and other winter hardy species is a prerequisite to increase subsequent freezing tolerance. Low temperatures upregulate the expression of C-repeat/dehydration-responsive element binding transcription factors (CBF/DREB1) which in turn induce the expression of COLD-REGULATED (COR) genes. We summarize evidence which indicates that the integration of these interactions is responsible for the dwarf phenotype and enhanced photosynthetic performance associated with cold-acclimated and CBF-overexpressing plants. Plants overexpressing CBFs but grown at warm temperatures mimic the cold-tolerant, dwarf, compact phenotype; increased photosynthetic performance; and biomass accumulation typically associated with cold-acclimated plants. In this review, we propose a model whereby the cold acclimation signal is perceived by plants through an integration of low temperature and changes in light intensity, as well as changes in light quality. Such integration leads to the activation of the CBF-regulon and subsequent upregulation of COR gene and GA 2-oxidase (GA2ox) expression which results in a dwarf phenotype coupled with increased freezing tolerance and enhanced photosynthetic performance. We conclude that, due to their photoautotrophic nature, plants do not rely on a single low temperature sensor, but integrate changes in light intensity, light quality, and membrane viscosity in order to establish the cold-acclimated state. CBFs appear to act as master regulators of these interconnecting sensing/signaling pathways.
Keywords: CBF, cold acclimation, photosynthesis, redox imbalance, gibberellins, abscisic acid, phytochromes
1. Introduction
Acclimation of cold-tolerant plant species to low, non-freezing temperatures increases the subsequent tolerance to freezing stress [1]. The biological variation exhibited by plant species with respect to their ability to cold acclimate and to develop freezing tolerance is genetically determined. Since plants are photoautotrophic, the ultimate energy source for the cold acclimation process is derived from the sunlight absorbed and transformed through the process of photosynthesis [2–5]. Cold or chilling stress refers to the exposure of plants to an abrupt shift from control, non-acclimated temperatures (20 to 25 °C) to low, non-freezing temperatures (0 to 5 °C) for a period of several hours to several days. Such a temperature shift induces a disruption of the plant’s overall homeostatic state. In contrast, cold acclimation is a long-term process occurring over several weeks to months where plant growth and development at a low, non-freezing temperature induces a new homeostatic state which typically is associated with a change in phenotype [3,4,6,7]. This new homeostatic state represents the cold-acclimated state. On the other hand, plant freezing tolerance is usually measured as LT50, the freezing temperature at which 50% of a plant population dies due to a short freeze stress event. This review is focused on the processes involved in establishing the new cold-acclimated state in cold-tolerant plants and photosynthetic microorganisms.
The process of cold acclimation is regulated by C-repeat/dehydration-responsive element binding transcription factors (CBF/DREB1) [8–15]. CBF/DREB1 transcription factors bind to the C-repeat/dehydration-responsive DNA regulatory elements in the promoters of COLD-REGULATED (COR) genes [14,16–18]. In Arabidopsis thaliana (L.) Heynh, cold acclimation or cold stress activate expression of three CBF genes (CBF1/DREB1b, CBF2/DREB1c, and CBF3/DREB1a) which, in addition to other genes, induce the expression of several COR genes [14,16,17,19–23]. Studies with the A. thaliana CBF2 null mutant in a Colombia (Col) background have shown that the freezing tolerance and the expression of CBF1 and CBF3 were increased by the absence of CBF2 [24], thus suggesting that CBF2 negatively regulates the expression of CBF1 and CBF3 [25]. However, another study which used A. thaliana plants from the Wassilewskija-2 (WS-2) background reported that all three CBF genes were positively involved in freezing tolerance and activation of COR genes [17].
The expression of CBF3 is positively regulated by the constitutively expressed ICE1 (inducer of CBF expression 1) gene, the product of which binds to multiple regulatory elements present in the CBF3 promoter and stimulates its transcription [8,12,14,26]. The action of ICE1, in turn, is tightly regulated by SIZ1, a SUMO (small ubiquitin-related modifier) E3 ligase, and HOS1 (high expression of osmotically responsive protein 1) which is a RING finger E3 ligase [12,14,27–29]. In non-acclimated A. thaliana plants, the HOS1 protein, which is located in the cytoplasm, causes ubiquitination and degradation of ICE1 [27,28]. However, when A. thaliana plants are exposed to low temperature conditions, the HOS1 protein relocates to the nucleus [28] and the SIZ1 protein sumoylates ICE1 protein which, in turn, activates AtCBF3 expression [29].
Overexpression of CBF genes in A. thaliana, canola (Brassica napus L.), tomato (Solanum lycopersicum L.) and poplar (Populus balsamifera subsp. trichocarpa) increased their freezing tolerance [16,30–32]. Interestingly, the increased freezing tolerance of CBF-overexpressing plants is associated with a dwarf phenotype and delayed flowering [16,30,31,33,34]. Similarly, cold acclimation of WT plants results in a dwarf phenotype [30,31,33,34]. Thus, the CBF regulon-induced freezing tolerance is associated with phenotypic changes that are analogous to growth and development events that are controlled by plant hormones and photoreceptors such as phytochromes [35]. In A. thaliana, there are five phytochrome genes (PHYA, PHYB, PHYC, PHYD and PHYE) [36]. Three of them, PHYB, PHYD and PHYE are involved in regulation of plant growth by light quality (red to far-red ratio) [37–40]. The phyB-deficient A. thaliana displays increased elongation, decreased leaf expansion, increased apical dominance, and early flowering [37]. PhyA is involved in light quality-regulated seed germination [41] and phyC is suspected to be involved in the red light inhibition of hypocotyl elongation [40,42]. Interestingly, A. thaliana WS ecotype was found to be a natural phyD deletion mutant [43], and Col and Landsberg erecta (Ler) ecotypes are known to exhibit divergent growth and development in response to light quality [44,45].
Plant growth and development is regulated by multiple plant hormones: gibberellins (GAs), auxins, cytokinins (CKs), brassinosteroids (BRs), abscisic acid (ABA), ethylene and, potentially, salicylic acid and jasmonic acid [46]. While the individual roles of plant hormones have long been established, they often overlap, because most, if not all, plant growth and development processes are regulated by several plant hormones via positive and negative interactions [47]. For example, auxins interact with GAs by upregulating the GA20ox and GA3ox expression, while downregulating GA2ox expression [48]. Cold acclimation of most plant species is associated with enhanced freezing tolerance and the phenotype of cold-acclimated plants is often characterized by dwarfism or, at least, a compact, rosette growth habit [14]. The classic example of the GA response is the dramatic stem elongation induced in short-day, rosette cabbage plants by the application of exogenous GA [49]. Thus, a dwarf or compact growth habit is a response to a decrease in endogenous GA levels [49]. Plants overexpressing CBF genes exhibit a dwarf phenotype regardless of the temperature treatment in A. thaliana [33], canola [31] and tomato [30]. Thus, the process of cold acclimation must involve changes in hormonal homeostasis. First, we dissect the involvement of plant hormones in cold acclimation, cold stress and freezing tolerance, and evaluate their interaction with the CBF regulon.
Second, we reported recently that overexpression of CBFs in Brassica napus cv. Westar not only induces a dwarf phenotype with enhanced freezing tolerance without prior exposure to low temperature, but concomitantly, also induces increased photosynthetic performance and water use efficiency combined with enhanced photosynthetic energy conversion efficiency and biomass accumulation [31,50,51]. In fact, unlike most dwarf plants, cold-acclimated plants exhibit a total plant biomass that is equal to or greater to control plants that exhibited the elongated phenotype [6,52,53]. Thus, we attempt to integrate the sensing of low temperatures via photosynthetic redox imbalance quantified as excitation pressure with the role(s) of CBFs, plant growth hormones and phytochromes in the governance not only of cold acclimation, phenotypic plasticity and freezing tolerance, but also photosynthetic performance and energy conversion efficiency.
2. CBF–Hormone Interactions, Cold Acclimation and Dwarf Phenotype
2.1. Gibberellins
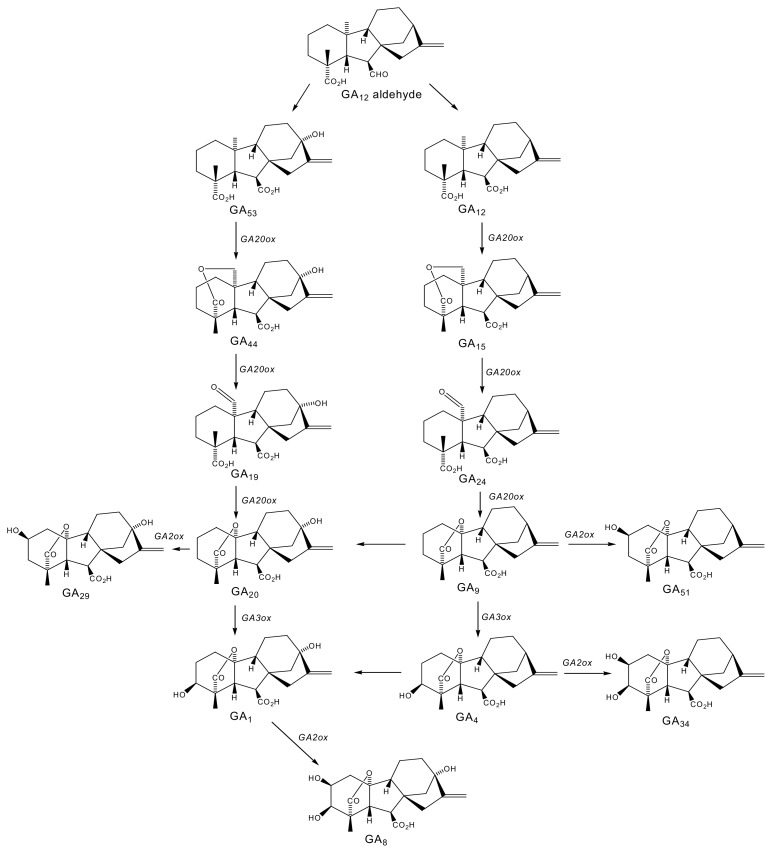
The application of exogenous growth-active GAs (such as GA1, GA3 or GA4) enhances shoot growth and mutants that are deficient in endogenous GAs, or have impaired GA signaling, exhibit a dwarf phenotype. Thus, GAs are promoters of stem elongation [48,54,55]. The biosynthetic pathway of GAs involves genes encoding for each of GA20-oxidase (GA20ox), GA3-oxidase (GA3ox) and GA2-oxidase (GA2ox) enzymes that control oxidation steps involving C-20 and each of the C-3β and C-2β-hydroxylations (Figure 1) [48]. A. thaliana plants overexpressing GA20ox or GA3ox produce higher levels of growth-active GAs and exhibit a large, extended phenotype [54]. In contrast, plants overexpressing GA2ox which is responsible for catabolism of growth-active GA1 and GA4 to their catabolite forms, GA8 and GA34 respectively, and catabolism of precursors to growth-active GAs, GA20 and GA9 to their catabolite forms, GA29 and GA51, respectively (Figure 1), exhibit a dwarf phenotype relative to wild-type (WT) plants [48,54,55]. It is well established that a change in temperature modifies endogenous GA levels [56–58], as well as the plant’s sensitivity to the application of exogenous growth-active GAs [59]. Endogenous GA levels decrease as temperature is lowered and this is associated with decreased shoot growth in carrot (Daucus carota L.) [60], wheat (Triticum aestivum L.) [61], Campanula isophylla [62], Dendranthema grandiflorum [63] and sunflower (Helianthus annuus L.) [58].
Figure 1.
Pathways of gibberellin biosynthesis: two putative pathways of gibberellin biosynthesis, the early 13-hydroxylation and early non-hydroxylation pathways that are likely utilized in vegetative tissues of many higher plants [48,54,70]. The GA9→GA20 and GA4→GA1 conversions are based on a limited number of examples in the literature, and thus may not be commonplace. Compliments of Ruichuan Zhang and Richard Pharis (University of Calgary, Calgary, AB, Canada).
Surprisingly, initial transcriptome microarray studies of A. thaliana seedlings exposed to low (4 °C) temperature indicated that genes involved in GA biosynthesis were not cold responsive [64–66]. However, Achard et al. [33] did show that exposure of A. thaliana plants to a 4 °C cold stress caused an increase in the expression levels of AtGA2ox3 and AtGA2ox6. Concomitantly, the cold-stressed plants exhibited lower endogenous levels of GA1 and GA4, and higher endogenous levels of GA8 and GA34[33]. It is important to note that the cold acclimation process is initiated only when the treatment temperature is lower than 10 °C. Exposure of A. thaliana seedlings initially grown at 22 °C to a lower temperature of 12 °C resulted in increased expression of the transcript level of AtGA3ox1 and decreased transcription of AtGA2ox2 [67]. Similarly, sunflower seedlings grown at 15 °C had higher endogenous GA1 levels and lower endogenous GA8 levels relative to seedlings grown at 20 °C [58].
Likewise, cold stress regulation of GA biosynthesis can differ as a function of a plant developmental stage. Exposure of imbibed A. thaliana seeds to a stratification temperature of 4 °C caused an increase in the expression of AtGA3ox1 transcript levels and was associated with increased endogenous GA1 and GA4 levels [68]. This is expected since stratification results in “germination” of seeds which would otherwise have remained “dormant”. However, this is contrary to cold regulation of GA biosynthesis in seedlings [33]. A comparison of CBF1 expression between maturing A. thaliana seeds and seedlings subjected to 4 °C cold treatment showed that, unlike seedlings, the maturing seeds did not increase their CBF1 expression [69]. However, there was an upregulation of AtGA2ox6 expression in maturing WT seeds subjected to cold treatment which was reduced in loss-of-function CBF lines [69]. Furthermore, no CBF1 expression was detected in imbibed seeds prior to the same cold treatment as received by mature seeds and seedlings [69]. Following the initiation of cold treatment, the imbibed seeds did eventually show CBF1 expression but with a lag in time and at significantly lower levels compared to that observed in seedlings. Concomitantly, the expression of COR15b in mature or imbibed seeds was not upregulated as it was in seedlings following a cold treatment [69].
