Abstract
Although neurodevelopmental impairment is a risk factor for poor cognitive and behavioral outcomes, associations between early and later functioning are only moderate in magnitude, and it is likely that other factors intervene to modify this trajectory. The current study tested the hypothesis that sensitive, stimulating caregiving would promote positive behavioral and cognitive outcomes among children who were at risk based on the results of a neurodevelopmental screener and a temperament inventory. The sample comprised 1,720 infants and toddlers from the National Survey of Child and Adolescent Well-Being, a longitudinal study of children who were involved with child welfare services. Children were first assessed between 3 and 24 months of age and subsequently 18 months later. Children who experienced improvements in the amount of sensitive, stimulating caregiving they received had positive cognitive and behavioral outcomes 18 months later, despite early levels of neurodevelopmental risk. The association between changes in caregiving quality and changes in children’s functioning was stronger for children who were removed from the care of their biological parents before the follow-up assessment than for children who remained in the care of biological parents, suggesting a causal role for caregiving quality on children’s outcomes.
Infants and toddlers who are victims of abuse and neglect are at risk for fine and gross motor impairments, language delay, and deficits in the regulation of behavior and emotion (De Bellis, 2001; Glaser, 2000; Rutter, O’Connor, & English and Romanian Adoptees Study Team, 2004). In the prenatal period, they are at risk for exposure to drugs of abuse (Bailey et al., 2004; Bunikowski et al., 1998; Kelley, 1992; Leventhal et al., 1997), poor prenatal care (Benedict, White, & Cornely, 1985), and obstetrical complications that arise if their mothers are victims of partner violence while pregnant (Bacchus, Mezey, & Bewley, 2004). In the postnatal period, experiencing abuse or neglect may result in traumatic brain injury, under-stimulation, malnutrition, and dysregulation of biological stress response systems (Barlow, Thomson, Johnson, & Minns, 2005; De Bellis, 2001; Glaser, 2000). Experiences of abuse and neglect that result in diffuse axonal injury (such as shaken baby syndrome), chronically elevated levels of stress chemicals, or protein deficiency may have especially severe consequences for the cognitive and social development of children under the age of 5 years (De Bellis, 2005; Levin, 2003; Rao & Georgieff, 2000), because this is an important period of neuronal proliferation and pruning, synaptogenesis, and rapid white matter development (Webb, Monk, & Nelson, 2001). This preliminary evidence of a particular period of vulnerability to child maltreatment has strong public health implications, as children under the age of 3 years comprise the largest group of abuse and neglect victims in the United States (US Department of Health and Human Services, 2005).
Although there are many reasons why maltreated children are at risk for neurodevelopmental impairment, surprisingly little research has been conducted on the neuropsychological functioning of abused and neglected children. Pears and Fisher (2005) reported that 3- to 6-year-old children with histories of maltreatment had significantly smaller head circumference and poorer visuospatial, language, and general cognitive functioning compared to community controls, although the two groups did not differ with respect to sensorimotor function, memory, or executive function. In a study of 1,542 infants and toddlers (3 months to 5 years of age) who were involved with child welfare services, over half scored below the normal range on the Denver Developmental Screening Test II (Frankenburg, Dodds, Archer, Shapiro, & Bresnick, 1992), and of these, 47% were mildly or significantly delayed according to their scores on the Bayley Scales of Infant Development II (Leslie et al., 2005). Rutter and colleagues (1998) assessed developmental delay among infants and toddlers (up to 42 months) who had been adopted by families in the United Kingdom from conditions of severe privation in institutional settings in Romania. Upon entry to the United Kingdom, the overwhelming majority of institution-reared children had mild to severe levels of developmental impairment. Finally, studies have shown that maltreatment is associated with language delay in children and adolescents (Eigsti & Cicchetti, 2004; McFayden & Kitson, 1996). Thus, although the extant literature is small, findings are consistent with the notion that experiences of abuse and neglect are associated with neurodevelopmental impairment.
Evidence of neurodevelopmental impairment early in a child’s life is of concern because motor, language, and self-regulatory impairments are associated with adverse cognitive and behavioral outcomes, regardless of whether neurodevelopmental functioning is measured directly (Aylward & Verhulst, 2000; Cannon et al., 2002; Jaffee et al., 2002; Moffitt, 1993; Murray et al., 2006; Shevell, Majnemer, Platt, Webster, & Birnbaum, 2004) or whether it is inferred from measures like difficult temperament (Rothbart & Bates, 1998) or low birth weight (Aylward, 2005), which is assumed to reflect cerebral damage in the neonatal period (Whitaker et al., 1997). However, other researchers have detected very weak continuities from measures of early neurodevelopmental functioning to later adjustment, particularly when neurodevelopmental functioning is assessed in the first year of life (Colombo, 1993; Molfese & Acheson, 1997; Rothbart & Bates, 1998).
One explanation for generally modest correlations is that the effects of neurodevelopmental impairment on subsequent cognitive and behavioral outcomes are moderated by environmental experiences. A growing body of evidence supports this hypothesis (e.g., Raine, 2002). At least two studies have shown that low birth weight predicts symptoms of inattention and hyperactivity in school-age children, but not when children have warm, supportive relationships with their mothers (Laucht, Esser, & Schmidt, 2001; Tully, Arseneault, Caspi, Moffitt, & Morgan, 2004). Prospective, longitudinal studies have shown that neuromotor impairment (Raine, Brennan, Mednick, & Mednick, 1996) and other indicators of neurodevelopmental impairment such as perinatal complications and difficult temperament (Arseneault, Tremblay, Boulerice, & Saucier, 2002; Brennan, Hall, Bor, Najman, & Williams, 2003) measured in the first 5 years of life increased risk for persistent aggressive, antisocial outcomes in middle childhood and adolescence, but only among children who were raised under conditions of adversity. Dramatic instances of cognitive catch-up at age 4 and 6 years were observed among children who had been raised in severely deprived institutional environments and who were later adopted into families in the United Kingdom, although the degree of catch-up depended on the duration of institutional rearing (Rutter & English and Romanian Adoptees [ERA] Study Team, 1998; O’Connor et al., 2000). Finally, Jacobson, Jacobson, Sokol, Chiodo, and Corobana (2004) detected adverse effects of prenatal alcohol exposure on children’s IQ when parents were low in emotional sensitivity and provided little cognitive stimulation, but not when emotional sensitivity and cognitive stimulation scores were high.
At least two aspects of the caregiving environment may be relevant in promoting developmentally appropriate functioning among children with neurodevelopmental impairments. First, cognitively stimulating environments may promote language development among at-risk children. For example, the amount of time that mothers spend talking to their infants is highly predictive of vocabulary growth (Huttenlocher, Haight, Bryk, Seltzer, & Lyons, 1991; Pan, Rowe, Singer, & Snow, 2005). Second, emotionally supportive environments may foster the emergence of behavioral control and emotion regulatory skills among at-risk children. For example, children who are temperamentally fearful and withdrawn successfully develop the ability to regulate their own conduct when mothers use gentle, inductive disciplinary techniques to modify children’s behavior. In contrast, temperamentally undercontrolled, “fearless” children learn to regulate their own conduct in the context of a warm, supportive relationship with a parent (Kochanska, 1997).
