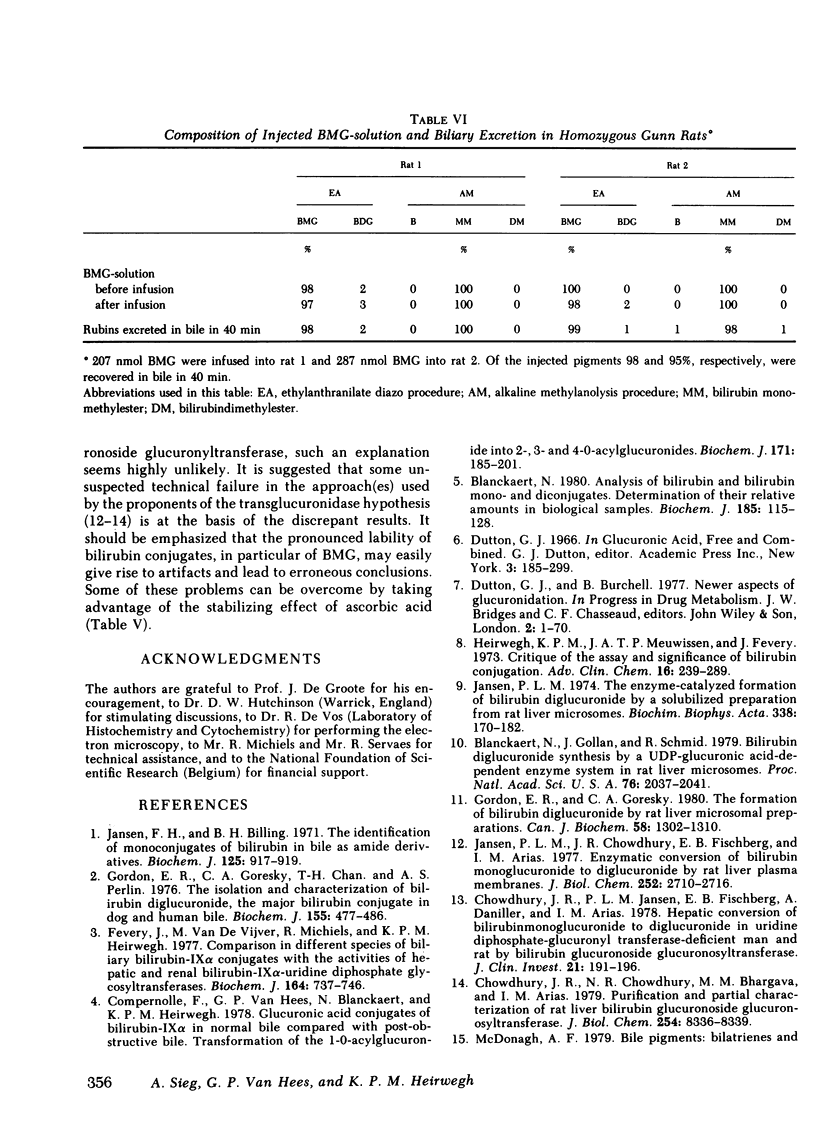
Abstract
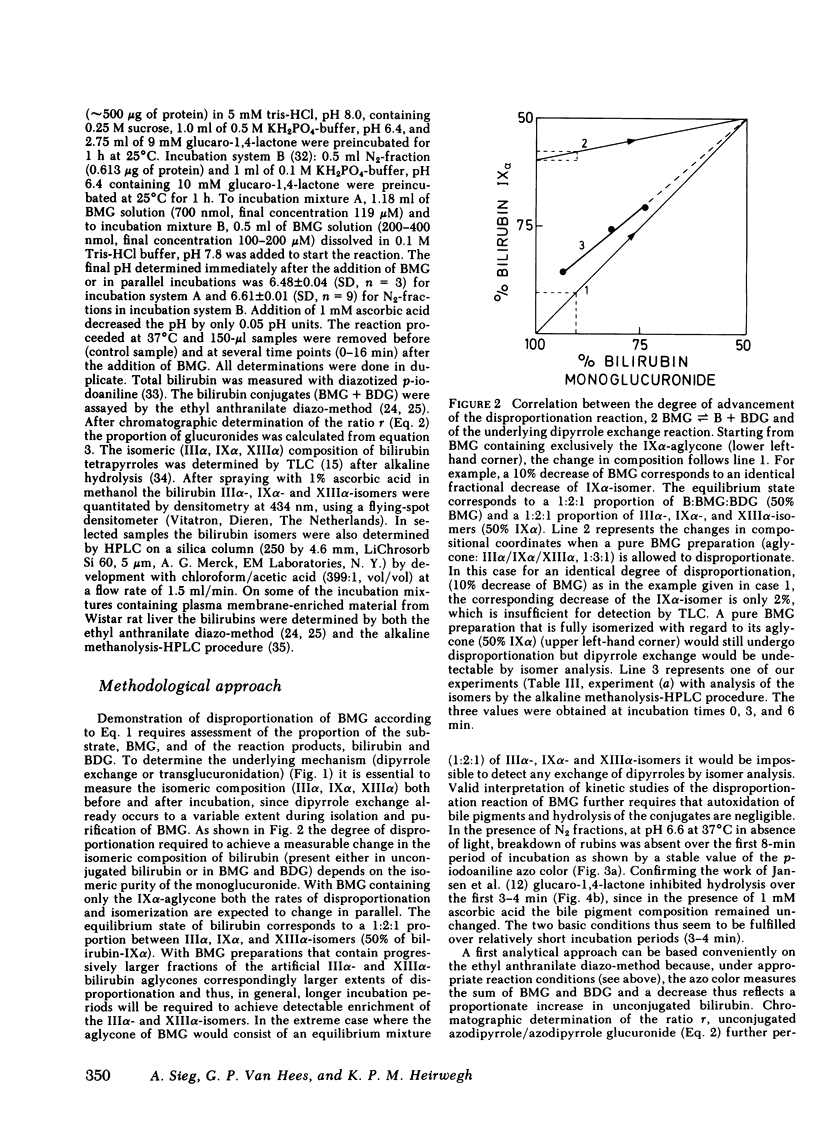
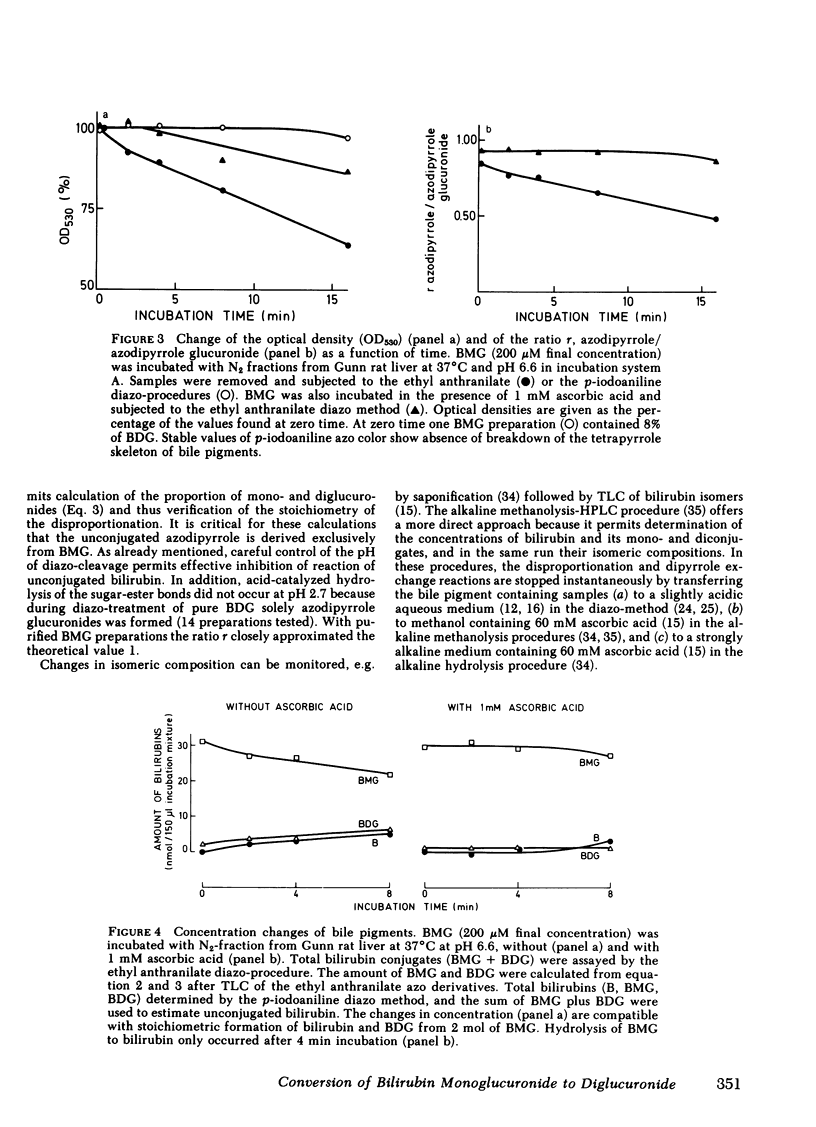
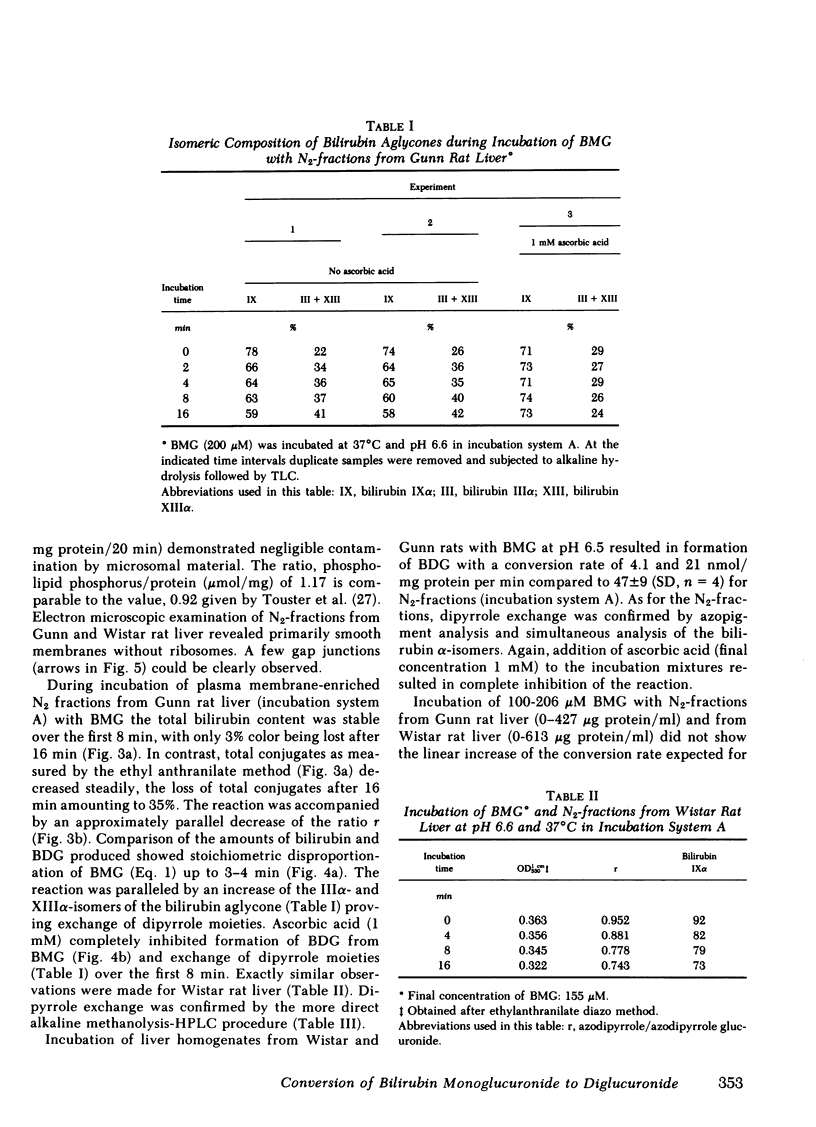
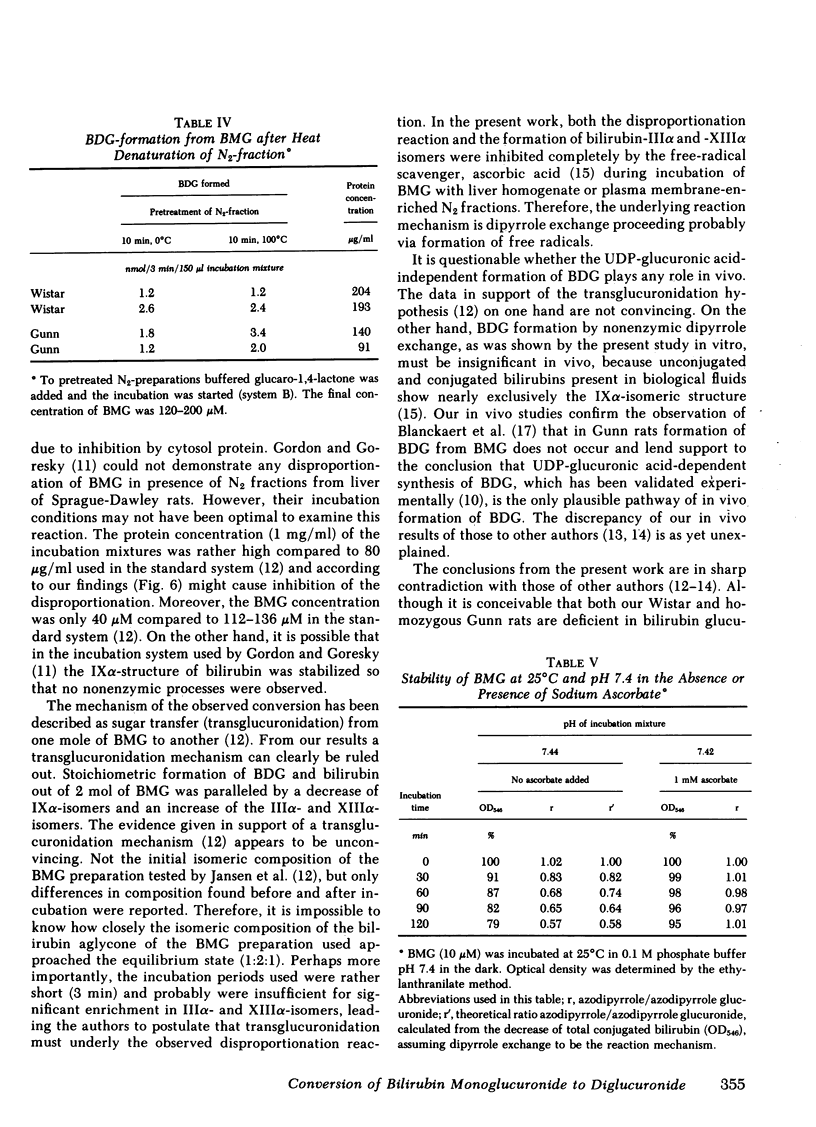
Two routes have been proposed for conversion of bilirubin monoglucuronide to the diglucuronide: glucuronyl transfer (a) from UDP-glucuronic acid to bilirubin monoglucuronide, catalyzed by a microsomal UDP-glucuronyltransferase, and (b) from one molecule of bilirubin monoglucuronide to another (transglucuronidation), catalyzed by an enzyme present in liver plasma membranes. The evidence regarding the role of the latter enzyme for in vivo formation of bilirubin diglucuronide is conflicting. We therefore decided to reexamine the transglucuronidation reaction in plasma membranes and to study the conversion of bilirubin monoglucuronide to diglucuronide in vivo. Purified bilirubin monoglucuronide was incubated with homogenates and plasma membrane-enriched fractions from liver of Wistar and Gunn rats. Stoichiometric formation of bilirubin and bilirubin diglucuronide out of 2 mol of bilirubin monoglucuronide was paralleled by an increase of the IIIα- and XIIIα-isomers of the bilirubin aglycone, thus showing that dipyrrole exchange, not transglucuronidation, is the underlying mechanism. Complete inhibition by ascorbic acid probably