The dwarf phenotype of CBF-overexpressing plants can be rescued by application of exogenous growth-active GAs, but not by application of other hormones which have been implied in shoot growth enhancement. Transgenic tomato plants overexpressing A. thaliana CBF1 are cold-tolerant and dwarf, the latter can be rescued by exogenous application of GA3[30]. Interestingly, the CBF1-overexpressing tomato plants treated with GA3 and thus exhibiting a normal, elongated phenotype retained the same cold tolerance as non-GA3-treated CBF1 plants [30]. However, it is likely that a reduction in the growth-active GA levels is necessary for cold or freezing tolerance since A. thaliana plants impaired in GA downstream signaling had reduced freezing tolerance. A. thaliana plants overexpressing CBF1 grown to the same developmental stage at 22 °C had increased AtGA2ox3 and AtGA2ox6 expression, as well as higher GA8 and GA34, and lower GA1 and GA4 levels [33]. Application of exogenous GA3 to CBF1 overexpressing A. thaliana plants rescued the dwarf phenotype [33]. Overexpression of cotton (Gossypium hirsutum)-derived GhDREB1 in A. thaliana delayed vegetative growth and flowering [34]. The initial, but not subsequent flowers, exhibited symptoms of male sterility such as shorter filaments and undehisced anthers relative to the WT type line [34]. Exposure of plants from this overexpressing line to low temperature resulted in higher tolerance to freezing stress than the WT. The dwarf phenotype of the A. thaliana line overexpressing GhDREB1 was rescued by exogenous application of GA3, but not by applications of growth-active auxin, BR or CK [34].
Phylogenetic studies in A. thaliana have shown that genes encoding the CBF family of transcription factors are closely related to dwarf and delayed-flowering1 (DDF1) gene [71]. Expression of RD29A/COR78 was also upregulated 9-fold in ddf1 plants, but not the expression of CBF3 genes [71]. Furthermore, both CBF and DDF1-overexpressing plants exhibit dwarf phenotypes [71–73]. Mutant ddf1 plants were reported to be GA-deficient and the dwarf phenotype could be rescued by application of exogenous GA3[71]. Furthermore, the endogenous GA1 and GA4 levels in ddf1 plants were lower than in WT background lines due to the repression of GA20ox genes [71]. A latter study by the same group confirmed that ddf1 plants have higher expression of AtGA2ox7 relative to WT [74]. Similarly, A. thaliana plants transformed with grape CBF1 (VrCBF1) exhibited increased expression of cold-regulated genes (AtCOR15a, AtRD29A, AtCOR6.6 and AtCOR47) and AtGA2ox7 which was associated with a dwarf phenotype and delayed flowering [75]. The latter was also associated with increased expression of AtFLC (FLOWERING LOCUS C), a floral repressor gene [75], confirming an earlier report of increased AtFLC expression and delayed flowering in A. thaliana plants overexpressing GhDREB1 [34].
There appears to be an inverse relationship between endogenous GA levels and CBF expression: an increase in CBF expression reduced the accumulation of growth-active GAs [33,75]. Furthermore, the application of exogenous GA3 to cotton seedlings exposed to 0 °C for 24 h inhibited the increase in expression of GhDREB1 observed for control seedlings [76]. Also, cold stress tolerance was strongly associated with increased expression of CBF1 and lower endogenous GA1 and GA4 levels in four bamboo species that exhibited natural variation in low temperature sensitivity [77]. The current model of CBF-GA interaction proposes that overexpression of CBF, either via cold induction or by transgenic means, stimulates the accumulation of DELLA proteins [33]. These growth-repressing proteins are positioned downstream in the GA signaling pathway and are deactivated by accumulation of growth-active GAs which, in turn, leads to an elongated phenotype [78]. The accumulation of DELLA proteins is likely a consequence of a reduction in growth-active GA levels caused by increased expression of GA2ox genes. Thus, the evidence is consistent with the thesis that CBF controls the expression of GA2ox genes in cold-acclimated plants [33].
2.2. Abscisic Acid
Involvement of ABA in plant cold acclimation and freezing tolerance has been known for some time due, in part, to the observations that the application of exogenous ABA could substitute for exposure to cold stress [79–85] and that cold stress induces increases in endogenous ABA levels [77,80,86–88]. Furthermore, A. thaliana mutant lines deficient or impaired in ABA biosynthesis showed reduced response to cold acclimation [88–90]. Nevertheless, there are also conflicting reports where the application of ABA to a wide range of plant species did not induce cold hardiness or freezing tolerance [91–95]. Thus, while cold-acclimation or cold-stress events usually do increase endogenous ABA levels, the role of ABA in these events remained unclear. The involvement of ABA in activating a cascade of downstream signaling events in response to a cold treatment has been evaluated. In A. thaliana plants, both cold treatment and application of exogenous ABA increases the expression of several COR genes including RAB18, RD29A (also known as COR78 or LTI78), KIN2 [92,96,97], as well as CBF1 [98]. Furthermore, in freezing-tolerant wild grapes (Vitis riparia Michx.) and freezing-sensitive cultivated grapes (Vitis vinifera L.) both cold (4 °C) and exogenous ABA treatments similarly activated the expression of CBF1 gene [99]. In contrast, none of the CBF genes responded strongly to exogenous ABA treatment in barley (Hordeum vulgare L.) [100]. The current consensus is that the activation of CBF and COR gene expression can be both ABA-dependent and ABA-independent [101–103].
However, even in the scenario of ABA-independent cold-induced activation of CBF gene expression [99,101–103], low temperatures do increase endogenous ABA accumulation [77,81,86]. This increase in endogenous ABA levels in cold-acclimated or cold-stressed plants can play a role in the resultant dwarf phenotype as well as cause the dwarf phenotype of CBF-overexpressing plants grown at optimal growth temperatures [31,51] since elevated ABA levels are usually associated with a decrease in shoot growth [104–106]. Historically, GAs and ABA have been reported to act antagonistically in the regulation of shoot growth and both plant hormones can downregulate each other’s biosynthetic genes [107]. In A. thaliana, ABA inhibits the expression of GA20ox and GA3ox during seed germination, and thus, reduces the levels of growth-active endogenous GAs [108]. In sunflower hypocotyls subjected to a range of low temperatures, there was an antagonistic association between endogenous ABA and GA20 and GA1, but not GA8 levels which would imply an antagonistic association between ABA, and GA20ox and GA3ox genes [58]. Similarly, in four bamboo species, cold tolerance was strongly associated with increased expression of CBF1 gene, higher endogenous ABA levels and lower endogenous GA1 and GA4 levels [77]. Thus, while the low temperature-mediated increase in CBF expression may occur independently of ABA, this plant hormone may still contribute to the reduced growth and the dwarf phenotype in CBF-overexpressing plants through its antagonistic interaction with CBF-dependent GA biosynthesis.
2.3. Cytokinins
Cytokinins (CKs) are involved mainly in the stimulation of cell division, lateral bud or shoot growth, leaf expansion and the prevention or deceleration of senescence [109]. Based on studies with CK-deficient plants it was postulated that CKs may act as negative regulators of abiotic stress signaling [110]. Abiotic stress can decrease CK content. A reduction in temperature stimulated the activity of cytokinin-oxidase and lowered endogenous CK levels in wheat [111]. A. thaliana plants with reduced CK biosynthesis due to the inactivation of the IPT gene were more tolerant to drought and salt stress than WT [112]. Also, these CK-deficient plants had reduced ABA levels and an increased sensitivity to exogenously applied ABA [112]. Thus, it is likely that CK homeostasis may play a role in plant responses to stress via changes in both ABA biosynthesis and the sensitivity of the plant to ABA [110].
The CK signal transduction pathway in A. thaliana involves histidine kinases (AHKs), which, upon activation, transfer the signal, via histidine phosphotransfer proteins (AHPs), to response regulators (ARRs), type-A and type-B [113,114]. Type-A response regulators inhibit type-B transcription factors through protein–protein interactions, whereas phosphorylation of type-B transcription factors regulates the expression of type-A ARRs and other genes [113,114]. A. thaliana plants overexpressing GhDREB1 were shown to be less sensitive to the exogenous addition of the cytokinin, benzyladenine (BA) [34]. Furthermore, plants overexpressing GhDREB1 had reduced expression of type-A and type-B ARR genes, whereas endogenous CK levels were not changed. This implies that GhDREB1 exerts a negative regulation of downstream CK signaling without affecting CK biosynthesis [34].
A. thaliana plants of the amp1 mutant line with increased endogenous CK levels [115] performed better than WT plants under continuous cold stress conditions by significantly increasing cell division [116]. The application of exogenous CK, zeatin, to WT plants mimicked the cold-induced amp1 phenotype under continuous cold stress conditions. However, untreated and zeatin-treated WT as well as amp1 plants exhibited similar levels of CBF and COR gene expression under continuous cold stress conditions [116]. Both cold stress and exogenous BA induced the expression of a subset of type-A ARR genes, whereas only cold stress, but not BA, induced the expression of CBF1, RD29A, and RD29B [117]. However, the cold-induced expression of type-A ARR was not associated with changes in endogenous CK levels. Furthermore, cold treatment of 35S:AtCKX2-2 plants with reduced CK levels did not result in changes of type-A ARR or CBF gene expression relative to wild-type plants [117]. Based on expression levels of type-A ARR and CBF in ahk single and double mutant lines, it was suggested that at least two CK-related AHKs, AHK2 and AHK3, are the mediators of cold stress response which activates expression of a subset of type-A ARR genes, whereas CBF expression is not connected to AHK or ARR expression [117]. Also, overexpressing ARR7 resulted in reduced freezing tolerance and insensitivity to applied ABA, whereas arr7 plants exhibited increased tolerance to freezing stress and hypersensitivity to ABA. This was further linked to ABA and it was concluded that AHK2, AHK3 and type-A ARRs inhibit ABA signaling, and thus, are negative regulators of the cold stress response in Arabidopsis thaliana [117]. In contrast, a more recent study reported that overexpression of ARR7 in Arabidopsis thaliana exhibited higher freezing tolerance than WT [118]. It appears that further research is needed to confirm a definitive relationship between CK levels and CBF expression.
2.4. Ethylene
Ethylene plays an important role in plant responses to a range of biotic and abiotic stresses [119]. Plant response to stress often causes increased ethylene production which is likely causal for shoot growth inhibition [119–121]. Increased ethylene biosynthesis has been implied in the enhancement of chilling or freezing tolerance in cucumber (Cucumis sativus L.), sunflower, tobacco (Nicotiana tabacum L.), tomato and winter rye (Secale cereal L.) [58,122–125]. Exogenous application of ethylene’s immediate precursor, 1-aminocyclopropane-1-carboxylic acid (ACC), to tobacco plants promoted freezing tolerance, whereas application of the ethylene biosynthesis inhibitor, aminoethoxyvinylglycine (AVG) or the ethylene receptor antagonist, AgNO3, decreased freezing tolerance [125]. In contrast, ethylene production was decreased by cold treatment in winter wheat and dwarf beans (Phaseolus vulgaris L.) [126,127]. Furthermore, reduction in ethylene production was associated with increased freezing tolerance in both mung bean (Vigna radiate L.) and A. thaliana [118,128]. The A. thaliana ethylene overproducer1 (eto1) mutant also exhibited reduced freezing tolerance relative to WT [118]. Furthermore, application of ACC to WT A. thaliana plants reduced freezing tolerance, whereas application of either AVG or AgNO3 increased freezing tolerance [118]. In line with the latter results, several ethylene insensitive mutant lines also exhibited enhanced freezing tolerance [118]. Therefore, the role of ethylene in cold acclimation and freezing tolerance appears to be complex and probably species dependent.
There is limited information on the potential interaction of ethylene with the CBF regulon, but this interaction may be negative. Plants overexpressing ETHYLENE INSENSITIVE3 (EIN3), a transcription factor positively regulating ethylene signaling [129–131], exhibited reduced freezing tolerance and had decreased CBF expression whereas ein3 plants exhibited increased freezing tolerance and increased expression of CBF relative to WT [118]. Furthermore, overexpression of CBF in A. thaliana also suppresses the responsiveness of leaves to ethylene, therefore delaying senescence and chlorophyll degradation [132]. Also, ethylene was suggested to be a negative regulator of CK signaling. Shi et al. reported that ethylene inhibited the expression of CBF and type-A ARR genes involved in CK signaling which caused reduced freezing tolerance [118].
2.5. Brassinosteroids
Brassinosteroids (BR) are mainly recognized for their involvement in stem etiolation [133], although applied growth-active BR, brassinolide (BL), can also promote stem elongation in light-grown plants [134–136]. There are several examples in the literature of exogenous BRs alleviating the effects of heat stress [135,137,138], likely via upregulation of ABA biosynthesis [139]. However, the involvement of BRs in the alleviation of cold stress is not well understood. Pretreatment with growth-active BRs did alleviate the effect of cold stress on chlorophyll content in corn (Zea mays L.) and increased A. thaliana tolerance to cold treatment [140–142]. Furthermore, the brassinosteroid-insensitive 1 (bri1) mutant with deficiency in BR signaling [143,144] was more tolerant, whereas the BRI1-overexpressing line was less tolerant to cold treatment compared with WT [142]. Both bri1 and BRI1 lines had higher basal expression levels of CBF genes under control temperature relative to WT [142]. Also, application of BR to non-stressed plants promoted the expression of COR15A [145]. Although this may suggest an involvement of BRs in CBF regulation, the cold-treated WT type, bri1 and BRI1 lines all showed similar increases in expression of both CBF and COR genes [142]. In contrast, A. thaliana seedlings overexpressing the BR biosynthetic gene AtDWF4 [146] were more tolerant to cold stress treatment and had higher expression of the COR15A gene [145]. Thus, a role of BRs in the regulation of CBFs and cold tolerance appears to be equivocal. Further research is required to elucidate the role of BRs in CBF regulation of cold tolerance.