In this study, biologically at-risk children were defined as those who scored in the moderate to high risk range on a neurodevelopmental screener in infancy and who were temperamentally unpredictable, showed low levels of positive affect, and were fussy and irritable. Resilience was defined as cognitive and behavioral functioning 18 months later that was better than expected, given initial levels of neurodevelopmental and temperamental risk, respectively. That is, behavioral and cognitive functioning are expected to be poor among children who were identified at an earlier point in time as having difficult temperament and neurodevelopmental impairment. Thus, resilient children will have higher levels of cognitive and behavioral functioning than would be predicted based on early biological risk factors. Formulated in this way, resilience to biological risk shares many features with resilience to psychosocial risk. First, risk is a probabilistic term, meaning that neither biological nor psychosocial risk factors are perfectly predictive of cognitive or behavioral outcomes. Second, resilience reflects positive adaptation in the face of earlier biological or psychosocial conditions that are typically associated with cognitive or behavioral maladaptation (Luthar, Cicchetti, & Becker, 2000). In the case of both biological and psychosocial risk factors, resilience may reflect self-organizing tendencies that enable the brain to adapt to new constraints (such as competent caregiving) by reorganizing neural systems that subserve cognition and behavior (e.g., Cicchetti & Curtis, 2006). Third, the likelihood that an individual will manifest positive adaptation despite conditions of biological or psychosocial risk will reflect the balance of risk and protective factors available to the organism (Cicchetti & Toth, 1995). These may include individual characteristics (across multiple levels of the organism from genetic to cognitive or emotional capacities) as well as familial and extrafamilial supports.
In the current study, the hypothesis was tested that improvements in caregiving would promote resilience in cognitive and behavioral domains among children who were at biological risk, as measured by performance on the neurodevelopmental screener and the temperament assessment. This hypothesis was tested in a sample of infants and toddlers, all of whom were reported to Child Protective Services (CPS) for allegations of abuse or neglect. Such children are expected to experience significant changes in the caregiving environment because they are likely to be removed from the care of their biological parents and placed in kin or nonkin foster settings, and because their parents are likely to be referred for services that target parenting skills or parent substance use or mental health problems.
Although a number of studies have shown that sensitive, stimulating parenting practices can protect at-risk children from poor outcomes, the causal nature of the association between parenting practices and children’s outcomes is often unclear. Two alternative explanations for this association are plausible. First, parents may engage in warm, cognitively stimulating behavior in response to a child’s positive temperament or the child’s capacity for learning, in which case the association would reflect an effect of the child on the caregiver rather than the reverse. Second, warm, stimulating parenting may be a marker for other factors (e.g., genetic factors) that causally influence children’s functioning. These alternative explanations were tested in the current study by (a) taking advantage of the longitudinal nature of the data to control for child effects on the caregiving environment and (b) focusing a subset of the analyses on children who were removed from the care of biological parents during the course of the study, thus breaking the link between the child’s genotype and the child’s rearing environment. This design is an example of a “natural experiment” in which, for some children, there was a discontinuity between the child’s rearing environment at earlier and later points in time (Rutter, Pickles, Murray, & Eaves, 2001).
Method
Sample
The National Survey of Child and Adolescent Well-Being (NSCAW) is a nationally representative sample of children in the United States who have had contact with CPS (Dowd et al., 2004). The cohort includes 5,501 children (50% female), less than 1 year to 14 years of age when sampled, who were subjects of child abuse or neglect investigations conducted by CPS from October 1999 to December 2000. The sample was selected using a two-stage stratified sample design. At the first stage, the United States was divided into nine sampling strata. Eight strata corresponded to the eight states with the largest child welfare caseloads and the ninth stratum consisted of the remaining 38 states and the District of Columbia. Within each of the nine strata, primary sampling units (PSUs) were formed and randomly selected. PSUs were defined as geographic areas that encompassed the population served by a single CPS agency (e.g., counties). At the second stage, equal numbers of children were selected from each PSU, regardless of PSU size. Children were selected from eight mutually exclusive and exhaustive domains such that the final sample adequately represented relevant combinations of (a) infants versus children age 1 to 14 years, (b) children receiving CPS-funded agency services versus children not receiving services, (c) children in out-of-home care versus children not in out-of-home care, and (d) children who were investigated for allegations of sexual abuse versus other forms of abuse or neglect. Additional information about the sample composition is available from Dowd and colleagues (2004).
Field staff completed 12 days of training on the protocol. At baseline (Wave 1), face-to-face interviews or assessments were conducted with children, their caregivers (e.g., biological parents, foster parents, custodial kin caregivers), their teachers (when children were school age), and their caseworkers (when applicable). At 12 months postbaseline (Wave 2), telephone interviews were conducted with current caregivers and caseworkers. At 18 and 36 months post-baseline (Waves 3 and 4), face-to-face interviews were conducted with children, current care-givers, teachers, and caseworkers. Of the 5,501 children assessed at baseline, 87% participated at Wave 2, 87% participated at Wave 3, and 85% participated at Wave 4. Current caregivers were paid $50 for their participation and children were given gift certificates worth $10–20.
The current analyses include the 1,720 children (47% female) who ranged in age from 3 to 24 months at Wave 1. Eighty-seven percent of these children participated at Wave 3. At Wave 1, 38% of the children were African American, 36% were White, 19% were Hispanic, and 6% were from other racial/ethnic groups. Caseworkers rated the most severe form of maltreatment children had experienced, based on the frequency, duration, and severity of maltreatment incidents. For 20% of children, the most severe form of maltreatment they experienced was physical abuse, for 4% it was sexual abuse, for 5% it was emotional abuse, for 60% it was neglect, and for 11% it was “other” forms of abuse, including abandonment, moral or legal maltreatment, educational maltreatment, exploitation, or other forms of maltreatment. Twenty-nine percent of children experienced multiple forms of maltreatment.
In order to generalize the results to the population of US children who have had contact with CPS, the data must be weighted. Analysis weights were calculated as the product of two probabilities: the probability that a PSU would be selected (first stage) and the probability that a child would be selected (second stage). Adjustments were made for missing data, nonresponse, and undercoverage. For further details, see Dowd and colleagues (2004). All subsequent analyses were adjusted for survey design using Stata 8.0 (StataCorp, 2001).
Measures
Bayley Infant Neurodevelopmental Screener (BINS)
The BINS (Aylward, 1995) is a screening tool used to identify infants between the ages of 3 and 24 months with potential developmental delays or neurological impairments. The BINS assesses basic neurological functions (of the central nervous system), receptive functions (sensation and perception), expressive functions (fine and gross motor skills, oral skills), and cognitive processes (memory and learning, thinking, and reasoning). The BINS has demonstrated validity (Aylward, 1995) and internal consistency reliability ranged from .73 to .84 for the various age groups (up to 24 months) in the NSCAW sample. Based on age-standardized total scores, children were categorized as being at low (15%), moderate (32%), or high (53%) risk for neurodevelopmental impairment. The moderate- and high-risk groups were combined for analyses. Scores falling in the moderate- to high-risk range at 6–24 months are indicative of clinically significant cognitive developmental impairment in verbal, perceptual, motor, quantitative, and memory domains at 36 months of age (Aylward & Verhulst, 2000) and other work has shown that when the Bayley Scales of Infant Development, 2nd Edition (Bayley, 1993), were used as the gold standard for measuring developmental delay, the BINS had optimal sensitivity (90%) when referral was made for BINS scores falling in the moderate-to high-risk range (Macias et al., 1998). The BINS was administered to children at Wave 1.