reflects intermediate formation of free radicals of dipyrrolic moieties. The reaction was nonenzymic because it proceeded independently of the protein concentration and heat denaturation of the plasma membranes did not result in decreased conversion rates. Collectively, these findings show spontaneous, nonenzymic dipyrrole exchange when bilirubin monoglucuronide is incubated in the presence of rat liver plasma membranes. Because bilirubin glucuronides present in biological fluids contain exclusively the bilirubin-IXα aglycone, formation of the diglucuronide from the monoglucuronide by dipyrrole exchange does not occur in vivo. Rapid excretion of unchanged bilirubin monoglucuronide in Gunn rat bile after injection of the pigment provides confirmatory evidence for the absence of a UDP-glucuronic acid-independent process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Blanckaert N. Analysis of bilirubin and bilirubin mono- and di-conjugates. Determination of their relative amounts in biological samples. Biochem J. 1980 Jan 1;185(1):115–128. doi: 10.1042/bj1850115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Fevery J., Heirwegh K. P., Compernolle F. Characterization of the major diazo-positive pigments in bile of homozygous Gunn rats. Biochem J. 1977 Apr 15;164(1):237–249. doi: 10.1042/bj1640237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Gollan J., Schmid R. Bilirubin diglucuronide synthesis by a UDP-glucuronic acid-dependent enzyme system in rat liver microsomes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2037–2041. doi: 10.1073/pnas.76.4.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Gollan J., Schmid R. Mechanism of bilirubin diglucuronide formation in intact rats: bilirubin diglucuronide formation in vivo. J Clin Invest. 1980 Jun;65(6):1332–1342. doi: 10.1172/JCI109797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Heirwegh K. P., Compernolle F. Synthesis and separation by thin-layer chromatography of bilirubin-IX isomers. Their identification as tetrapyrroles and dipyrrolic ethyl anthranilate azo derivatives. Biochem J. 1976 May 1;155(2):405–417. doi: 10.1042/bj1550405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Kabra P. M., Farina F. A., Stafford B. E., Marton L. J., Schmid R. Measurement of bilirubin and its monoconjugates and diconjugates in human serum by alkaline methanolysis and high-performance liquid chromatography. J Lab Clin Med. 1980 Aug;96(2):198–212. [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R., Bhargava M. M., Arias I. M. Purification and partial characterization of rat liver bilirubin glucuronoside glucuronosyltransferase. J Biol Chem. 1979 Sep 10;254(17):8336–8339. [PubMed] [Google Scholar]
