Abstract
Histamine is known to have a profound effect on capillary permeability in nonrenal tissues and this effect is presumably mediated by cyclic (c)AMP. Because in our previous experiments we found that histamine stimulates cAMP accumulation in glomeruli (Torres, V. E., T. E. Northryn, R. M. Edwards, S. V. Shah, and T. P. Dousa. 1978. Modulation of cyclic nucleotides in isolated rat glomeruli. J. Clin. Invest.62: 1334.), we now explored whether this amine is formed in renal tissue, namely in glomeruli, and whether its renal metabolism is altered in experimental nephrosis induced by puromycin aminonucleoside (PA) in rats. In normal rats, histamine content was higher (Δ + 240%) in cortex than in medulla. In glomeruli isolated from renal cortex, histamine content was significantly higher (Δ + 260%) than in tubules. Incubation of isolated glomeruli with l-histidine resulted in a time-dependent increase of histamine content in glomeruli, but no change was found in tubules. The increase in glomerular histamine was blocked by the histidine decarboxylase inhibitor bromocresine. In rats with PA nephrosis induced by a single intraperitoneal injection of PA (15 mg/100 g body wt) urinary excretion of histamine was markedly increased (>Δ + 200%), but control rats did not differ from rats with PA nephrosis in urinary excretions of l-histidine and of creatinine. At the peak of proteinuria (day 9 after injection of PA) the plasma level of histamine was slightly elevated, and plasma histidine slightly decreased in animals that developed PA nephrosis. The content of histamine was markedly higher and the level of histidine was significantly lower in the renal cortex of PA-nephrotic rats as compared with controls; PA-nephrotic and control rats did not differ in the content of histidine and histamine in the liver. In addition, the content of histamine was higher in glomeruli isolated from PA-nephrotic rats; lesser difference was found in cortical tubules.
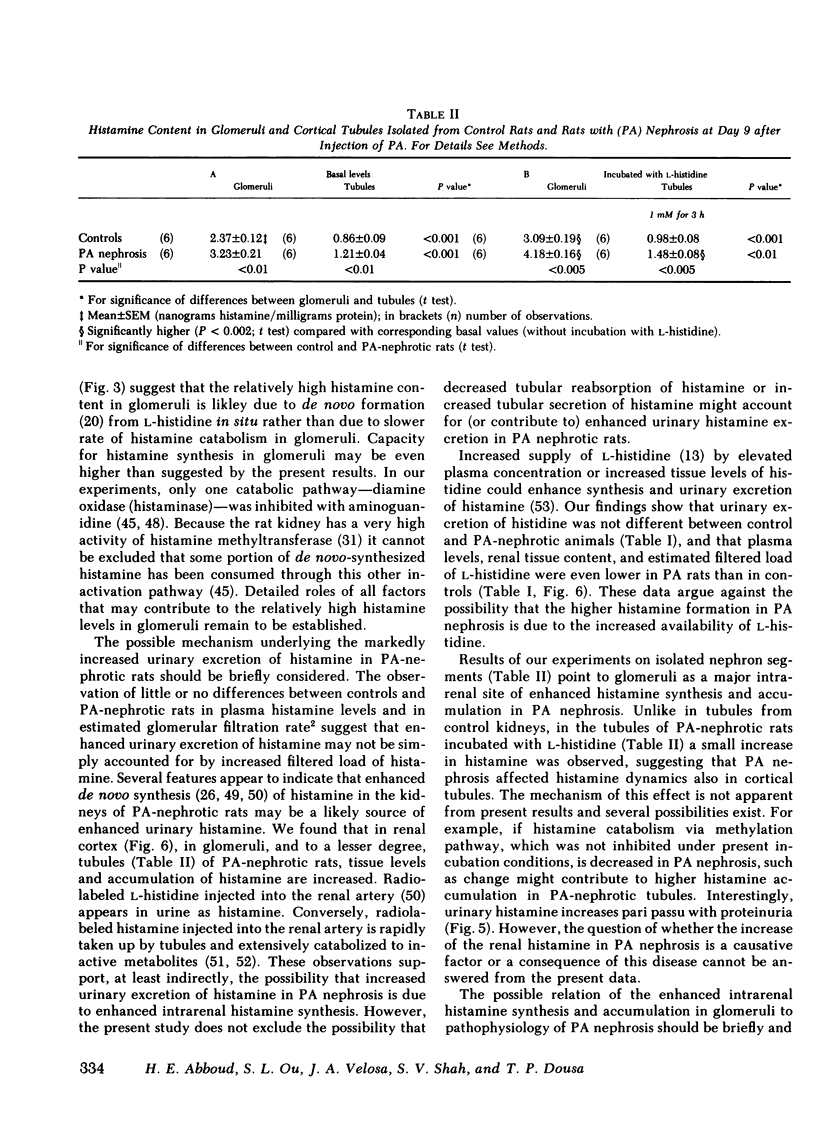
The results further indicate that PA-nephrotic rats have higher content of histamine in the renal cortex, predominently in glomeruli with increased urinary histamine excretion. The elevated renal cortical histamine is not due to higher availability of histamine precursor l-histidine. Results thus show that glomeruli are a major site of intrarenal histamine synthesis and accumulation, and also suggest that abnormal renal metabolism of this amine in PA nephrosis may be related, as a cause or as a consequence, to the pathogenesis of this disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E., Shah S. V., Dousa T. P. Effects of dexamethasone on cyclic nucleotide accumulation in glomeruli. J Lab Clin Med. 1979 Nov;94(5):708–717. [PubMed] [Google Scholar]
- Almeida A. P., Flye W., Deveraux D., Horakova Z., Beaven M. A. Distribution of histamine and histaminase (diamine oxidase)d in blood of various species. Comp Biochem Physiol C. 1980;67C(2):187–190. doi: 10.1016/0306-4492(80)90014-3. [DOI] [PubMed] [Google Scholar]