Difficult temperament
The temperament scales used in NSCAW were developed for use in the National Longitudinal Survey of Youth using a variety of existing instruments (Mott, Baker, Ball, Keck, & Lenhart, 1995). At Wave 1, caregivers whose infants were under 24 months of age were asked to report how often their infants exhibited specific behaviors (e.g., “During the day, how often does [NAME] get fussy and irritable?” and “How often do you have trouble soothing [NAME] when he/she is tired or upset?”). Responses ranged from 1 = never or almost never to 5 = almost always. For infants younger than 12 months at Wave 1, difficult temperament was the sum of the following sub-scales: positive affect, friendliness, and predictability (all reverse scored) as well as fearfulness. For 12- to 23-month-olds, difficult temperament was the sum of the positive affect and friendliness subscales (reverse scored) and the fearfulness subscale. Difficult temperament scale scores were standardized to account for the fact that different versions of the scale were calculated depending on whether children were younger or older than 12 months of age at Wave 1 (M = 0.007, SE = 0.06).1 The reliability of the difficult temperament scale was α = .62.
Home Observation for Measurement of the Environment—Short Form (HOME-SF)
The HOME-SF measures the quality and quantity of cognitive stimulation and emotional support that children receive in the home environment and is valid for children from birth to 10 years of age (Caldwell & Bradley, 1984). The number of items ranges from 20 to 24 depending on the child’s age. Interviewers indicate whether specific caregiver behaviors or physical conditions are observed in the home or if they are reported by caregivers. The cognitive stimulation sub-scale (9 items for 0- to 2-year-olds; 14 items for 3- to 5-year-olds) includes questions and observations about the physical environment (e.g., interviewer notes whether there are safe play spaces) and provision of stimulating materials and activities for the child (e.g., interviewer indicates whether caregiver provided toys or interesting activities for the child during the visit). The emotional support subscale (9 items for 0- to 2-year-olds, 12 items for 3- to 5-year-olds) includes questions and observations about physical affection (e.g., interviewer indicates whether caregiver caressed, kissed, or hugged child during the visit) and the caregiver’s response to the child’s behavior (e.g., “In the last week, how many times, if any, have you had to spank [NAME]?). Internal consistency reliabilities are not reported for the HOME-SF because the measure is an index of observed and reported behaviors and index items are not expected to be interrelated. The HOME was administered at Waves 1 and 3. Cognitive stimulation (Wave 1 M = 0.23, SE = 0.04; Wave 3 M = 0.02, SE = 0.06) and emotional support (Wave 1 M = −0.05, SE = 0.06; Wave 3 M = 0.007, SE = 0.05) scores were standardized because different versions of the HOME were administered at Wave 3 depending on whether children were younger or older than 3 years.
Preschool Language Scale (PLS-3)
The PLS-3 (Zimmerman, Steiner, & Pond, 1992) measures language development in children from birth to 6 years of age. The PLS-3 includes 48 items that measure auditory comprehension and 48 items that measure expressive communication. These scores were age standardized and summed to create a total language score that was then converted to a normalized score (M = 90.39, SE = 0.95). The PLS-3 has demonstrated predictive and concurrent validity as well as high interrater agreement (89%), good test–retest reliability (.91–.94 for the total language score), and good internal consistency reliability (α = .87; Zimmerman et al., 1992). The PLS-3 was administered to children at the Wave 3 interview.
Children’s problem behaviors
Problem behaviors were assessed at Wave 3 by caregivers who completed the Child Behavior Checklist (CBCL 2/3; Achenbach, 1991) for children 2 to 3 years of age and the CBCL 4/16 (Achenbach, 1991) for children 4 years and older. The CBCL assesses children’s competencies as well as eight problem behavior syndromes including withdrawn behavior, thought problems, social problems, attentional problems, aggressive behavior, delinquent behavior, somatic complaints, and anxious/depressed behavior. Caregivers indicate whether any of 100 (CBCL 2/3) or 113 (CBCL 4/16) items apply to their child (0 = never to 2 = always). Scores on the syndrome scales were summed to create a total problem behaviors score. The analyses reported in this paper rely on the age- and gender-standardized total problem behaviors T score (M = 55.38, SE = 0.39). The internal consistency reliability for the Total Problem Behaviors Scale was α = .95 for 2- to 3-year-olds and α = .96 for 4- to 16-year-olds. Children who were not yet 24 months of age by Wave 3 (n = 679) were missing CBCL data.
Income
Total annual income from all sources was reported by caregivers at Waves 1 and 3. Responses ranged from 1 ($0 to $9,999) to 5 ($40,000 or higher). Average incomes at Wave 1 (M = 2.62, SE = 0.08) and at Wave 3 (M = 2.67, SE = 0.08) ranged from $10,000 to $29,999.
Results
The results are presented in four parts. First, associations among the measures of biological risk, the caregiving environment, and children’s language abilities and problem behaviors are described. Second, measures of resilient functioning are derived in two domains: language development and children’s problem behaviors. Third, analyses are presented that test whether improvements in the amount of cognitive stimulation and emotional support provided to children by caregivers predicted better than expected language skills and behavior, respectively, in children. Fourth, the hypothesis is tested that the association between changes in the caregiving environment and changes in children’s functioning will be significant regardless of whether children resided with biological parents.
What characterizes children with neurodevelopmental impairment and difficult temperament?
As shown in Table 1, moderate to high risk for neurodevelopmental impairment (as identified by the neurodevelopmental screener) was positively correlated with difficult temperament. Risk for neurodevelopmental impairment and difficult temperament were negatively correlated with language ability and positively correlated with problem behaviors. Moreover, these biological risk factors were associated with lower levels of cognitive stimulation at Wave 1 and lower levels of emotional support at Wave 3.
Table 1.
Associations among measures of neurodevelopmental risk status, difficult temperament, HOME scores, language ability, and problem behaviors
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Neurodev. risk | 1.00 | |||||||
| 2. Difficult temperament | .14*** | 1.00 | ||||||
| 3. Cognitive stim. (Wave 1) | −.15*** | −.11*** | 1.00 | |||||
| 4. Emot. supp. (Wave 1) | −.05 | −.03 | .32*** | 1.00 | ||||
| 5. Cognitive stim. (Wave 3) | −.04 | −.17*** | .19*** | .14*** | 1.00 | |||
| 6. Emot. supp. (Wave 3) | −.11*** | −.07** | .09*** | .23*** | .38*** | 1.00 | ||
| 7. Language ability | −.20*** | −.18*** | .15*** | .08*** | .21*** | .18*** | 1.00 | |
| 8. Problem behavior | .07* | .33*** | .00 | −.15*** | −.22*** | −.24*** | .01 | 1.00 |
p ≥ .05.
p ≥.01.
p ≥.001.
Deriving measures of resilient functioning
Resilient children were identified as those who were doing better than expected with respect to language development and behavior at Wave 3 given early-emerging neurodevelopmental and temperamental vulnerabilities. To identify children who had better than expected language abilities at Wave 3, language scores measured at Wave 3 were regressed on neurodevelopmental impairment scores measured at Wave 1 and the residualized scores were saved (range = −44.89 to 63.80). Residualized scores (as measures of change over time) are preferable to difference change scores because they take into account potential differences in variance at the two time points. Children with moderate to high risk for neurodevelopmental impairment had lower language scores at Wave 3 (b = −9.69, SE = 2.87, p < .001) compared with children who were at low risk for neurodevelopmental impairment. Positive residualized scores indicate that children had higher than expected language scores at Wave 3, given their Wave 1 scores on the BINS and negative residualized scores indicate that children had lower than expected language scores at Wave 3. Forty-seven percent of children had better language scores than expected at Wave 3 given baseline neurodevelopmental impairment.