- Chowdhury J. R., Jansen P. L., Fischberg E. B., Daniller A., Arias I. M. Hepatic conversion of bilirubin monoglucuronide to diglucuronide in uridine diphosphate-glucuronyl transferase-deficient man and rat by bilirubin glucuronoside glucuronosyltransferase. J Clin Invest. 1978 Jul;62(1):191–196. doi: 10.1172/JCI109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Blanckaert N., Heirwegh K. P. Glucuronic acid conjugates of bilirubin-IXalpha in normal bile compared with post-obstructive bile. Transformation of the 1-O-acylglucuronide into 2-, 3-, and 4-O-acylglucuronides. Biochem J. 1978 Apr 1;171(1):185–201. doi: 10.1042/bj1710185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fevery J., Van de Vijver M., Michiels R., Heirwegh K. P. Comparison in different species of biliary bilirubin-IX alpha conjugates with the activities of hepatic and renal bilirubin-IX alpha-uridine diphosphate glycosyltransferases. Biochem J. 1977 Jun 15;164(3):737–746. doi: 10.1042/bj1640737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. R., Goresky C. A., Chang T. H., Perlin A. S. The isolation and characterization of bilirubin diglucuronide, the major bilirubin conjugate in dog and human bile. Biochem J. 1976 Jun 1;155(3):477–486. doi: 10.1042/bj1550477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. R., Goresky C. A. The formation of bilirubin diglucuronide by rat liver microsomal preparations. Can J Biochem. 