2.6. Salicylic Acid
Salicylic acid (SA) has been mainly associated with the activation of plant defense mechanisms against pathogen attack [147]. However, application of exogenous SA can affect the physiology, metabolism and reproductive development of some higher plants [148]. At higher exogenous concentrations, SA often inhibits growth [149], but at low, near-physiological concentrations it may promote biomass accumulation [150]. Pretreatment of plants with exogenous SA effectively alleviated some of the cold stress-induced effects in several species such as banana (Musa acuminate L.), corn, cucumber, peach (Prunus persica [L.] Batch.), potato (Solanum tuberosum L.), rice (Oryza sativa L.) and tomato plants [151–160]. Cold treatment increased endogenous SA accumulation in A. thaliana WT, but mutant lines with reduced SA biosynthesis (NahG and eds5) responded to cold treatment with increased growth relative to WT [116,161–163]. In contrast, the SA overproducing line, cpr1, was less tolerant to cold stress and exhibited acceleration in the development of a dwarf phenotype [163]. However, the expression of CBF genes in cold-treated WT and NahG mutant lines was similar [116]. A study with the SA overproducing lines, siz1 and acd6 which exhibit a dwarf phenotype, confirmed the decrease in tolerance to cold stress associated with higher endogenous SA accumulation and linked it to a decrease in CBF gene expression [164].
3. Light, Cold Acclimation and Freezing Tolerance
The light-dependence of plant cold acclimation and freezing tolerance is not new [6,165]. However, confusion persists in the literature with respect to the role of light quality, sensed through plant photoreceptors such as phytochrome and cryptochrome, versus the role of light intensity, sensed through redox imbalances associated with photosynthetic electron transport. The former initiates and governs processes involved in plant photomorphogenesis while the latter process results in alterations in the structure and function of the chloroplast photosynthetic apparatus through the process of photoacclimation [2,166–170]. When uncoupled, both light quality and light intensity signals were shown to differently regulate plant hormone biosynthesis and thus plant phenotype [171,172]. Walters et al. [173] reported that photomorphogenic mutants of A. thaliana exhibited photoacclimation and concluded that photoreceptors do not play a major role in photoacclimation. However, a det1 signal transduction mutant did exhibit a defect in photoacclimation from which they concluded that there must be cross-talk between photoreceptor-regulated responses and regulatory components involved in photoacclimation in A. thaliana [173].
3.1. Light Quality, Photoreceptors and Cold Acclimation
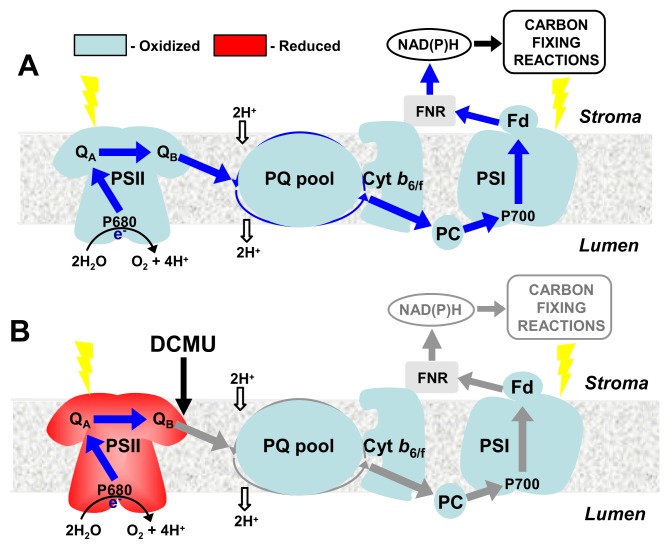
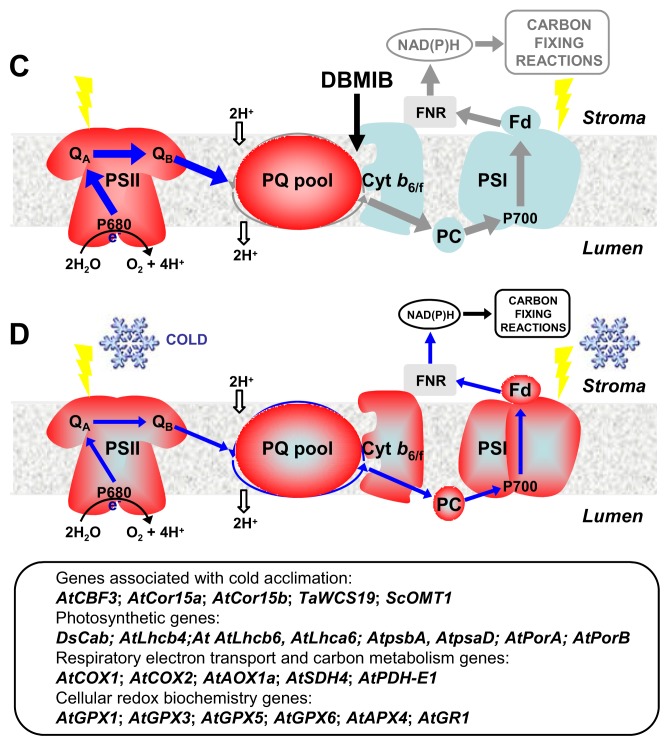
Pogson and co-workers [174,175] differentiate sensing/signaling associated with changes in light quality through photoreceptors involved in photomorphogenesis as “biogenic signals” versus sensing/signaling associated with changes in light intensity through photosynthetic electron transport in mature chloroplasts as “operational signals”. For example, the former are involved in chloroplast biogenesis and govern the proper biosynthesis and assembly of thylakoid membranes and their constituent membrane protein complexes whereas the latter are required to ensure energy balance and cellular homeostasis in an environment where light intensity, temperature, water and nutrient availability constantly fluctuate [4,5,176]. Kim et al. reported that phytochrome regulates low temperature-induced gene expression of COR15a through CBFs in A. thaliana [177]. In an attempt to eliminate the possible role of light-mediated gene expression through photosynthesis, these authors reported that inhibition of photosystem II (PSII) photochemistry by DCMU [3-(3,4-dichlorophenyl)-1,1-dimethyl urea] did not affect CBF gene expression. On this basis, they concluded that changes in photosynthetic redox potential did not contribute to the light regulation of CBF and COR gene expression during cold acclimation of A. thaliana [177]. However, the experimental design employed by Kim et al. [177] is seriously flawed for the following reasons. First, the reduction state of the PQ (plastoquinone) pool of the photosynthetic electron transport chain is considered an important sensor which governs nuclear gene expression through retrograde signaling [174,178–182]. In the presence of DCMU, although electron transfer from PSII to the PQ pool is inhibited, the PQ pool is oxidized as a consequence of electron transfer from the PQ pool to PSI via the cytochrome b6/f complex and plastocyanin (Figure 2B) [4,5]. To assess the effects of a reduced PQ pool, one must measure the effects of the electron transport inhibitor, DBMIB (2,5-dibromo-3-methyl-6-isopropylbenzoquinone) which is known to block photosynthetic electron transport at the cytochrome b6/f complex [4,5]. Thus, in the light and in the presence of DBMIB, the PQ pool remains reduced because PSII transfers electrons into the PQ pool, but electrons cannot exit the PQ pool because electron transfer through the cytochrome b6/f complex to PSI is blocked (Figure 2C). Thus, one must modulate the redox state of the PQ pool with both DCMU and DBMIB separately. Second, in addition to the PQ pool, the acceptor-side of PSI is also an important generator of redox signals that emanate from photosynthetic electron transport [5,183,184]. Since Kim et al. [177] failed to test the effects of DBMIB on CBF expression, the conclusions of their photosynthetic electron transport inhibition studies remain equivocal. In Figure 2D, we present a few specific examples of photosynthetic and non-photosynthetic genes shown to be regulated by changes in the redox state of the photosynthetic electron transport chain in wheat (Triticum aestivum, Ta), rye (Secale cereals, Sc), Dunaliella salina (Ds), and Arabidopsis thaliana (At). These include photosynthetic (DsCab; AtLhcb4;At AtLhcb6, AtLhca6; AtpsbA, AtpsaD; AtPorA; AtPorB) as well as non-photosynthetic genes previously associated with cold acclimation (AtCBF3; AtCor15a; AtCor15b; TaWCS19; ScOMT1), respiratory electron transport and carbon metabolism (AtCOX1; AtCOX2; AtAOX1a; AtSDH4; AtPDH-E1), as well as cellular redox biochemistry (AtGPX1; AtGPX3; AtGPX5; AtGPX6; AtAPX4; AtGR1). Cab and Lhcb is the gene family encoding the major light harvesting proteins of PSII whereas Lhca represents the gene family encoding the major light harvesting proteins of PSI. psbA is the gene encoding the reaction centre polypeptide associated with PSII whereas psaD encodes the D subunit of PSI reaction centres. PorA and PorB are the genes encoding protochlorophyllide oxidoreductase A and B. CBF3 is the gene encoding the C-repeat binding transcription factor 3 and Cor15a and Cor15b are cold-regulated genes 15a and 15b. WCS19 is the wheat cold-regulated gene 19 whereas OMT1 is the gene encoding O-methyltransferase1. COX1 and COX2 represent genes encoding subunits of mitochondrial cytochrome oxidase and AOX1a, the alternative oxidase1a of mitochondrial electron transport. SDH4 is the gene encoding succinate dehydrogenase 4 of the mitochondrial electron transport and PDH the gene encoding the E1 subunit of the pyruvate dehydrogenase complex. Genes involved in redox biochemistry include glutathione peroxidase (GPX), ascorbate peroxidase (APX) and glutathione–disulfide reductase1 GR. Thus, in contrast to the report of Kim et al. [177], CBF3 and Cor genes are sensitive to the redox state of the chloroplast.
Figure 2.
Simplified overview of possible effects of specific inhibitors and cold stress on the redox state of the photosynthetic electron transport chain components. (A) During growth and development of plants under optimal temperature conditions, the PQ pool and all components of the photosynthetic electron transport chain remain preferentially oxidized (light blue) because the rate of consumption of photosynthetic electrons through metabolic sinks (carbon fixing reactions) keeps pace with the rate at which PSII undergoes charge separation to reduce the PQ pool. Under these conditions, the linear photosynthetic electron flow (dark blue arrows) from PSII (water splitting) to PSI (NADP+ generation) dominates and is fully operational; (B) Adding DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea], a selective inhibitor at the QB binding site of PSII, to chloroplast membranes or intact leaves, blocks the linear electron transport rendering photosystem II (PSII) components more reduced (red), while all components downstream of PSII remain oxidized due electron consumption by photo-oxidized PSI; (C) The specific inhibitor of Cytb6/f complex (Q cycle) DBMIB (2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone) causes a reduction of PSII complex as well as the PQ pool, whereas the components downstream of PQ remain oxidized; (D) Exposure of plants to cold stress results in lower demand for electrons required for carbon fixing reactions. Cold stress imposes thermodynamic limitations in the rates of consumption of photosynthetically-generated electrons by the carbon fixation reactions on the acceptor side of PSI which increases the reduction state of PQ pool and all components of the photosynthetic electron transport chain. Such a reduction state is quantified in vivo as excitation pressure using chlorophyll fluorescence induction. Excitation pressure reflects the relative redox state of QA, the first stable quinone electron acceptor in the PSII reaction centre. Under cold stress conditions (Figure 2D), the linear photosynthetic electron flow between PSII and PSI is partially restricted relative to controls (Figure 2A).
However, there is considerable evidence that phytochromes also regulate the CBF regulon and that light quality is an important factor that governs cold acclimation and freezing tolerance [35,177,185–187]. Kim et al. [177] examined the integration of light and cold acclimation signaling pathways in transgenic A. thaliana containing four copies of a CRT/DRE element. The authors reported that phyB induced CRT/DRE reporter expression from which they concluded that light signals perceived through phytochromes regulate the CBF regulon and plant-freezing tolerance in a positive manner [177]. However, Franklin and Whitelam [186] reported that phytochromes repress rather than induce the expression of genes associated with the CBF regulon.
Furthermore, phytochrome regulation of the CBF regulon was dependent upon the ambient temperature [186]. Although exposure of A. thaliana to 16 °C resulted in a phytochrome-dependent upregulation of COR genes and an increase in freezing tolerance, this phenomenon was not observed at 22 °C [35,186]. Further mutant analysis indicated that phyB and phyD were both involved in the repression of the CBF regulon which counteracted the low temperature enhanced CBF expression as well as COR expression [186]. Kidokoro et al. [187] showed that phyB-regulated PIF7 (phytochrome-interacting factor 7) binds specifically to the G-box of the DREB1C promoter and functions as a transcriptional repressor for DREB1C expression when plants are not subject to abiotic stress.
Fowler et al. [185] reported that the low temperature-dependent expression of AtCBF1, AtCBF2 and AtCBF3 was dependent on the time during the photoperiod that Arabidopsis thaliana was exposed to the low temperature. The highest levels of expression were observed 4h into the photoperiod whereas the lowest were detected at the end of the photoperiod [185]. Consequently, the results of Fowler et al. [185] and Franklin and Whitelam [186] regarding the photoperiod-dependent expression of CBFs and the CBF regulon are consistent with the thesis that cold acclimation and plant freezing tolerance are regulated by the circadian clock from which Franklin [35] concludes that phytochromes may act as cellular temperature sensors. Furthermore, published evidence suggests that the photoreceptor- and temperature-signaling pathways involved in cold acclimation may converge with GA regulated-signaling pathways [171,172,188,189]. Indeed, the response of plants to light quality signaling is temperature- and GA-dependent [58]. Clearly, cold acclimation must be the result of a complex network of signaling pathways controlled by light quality, low temperature and plant hormones.