To identify children who were doing better than expected with respect to problem behaviors at Wave 3, total problem behavior scores measured at Wave 3 were regressed on difficult temperament scores assessed at Wave 1 and the residualized scores were saved (range = −32.96 to 37.86). Higher ratings of difficult temperament at Wave 1 predicted higher problem behavior scores at Wave 3 (b = 3.29, SE = 0.81, p < 001). Positive residualized scores indicate that children had higher than expected problem behavior scores at Wave 3, given their Wave 1 scores on the temperament assessment. Negative residualized scores indicate that children had lower than expected behavior problem scores at Wave 3.2 Fifty-one percent of children had lower behavior problem scores than expected at Wave 3 given baseline difficult temperament.3
Do improvements in the quality of the home environment predict better than expected language abilities and behavior in children?
Children who experienced improvements in the quality of their home environment were defined as those whose HOME cognitive stimulation or emotional support scores were better than expected at Wave 3 given scores at Wave 1. To estimate improvements in the quality of the home environment, Wave 3 cognitive stimulation and emotional support scores were regressed, respectively, on the Wave 1 versions of these measures. Cognitive stimulation (b = 0.25, SE = 0.05, p < .001) and emotional support (b = 0.24, SE = 0.04, p < .001) at Wave 1 significantly predicted cognitive stimulation and emotional support at Wave 3. To identify children who experienced improvements in their home environment, the residualized scores were saved (range = −5.24 to 2.04 for cognitive stimulation, range = −4.00 to 2.24 for emotional support). Positive scores indicate that children were experiencing more cognitive stimulation or emotional support than expected at Wave 3; negative scores indicate that children were experiencing less cognitive stimulation or emotional support than expected at Wave 3.
Although the hypothesis was that improvements in the quality of the home environment would predict better than expected functioning, it is possible that the direction of effects is reversed and that caregiving behaviors change in response to children’s behavior or abilities. To test this possibility, two sets of ordinary least squares (OLS) regression analyses were conducted. The first set showed that Wave 1 cognitive stimulation scores predicted better than expected language scores at Wave 3 (b = 2.49, SE = 1.05, p < .05) and that Wave 1 emotional support scores predicted better than expected behavior at Wave 3 (b = −1.71, SE = 0.79, p < .05). In contrast, the second set of regression analyses showed that neurodevelopmental risk at Wave 1 was not predictive of changes in cognitive stimulation (b = 0.05, SE = 0.13, p = .69) nor was difficult temperament predictive of changes in emotional support (b = −0.09, SE = 0.05, p = .07), although there was a trend in this direction. Thus, the results of these analyses are more consistent with the hypothesis that children’s functioning is influenced by the caregiving environment than the alternative hypothesis that the caregiving environment is influenced by children’s behavior or abilities.
Finally, OLS regression analyses were conducted to test whether improvements in the quality of the caregiving environment predicted resilience in the language and problem behavior domains given early measures of biological risk. Regressing the residualized language scores on the residualized cognitive stimulation scores demonstrated that improvements in the amount of cognitive stimulation provided to children by caregivers were associated with better than expected language scores at Wave 3 (b = 3.13, SE = 0.93, p < .001). Similarly, improvements in caregivers’ emotional support were associated with lower than expected levels of problem behaviors in children at Wave 3 (b = −1.85, SE = 0.84, p < .05). Thus, improvements in the quality of the home environment were associated with changes for the better in children’s language abilities and behavior.
Gender differences in risk for neurodevelopmental impairment, cognitive stimulation, and emotional support were statistically nonsignificant, although there was a trend for girls to have higher difficult temperament scores than boys (b = 0.23, SE = 0.12, p < .06). An additional analysis was conducted to test whether the effect of changes in cognitive stimulation or emotional support on children’s functioning differed for girls and boys. Neither the effect of changes in cognitive stimulation on language ability (binteraction = 0.91, SE = 1.84, p = .62) nor the effect of changes in emotional support on behavior (binteraction = −1.00, SE = 1.51, p = .51) varied as a function of gender.
How does removing children from the care of biological parents affect children’s functioning?
Only children who were residing with a biological parent at Wave 1 were included in this analysis (n = 818). Of these, 49 were removed from the care of their biological parents between Waves 1 and 3. At Wave 3, these children were living with foster parents (41%), adoptive parents (4%), other nonrelatives (8%), grandparents (35%), or other relatives (12%).
A hierarchical OLS regression was conducted to test the main and interactive effects of (a) removal from the care of biological parents and (b) changes in cognitive stimulation or emotional sensitivity on children’s language abilities and problem behaviors. This analysis controlled for family income at Wave 3 because children who were removed from the care of biological parents between Waves 1 and 3 had significantly higher family incomes at Wave 3 (M = 3.98, SE = 0.18) than children who remained in the care of biological parents (M = 2.33, SE = 0.09; b = 1.65, SE = 0.20, p < .001). The groups did not differ with respect to family income at Wave 1 (b = −0.48, SE = 0.33, p = .14).
As shown in the top portion of Table 2, improvements in the amount of cognitive stimulation provided to children were associated with higher than expected language scores 18 months later. Removal from the care of biological parents had no effect on language scores at Wave 3, although income was positively associated with better than expected language scores. However, removal from the care of biological parents moderated the effect of change in cognitive stimulation scores on children’s language abilities (Figure 1).4 Analysis of the simple slopes indicated that the effect of changes in cognitive stimulation on children’s language abilities was stronger when children were removed from the care of biological parents (t = 2.86, p = .004) than when they remained in the care of biological parents (t = 2.30, p = .02). Removal from the care of biological parents was associated with higher than expected language scores when there were improvements in the amount of cognitive stimulation provided to children, but with lower than expected language scores when there were decrements in the amount of cognitive stimulation provided to children. This model accounted for 8.5% of the variance in children’s language ability.
Figure 1.
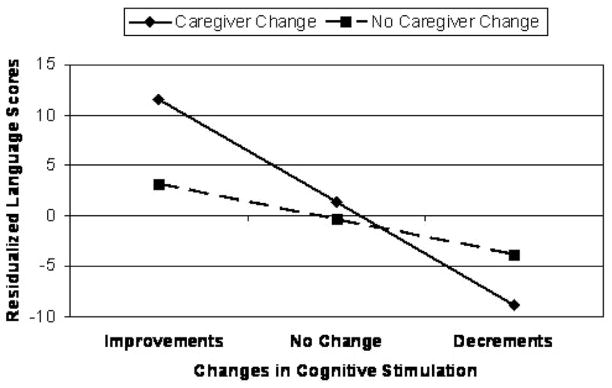
Removal from the care of biological parents moderates the effect of changes in cognitive stimulation on children’s language abilities.
As shown in the bottom portion of Table 2, there was a trend suggesting that improvements in caregivers’ emotional sensitivity was associated with lower than expected problem behaviors in children at 18 months. Neither removal from the care of biological parents nor family income was associated with children’s problem behaviors. However, there was a trend suggesting that removal from the care of biological parents moderated the effect of changes in emotional sensitivity on children’s problem behaviors such that the effect of changes in emotional sensitivity on changes in children’s problem behaviors was stronger among children who were removed from the care of biological parents than among children who were not. The final model accounted for 5.5% of the variance in children’s behavior.