- Ambrose J. A., Crimm A., Burton J., Paullin K., Ross C. Fluorometric determination of histidine. Clin Chem. 1969 May;15(5):361–366. [PubMed] [Google Scholar]
- BROWN D. D., TOMCHICK R., AXELROD J. The distribution and properties of a histamine-methylating enzyme. J Biol Chem. 1959 Nov;234:2948–2950. [PubMed] [Google Scholar]
- Banks R. O., Fondacaro J. D., Schwaiger M. M., Jacobson E. D. Renal histamine H1 and H2 receptors: characterization and functional significance. Am J Physiol. 1978 Dec;235(6):F570–F575. doi: 10.1152/ajprenal.1978.235.6.F570. [DOI] [PubMed] [Google Scholar]
- Beaven M. A. Histamine (second of two parts). N Engl J Med. 1976 Feb 5;294(6):320–325. doi: 10.1056/NEJM197602052940608. [DOI] [PubMed] [Google Scholar]
- Beaven M. A. Histamine: its role in physiological and pathological processes. Monogr Allergy. 1978;13:1–113. [PubMed] [Google Scholar]
- Beaven M. A., Jacobsen S. A new assay for histaminase activity: measurement of tritiated water from beta (side chain label)-H 3-histamine. J Pharmacol Exp Ther. 1971 Jan;176(1):52–64. [PubMed] [Google Scholar]
- Beaven M. A., Jacobsen S., Horáková Z. Modification of the enzymatic isotopic assay of histamine and its application to measurement of histamine in tissues, serum and urine. Clin Chim Acta. 1972 Mar;37:91–103. doi: 10.1016/0009-8981(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Bignold L. P., Lykke A. W. Time courses and refractoriness of enhanced vascular permeability induced by histamine, serotonin and bradykinin in synovialis of the rat. Experientia. 1979 Dec 15;35(12):1645–1647. doi: 10.1007/BF01953245. [DOI] [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Robertson C. R., Brenner B. M., Troy J. L., Willis W. T. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J Clin Invest. 1977 Jul;60(1):152–161. doi: 10.1172/JCI108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Bökesoy T. A., Türker R. K. The presence of histamine H2-receptors in rabbit kidney. Arch Int Pharmacodyn Ther. 1974 May;209(1):144–149. [PubMed] [Google Scholar]
- Campbell W. B., Itskovitz H. D. Effect of histamine and antihistamines on renal hemodynamics and functions in the isolated perfused canine kidney. J Pharmacol Exp Ther. 1976 Sep;198(3):661–667. [PubMed] [Google Scholar]
- Diamant S., Shafrir E. Lipogenesis in aminonucleoside-induced nephrotic syndrome. Biochim Biophys Acta. 1974 Sep 19;360(3):241–251. doi: 10.1016/0005-2760(74)90053-8. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Shah S. V., Abboud H. E. Potential role of cyclic nucleotides in glomerular pathophysiology. Adv Cyclic Nucleotide Res. 1980;12:285–299. [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961 Nov 1;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigen L. P., Chapnick B. M., Flemming J. E., Kadowitz P. J. Prostaglandins: renal vascular responses to bradykinin, histamine, and nitroglycerine. Am J Physiol. 1978 Apr;234(4):H496–H502. doi: 10.1152/ajpheart.1978.234.4.H496. [DOI] [PubMed] [Google Scholar]
- Glasser R. J., Velosa J. A., Michael A. F. Experimental model of focal sclerosis. I. Relationship to protein excretion in aminonucleoside nephrosis. Lab Invest. 1977 May;36(5):519–526. [PubMed] [Google Scholar]