3.2. Light Intensity, Photosynthesis and Cold Acclimation
Cold-tolerant species such as winter wheat, winter rye, barley, spinach, canola and A. thaliana exhibit an increase in photosynthetic capacity through upregulation of carbon metabolism during cold acclimation [2,51,53,190–194]. The upregulation of carbon metabolism in cold-tolerant plant species is associated with increased expression and subsequent activities of the CO2-fixing enzyme Rubisco (ribulose-1,5-bisphosphate carboxylase oxygenase) [51,53,195,196], as well as enhanced activities of the cytosolic sucrose biosynthetic enzymes, cFBPase (cytosolic fructose-1,6-bisphosphatase) and SPS (sucrose phosphate synthase) [31,51,53,191,196–198] in response to low growth temperature. In addition, winter cultivars of wheat [197,199] and A. thaliana [193] enhance sink capacity and concomitant sucrose export to the sinks during cold acclimation [200]. Furthermore, most cereals stimulate carbon storage as fructans in the crown tissue, as well as in the leaf vacuoles during cold acclimation [201,202]. Consequently, cold acclimation of winter wheat, winter canola [191] and A. thaliana [193] results in enhanced Pi cycling [203,204] and increased capacity for RuBP (ribulose-1,5-bisphosphate) regeneration [205] through increased utilization of phosphorylated intermediates. Furthermore, cold acclimation results in the suppression of photorespiration, thus, diverting ATP and NADPH from oxygenation to carboxylation in winter wheat [197] and also increases water use efficiency by about two-fold in winter wheat, rye and canola [51,199]. Consequently, it was suggested that cold acclimation of winter wheat and rye leads to a feed-forward upregulation of photosynthetic carbon assimilation through the global reprogramming of photosynthetic carbon metabolism [191,193,195,196]. This is supported by a detailed, comparative metabolomics study of the cold-acclimated versus non-acclimated A. thaliana [206]. In addition, the cold acclimation-induced stimulation in photosynthetic capacity is positively correlated with the development of freezing tolerance measured as LT50 [207], as well as an increased resistance to low temperature-induced photoinhibition in spinach [208,209], winter rye and winter wheat [207,210,211], A. thaliana [212] and canola [31].
These adjustments at the physiological, biochemical and molecular levels are associated with coordinated changes in leaf anatomy and plant phenotype [6,51,53,196,213] and induction of freezing tolerance in cold-tolerant species [8,31,198,211,214,215]. With respect to phenotypic plasticity, cold acclimation of winter wheat, winter rye, canola and spinach as well as A. thaliana not only results in the dwarf, compact growth habit but also altered leaf morphology and anatomy with increased leaf thickness and specific leaf weight relative to the elongated phenotype of their non-acclimated counterparts [31,51,53,196,209,216–218]. The increased leaf thickness associated with the cold-acclimated state can be accounted for by either increases in leaf mesophyll cell size [216,217] and/or increases in the number of palisade mesophyll layers [51,209]. At the ultrastructural level, cold-acclimated winter rye and A. thaliana exhibit an apparent increase in cytoplasmic volume and an apparent decrease in vacuolar volume [196,219]. This is accompanied by an increase in the content of sucrose and structural carbohydrates [191,196,202,204,218,220] such that the total plant biomass of the dwarf, cold-acclimated winter cultivars is equal to or greater than the nonacclimated plants which exhibit an apparently larger, elongated phenotype [6,52,53]. However, a comparable dwarf phenotype can be generated by growth of plants at warm temperatures but high light. In addition, cold acclimation of winter wheat and rye under low light conditions results in the elongated phenotype [2,6]. The historical assumption that the dwarf phenotype associated with cold acclimation reflects a growth response to low temperature is incorrect because the generation of dwarf phenotype is mimicked by growth under high light conditions. The dwarf phenotype, the enhanced photosynthetic performance and decreased susceptibility to photoinhibition of winter hardy plants previously assumed to be regulated by low temperature, is in fact, regulated by the redox state of the chloroplast measured as excitation pressure [2,6].
All photoautotrophs are predisposed to maintain a balance between energy trapped by temperature-insensitive photochemical reactions (energy source) and the energy utilized through temperature-dependent metabolism, growth and development (energy sinks) [2,4,169]. An imbalance between energy trapped through photochemistry versus energy either utilized through biochemistry or dissipated through nonphotochemical quenching (NPQ) will occur whenever the rate at which the energy absorbed through PSII exceeds the rates of temperature-dependent nonphotochemical processes and metabolic electron sink capacity. Such an imbalance is defined as excitation pressure which is a measure of the relative redox state of QA, the first stable quinone electron acceptor of PSII reaction centres and reflects modulation of the redox state of the PQ pool and components of the intersystem photosynthetic electron transport chain (Figure 2D) [2,4,169]. The modulation of excitation pressure by acclimation to high light is mimicked by acclimation to low temperature [2,4,5]. Consequently, we have shown that excitation pressure governs the reversible conversion of the dwarf versus elongated phenotypes associated with cold acclimation in wheat and rye [2,4,6], high light versus low light phenotypes associated with cold acclimation and photoacclimation of the green algae Chlorella vulgaris and Dunaliella salina [2] and the cyanobacterium, Plectonema boryanum [221], as well as the extent of green and white sectoring in the Arabidopsis thaliana variegation mutant, immutans [222]. Since the high and low light phenotypes can be generated by chemical manipulation of the redox state of the PQ pool through inhibition of photosynthetic electron transport by either DCMU or DBMIB [176], this indicates that the phenotypic response to excitation pressure occurs independently of phytochrome. Thus, redox energy imbalances detected through modulation of excitation pressure within the chloroplast and the photosynthetic apparatus represents an extremely sensitive mechanism for all photoautotrophs to sense changes in temperature since photosynthetic redox imbalances affect nuclear gene expression through chloroplast retrograde regulation [174,178–182]. Such an “operational” redox signal is critically important for the maintenance of a functional photosynthetic apparatus in mature chloroplasts upon exposure to a changing abiotic environment [174,175].
An Arabidopsis thaliana genome-wide microarray analysis of gene expression in response to excitation pressure indicated that a total of 2489 transcripts, that is 10.9% of the total probes monitored on the Affymetrix Arabidopsis ATH1 Genome Array, were regulated by excitation pressure of which 817 were upregulated and 1672 were downregulated [5,223]. Figure 2D provides a few examples of photosynthetic and non-photosynthetic genes shown to be regulated by changes in excitation pressure in wheat (Triticum aestivum, Ta), rye (Secale cereals, Sc), Dunaliella salina (Ds), and Arabidopsis thaliana (At).
4. CBFs and Photosynthetic Performance
As discussed above, plant CBFs are integral components in the induction of the cold-acclimated state, freezing tolerance and phenotypic plasticity. The cold acclimation-induced adjustments at the physiological, morphological, biochemical and molecular levels of winter wheat and winter rye are associated with an enhanced capacity to utilize absorbed light energy and convert it to biomass and grain yield [50,51]. The apparent quantum requirements to close 50% of the PSII reaction centres and the apparent quantum requirements to induce one unit of NPQ under ambient CO2 conditions were about 35% and 50% higher in cold-acclimated Norstar wheat relative to its non-acclimated counterpart [50,53]. This was coupled to a 45% increase in dry biomass accumulation relative to non-acclimated counterpart [50]. Concomitantly, the cold-acclimated plants dissipate less energy as heat through NPQ because of the enhanced capacity to utilize and store absorbed energy as biomass. This means that in the cold-acclimated state, the winter cereals are able to trap a greater percentage of the incident radiation through photochemistry and convert this energy into biomass and seed production. Comparable results for cold acclimation-induced increases in biomass and concomitant increases in the apparent quantum requirements for PSII closure and NPQ induction under ambient CO2 conditions were reported for Musketeer winter rye [51,53].
What regulates the increased energy conversion efficiency observed for cold-acclimated winter cereals? Compared to non-acclimated wild-type (WT), the BnCBF17-overexpresser (BnCBF17-OE) grown at 20 °C mimicked cold-acclimated WT canola with respect to increased biomass and specific leaf weight (SLW), compact dwarf phenotype, increased light saturated rates of photosynthesis and photosynthetic electron transport, improved water use efficiency (WUE) and enhanced levels of key Calvin Cycle enzymes and components of photosynthetic electron transport, at ambient CO2[51,53]. BnCBF17-overexpression as well as cold acclimation of WT canola increased the dry biomass by 25%–35% relative to non-acclimated WT at ambient CO2 which were associated with increased amount of key regulatory photosynthetic enzymes such as stromal-localized Rubisco (rbcL) and cFBPase important in regulating sucrose biosynthesis [51]. These results for the BnCBF17-OE and cold-acclimated canola are comparable to the enhanced photosynthetic performance reported for cold-acclimated winter wheat and winter rye [53] and consistent with the report of Savitch et al. [31] who reported that the BnCBF17-OE exhibited significant enhancement in the gene expression as well as enzyme activities of Rubisco, SPS and cFBPase. Concomitantly, this was associated with an increased quantum requirement to close PSII reaction centres and to induce energy dissipation by NPQ in cold-acclimated and the BnCBF17-OE relative to WT controls. This indicates that compared to non-acclimated WT, BnCBF17-OE as well as cold-acclimated WT canola exhibit a comparable enhanced capacity to utilize absorbed light energy and convert it to biomass with a concomitant decreased reliance on NPQ to dissipate absorbed energy for photoprotection. Tobacco plants overexpressing A. thaliana CBF exhibited increased freezing tolerance and had a higher photochemical efficiency of PSII during cold acclimation period relative to WT plants [224]. Genetic alterations which influence chloroplast development, such as those regulated by MutS HOMOLOG1 (MSH1), induced programmed changes in phenotypic plasticity of six different plant species [225]. Recently, we have shown by genome-wide transcriptome analyses of A. thaliana that CBF3 transcript levels are regulated by excitation pressure (Figure 2D) [223]. We conclude that CBFs are critically important factors, not only in the regulation of plant freezing tolerance and phenotypic plasticity, but also in the governance of photosynthetic performance and energy conversion efficiency. Therefore, we suggest that CBFs may represent master regulators which integrate phenotypic plasticity, cold acclimation and hormonal homeostasis with photosynthetic performance and energy conversion efficiency which results in enhanced biomass production and seed grain yield [51] under suboptimal growth conditions.
5. CBFs as Master Regulators
Historically, research focused on cold acclimation and freezing tolerance has focused on changes in the physical structure of the cell membrane as a primary sensor involved in acclimation to low temperature [8,165,216,226]. Low temperature-induced increase in cell membrane viscosity has been shown to activate two-component histidine kinases which stimulate the expression of specific fatty acid desaturases involved in the modulation of membrane fluidity in cyanobacteria [227,228]. Modification of the physical properties of the cell membranes also activates Ca2+ channels which results in the accumulation of Ca2+ in the cytosol. Such a low temperature-induced Ca2+ signal occurs rapidly upon exposure to low temperature stress [229–231]. This accumulation of Ca2+ in the cytosol activates a protein kinase which phosphorylates ICE1 [8] which is necessary to induce the expression of CBFs and initiate the expression of COR genes necessary to acquire freezing tolerance [8,14,15–17,35,186,232,233]. A unique feature of all plants and photosynthetic micro-organisms is that they are photoautotrophic. Thus, these organisms are dependent upon light both as a signal to regulate plant development and reproduction through photoreceptors such as the phytochromes and cryptochromes [234], as well as an energy source that regulates primary C, N and S assimilation [5,235,236]. The regulation of the phytochrome-dependent, photomorphogenic signal transduction pathways [35,186], as well as the retrograde sensing/signaling pathways between the chloroplast and the nucleus involved in remodeling of the photosynthetic apparatus in response to changes in excitation pressure, are both signaling networks which govern plant plasticity in response to an ever-changing environment. Consequently, we suggest that plants do not exhibit a single low temperature sensor, but rather, they integrate information regarding changes in temperature through changes in the redox state of the photosynthetic electron transport chain, phytochrome, as well as specific cell membrane, low temperature sensors in order to establish the cold-acclimated state.
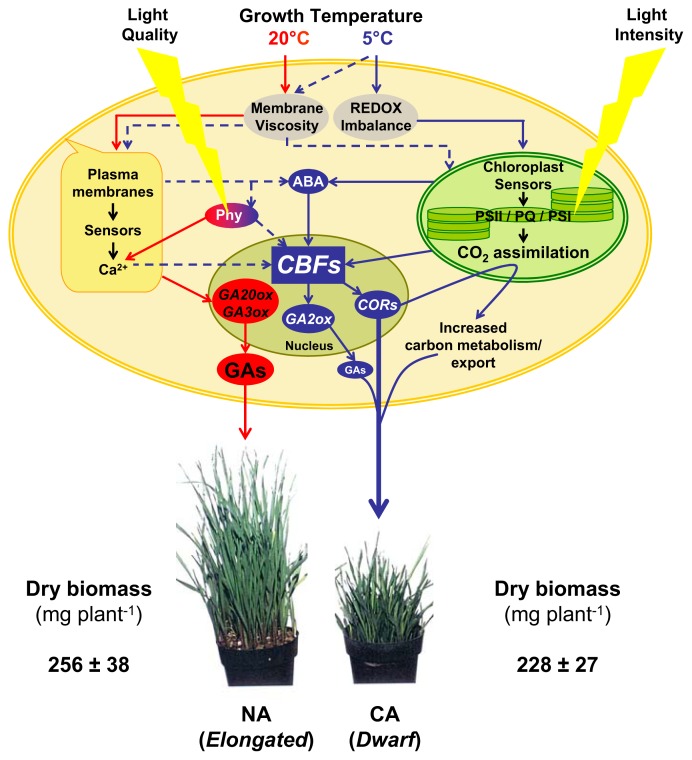
There appears to be a consensus in the literature that ICE1 stimulates AtCBF3 transcription which, in turn, activates the COR family required for cold acclimation. Concomitantly, this cold acclimation process results in the development of a dwarf phenotype [14,16–18]. Overexpression of CBFs mimics the cold acclimation process, as well as the phenotypic plasticity and enhanced photosynthetic performance associated with cold acclimation [2,6,14,16–18,31,51,53]. We reported that COR gene expression, the development of the dwarf plant phenotype, in addition to photosynthetic performance are regulated by the chloroplast redox status measured as excitation pressure [2,6]. Furthermore, Figure 2D illustrates that the expression of AtCBF3, AtCor15a, AtCor15b appeared to be governed by chloroplast excitation pressure modulated either by low temperature or high light. Thus, in Figure 3, we present a simplified schematic model which attempts to illustrate a central role for CBFs in integrating input signals generated by changes in plasma membrane viscosity due to low temperature, as well as changes in light quality sensed through the photoreceptor, phytochrome, but also input signals due to changes in chloroplast redox imbalance, generated either by modulation of growth temperature and/or growth irradiance.