Table 2.
OLS regression analysis predicting language functioning and problem behaviors at Wave 3
| Predictor Variables | Language Ability
|
|||
|---|---|---|---|---|
| Model 1
|
Model 2
|
|||
| b | SE | b | SE | |
| Change in cognitive stimulation | 3.03* | 1.21 | 2.83* | 1.23 |
| Removal from care of biol. parents | −1.11 | 3.60 | −3.27 | 2.97 |
| Family income (Wave 3) | 2.94*** | 0.79 | 2.98*** | 0.79 |
| Removal from care × change in cognitive stimulation | 7.82* | 3.92 | ||
| R2 | 8.2% | 8.5% | ||
|
| ||||
| Problem Behaviors
|
||||
| Model 1
|
Model 2
|
|||
| b | SE | b | SE | |
| Change in emotional support | −1.77† | 0.95 | −1.60 | 0.99 |
| Removal from care of biol. parents | −3.95 | 3.76 | −1.53 | 2.85 |
| Family income (Wave 3) | −0.61 | 0.62 | −0.65 | 0.62 |
| Removal from care × change in emotional support | −3.66† | 2.07 | ||
| R2 | 5.1% | 5.5% | ||
p < .08.
p < .05.
p < .01.
p < .001.
Discussion
Children who are victims of abuse and neglect are at risk for neurodevelopmental impairment and related regulatory deficits. Indeed, 85% of the infants and toddlers in the NSCAW sample were at moderate to high risk for neurodevelopmental impairment as indicated by their performance on a neurodevelopmental screener at 3 to 24 months of age and risk for neurodevelopmental impairment was associated with difficult temperament. These early biological risks were predictive of poor language abilities and elevated levels of problem behaviors in children 18 months later. Nevertheless, some children were doing better than expected on these measures of child functioning. Specifically, children whose caregiving environments improved over the 18-month period with respect to the amount of cognitive stimulation and emotional support they were receiving had better than expected language abilities and behavior. These findings illustrate the complex interplay between social experiences and brain development wherein the brain’s malleability to experiences like abuse or neglect can result in maladaptive outcomes such as neurodevelopmental impairment and its malleability to experiences encountered in the average expectable environment can result in improvements in language abilities or behavior, even given initial biological and social constraints (Cicchetti & Curtis, 2006).
The data were most consistent with a model in which the caregiving environment predicted changes in children’s functioning, rather than the reverse. That is, children’s characteristics did not predict changes in caregiving, suggesting that child effects, if present at all, were not persistent across the 18 months of the study. Moreover, the study took advantage of a natural experiment, in which some children were removed from the care of biological parents and changes in cognitive stimulation and emotional support were measured. The association between changes in the caregiving environment and changes in children’s functioning was significant and, in fact, stronger, when children were removed from the care of biological parents. This “natural experiment” demonstrates two things. First, it further minimizes the possibility of child effects on the caregiving environment because it establishes that the same child was, in many cases, experiencing different kinds of care from different caregivers. Second, it minimizes the possibility that the association between changes in the caregiving environment and changes in children’s functioning can be accounted for by an unmeasured genetic factor because removal from the care of biological parents breaks the link between the person providing the child’s rearing environment and the person providing the child’s genotype (although some children were living with second degree or more distant relatives).
Implications for research and theory
Like other studies that situate children’s development within a biopsychosocial framework (e.g., Raine, 2002), the current study found that early biological vulnerabilities increased risk for poor cognitive and behavioral outcomes only when children were exposed to ongoing adversity (e.g., decrements in the quality of the caregiving environment). These findings are promising because they suggest targets for intervention, generate hypotheses about the mechanisms by which biological vulnerabilities and social experiences jointly affect children’s functioning, and suggest that biological vulnerabilities do not invariably compromise development. Indeed, these findings are consistent with data from studies of low- and middle-income children in showing that efforts by caregivers to speak and read to children and to be responsive to children’s role play, vocalizations, and exploration of objects can promote children’s language growth (Pan et al., 2005; Tamis-LeMonda, Bornstein, & Baumwell, 2001). Moreover, these findings extend this work by demonstrating that the association is present in children with moderate to severe levels of neurodevelopmental impairment. The findings are also consistent with a body of literature that finds that the fit between a child’s temperament and the type of parenting the child experiences (rather than either factor alone) is predictive of children’s behavioral trajectories (Rothbart & Bates, 1998).
As reviewed by Cicchetti and Curtis (2006), experiences like competent caregiving are likely to lead to changes in brain structure and function (and, ultimately, behavior) by altering regulation of gene expression (e.g., via epigenetic processes such as methylation) and protein phosphorylation (inducing posttranslational modifications to protein products). For example, Meaney and colleagues (Caldji et al., 1998; Francis, Diorio, Liu, & Meaney, 1999; Liu et al., 1997) have demonstrated that relatively greater amounts of time rat mothers spend licking and grooming their pups and engaging in arched back nursing in the first week of life have long-term effects on reducing fearful behavior and hypothalamic–pituitary–adrenal axis responsivity when offspring are subjected to stressors in adulthood. These observed differences in stress responsivity are mediated by changes in gene expression in brain regions associated with fear and stress responsivity (Caldji, Diorio, & Meaney, 2003; Francis et al., 1999). Weaver et al. (2004) showed that high levels of licking, grooming, and arched back nursing caused increases in offspring hippocamapal glucocorticoid receptor gene expression, and that these increases were the result of stable changes in DNA methylation patterns that were established in the first week of life. Crucially, these methylation patterns were reversible in adulthood. Thus, administration of enzymes that altered DNA receptivity to transcription factor binding caused the upregulation of hippocampal glucocorticoid receptor gene expression in the adult offspring of mothers who had engaged in low levels of licking, grooming, and arched back nursing and a concomitant reversal in the adrenocortical response to stress (Weaver et al., 2004). Much more research is required to demonstrate whether reversals in DNA methylation patterns and subsequent changes in gene expression can arise from social experiences and whether similar processes underlie cognitive and behavioral changes among at-risk human children who are subjected to remedial intervention.
Future research should also explore the limits of the brain to reorganize in the face of new experiences. As reviewed by Curtis and Cicchetti (2003), the brain is not infinitely plastic and certain changes (e.g., structural changes resulting from traumatic head injury, as in some cases of physical abuse) may be irreversible, even when the caregiving environment improves dramatically. This suggests that, depending on the cause and timing of brain insults that resulted in neurodevelopmental impairment, some children in the NSCAW sample may have shown greater recovery in language and behavioral functioning than others. Although levels of neurodevelopmental impairment and difficult temperament were similar across maltreatment groups (as were rates of resilience), it is possible that different forms of maltreatment are associated with more or less lasting changes in the brain. However, given that different forms of maltreatment often co-occur and studies have rarely shown specific associations between maltreatment sequelae (e.g., malnutrition resulting from food neglect or traumatic head injury resulting from physical abuse) and cognitive and behavioral outcomes, it seems unlikely that the brain’s ability to reorganize in the face of remedial social experiences will depend on the type of maltreatment a child experienced. Rather, it seems more plausible that the severity and duration of maltreatment, regardless of subtype, will be most predictive of plasticity.