- Heald J. I., Hollis T. M. Histidine decarboxylase-mediated histamine synthesis in glomeruli from rat kidneys. Am J Physiol. 1976 May;230(5):1349–1353. doi: 10.1152/ajplegacy.1976.230.5.1349. [DOI] [PubMed] [Google Scholar]
- Henningsson S. S., Rosengren E. Distribution of histidine decarboxylase in the pregnant mouse kidney. Q J Exp Physiol Cogn Med Sci. 1971 Jul;56(3):156–159. doi: 10.1113/expphysiol.1971.sp002114. [DOI] [PubMed] [Google Scholar]
- Horakova Z., Keiser H. R., Beaven M. A. Blood and urine histamine levels in normal and pathological states as measured by a radiochemical assay. Clin Chim Acta. 1977 Sep 1;79(2):447–456. doi: 10.1016/0009-8981(77)90441-7. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Brenner B. M. Mechanisms of action of hisamine and histamine antagonists on the glomerular microcirculation in the rat. Circ Res. 1979 Dec;45(6):737–745. doi: 10.1161/01.res.45.6.737. [DOI] [PubMed] [Google Scholar]
- Juhlin L. Determination of histamine in small biopsies and histological sections. Acta Physiol Scand. 1967 Sep;71(1):30–36. doi: 10.1111/j.1748-1716.1967.tb03706.x. [DOI] [PubMed] [Google Scholar]
- Kahn A., Brachet E. The permeability coefficient of albumin of the isolated rat mesentery. Modification by some mediators of inflammation, cyclic AMP and calcium. Biochim Biophys Acta. 1979 Dec 3;588(2):219–231. doi: 10.1016/0304-4165(79)90205-8. [DOI] [PubMed] [Google Scholar]
- LINDELL S. E., SCHAYER R. W. Formation of histamine in the kidney of the dog. Br J Pharmacol Chemother. 1958 Mar;13(1):89–90. doi: 10.1111/j.1476-5381.1958.tb00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDELL S. E., SCHAYER R. W. Metabolism of injected [14C] histamine in the kidney of the dog. Br J Pharmacol Chemother. 1958 Mar;13(1):52–53. doi: 10.1111/j.1476-5381.1958.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mallick N. P. The pathogenesis of minimal change nephropathy. Clin Nephrol. 1977 Mar;7(3):87–95. [PubMed] [Google Scholar]
- Maśliński C. Histamine and its metabolism in mammals. Part II: Catabolism of histamine and histamine liberation. Agents Actions. 1975 Aug;5(3):183–225. doi: 10.1007/BF02026434. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mortillaro N. A., Granger D. N., Kvietys P. R., Rutili G., Taylor A. E. Effects of histamine and histamine antagonists on intestinal capillary permeability. Am J Physiol. 1981 May;240(5):G381–G386. doi: 10.1152/ajpgi.1981.240.5.G381. [DOI] [PubMed] [Google Scholar]
- Rasio E. A. Metabolic control of permeability in isolated mesentery. Am J Physiol. 1974 Apr;226(4):962–968. doi: 10.1152/ajplegacy.1974.226.4.962. [DOI] [PubMed] [Google Scholar]
- Renkin E. M., Carter R. D., Joyner W. L. Mechanism of the sustained action of histamine and bradykinin on transport of large molecules across capillary walls in the dog paw. Microvasc Res. 1974 Jan;7(1):49–60. doi: 10.1016/0026-2862(74)90036-3. [DOI] [PubMed] [Google Scholar]