Figure 3.
CBFs as integrators of cold acclimation: a schematic model of plant responses to growth temperature of 20 °C and 5 °C (see text for details). ABA—abscisic acid; CA—cold-acclimated; CBFs—C-repeat binding factors; COR—cold regulated; Gas—growth-active gibberellins; GA2ox—GA2 oxidase; GA3ox—GA3 oxidase; GA20ox—GA20 oxidase; NA—non-acclimated; Phy—phytochromes; PQ—plastoquinone; PSI or PSII—photosystem I or II.
Growth of winter rye plants at 20 °C (“red” pathway) is likely regulated by photoreceptors such as phytochrome as well as the plasma membrane and translated in the downstream mode with Ca2+ as second messenger which ultimately leads to the appearance of a typical elongated phenotype. This is dependent upon the upregulation of GA20ox and GA3ox genes which result in high levels of growth-active GAs. In contrast, growth of winter rye plants at a cold acclimation temperature of 5 °C (the “blue” pathway) to a comparable developmental stage as 20 °C-grown plants is sensed by changes in plasma membrane viscosity (dashed blue line pathway), phytochrome and chloroplast redox imbalance (solid blue line pathway). The increase in plasma membrane viscosity increases Ca2+ levels which eventually leads to upregulation of ABA biosynthesis. ABA biosynthesis may also be induced by a redox imbalance in chloroplast which causes changes in the redox state of the photosynthetic electron transport chain (PSII/PQ/PSI). The increase in ABA biosynthesis activates CBF gene expression which can also be activated independently of ABA, likely directly by chloroplast redox imbalance. Upregulation of CBF genes causes an increase in the expression of COR and GA2ox genes. Upregulation of GA2ox genes reduces the amount of growth-active GA genes which is integral in the generation of the dwarf phenotype. Upregulation of COR genes not only increases cold and freezing tolerance, but also enhances photosynthetic performance via its effect on increased CO2 assimilation, photosynthetic carbon metabolism and carbon export. This results in plants which exhibit a dwarf phenotype at 5 °C and a dry biomass comparable to plants grown at 20 °C. The comparable dry mass between the elongated and dwarf phenotype is accounted for by increased leaf thickness, decreased cellular water content and increased size of crown tissue in cold-acclimated versus non-acclimated plants [2]. Although further research is necessary, we believe that the proposed model provides a framework through which the model can be tested experimentally.
6. Conclusions
In summary, we suggest that CBFs are master regulators of cold acclimation which integrate both the upstream and downstream signals. We propose that input sensors including chloroplast redox imbalance, phytochromes, as well as membrane viscosity are integrated by CBFs, likely directly via upregulation of either ABA metabolism, or indirectly through chloroplast redox imbalance. Furthermore, we propose that upregulation of CBF genes regulates the phenotype and physiological properties of cold-acclimated plants via the two output signals: upregulation of COR genes to induce freezing tolerance and photosynthetic and respiratory genes involved in carbon metabolism, in addition to the upregulation of GA2ox genes to remove growth-active GAs. This results in a dwarf phenotype that exhibits enhanced photosynthetic performance and high biomass coupled with enhanced seed yield [51]. We cannot discount the possible roles of other plant hormones in increased freezing tolerance following a period of cold acclimation. SIZ1-induced inhibition of SA accumulation may be necessary for cold acclimation process [164], and both CKs and BRs may be involved in CBF-regulated cold acclimation. However, a consensus regarding the regulation of CBFs by SA, CKs and BRs remains to be established. We suggest that, due to their photoautotrophic nature, plants probably do not exhibit a single low temperature sensor, but rather, through an integrated network, information regarding changes in temperature, light intensity and light quality are sensed through chloroplast redox imbalance, photoreceptors such as phytochrome, as well as specific cell membrane, low temperature sensors in order to establish the cold-acclimated state. This is consistent with the thesis that the chloroplast has a dual function: not only is it the primary energy transformer for all photoautotrophs, it also acts as a major sensor for detecting changes in the environment [2].
Acknowledgments
We would like to acknowledge the financial support of the KEMPE Foundation, Sweden (VMH, LVK, NPAH), Natural Sciences and Engineering Research Council of Canada (NPAH), the Canada Research Chairs Program (NPAH) and the Canada Foundation for Innovation (NPAH).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Thomashow M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 2.Hüner N.P.A., Öquist G., Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- 3.Wilson K.E., Ivanov A.G., Öquist G., Grodzinski B., Sarhan F., Hüner N.P.A. Energy balance, organellar redox status, and acclimation to environmental stress. Can. J. Bot. 2006;84:1355. –1370. [Google Scholar]
- 4.Hüner N.P.A., Bode R., Dahal K., Hollis L., Rosso D., Krol M., Ivanov A.G. Chloroplast redox imbalance governs phenotypic plasticity: The “grand design of photosynthesis” revisited. Front. Plant Sci. 2012;3 doi: 10.3389/fpls.2012.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hüner N.P.A., Bode R., Dahal K., Busch F.A., Possmayer M., Szyszka B., Rosso D., Ensminger I., Krol M., Ivanov A.G., et al. Shedding some light on cold acclimation, cold adaptation, and phenotypic plasticity. Can. J. Bot. 2013;91:127–136. [Google Scholar]
- 6.Gray G.R., Chauvin L.-P., Sarhan F., Hüner N.P.A. Cold acclimation and freezing tolerance (A complex interaction of light and temperature) Plant Physiol. 1997;114:467–474. doi: 10.1104/pp.114.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theocharis A., Clement C., Barka E.A. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012;235:1091–1105. doi: 10.1007/s00425-012-1641-y. [DOI] [PubMed] [Google Scholar]
- 8.Chinnusamy V., Zhu J, Zhu J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Guy C., Kaplan F., Kopka J., Selbig J., Hincha D.K. Metabolomics of temperature stress. Physiol. Plant. 2008;132:220–235. doi: 10.1111/j.1399-3054.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 10.Penfield S. Temperature perception and signal transduction in plants. New Phytol. 2008;179:615–628. doi: 10.1111/j.1469-8137.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- 11.Galiba G., Vagujfalvi A., Li C.X., Soltesz A., Dubcovsky J. Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Sci. 2009;176:12–19. [Google Scholar]
- 12.Hua J. From freezing to scorching, transcriptional responses to temperature variations in plants. Curr. Opin. Plant Biol. 2009;12:568–573. doi: 10.1016/j.pbi.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Ruelland E., Vaultier M.N., Zachowski A., Hurry V. Cold signalling and cold acclimation in plants. Adv. Bot. Res. 2009;49:35–150. [Google Scholar]
- 14.Thomashow M.F. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010;154:571–577. doi: 10.1104/pp.110.161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina J., Catala R., Salinas J. The CBFs: Three Arabidopsis transcription factors to cold acclimate. Plant Sci. 2011;180:3–11. doi: 10.1016/j.plantsci.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Jaglo-Ottosen K.R., Gilmour S.J., Zarka D.G., Schabenberger O., Thomashow M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- 17.Gilmour S.J., Fowler S.G., Thomashow M.F. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol. Biol. 2004;54:767–781. doi: 10.1023/B:PLAN.0000040902.06881.d4. [DOI] [PubMed] [Google Scholar]
- 18.Lee C.M., Thomashow M.F. Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2012;109:15054–15059. doi: 10.1073/pnas.1211295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockinger E.J., Gilmour S.J., Thomashow M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl. Acad. Sci. USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmour S.J., Zarka D.G., Stockinger E.J., Salazar M.P., Houghton J.M., Thomashow M.F. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina J., Bargues M., Terol J., Perez-Alonso M., Salinas J. The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol. 1999;119:463–470. doi: 10.1104/pp.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novillo F., Medina J., Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc. Natl. Acad. Sci. USA. 2007;104:21002–21007. doi: 10.1073/pnas.0705639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novillo F., Alonso J.M., Ecker J.R., Salinas J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2004;101:3985–3990. doi: 10.1073/pnas.0303029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novillo F., Medina J., Rodriguez-Franco M., Neuhaus G., Salinas J. Genetic analysis reveals a complex regulatory network modulating CBF gene expression and Arabidopsis response to abiotic stress. J. Exp. Bot. 2012;63:293–304. doi: 10.1093/jxb/err279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinnusamy V., Ohta M., Kanrar S., Lee B.H., Hong X., Agarwal M., Zhu J.K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H., Xiong L., Gong Z., Ishitani M., Stevenson B., Zhu J.K. The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo–cytoplasmic partitioning. Genes Dev. 2001;15:912–924. doi: 10.1101/gad.866801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong C.H., Agarwal M., Zhang Y., Xie Q., Zhu J.K. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA. 2006;103:8281–8286. doi: 10.1073/pnas.0602874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura K., Jin J.B., Lee J., Yoo C.Y., Stirm V., Miura T., Ashworth E.N., Bressan R.A., Yun D.J., Hasegawa P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19:1403–1414. doi: 10.1105/tpc.106.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh T.H., Lee J.T., Yang P.T., Chiu L.H., Charng Y.Y., Wang Y.C., Chan M.T. Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 2002;129:1086–1094. doi: 10.1104/pp.003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savitch L.V., Allard G., Seki M., Robert L.S., Tinker N.A., Hüner N.P.A., Shinozaki K., Singh J. The effect of overexpression of two Brassica CBF/DREB1-like transcription factors on photosynthetic capacity and freezing tolerance in Brassica napus. Plant Cell Physiol. 2005;46:1525–1539. doi: 10.1093/pcp/pci165. [DOI] [PubMed] [Google Scholar]
- 32.Benedict C., Skinner J.S., Meng R., Chang Y., Bhalerao R., Hüner N.P.A., Finn C.E., Chen T.H.H., Hurry V. The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ. 2006;29:1259–1272. doi: 10.1111/j.1365-3040.2006.01505.x. [DOI] [PubMed] [Google Scholar]
- 33.Achard P., Gong F., Cheminant S., Alioua M., Hedden P., Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008;20:2117–2129. doi: 10.1105/tpc.108.058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J.-G., Yang M., Liu P., Yang G.-D., Wu C.-A., Zheng C.-C. GhDREB1 enhances abiotic stress tolerance, delays GA-mediated development and represses cytokinin signalling in transgenic Arabidopsis. Plant Cell Environ. 2009;32:1132–1145. doi: 10.1111/j.1365-3040.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 35.Franklin K.A. Light and temperature signal crosstalk in plant development. Curr. Opin. Plant Biol. 2009;12:63–68. doi: 10.1016/j.pbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Clack T., Mathews S., Sharrock R.A. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- 37.Devlin P.F., Halliday K.J., Harberd N.P., Whitelam G.C. The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: Novel phytochromes control internode elongation and flowering time. Plant J. 1996;10:1127–1134. doi: 10.1046/j.1365-313x.1996.10061127.x. [DOI] [PubMed] [Google Scholar]
- 38.Devlin P.F., Patel S.R., Whitelam G.C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1487. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devlin P.F., Robson P.R.H., Patel S.R., Goosey L., Sharrock R.A., Whitelam G.C. Phytochrome D acts in the shade avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franklin K.A., Davis S.J., Stoddart W.M., Vierstra R.D., Whitelam G.C. Mutant analyses define multiple roles for phytochrome C in Arabidopsis photomorphogenesis. Plant Cell. 2003;15:1981–1989. doi: 10.1105/tpc.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinomura T., Nagatani A., Chory J., Furuya M. The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A. Plant Physiol. 1994;104:363–371. doi: 10.1104/pp.104.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monte E., Alonso J.M., Ecker J.R., Zhang Y., Li X., Young J., Austin-Phillips S., Quail P.H. Isolation and characterization of PHYC mutants in Arabidopsis reveals complex crosstalk between phytochrome signalling pathways. Plant Cell. 2003;15:1962–1980. doi: 10.1105/tpc.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aukerman M.J., Hirschfeld M., Wester L., Weaver M., Clack T., Amasino R.M., Sharrock R.A. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanovsky M.J., Casal J.J., Luppi J.P. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signal transduction that correspond to very-low-fluence and high-irradiance responses. Plant J. 1997;12:659–667. doi: 10.1046/j.1365-313x.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- 45.Kurepin L.V., Walton L.J., Hayward A., Emery R.J.N., Pharis R.P., Reid D.M. Interactions between plant hormones and light quality signaling in regulating the shoot growth of Arabidopsis thaliana seedlings. Can. J. Bot. 2012;90:237–246. [Google Scholar]
- 46.Davies P.J. The Plant Hormones: Their Nature, Occurrence, and Functions. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! 3rd ed. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2010. pp. 1–15. [Google Scholar]