Other research suggests that the capacity for remedial experiences to result in neural reorganization is conditioned on individual differences that may include genotype and the developmental period in which such experiences are encountered. For example, Rutter and colleagues (2004) have illustrated that experiences of gross neglect can result in lasting cognitive and socioemotional deficits that are irreversible by later experiences. However, this work has also demonstrated that many children show remarkable cognitive, behavioral, and physical catch-up upon removal from conditions of severe privation. Moreover, even though children who experience longer bouts of adversity manifest more persistent impairments (Rutter et al., 2004), there is little evidence that the first 3 years of life are a critical period for cognitive or socioemotional development (Bruer, 2001). For example, Duyme, Dumaret, and Tomkiewicz (1999) studied changes in IQ among children who had been abused or neglected in infancy and who were adopted between the ages of 4 and 6 years. Although these children had, on average, below-average IQ scores prior to adoption (M = 77), IQ scores increased dramatically byearly adolescence, particularlyamong children who were adopted by high socioeconomic status families (M = 98). These data suggest that although the brain may be optimally responsive to environmental remediation within the first 3 years of life, developmental trajectories can still be modified by environmental experiences at later points in time.
Implications for practice
The findings reported here demonstrate that specific caregiving behaviors can lead to better than expected cognitive and behavioral outcomes among children who are at biological risk. However, parents of biologically vulnerable children are disproportionately likely to have mental health or substance use problems and to be living in conditions of considerable adversity (Moffitt, 1993). These parents may find it especially difficult to provide the kind of cognitive stimulation or emotional support that promotes resilience in children. Consistent with this possibility, changes in the amount of cognitive stimulation provided to children were more weakly associated with children’s language abilities in families in which children were not removed from the care of biological parents versus families in which they were. It is possible that the potential for change in some families (and the effects of change on children’s functioning) may be limited by characteristics of the parents or by the broader family context (Belsky, 1993; Jaffee, 2005).
Although some researchers have demonstrated that removal from the care of abusive or neglectful biological parents is associated with improvements in children’s well-being (Davidson-Arad, Englechin-Segal, & Wozner, 2003), there remains considerable disagreement over how best to protect children from violence by family members (Erickson, 2000). Federal child protection policy in the United States has swung from a focus on family preservation (e.g., the 1980 Adoption Assistance and Child Welfare Act and federal funding in 1993 for the Family Preservation and Support Program) to a focus on the child’s health and safety (e.g., the 1997 Adoption and Safe Families Act). Data are needed to inform policy about children exposed to family violence. The current analysis demonstrated one domain in which infants and toddlers benefited from being removed from the care of abusive biological parents. Critically, these benefits only accrued to children who experienced improvements in the quality of the caregiving they received. When the quality of the caregiving they received worsened, they showed poorer (or as poor) functioning compared with children who remained in the care of biological parents. Although child welfare workers expend considerable resources to ensure the safety of kin and nonkin foster care placements, more data is needed on how foster and biological parents can be helped to promote positive outcomes in maltreated children who are likely to be vulnerable to cognitive, motor, and socioemotional deficits. Multisystemic interventions that target foster parents, children, and biological parents have been shown to increase the success rate of permanent placements (i.e., the child’s final nonfoster care placement; Fisher, Burraston, & Pears, 2005) and successful permanent placements are predictive of children’s psychological well-being (Rubin, O’Reilly, Luan, & Localio, 2007), but more research is clearly needed to uncover the mechanisms by which permanent placements improve children’s psychological well-being.
Strengths and limitations
This study is characterized by a number of limitations. First, change over time in children’s functioning and the quality of the child’s environment was estimated within a relatively brief period (18 months). On the one hand, the first 2–3 years of life are a period of rapid brain development and changes in the child’s environment over this period may have especially strong influences on the child’s functioning. On the other hand, the findings may not replicate over longer periods of time. For example, children with perinatal or early traumatic brain injury (which many of the neurodevelopmentally impaired children may have had) have shown delays in language learning when tested before age 4 years, but not after that point (Levin, 2003), a phenomenon that might result from school attendance (Huttenlocher, Levine, & Vevea, 1998) or early interventions like Head Start. Nevertheless, the child who enters school with deficits in vocabulary size remains at risk for reading problems during the primary school years (Snow, Burns, & Griffin, 1998) suggesting that even when children “catch up” with respect to language abilities, they may still be experiencing difficulties relative to other children in other domains.
Second, these analyses demonstrated that a positive caregiving environment predicted better than expected child functioning, but these relative changes do not necessarily mean that children were functioning in the normal range by Wave 3 nor do they mean that observed changes reflected transitions from clinically significant to normative states. For example, although the average language score was 102.54 among children who demonstrated better than expected language abilities at Wave 3 (the PLS is normed to have a mean of 100), scores ranged from 87 to 150. All problem behavior scores among children who demonstrated better than expected behavior at Wave 3 (M = 43.87, range = 24–58) fell below the cutoff of 60 indicating borderline or clinically significant problem behaviors. Together, these data suggest that some children were still not functioning in the normal range by Wave 3, although they were doing better than expected, and that other children simply showed improvements in already normal functioning. In addition, the variance in children’s language and behavior that was accounted for by changes in the care-giving environment was small (<10%) as were effect sizes for associations between language abilities, problem behaviors, and measures of cognitive stimulation, emotional support, and early biological risk. That said, most of these associations were at least as large as the association between SAT scores and subsequent college grade point average or the association between reduced blood flow and subsequent thrombosis (Meyer et al., 2001).
Third, the NSCAW sample is representative of children who have had contact with child welfare services in the United States, but it is not representative of the overall population of US children. Although the NSCAW sample was ideal for identifying large numbers of children who were at risk for neurodevelopmental impairment, it remains to be seen whether the findings will replicate in a non-high-risk sample.
Fourth, although neurodevelopmental impairment and difficult temperament were identified as biological risks, their etiology is likely to be both biological (e.g., genetic) and environmental (e.g., intrauterine, perinatal, or postnatal risks). Nevertheless, they are described as biological risks because they are at least partly biologically based characteristics of children.
Fifth, children would have experienced a range of changes when they were removed from the care of biological parents, including changes in cognitive stimulation and emotional support, as well as changes in family income, neighborhood characteristics, and caregiver characteristics. Given all these possible changes, it is impossible to say with certainty that changes in cognitive stimulation per se influenced children’s language ability. Although family income was controlled in the analyses (and the effect of changes in cognitive stimulation remained statistically significant), other family and caregiver characteristics were not included in the models. That said, to the extent that these other characteristics influence children’s functioning, they are likely to do so via their effects on parenting (Belsky & Jaffee, 2005; Conger et al., 1992; McLoyd, Jayartane, Ceballo, & Borquez, 1994; Yeung, Linver, & Brooks-Gunn, 2002).
This investigation of resilience among biologically at-risk children also has a number of strengths. The NSCAW sample comprised a large number of neurodevelopmentally at-risk children who were followed over time. The longitudinal nature of the data facilitated analyses that clarified the direction of effects between changes in the caregiving environment and changes in children’s functioning. Moreover, the fact that some children experienced a turning point in their living arrangements over the 18-month time frame of the study provided a natural experiment in which changes in the quality of children’s care coincided with their removal from the care of biological parents. The findings from this study suggest that interventions targeted at neurodevelopmentally impaired infants and toddlers, many of whom will be victims of abuse and neglect, are likely to promote positive behavioral and cognitive outcomes when caregivers are taught to provide adequate cognitive stimulation and when they can foster warm, supportive relationships with children.