- SCHAYER R. W., WU K. Y. T., SMILEY R. L. Sources of urinary histamine in the rat. Am J Physiol. 1954 Dec;179(3):481–485. doi: 10.1152/ajplegacy.1954.179.3.481. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Shaff R. E., Beaven M. A. Increased sensitivity of the enzymatic isotopic assay of histamine: measurement of histamine in plasma and serum. Anal Biochem. 1979 Apr 15;94(2):425–430. doi: 10.1016/0003-2697(79)90385-3. [DOI] [PubMed] [Google Scholar]
- Shah S. V., Kempson S. A., Northrup T. E., Dousa T. P. Renal adaptation to a low phosphate diet in rats. J Clin Invest. 1979 Oct;64(4):955–966. doi: 10.1172/JCI109562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V., Northrup T. E., Hui Y. S., Dousa T. P. Action of serotonin (5-hydroxytryptamine) on cyclic nucleotides in glomeruli of rat renal cortex. Kidney Int. 1979 May;15(5):463–472. doi: 10.1038/ki.1979.62. [DOI] [PubMed] [Google Scholar]
- Shimamura T. Effects of inflammatory mediators on the glomerular localization of intravenously administered ferritins. Experientia. 1978 Sep 15;34(9):1196–1197. doi: 10.1007/BF01922954. [DOI] [PubMed] [Google Scholar]
- Slorach S. A., Uvnäs B. Amine formation by rat mast cells in vitro. Acta Physiol Scand. 1968 Aug;73(4):457–470. doi: 10.1111/j.1365-201x.1968.tb10885.x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Baldessarini R. J., Axelrod J. A sensitive and specific enzymatic isotopic assay for tissue histamine. J Pharmacol Exp Ther. 1966 Sep;153(3):544–549. [PubMed] [Google Scholar]
- Taylor K. M., Snyder S. H. Dynamics of the regulation of histamine levels in mouse brain. J Neurochem. 1972 Feb;19(2):341–354. doi: 10.1111/j.1471-4159.1972.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Snyder S. H. Isotopic microassay of histamine, histidine, histidine decarboxylase and histamine methyltransferase in brain tissue. J Neurochem. 1972 May;19(5):1343–1358. doi: 10.1111/j.1471-4159.1972.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Torres V. E., Northrup T. E., Edwards R. M., Shah S. V., Dousa T. P. Modulation of cyclic nucleotides in islated rat glomeruli: role of histamine, carbamylcholine, parathyroid hormone, and angiotensin-II. J Clin Invest. 1978 Dec;62(6):1334–1343. doi: 10.1172/JCI109254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux R. Mast cell increase in the duodenum and kidney of magnesium-deficient rats. Lab Invest. 1975 Jul;33(1):80–87. [PubMed] [Google Scholar]
- Velosa J. A., Glasser R. J., Nevins T. E., Michael A. F. Experimental model of focal sclerosis. II. Correlation with immunopathologic changes, macromolecular kinetics, and polyanion loss. Lab Invest. 1977 May;36(5):527–534. [PubMed] [Google Scholar]
- Venkatachalam M. A., Rennke H. G. The structural and molecular basis of glomerular filtration. Circ Res. 1978 Sep;43(3):337–347. doi: 10.1161/01.res.43.3.337. [DOI] [PubMed] [Google Scholar]
- Zeiger R. S., Yurdin D. L., Twarog F. J. Histamine metabolism. I. Thin-layer radiochromatographic assays for histaminase and histidine decarboxylase enzyme activities. J Lab Clin Med. 1976 Jun;87(6):1065–1074. [PubMed] [Google Scholar]