- 47.Davies P.J. Regulatory Factors in Hormone Action: Level, Location and Signal Transduction. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! 3rd ed. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2010. pp. 16–35. [Google Scholar]
- 48.Sponsel V.M., Hedden P. Gibberellin Biosynthesis and Inactivation. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! 3rd ed. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2010. pp. 63–94. [Google Scholar]
- 49.Taiz L., Zeiger E. Plant Physiology. 4th ed. Chapter 20. Sinauer Associates Inc; Sunderland, MA, USA: 2006. Gibberellins; p. 511. [Google Scholar]
- 50.Dahal K. Ph.D. Thesis. Western University; London, Canada: Jun, 2012. Plasticity in Photosynthetic Performance and Energy Utilization Efficiency in Triticum aestivum L., Secale cereale L. and Brassica napus in Response to Low Temperature and High CO2. [Google Scholar]
- 51.Dahal K., Gadapati W., Savitch L.V., Singh J., Hüner N.P.A. Cold acclimation and BnCBF17-over-expression enhance photosynthetic performance and energy conversion efficiency during long-term growth of Brassica napus under elevated CO2 conditions. Planta. 2012;236:1639–1652. doi: 10.1007/s00425-012-1710-2. [DOI] [PubMed] [Google Scholar]
- 52.Krol M., Griffith M., Hüner N.P.A. An appropriate physiological control for environmental temperature studies: Comparative growth kinetics for winter rye. Can. J. Bot. 1984;62:1062–1068. [Google Scholar]
- 53.Dahal K., Kane K., Gadapati W., Webb E., Savitch L.V., Singh J., Sharma P., Sarhan F., Longstaffe F.J., Grodzinski B., et al. The effects of phenotypic plasticity on photosynthetic performance in winter rye, winter wheat and Brassica napus. Physiol. Plant. 2012;144:169–188. doi: 10.1111/j.1399-3054.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- 54.Sun T.P. Gibberellin Signal Transduction in Stem Elongation & Leaf Growth. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! revised 3rd ed. Springer Dordrecht Heidelberg; London, New York: 2010. pp. 308–328. [Google Scholar]
- 55.Kurepin L.V., Ozga J.A., Zaman M., Pharis R.P. The physiology of plant hormones in cereal, oilseed and pulse crops. Prairie Soils Crops. 2013;6:7–23. [Google Scholar]
- 56.Reid D.M., Pharis R.P., Roberts D.W.A. Effects of four temperature regimens on the gibberellin content of winter wheat cv. Kharkov. Physiol. Plant. 1973;30:53–57. [Google Scholar]
- 57.Rood S.B., Major D.J., Pharis R.P. Low temperature eliminates heterosis for growth and gibberellin content in maize. Crop Sci. 1985;25:1063–1068. [Google Scholar]
- 58.Kurepin L.V., Walton L.J., Pharis R.P., Emery R.J.N., Reid D.M. Interactions of temperature and light quality on phytohormone-mediated elongation of Helianthus annuus hypocotyls. Plant Growth Regul. 2011;64:147–154. [Google Scholar]
- 59.Sarkar S., Perras M.R., Falk D.E., Zhang R., Pharis R.P., Fletcher R.A. Relationship between gibberellins, height, and stress tolerance in barley (Hordeum vulgare L.) seedlings. Plant Growth Regul. 2004;42:125–135. [Google Scholar]
- 60.Hiller L.K., Kelly W.C., Powell L.E. Temperature interactions with growth regulators and endogenous gibberellin-like activity during seedstalk elongation in carrots. Plant Physiol. 1979;63:1055–1061. doi: 10.1104/pp.63.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinthus M.J., Gale M.D., Appleford N.E.J., Lenton J.R. Effect of temperature on gibberellin (GA) responsiveness and on endogenous GA1 content of tall and dwarf wheat genotypes. Plant Physiol. 1989;90:854–859. doi: 10.1104/pp.90.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen E., Eilertsen S., Ernsten A., Juntilla O., Moe R. Thermoperiodic control of stem elongation and endogenous gibberellins in Campanula isophylla. J. Plant Growth Regul. 1996;15:167–171. [Google Scholar]
- 63.Nishijima T., Nonaka M., Koshioka M., Ikeda H., Douzono M., Yamazaki H., Mander L.N. Role of gibberellins in the thermoperiodic regulation of stem elongation in Dendranthema grandiflorum tzvelev. Biosci.Biotechnol. Biochem. 1997;61:1362–1366. [Google Scholar]
- 64.Fowler S., Thomashow M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kreps J.A., Wu Y., Chang H.S., Zhu T., Wang X., Harper J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seki M., Narusaka M., Ishida J., Nanjo T., Fujita M., Oono Y., Kamiya A., Nakajima M., Enju A., Sakurai T., Satou M., Akiyama K., Taji T., Yamaguchi-Shinozaki K., Carninci P., Kawai J., Hayashizaki Y., Shinozaki K. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002;31:279–292. doi: 10.1046/j.1365-313x.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- 67.Provart N.J., Gil P., Chen W., Han B., Chang H.-S., Wang X., Zhu T. Gene expression phenotypes of Arabidopsis associated with sensitivity to low temperatures. Plant Physiol. 2003;132:893–906. doi: 10.1104/pp.103.021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamauchi Y., Ogawa M., Kuwahara A., Hanada A., Kamiya Y., Yamaguchi S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell. 2004;16:367–378. doi: 10.1105/tpc.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kendall S.A., Hellwege A., Marriot P., Whalley C., Graham I.A., Penfield S. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell. 2011;23:2568–2580. doi: 10.1105/tpc.111.087643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nadeau C.D., Ozga J.A., Kurepin L.V., Jin A., Pharis R.P., Reinecke D.M. Tissue-specific regulation of gibberellin biosynthesis in developing pea seeds. Plant Physiol. 2011;156:897–912. doi: 10.1104/pp.111.172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magome H., Yamaguchi S., Hanada A., Kamiya Y., Oda K. drarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J. 2004;377:720–729. doi: 10.1111/j.1365-313x.2003.01998.x. [DOI] [PubMed] [Google Scholar]
- 72.Kasuga M., Liu Q., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- 73.Gilmour S.J., Sebolt A.M., Salazar M.P., Everard J.D., Thomashow M.F. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000;124:1854–1865. doi: 10.1104/pp.124.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magome H., Yamaguchi S., Hanada A., Kamiya Y., Oda K. The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J. 2008;56:613–626. doi: 10.1111/j.1365-313X.2008.03627.x. [DOI] [PubMed] [Google Scholar]
- 75.Siddiqua M., Nassuth A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ. 2011;34:1345–1359. doi: 10.1111/j.1365-3040.2011.02334.x. [DOI] [PubMed] [Google Scholar]
- 76.Shan D.-P., Huang J.-G., Yang Y.-T., Guo Y.-H., Wu C.-A., Yang G.-D., Gao Z., Zheng C.C. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007;176:70–81. doi: 10.1111/j.1469-8137.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhang F., Wan X.Q., Zhang H.Q., Liu G.L., Jiang M.Y., Pan Y.Z., Chen Q.B. The effect of cold stress on endogenous hormones and CBF1 homolog in four contrasting bamboo species. J. For. Res. 2012;17:72–78. [Google Scholar]
- 78.Peng J., Carol P., Richards D.E., King K.E., Cowling R.J., Murphy G.P., Harberd N.P. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irving R.M., Lanphear F.O. Regulation of cold hardiness in Acer negundo. Plant Physiol. 1968;43:9–13. doi: 10.1104/pp.43.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rikin A., Atsmon D., Gitler C. Chilling injury in cotton (Gossypium hirsutum L.): Prevention by abscisic acid. Plant Cell Physiol. 1979;20:1537–2546. [Google Scholar]
- 81.Rikin A., Waldman M., Richmond A.E., Dovrat A. Hormonal regulation of morphogenesis and cold resistance. I. Modifications by abscisic acid and by gibberellic acid in alfalfa (Medicago sativa L.) seedlings. J. Exp.Bot. 1975;16:175–183. [Google Scholar]
- 82.Chen T.H.H., Gusta L.V. Abscisic acid-induced freezing resistance in cultured plant cells. Plant Physiol. 1983;73:71–75. doi: 10.1104/pp.73.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lalk I., Dorfling K. Hardening, abscisic acid, praline and freezing resistance in two winter wheat varieties. Physiol. Plant. 1985;63:287–292. [Google Scholar]
- 84.Xing W., Rajashekar C.B. Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ. Exp. Bot. 2001;46:21–28. doi: 10.1016/s0098-8472(01)00078-8. [DOI] [PubMed] [Google Scholar]
- 85.Nayyar H., Bains T., Kumar S. Low temperature induced floral abortion in chickpea: Relationship to abscisic acid and cryoprotectants in reproductive organs. Environ. Exp. Bot. 2004;52:219–231. [Google Scholar]
- 86.Dale J., Campbell W.F. Response of tomato plants to stressful temperatures: Increase in abscisic acid concentrations. Plant Physiol. 1981;67:26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lang V., Mantyla E., Welin B., Sundberg B., Palva E.T. Alterations in water status, endogenous abscisic acid content, and expression of rab18 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol. 1994;104:1341–1349. doi: 10.1104/pp.104.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mantyla E., Lang V., Palva E.T. Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol. 1995;107:141–148. doi: 10.1104/pp.107.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gilmour S.J., Thomashow M.F. Cold acclimation and cold-regulated gene expression in ABA mutants of Arabidopsis thaliana. Plant Mol. Biol. 1991;17:1233–1240. doi: 10.1007/BF00028738. [DOI] [PubMed] [Google Scholar]
- 90.Xiong L., Lee B.H., Ishitani M., Lee H., Zhu J.K. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J. Biol. Chem. 2002;56:277–289. doi: 10.1074/jbc.M109275200. [DOI] [PubMed] [Google Scholar]
- 91.Fuchigami L.H., Evert D.R., Weiser C.J. A translocatable cold hardiness promoter. Plant Physiol. 1971;47:164–167. doi: 10.1104/pp.47.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fayyaz M.M., McCown B.H., Beck G.H. Effect of temperature, photoperiod and several growth substances on the cold hardiness of Chrysanthemum morifolium rhizome. Physiol. Plant. 1978;44:73–76. [Google Scholar]
- 93.Gusta L.V., Fowler D.B., Tyler N.J. The effect of abscisic acid and cytokinins on the cold hardiness of winter wheat. Can. J. Bot. 1982;60:301–305. [Google Scholar]
- 94.Holubowicz T., Cummins J.N., Forsline P.L. Responses of Malus clones to programmed low-temperature stresses in late winter. J. Am. Soc. Hortic. Sci. 1982;107:492–496. [Google Scholar]
- 95.Holubowicz T., Pieniazek J., Khamis M.A. Plant Hardiness and Freezing Stress. Academic Press; New York, NY, USA: 1982. Modification of Frost Resistance of Fruit Plants by Applied Growth Regulators; pp. 541–559. [Google Scholar]
- 96.Lang V., Palva E.T. The expression of rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol. Biol. 1992;20:951–962. doi: 10.1007/BF00027165. [DOI] [PubMed] [Google Scholar]
- 97.Kurkela S., Franck M. Structure and expression of kin2, one of two cold- and ABA-induced genes of Arabidopsis thaliana. Plant Mol. Biol. 1992;19:689–692. doi: 10.1007/BF00026794. [DOI] [PubMed] [Google Scholar]
- 98.Knight H., Zarka D.G., Okamoto H., Thomashow M.F., Knight M.R. Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol. 2004;135:1710–1717. doi: 10.1104/pp.104.043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao H., Siddiqua M., Braybrook S., Nassuth A. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ. 2006;29:1410–1421. doi: 10.1111/j.1365-3040.2006.01524.x. [DOI] [PubMed] [Google Scholar]
- 100.Skinner J.S., von Zitzewitz J., Szucs P., Marquez-Cedillo L., Filichkin T., Amundsen K., Stockinger E.J., Thomasow M.F., Chen T.N.N., Hayes P.M. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Mol. Biol. 2005;59:533–551. doi: 10.1007/s11103-005-2498-2. [DOI] [PubMed] [Google Scholar]
- 101.Shinozaki K., Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000;3:217–223. [PubMed] [Google Scholar]
- 102.Gusta L.V., Trischuk R., Weiser C.J. Plant cold acclimation: The role of abscisic acid. J. Plant Growth Regul. 2005;24:308–318. [Google Scholar]
- 103.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu.Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 104.Trewavas A.J., Jones H.G. An Assessment of the Role of Aba in Plant Development. In: Davies W.J., Jones H.G., editors. Abscisic Acid: Physiology and Biochemistry. Bios Scientific Publishers; Oxford, UK: 1991. pp. 169–188. [Google Scholar]
- 105.Munns R., Cramer G.R. Is coordination of leaf and root growth mediated by abscisic acid? Plant Soil. 1996;185:33–49. [Google Scholar]
- 106.Schwartz S.H., Zeevaart J.A.D. Abscisic Acid Biosynthesis and Metabolism. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! 3rd ed. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2010. pp. 137–155. [Google Scholar]
- 107.Zentella R., Zhang Z.L., Park M., Thomas S.G., Endo A., Murase K., Fleet C.M., Jikumaru Y., Nambara E., Kamiya Y., et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell. 2007;19:3037–3057. doi: 10.1105/tpc.107.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toh S., Imamura A., Watanabe A., Nakabayashi K., Okamoto M., Jikumaru Y., Hanada A., Aso Y., Ishiyama K., Tamura N., et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008;146:1368–1385. doi: 10.1104/pp.107.113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roef L., van Onckelen H. Cytokinin Regulation of the Cell Division Cycle. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! 3rd ed. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2010. pp. 241–261. [Google Scholar]