Acknowledgments
Many thanks to Louise Arseneault, Andrea Maikovich, and Tom Price for their helpful comments on an earlier version of this manuscript. This work was supported by Grant R01 HD050691 from the National Institute of Child Health and Human Development. This document includes data from the National Survey of Child and Adolescent Well-Being, which was developed under contract with the Administration on Children, Youth, and Families, United States Department of Health and Human Services. The information and opinions expressed herein are solely those of the author.
Footnotes
The standard errors are the estimated variation in the population after accounting for sample stratification.
Resilient functioning in the language and problem behavior domains was defined with respect to early levels of neurodevelopmental risk and difficult temperament, respectively, because risk for neurodevelopmental impairment was more strongly related to children’s language ability than their problem behaviors, and difficult temperament was more strongly related to children’s problem behaviors than their language ability.
Logistic and OLS regression analyses were conducted to compare physically abused and neglected children (the two most common groups) on the prevalence of neurodevelopmental impairment, mean difficult temperament scores, and the prevalence of better than expected language and behavior problem scores. None of these differences were statistically significant.
The variables were essentially already centered when they were entered in the model (residualized cognitive stimulation M = 0.03, residualized emotional support M = −0.01).
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Arseneault L, Tremblay R, Boulerice B, Saucier JF. Obstetrical complications and violent delinquency: Testing two developmental pathways. Child Development. 2002;73:496–508. doi: 10.1111/1467-8624.00420. [DOI] [PubMed] [Google Scholar]
- Aylward GP. Bayley Infant Neurodevelopmental Screener. San Antonio, TX: Psychological Corporation; 1995. [Google Scholar]
- Aylward GP. Neurodevelopmental outcomes in infants born prematurely. Developmental and Behavioral Pediatrics. 2005;26:427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- Aylward GP, Verhulst SJ. Predictive utility of the Bayley Infant Neurodevelopmental Screener (BINS) risk status classifications: Clinical interpretation and application. Developmental Medicine and Child Neurology. 2000;42:25–31. doi: 10.1017/s0012162200000062. [DOI] [PubMed] [Google Scholar]
- Bacchus L, Mezey G, Bewley S. Domestic violence: Prevalence in pregnant women and associations with physical and psychological health. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2004;113:6–11. doi: 10.1016/S0301-2115(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Bailey BN, Delaney-Black V, Covington CY, Ager J, Janisse J, Hannigan JH, et al. Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. American Journal of Obstetrics and Gynecology. 2004;191:1037–1043. doi: 10.1016/j.ajog.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Barlow KM, Thomson E, Johnson D, Minns RA. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116:174–185. doi: 10.1542/peds.2004-2739. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- Belsky J. Etiology of child maltreatment: A developmental–ecological analysis. Psychological Bulletin. 1993;114:413–434. doi: 10.1037/0033-2909.114.3.413. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jaffee SR. The multiple determinants of parenting. In: Cicchetti D, Cohen DJ, editors. Handbook of developmental psychopathology: Vol. 3. Risk, disorder, and adaptation. 2. New York: Wiley; 2005. pp. 38–85. [Google Scholar]
- Benedict MI, White RB, Cornely DA. Maternal and perinatal risk factors and child abuse. Child Abuse & Neglect. 1985;9:217–224. doi: 10.1016/0145-2134(85)90014-6. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Hall J, Bor W, Najman JM, Williams G. Integrating biological and social processes in relation to early-onset persistent aggression in boys and girls. Developmental Psychology. 2003;39:309–323. [PubMed] [Google Scholar]
- Bruer JT. A critical and sensitive period primer. In: Bailey DB, Bruer JT, Symons FJ, Lichtman JW, editors. Critical thinking about critical periods. Baltimore, MD: Paul H. Brookes; 2001. pp. 3–26. [Google Scholar]
- Bunikowski R, Grimmer I, Heiser A, Metze B, Schafer A, Obladen M. Neurodevelopmental outcome after prenatal exposure to opiates. European Journal of Pediatrics. 1998;157:724–730. doi: 10.1007/s004310050923. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABAA receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:150–159. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley RH. Home observation for measurement of the environment. Little Rock, AR: University of Arkansas at Little Rock; 1984. [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophrenia: Results from a longitudinal birth cohort study. Archives of General Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Curtis WJ. The developing brain and neural plasticity: Implications for normality, psychopathology, and resilience. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2. New York: Wiley; 2006. pp. 1–64. [Google Scholar]
- Cicchetti D, Toth SL. A developmental psychopathology perspective on child abuse and neglect. Journal of the American Academy of Child Psychiatry. 1995;34:541–565. doi: 10.1097/00004583-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant cognition: Predicting later intellectual functioning. Thousand Oaks CA: Sage; 1993. [Google Scholar]
- Conger RD, Conger KJ, Elder GHJ, Lorenz FO, Simons RL, Whitbeck LB. A family process model of economic hardship and adjustment of early adolescent boys. Child Development. 1992;63:526–541. doi: 10.1111/j.1467-8624.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Moving research on resilience into the 21st century: Theoretical and methodological considerations in examining the biological contributors to resilience. Development and Psychopathology. 2003;15:773–810. doi: 10.1017/s0954579403000373. [DOI] [PubMed] [Google Scholar]
- Davidson-Arad B, Englechin-Segal D, Wozner Y. Short-term follow-up of children at risk: Comparison of the quality of life of children removed from home and children remaining at home. Child Abuse & Neglect. 2003;27:733–750. doi: 10.1016/s0145-2134(03)00113-3. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. The psychobiology of neglect. Child Maltreatment. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- Dowd K, Kinsey S, Wheeless S, Thissen R, Richardson J, Suresh R, et al. National Survey of Child and Adolescent Well-Being (NSCAW): Combined Waves 1–4 data user’s manual. Durham, NC: Research Triangle Institute; 2004. [Google Scholar]
- Duyme M, Dumaret AC, Tomkiewicz S. How can we boost IQs of “dull children”?: A late adoption study. Proceedings of the National Academy of Sciences USA. 1999;96:8790–8794. doi: 10.1073/pnas.96.15.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti IM, Cicchetti D. The impact of child maltreatment on expressive syntax at 60 months. Developmental Science. 2004;7:88–102. doi: 10.1111/j.1467-7687.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- Erickson PE. Federal child abuse and child neglect policy in the United States since 1974: A review and critique. Criminal Justice Review. 2000;25:77–92. [Google Scholar]
- Fisher PA, Burraston B, Pears K. The Early Intervention Foster Care Program: Permanent placement outcomes from a randomized trial. Child Maltreatment. 2005;10:61–71. doi: 10.1177/1077559504271561. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: A Major Revision and Restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91–97. [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain—A review. Journal of Child Psychology and Psychiatry. 2000;41:97–116. [PubMed] [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, Lyons T. Early vocabulary growth: Relation to language input and gender. Developmental Psychology. 1991;27:236–248. [Google Scholar]