1980 Nov;58(11):1302–1310. doi: 10.1139/o80-175. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Fevery J., Meuwissen J. A., De Groote J., Compernolle F., Desmet V., Van Roy F. P. Recent advances in the separation and analysis of diazo-positive bile pigments. Methods Biochem Anal. 1974;22:205–250. doi: 10.1002/9780470110423.ch5. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Meuwissen J. A., Fevery J. Critique of the assay and significance of bilirubin conjugation. Adv Clin Chem. 1973;16:239–288. doi: 10.1016/s0065-2423(08)60347-9. [DOI] [PubMed] [Google Scholar]
- Jansen F. H., Billing B. H. The identification of monoconjugates of bilirubin in bile as amide derivatives. Biochem J. 1971 Dec;125(3):917–919. doi: 10.1042/bj1250917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen P. L., Chowdhury J. R., Fischberg E. B., Arias I. M. Enzymatic conversion of bilirubin monoglucuronide to diglucuronide by rat liver plasma membranes. J Biol Chem. 1977 Apr 25;252(8):2710–2716. [PubMed] [Google Scholar]
- Jansen P. L. The isomerisation of bilirubin monoglucuronide. Clin Chim Acta. 1973 Dec 12;49(2):233–240. doi: 10.1016/0009-8981(73)90296-9. [DOI] [PubMed] [Google Scholar]