- 110.Ha S., Vankova R., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.S.P. Cytokinins: Metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 2012;17:172–179. doi: 10.1016/j.tplants.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 111.Veselova S.V., Farhutdinov R.G., Veselov S.Y., Kudoyarova G.R., Veselov D.S., Hartung W. The effect of root cooling on hormone content, leaf conductance and root hydraulic conductivity of durum wheat seedlings (Triticum durum L.) J. Plant Physiol. 2005;162:21–26. doi: 10.1016/j.jplph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 112.Nishiyama R., Watanabe Y., Fujita Y., Le D.T., Kojima M., Werner T., Vankova R., Yamaguchi-Shinozaki K., Shinozaki K., Kakimoto T., et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 2011;23:2169–2183. doi: 10.1105/tpc.111.087395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sakakibara H. Cytokinin Biosynthesis Metabolism. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! 3rd ed. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2010. pp. 95–114. [Google Scholar]
- 114.Hwang I., Sheen J., Muller B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012;63:353–380. doi: 10.1146/annurev-arplant-042811-105503. [DOI] [PubMed] [Google Scholar]
- 115.Chaudhury A.M., Letham S., Craig S., Dennis E.S. amp1—A mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993;4:907–916. [Google Scholar]
- 116.Xia J., Zhao H., Liu W., Li L., He Y. Role of cytokinin and salicylic acid in plant growth at low temperatures. Plant Growth Regul. 2009;57:211–221. [Google Scholar]
- 117.Jeon J., Kim N.Y., Kim S., Kang N.Y., Novak O., Ku S.J., Cho C., Lee D.J., Lee E.J., Strnad M., et al. A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J. Biol. Chem. 2010;285:23371–23386. doi: 10.1074/jbc.M109.096644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi Y., Tian S., Hou L., Huang X., Zhang X., Guo H., Yang C. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell. 2012;24:2578–2595. doi: 10.1105/tpc.112.098640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abeles F.B., Morgan P.W., Saltveit M.E. Ethylene in Plant Biology. 2nd ed. Academic Press; New York, NY, USA: 1992. [Google Scholar]
- 120.Kurepin L.V., Walton L.J., Reid D.M. Interaction of red to far red light ratio and ethylene in regulating stem elongation of Helianthus annuus. Plant Growth Regul. 2007;51:53–61. [Google Scholar]
- 121.Kurepin L.V., Walton L.J., Yeung E.C., Chinnappa C.C., Reid D.M. The interaction of light irradiance with ethylene in regulating growth of Helianthus annuus shoot tissues. Plant Growth Regul. 2010;62:43–50. [Google Scholar]
- 122.Wang C.Y., Adams D.O. Chilling-induced ethylene production in cucumbers (Cucumis sativus L.) Plant Physiol. 1982;69:424–427. doi: 10.1104/pp.69.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ciardi J.A., Deikman J., Orzolek M.D. Increased ethylene synthesis enhances chilling tolerance in tomato. Physiol. Plant. 1997;101:333–340. [Google Scholar]
- 124.Yu X.M., Griffith M., Wiseman S.B. Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol. 2001;126:1232–1240. doi: 10.1104/pp.126.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z., Huang R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010;73:241–249. doi: 10.1007/s11103-010-9609-4. [DOI] [PubMed] [Google Scholar]
- 126.Field R.J. The role of 1-aminocyclopropane-1-carboxylic acid in the control of low temperature induced ethylene production in leaf tissue of Phaseolus vulgaris L. Ann. Bot. 1984;54:61–67. [Google Scholar]
- 127.Machacckova I., Hanisova A., Krekule J. Levels of ethylene, ACC, MACC, ABA and proline as indicators of cold hardening and frost resistance in winter wheat. Physiol. Plant. 1989;76:603–607. [Google Scholar]
- 128.Collins G.G., Nie X., Saltveit M.E. Heat shock increases chilling tolerance of mung bean hypocotyls tissues. Physiol. Plant. 1995;89:117–124. [Google Scholar]
- 129.Chang C., Kwok S.F., Bleecker A.B., Meyerowitz E.M. Arabidopsis ethylene-response gene ETR1: Similarity of product to two component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 130.Bleecker A.B., Esch J.J., Hall A.E., Rodriguez F.I., Binder B.M. The ethylene-receptor family from Arabidopsis—Structure and function. Philos. Trans. R. Soc. B. 1998;353:1405–1412. doi: 10.1098/rstb.1998.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Solano R., Stepanova A., Chao Q., Ecker J.R. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sharabi-Schwager M., Samach A., Porat R. Overexpression of the CBF2 transcriptional activator in Arabidopsis suppresses the responsiveness of leaf tissue to the stress hormone ethylene. Plant Biol. 2010;12:630–638. doi: 10.1111/j.1438-8677.2009.00255.x. [DOI] [PubMed] [Google Scholar]
- 133.Nagata N., Min Y.K., Nakano T., Asami T., Yoshida S. Treatment of dark-grown Arabidopsis thaliana with a brassinosteroid biosynthesis inhibitor, brassinazole, induces some characteristics of light-grown plants. Planta. 2000;211:781–790. doi: 10.1007/s004250000351. [DOI] [PubMed] [Google Scholar]
- 134.Zullo M.A.T., Adam G. Brassinosteroid phytohormones—Structure, bioactivity and applications. Braz. J. Plant Physiol. 2002;14:143–181. [Google Scholar]
- 135.Choe S. Brassinosteroid Biosynthesis Metabolism. In: Davies P.J., editor. Plant Hormones: Biosynthesis, Signal Transduction and Action! 3rd ed. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2010. pp. 156–178. [Google Scholar]
- 136.Kurepin L.V., Joo S.H., Kim S.K., Pharis R.P., Back T.G. Interaction of brassinosteroids with light quality and plant hormones in regulating shoot growth of young sunflower and Arabidopsis seedlings. J. Plant Growth Regul. 2012;31:156–164. [Google Scholar]
- 137.Wilen R.W., Sacco M., Gusta L.V., Krishna P. Effects of 24-epibrassinolide on freezing and thermotolerance of bromegrass (Bromus inermis) cell cultures. Physiol. Plant. 1995;95:195–202. [Google Scholar]
- 138.Khripach V.A., Zhabinskii V.N., de Groot A.E. Brassinosteroids: A New Class of Plant Hormones. Academic Press; San Diego, CA, USA: 1999. pp. 263–277. [Google Scholar]
- 139.Kurepin L.V., Qaderi M.M., Back T.G., Reid D.M., Pharis R.P. A rapid effect of applied brassinolide on abscisic acid concentrations in Brassica napus leaf tissue subjected to short-term heat stress. Plant Growth Regul. 2008;55:165–167. [Google Scholar]
- 140.He R.Y., Wang G.Y., Wang S.X. Effect of Brassinolide on Growth and Chilling Resistance of Maize Seedlings. In: Cutler H.G., Yokoda T., Adam G., editors. Brassinosteroids: Chemistry, Bioactivity and Applications. American Chemical Society; Washington, DC, USA: 1991. pp. 220–230. [Google Scholar]
- 141.Kagale S., Divi U.K., Krochko J.E., Keller W.A., Krishna P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta. 2007;225:353–364. doi: 10.1007/s00425-006-0361-6. [DOI] [PubMed] [Google Scholar]
- 142.Kim S.Y., Kim B.H., Lim C.J., Lim C.O., Nam K.H. Constitutive activation of stress-inducible genes in a brassinosteroid-insensitive 1 (bri1) mutant results in higher tolerance to cold. Physiol. Plant. 2010;138:191–204. doi: 10.1111/j.1399-3054.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z.Y., Seto H., Fujioka S., Yoshida S., Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- 144.Li J., Wen J., Lease K.A., Doke J.T., Tax F.E., Walker J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 145.Divi U.K., Krishna P. Overexpression of the brassinosteroid biosynthetic gene AtDWF4 in Arabidopsis seeds overcomes abscisic acid-induced inhibition of germination and increases cold tolerance in transgenic seedlings. J. Plant Growth Regul. 2010;29:385–393. [Google Scholar]
- 146.Kim H.B., Kwon M., Ryu H., Fujioka S., Takatsuto S., Yoshida S., An C.S., Lee I., Hwang I., Choe S. The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis. Plant Physiol. 2006;140:548–557. doi: 10.1104/pp.105.067918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Raskin I. Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:439–463. [Google Scholar]
- 148.Raskin I. Salicylic Acid. In: Davies P.J., editor. Plant Hormones, Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1995. pp. 188–205. [Google Scholar]
- 149.Schettel N.L., Balke N.E. Plant growth response to several allelopathic chemicals. Weed Sci. 1983;31:293–298. [Google Scholar]
- 150.Hayat Q., Hayat S., Irfan M., Ahmad A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010;68:14–25. [Google Scholar]
- 151.Janda T., Szalai G., Antunovics Z.S., Horvath E., Paldi E. Effect of benzoic acid and aspirin on chilling tolerance and photosynthesis in young maize plants. Maydica. 2000;45:29–33. [Google Scholar]
- 152.Janda T., Szalai G., Tari I., Paldi E. Hydroponic treatment with salicylic acid decreases the effect of chilling injury in maize (Zea mays L.) plants. Planta. 1999;208:175–180. [Google Scholar]
- 153.Ding C.K., Wang C.Y., Gross K.C., Smith D.L. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002;214:895–901. doi: 10.1007/s00425-001-0698-9. [DOI] [PubMed] [Google Scholar]
- 154.Kang G.Z., Wang C.H., Sun G.C., Wang Z.X. Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ. Exp. Bot. 2003;50:9–15. [Google Scholar]
- 155.Kang H.M., Saltveit M.E. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Plant. 2002;115:571–576. doi: 10.1034/j.1399-3054.2002.1150411.x. [DOI] [PubMed] [Google Scholar]
- 156.Kurepin L.V., Dahal K.P., Zaman M., Pharis R.P. Interplay between Environmental Signals and Endogenous Salicylic Acid Concentration. In: Hayat S., Ahmad A., Alyemini M.N., editors. Salicylic Acid: Plant Growth and Development. Springer Science+Business Media B.V.; Dordrecht, The Netherlands: 2013. pp. 61–82. [Google Scholar]
- 157.Farooq M., Aziz T., Basra S.M.A., Cheema M.A., Rehman H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 2008;194:161–168. [Google Scholar]
- 158.Lei T., Feng H., Sun X., Dai Q.-L., Zhang F., Liang H.-G., Lin H.-H. The alternative pathway in cucumber seedlings under low temperature stress was enhanced by salicylic acid. Plant Growth Regul. 2010;60:35–42. [Google Scholar]
- 159.Mora-Herrera M.E., Lopez-Delgado H., Castillo-Morales A., Foyer C.H. Salicylic acid and H2O2 function by independent pathways in the induction of freezing tolerance in potato. Physiol. Plant. 2005;125:430–440. [Google Scholar]
- 160.Wang L., Chen S., Kong W., Li S., Archbold D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006;41:244–251. [Google Scholar]
- 161.Bowling S.A., Clarke J.D., Liu Y., Klessig D.F., Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Falk A., Feys B.J., Frost L.N., Jones J.D., Daniels M.J., Parker J.E. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA. 1999;96:3292–3297. doi: 10.1073/pnas.96.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Scott I.M., Clarke S.M., Wood J.E., Mur L.A.J. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004;135:1040–1049. doi: 10.1104/pp.104.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miura K., Ohta M. SIZ1, a small ubiquitin-related modifier ligase, controls cold signaling through regulation of salicylic acid accumulation. J. Plant Physiol. 2010;167:555–560. doi: 10.1016/j.jplph.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 165.Levitt J. Chilling, Freezing, and High Temperature Stresses. Vol. 1. Academic Press; New York, NY, USA: 1980. Responses of Plants to Environmental Stresses; p. 497. [Google Scholar]
- 166.Demmig-Adams B, Adams W.W., III Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992;43:599–626. [Google Scholar]
- 167.Anderson J.M., Chow W.S., Park Y.-I. The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynth. Res. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- 168.Fey V., Wagner R., Brautigam K., Pfannschmidt T. Photosynthetic redox control of nuclear gene expression. J. Exp. Bot. 2005;56:1491–1498. doi: 10.1093/jxb/eri180. [DOI] [PubMed] [Google Scholar]
- 169.Ensminger I., Busch F., Hüner N.P.A. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006;126:28–44. [Google Scholar]
- 170.Murchie E.H., Pinto M., Horton P. Agriculture and the new challenges for photosynthesis research. New Phytol. 2009;181:532–552. doi: 10.1111/j.1469-8137.2008.02705.x. [DOI] [PubMed] [Google Scholar]
- 171.Kurepin L.V., Emery R.J.N., Pharis R.P., Reid D.M. The interaction of light quality and irradiance with gibberellins, cytokinins and auxin in regulating growth of Helianthus annuus hypocotyls. Plant Cell Environ. 2007;30:147–155. doi: 10.1111/j.1365-3040.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- 172.Kurepin L.V., Emery R.J.N., Pharis R.P., Reid D.M. Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: Putative roles for plant hormones in leaf and internode growth. J. Exp. Bot. 2007;58:2145–2157. doi: 10.1093/jxb/erm068. [DOI] [PubMed] [Google Scholar]
- 173.Walters R.G., Rogers J.J.M., Shephard F., Horton P. Acclimation of Arabidopsis thaliana to the light environment: The role of photoreceptors. Planta. 1999;209:517–527. doi: 10.1007/s004250050756. [DOI] [PubMed] [Google Scholar]
- 174.Pogson B.J., Woo N.S., Förster B., Small I.D. Plastid signalling to the nucleus and beyond. Trends Plant Sci. 2008;13:602–609. doi: 10.1016/j.tplants.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 175.Estavillo G.M., Chan K.X., Phua S.Y., Pogson B.J. Reconsidering the nature and mode of action of metabolite retrograde signals from the chloroplast. Front. Plant Physiol. 2013;3 doi: 10.3389/fpls.2012.00300. doi:310.3389/fpls.2012.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wilson K.E., Krol M., Hüner N.P.A. Temperature-induced greening of Chlorella vulgaris. The role of the cellular energy balance and zeaxanthin-dependent nonphotochemical quenching. Planta. 2003;217:616–627. doi: 10.1007/s00425-003-1021-8. [DOI] [PubMed] [Google Scholar]
- 177.Kim H.-J., Kim Y.-K., Park J.-Y., Kim J. Light signalling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana. Plant J. 2002;2:693–704. doi: 10.1046/j.1365-313x.2002.01249.x. [DOI] [PubMed] [Google Scholar]
- 178.Nott A., Jung H.-S., Koussevitzky S., Chory J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006;57:739–759. doi: 10.1146/annurev.arplant.57.032905.105310. [DOI] [PubMed] [Google Scholar]