- Huttenlocher J, Levine S, Vevea J. Environmental input and cognitive growth: A study using time-period comparisons. Child Development. 1998;69:1012–1029. [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual functioning. Alcoholism: Clinical and Experimental Research. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jaffee SR. Family violence and parent psychopathology: Implications for children’s socioemotional development and resilience. In: Goldstein S, Brooks R, editors. Handbook of resilience in children. New York: Kluwer; 2005. pp. 149–163. [Google Scholar]
- Jaffee SR, Moffitt TE, Caspi A, Fombonne E, Poulton R, Martin J. Differences in early childhood risk factors for juvenile-onset and adult-onset depression. Archives of General Psychiatry. 2002;59:215–222. doi: 10.1001/archpsyc.59.3.215. [DOI] [PubMed] [Google Scholar]
- Kelley SJ. Parenting stress and child maltreatment in drug-exposed children. Child Abuse & Neglect. 1992;16:317–328. doi: 10.1016/0145-2134(92)90042-p. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Multiple pathways to conscience for children with different temperaments: From toddler-hood to age 5. Developmental Psychology. 1997;35:228–240. doi: 10.1037//0012-1649.33.2.228. [DOI] [PubMed] [Google Scholar]
- Laucht M, Esser G, Schmidt M. Developmental outcome of infants born with biological and psychosocial risks. Journal of Child Psychiatry. 2001;38:843–853. doi: 10.1111/j.1469-7610.1997.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Leslie LK, Gordon JN, Meneken L, Premji K, Michelmore KL, Ganger W. The physical, developmental, and mental health needs of young children in child welfare by initial placement type. Developmental and Behavioral Pediatrics. 2005;26:177–185. doi: 10.1097/00004703-200506000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal JM, Forsyth BW, Qi K, Johnson L, Scroeder D, Votto N. Maltreatment of children born to women who used cocaine during pregnancy: A population-based study. Pediatrics. 1997;100:E7. doi: 10.1542/peds.100.2.e7. [DOI] [PubMed] [Google Scholar]
- Levin HS. Neuroplasticity following non-penetrating traumatic brain injury. Brain Injury. 2003;17:665–674. doi: 10.1080/0269905031000107151. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: A critical evaluation and guidelines for future work. Child Development. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias MM, Saylor CF, Greer MK, Charles JM, Bell N, Katikaneni LD. Infant screening: The usefulness of the Bayley Infant Neurodevelopmental Screener and the Clinical Adaptive Test/Clinical Linguistic Auditory Milestone Scale. Developmental and Behavioral Pediatrics. 1998;19:155–161. doi: 10.1097/00004703-199806000-00002. [DOI] [PubMed] [Google Scholar]
- McFayden RG, Kitson WJH. Language comprehension and expression among adolescents who have experienced childhood physical abuse. Journal of Child Psychology and Psychiatry. 1996;37:551–562. doi: 10.1111/j.1469-7610.1996.tb01441.x. [DOI] [PubMed] [Google Scholar]
- McLoyd VC, Jayartane TE, Ceballo R, Borquez J. Unemployment and work interruption among African American single mothers: Effects on parenting and adolescent socioemotional functioning. Child Development. 1994;65:562–589. [PubMed] [Google Scholar]
- Meyer GJ, Finn SE, Eyde LD, Kay GG, Moreland KL, Dies RR, et al. Psychological testing and psychological assessment. American Psychologist. 2001;56:128–165. [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Molfese VJ, Acheson S. Infant and preschool mental and verbal abilities: How are infant scores related to preschool scores? International Journal of Behavioral Development. 1997;20:595–607. [Google Scholar]
- Mott FL, Baker PC, Ball DE, Keck CC, Lenhart SM. The NLSY children 1992: Description and evaluation. Columbus, OH: Ohio State University Center for Human Resource Research; 1995. [Google Scholar]
- Murray GK, Jones PB, Moilanen K, Veijola J, Miettunen J, Cannon TD, et al. Infant motor development and adult cognitive functions in schizophrenia. Schizophrenia Research. 2006;81:65–74. doi: 10.1016/j.schres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Rutter M, Beckett C, Keaveney L, Kreppner JM English and Romanian Adoptees Study Team. The effects of global severe privation on cognitive competence: Extension and longitudinal follow-up. Child Development. 2000;71:376–390. doi: 10.1111/1467-8624.00151. [DOI] [PubMed] [Google Scholar]
- Pan BA, Rowe ML, Singer JD, Snow CE. Maternal correlates of growth in toddler vocabulary production in low-income families. Child Development. 2005;76:763–782. doi: 10.1111/j.1467-8624.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Pears K, Fisher PA. Developmental, cognitive, and neuropsychological functioning in preschool-aged foster children: Associations with prior maltreatment and placement history. Developmental and Behavioral Pediatrics. 2005;26:112–122. doi: 10.1097/00004703-200504000-00006. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Brennan P, Mednick B, Mednick SA. High rates of violence, crime, academic problems, and behavioral problems in males with both early neuromotor deficits and unstable family environments. Archives of General Psychiatry. 1996;53:544–549. doi: 10.1001/archpsyc.1996.01830060090012. [DOI] [PubMed] [Google Scholar]
- Rao R, Georgieff MK. Early nutrition and brain development. In: Nelson CA, editor. The Minnesota Symposia on Child Psychology: Vol. 31. The effects of early adversity of neurobehavioral development. Mahwah, NJ: Erlbaum; 2000. pp. 1–30. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Eisenberg N, editor. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 5. New York: Wiley; 1998. pp. 105–176. [Google Scholar]
- Rubin DM, O’Reilly A, Luan X, Localio AR. The impact of placement stability on behavioral well-being for children in foster care. Pediatrics. 2007;119:336–344. doi: 10.1542/peds.2006-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M English and Romanian Adoptees (ERA) Study Team. Developmental catch-up, and deficit, following adoption after severe global early privation. Journal of Child Psychology and Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- Rutter M, O’Connor TG English and Romanian Adoptees Study Team. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40:81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves L. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Shevell M, Majnemer A, Platt RW, Webster R, Birnbaum R. Developmental and functional outcomes at school age of preschool children with global developmental delay. Journal of Child Neurology. 2004;20:648–654. doi: 10.1177/08830738050200080301. [DOI] [PubMed] [Google Scholar]
- Snow CE, Burns S, Griffin P. Preventing reading difficulties in young children. Washington, DC: National Academy Press; 1998. [Google Scholar]
- StataCorp. Stata statistical software: Release 7.0. College Station, TX: Stata Corporation; 2001. [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, Baumwell L. Maternal responsiveness and children’s achievement of language milestones. Child Development. 2001;72:748–767. doi: 10.1111/1467-8624.00313. [DOI] [PubMed] [Google Scholar]
- Tully LA, Arseneault L, Caspi A, Moffitt TE, Morgan J. Does maternal warmth moderate the effects of birth weight on twins’ attention-deficit/hyperactivity disorder (ADHD) symptoms and low IQ? Journal of Consulting and Clinical Psychology. 2004;72:218–226. doi: 10.1037/0022-006X.72.2.218. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Child Maltreatment 2003. Retrieved February 13, 2006, from http://www.acf.hhs.gov/programs/cb/pubs/cm03/index.htm.
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Monk CS, Nelson CA. Mechanisms of postnatal neurobiological development: Implications for human development. Developmental Neuropsychology. 2001;19:147–171. doi: 10.1207/S15326942DN1902_2. [DOI] [PubMed] [Google Scholar]
- Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Torre C, et al. Psychiatric outcomes in low-birth-weight children at age 6 years: Relation to neonatal cranial ultrasound abnormalities. Archives of General Psychiatry. 1997;54:847–856. doi: 10.1001/archpsyc.1997.01830210091012. [DOI] [PubMed] [Google Scholar]
- Yeung WJ, Linver MR, Brooks-Gunn J. How money matters for young children’s development: Parental investment and family processes. Child Development. 2002;73:1861–1879. doi: 10.1111/1467-8624.t01-1-00511. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scales—3. San Antonio, TX: Psychological Corporation; 1992. [Google Scholar]