- Ostrow J. D., Boonyapisit S. T. Inaccuracies in measurement of conjugated and unconjugated bilirubin in bile with ethyl anthranilate diazo and solvent-partition methods. Biochem J. 1978 Jul 1;173(1):263–267. doi: 10.1042/bj1730263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow J. D. Photocatabolism of labeled bilirubin in the congenitally jaundiced (Gunn) rat. J Clin Invest. 1971 Mar;50(3):707–718. doi: 10.1172/JCI106541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- SCHMID R., AXELROD J., HAMMAKER L., SWARM R. L. Congenital jaundice in rats, due to a defect in glucuronide formation. J Clin Invest. 1958 Aug;37(8):1123–1130. doi: 10.1172/JCI103702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID R., HAMMAKER L. METABOLISM AND DISPOSITION OF C14-BILIRUBIN IN CONGENITAL NONHEMOLYTIC JAUNDICE. J Clin Invest. 1963 Nov;42:1720–1734. doi: 10.1172/JCI104858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F. P., Meuwissen J. A., De Meuter F., Heirwegh K. P. Determination of bilirubin in liver homogenates and serum with diazotized p-iodoaniline. Clin Chim Acta. 1971 Jan;31(1):109–118. doi: 10.1016/0009-8981(71)90367-6. [DOI] [PubMed] [Google Scholar]
- Weaver R. A., Boyle W. Purification of plasma membranes of rat liver. Application of zonal centrifugation to isolation of cell membranes. Biochim Biophys Acta. 1969 Apr;173(3):377–388. doi: 10.1016/0005-2736(69)90003-0. [DOI] [PubMed] [Google Scholar]