- 179.Fernandez A.P., Strand A. Retrograde signaling and plant stress: Plastid signals initiate cellular stress responses. Curr. Opin. Plant Biol. 2008;11:509–513. doi: 10.1016/j.pbi.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 180.Jung H.-S., Chory J. Signaling between chloroplasts and the nucleus: Can a systems biology approach bring clarity to a complex and highly regulated pathway? Plant Physiol. 2010;152:453–459. doi: 10.1104/pp.109.149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Foyer C.H., Neukermans J., Queval G., Noctor G., Harbinson J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012;63:1637–1661. doi: 10.1093/jxb/ers013. [DOI] [PubMed] [Google Scholar]
- 182.Munné-Bosch S., Queval G., Foyer C.H. The impact of global change factors on redox signaling underpinning stress tolerance. Plant physiol. 2013;161:5–19. doi: 10.1104/pp.112.205690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dietz K.-J. Redox signal integration: From stimulus to networks and genes. Physiol. Plant. 2008;133:459–468. doi: 10.1111/j.1399-3054.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 184.Dietz K.-J., Pfannschmidt T. Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant physiol. 2011;155:1477–1485. doi: 10.1104/pp.110.170043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fowler S.G., Cook D., Thomashow M.F. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 2005;137:961–968. doi: 10.1104/pp.104.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Franklin K.A., Whitelam G.C. Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat. Genet. 2007;39:1410–1413. doi: 10.1038/ng.2007.3. [DOI] [PubMed] [Google Scholar]
- 187.Kidokoro S., Maruyama K., Nakashima K., Imura Y., Narusaka Y., Shinwari Z.K., Osakabe Y., Fujita Y., Mizoi J., Shinozaki K., et al. The phytochrome-interacting factor PIF7 negatively regulates DREB1 expression under circadian control in Arabidopsis. Plant Physiol. 2009;151:2046–2057. doi: 10.1104/pp.109.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.De Lucas M., Daviere J.M., Rodrıguez-Falcon M., Pontin M., Iglesias-Pedraz J.M., Lorrain S., Fankhauser C., Blasquez M.A., Titarenko E., Prat S. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–486. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- 189.Feng S., Martinez C., Gusmaroli G., Wang Y., Zhou J., Wang F., Chen L., Yu L., Iglesias-Pedraz J.M., Kircher S. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hurry V.M., Hüner N.P.A. Low growth temperature effects a differential inhibition of photosynthesis in spring and winter wheat. Plant Physiol. 1991;96:491–497. doi: 10.1104/pp.96.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hurry V.M., Strand A., Tobiaeson M., Gardeström P., Öquist G. Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiol. 1995;109:697–706. doi: 10.1104/pp.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hurry V., Strand Å., Furbank R., Stitt M. The role of inorganic phosphate in the developpment of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutantsof Arabidopsis thaliana. Plant J. 2000;24:383–396. doi: 10.1046/j.1365-313x.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- 193.Stitt M., Hurry V. A plant for all seasons: Alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr. Opin. Plant Biol. 2002;5:199–206. doi: 10.1016/s1369-5266(02)00258-3. [DOI] [PubMed] [Google Scholar]
- 194.Öquist G., Hüner N.P.A. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003;54:329–355. doi: 10.1146/annurev.arplant.54.072402.115741. [DOI] [PubMed] [Google Scholar]
- 195.Hurry V.M., Malmberg G., Gardeström P., Öquist G. Effects of a short-term shift to low temperature and of long-term cold hardening on photosynthesis and ribulose 1,5-bisphosphate carboxylase/oxygenase and sucrose phosphate synthase activity in leaves of winter rye (Secale cereale L.) Plant Physiol. 1994;106:983–990. doi: 10.1104/pp.106.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Strand A., Hurry V., Henkes S., Hüner N.P.A., Gustafsson P., Gardestrom P., Stitt M. Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol. 1999;119:1387–1397. doi: 10.1104/pp.119.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Savitch L.V., Leonardos E.D., Krol M., Jansson S., Grodzinski B., Hüner N.P.A., Öquist G. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ. 2002;25:761–771. [Google Scholar]
- 198.Rapacz M., Wolanin B., Hura K., Tyrka M. The effects of cold acclimation on photosynthetic apparatus and the expression of COR14b in four genotypes of barley (Hordeum vulgare) contrasting in their tolerance to freezing and high-light treatment in cold conditions. Ann. Bot. 2008;101:689–699. doi: 10.1093/aob/mcn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leonardos E.D., Savitch L.V., Hüner N.P.A., Öquist G., Grodzinski B. Daily photosynthetic and C-export patterns in winter wheat leaves during cold stress and acclimation. Physiol. Plant. 2003;117:521–531. doi: 10.1034/j.1399-3054.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 200.Lundmark M., Cavaco A.M., Trevanion S., Hurry V. Carbon partitioning and export in transgenic Arabidopsis thaliana with altered capacity for sucrose synthesis grown at low temperature: A role for metabolite transporters. Plant Cell Environ. 2006;29:1703–1714. doi: 10.1111/j.1365-3040.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 201.Pollock C.J., Cairns A.J. Fructan metabolism in grasses and cereals. Annu. Rev. Plant Biol. 1991;42:77–101. [Google Scholar]
- 202.Savitch L.V., Harney T., Hüner N.P.A. Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimation. Physiol. Plant. 2000;108:270–278. [Google Scholar]
- 203.Hurry V.M., Gardeström P., Öquist G. Reduced sensitivity to photoinhibition following frost-hardening of winter rye is due to increased phosphate availability. Planta. 1993;190:484–490. [Google Scholar]
- 204.Strand A., Foyer C.H., Gustafsson P., Gardestrom P., Hurry V. Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant Cell Environ. 2003;26:523–535. [Google Scholar]
- 205.Hurry V., Keerberg O., Pärnik T., Öquist G., Gardeström P. Effect of cold hardening on the components of respiratory decarboxylation in the light and in the dark in leaves of winter rye. Plant Physiol. 1996;111:713–719. doi: 10.1104/pp.111.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gray G.R., Heath D. A global reorganization of the metabolome in Arabidopsis during cold acclimation is revealed by metabolic finger printing. Physiol. Plant. 2005;124:236–248. [Google Scholar]
- 207.Öquist G., Hurry V.M., Hüner N.P.A. The temperature dependence of the redox state of QA and the susceptibility of photosynthesis to photoinhibition. Plant Physiol. Biochem. 1993;31:683–691. [Google Scholar]
- 208.Krause G.H. Photoinhibition of photosynthesis: An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988;74:566–574. [Google Scholar]
- 209.Boese S.R., Hüner N.P.A. Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol. 1990;94:1830–1836. doi: 10.1104/pp.94.4.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gray G.R., Savitch L.V., Ivanov A.G., Hüner N.P.A. Photosystem II excitation pressure and development of resistance to photoinhibition: II. Adjustment of photosynthetic capacity in winter wheat and winter rye. Plant Physiol. 1996;110:61–71. doi: 10.1104/pp.110.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pocock T.H., Hurry V., Savitch L.V., Hüner N. Susceptibility to low-temperature photoinhibition and the acquisition of freezing tolerance in winter and spring wheat: The role of growth temperature and irradiance. Physiol. Plant. 2001;113:499–506. [Google Scholar]
- 212.Savitch L.V., Barker-Åström J., Ivanov A.G., Hurry V., Öquist G., Hüner N.P.A., Gardeström P. Cold acclimation of Arabidopsis thaliana results in incomplete recovery of photosynthetic capacity, associated with an increased reduction of the chloroplast stroma. Planta. 2001;214:295–303. doi: 10.1007/s004250100622. [DOI] [PubMed] [Google Scholar]
- 213.Hüner N.P.A., Hopkins W.G. Growth and development of winter rye at cold-hardening temperatures results in thylakoid membranes with increased sensitivity to low concentrations of osmoticum. Physiol. Plant. 1985;64:468–476. [Google Scholar]
- 214.Sarhan F., Ouellet F., Vazquez-Tello A. The wheat wcs120 gene family. A useful model to understand the molecular genetics of freezing tolerance in cereals. Physiol. Plant. 1997;101:439–445. [Google Scholar]
- 215.Thomashow M.F. So what’s new in the field of plant cold acclimation? Lots! Plant Physiol. 2001;125:89–93. doi: 10.1104/pp.125.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hüner N.P.A., Palta J.P., Li P.H., Carter J.V. Anatomical changes in leaves of puma rye in response to growth at cold-hardening temperatures. Bot. Gaz. 1981;142:55–62. [Google Scholar]
- 217.Gorsuch P.A., Pandey S., Atkin O.K. Temporal heterogeneity of cold acclimation phenotypes in Arabidopsis leaves. Plant Cell Environ. 2010;33:244–258. doi: 10.1111/j.1365-3040.2009.02074.x. [DOI] [PubMed] [Google Scholar]
- 218.Gorsuch P.A., Pandey S., Atkin O.K. Thermal de-acclimation: How permanent are leaf phenotypes when cold-acclimated plants experience warming? Plant Cell Environ. 2010;33:1124–1137. doi: 10.1111/j.1365-3040.2010.02134.x. [DOI] [PubMed] [Google Scholar]
- 219.Hüner N.P.A., Elfman B., Krol M., MacIntosh A. Growth and development at cold hardening temperatures. Chloroplast ultrastructure, pigment content and composition. Can. J. Bot. 1984;62:53–60. [Google Scholar]
- 220.Guy C.L., Huber J.L.A., Huber S.C. Sucrose phosphate synthase and sucrose accumilation at low temperature. Plant Physiol. 1992;100:502–508. doi: 10.1104/pp.100.1.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Miśkiewicz E., Ivanov A.G., Williams J.P., Khan M.U., Falk S., Hüner N.P.A. Photosynthetic acclimation of the filamentous cyanobacterium, Plectonema boryanum UTEX 485, to temperature and light. Plant Cell Physiol. 2000;41:767–775. doi: 10.1093/pcp/41.6.767. [DOI] [PubMed] [Google Scholar]
- 222.Rosso D., Bode R., Li W., Krol M., Saccon D., Wang S., Schillaci L., Rodermel S.R., Maxwell D.P., Hüner N.P.A. Photosynthetic redox imbalance governs leaf sectoring in the Arabidopsis thaliana variegation mutants immutans, spotty, var1, and var2. Plant Cell. 2009;21:3473–3492. doi: 10.1105/tpc.108.062752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bode R. Ph.D. Thesis. Western University; London, Canada: Jan, 2013. Effects of excitation pressure on variegation and global gene expression in Arabidopsis thaliana. [Google Scholar]
- 224.Yang J.S., Wang R., Meng J.J., Bi Y.P., Xu P.L., Guo F., Wan S.B., He Q.W., Li X.G. Overexpression of Arabidopsis CBF1 gene in transgenic tobacco alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. J. Plant Physiol. 2010;167:534–539. doi: 10.1016/j.jplph.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 225.Xu Y.Z., Santamaria R.R., Virdi K.S., Arrieta-Montiel M.P., Razvi F., Li S., Ren G., Yu B., Alexander D., Guo L., et al. The chloroplast triggers developmental reprogramming when MUTS HOMOLOG1 is suppressed in plants. Plant Physiol. 2012;159:710–720. doi: 10.1104/pp.112.196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Murata N., Los D.A. Membrane fluidity and temperature perception. Plant Physiol. 1997;115:875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Los D.A., Murata N. Sensing and Response to Low Temperature in Cyanobacteria. In: Storey K.B., Storey J.M., editors. Sensing, signaling and cell adaptation: Cell and Molecular Responses to Stress. Vol. 3. Elsevier Press; Amsterdam, Holland: 2002. pp. 139–153. [Google Scholar]
- 228.Xin Z. Acquired Freezing Tolerance in Higher Plants: The Sensing and Molecular Responses to Low Nonfreezing Temperatures. In: Storey K.B., Storey J.M., editors. Sensing, Signaling and Cell Adaptation: Cell and Molecular Responses to Stress. Vol. 3. Elsevier Press; Amsterdam, Holland: 2002. pp. 121–137. [Google Scholar]
- 229.Monroy A.F., Sangwan V., Dhindsa R.S. Low temperature signal transduction during cold acclimation: Protein phosphatase 2A as an early target for cold-inactivation. Plant J. 1998;13:653–660. [Google Scholar]
- 230.Plieth C., Hansen U.F., Knight H., Knight M.R. Temperature sensing by plants: The primary characteristics of signal perception and calcium response. Plant J. 1999;18:491–497. doi: 10.1046/j.1365-313x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 231.Örvar B.L., Sangwan V., Omann F., Dhindsa R.S. Early steps in cold sensing by plant cells: The role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23:785–794. doi: 10.1046/j.1365-313x.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 232.Zarka D.G., Vogel J.T., Cook D., Thomashow M.F. Cold induction of Arabidopsis CBF genes involves multiple ICE (Inducer of CBF Expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol. 2003;133:910–918. doi: 10.1104/pp.103.027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Benedict C., Geisler M., Trygg J., Hüner N.P.A., Hurry V. Consensus by democracy. Using meta-analyses of microarray and genomic data to model the cold acclimation signaling pathway in Arabidopsis. Plant Physiol. 2006;141:1219–1232. doi: 10.1104/pp.106.083527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Waters M.T., Langdale J.A. The making of a chloroplast. EMBO J. 2009;28:2861–2873. doi: 10.1038/emboj.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Foyer C.H., Noctor G. Photosynthetic Nitrogen Assimilation: Inter-Pathway Control and Signalling. In: Foyer C.H., Noctor G., editors. Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism. Vol. 12. Kluwer; Amsterdam, Holland: 2002. pp. 1–22. [Google Scholar]
- 236.Hüner N.P.A., Grodzinski B. Photosynthesis and Photoautotrophy. In: Moo-Young M., editor. Comprehensive Biotechnology. Vol. 1. Elsevier; London, UK: 2011. pp. 315–322. [Google Scholar]