Abstract
Objectives
To evaluate the efficacy and safety of adjunctive aripiprazole compared with standard antidepressant therapy (ADT) for older patients with major depressive disorder (MDD) who demonstrated an incomplete response to standard antidepressant monotherapy.
Methods
Data from three similar 14-week studies (an 8-week prospective ADT treatment phase and a 6-week randomized, double-blind phase) of aripiprazole augmentation were pooled for this post hoc analysis. Two age groups were defined: younger patients (aged 18–49 years) and older patients (aged 50–67 years). The older patient group was further divided into three subgroups: 50–55, 56–60, and 61–67 years. The efficacy endpoint was the mean change in Montgomery–Åsberg Depression Rating Scale (MADRS) total score from end of the prospective phase (Week 8) to endpoint (Week 14, last observation carried forward (LOCF)). Remission was defined as MADRS total score ≤10 at endpoint.
Results
Four hundred and nine older patients (placebo, n = 198; aripiprazole, n = 211) and 679 younger patients (placebo, n = 341; aripiprazole, n = 338) were included in this analysis. Older patients receiving aripiprazole demonstrated significantly greater improvement in MADRS total score versus placebo at Week 14 (−10.0 vs. −6.4; p < 0.001; LOCF), similar to the improvement seen in younger patients. Remission rates were significantly higher with aripiprazole versus placebo in older (32.5% vs. 17.1%; p < 0.001) and younger (26.9% vs. 16.4%; p < 0.001) patients. Akathisia was the most common adverse event in both the older (17.1%) and younger (26.0%) patient groups.
Conclusions
Adjunctive aripiprazole was effective in improving depressive symptoms in older patients, 50–67 years, with MDD who have had an inadequate response to standard antidepressant medication.
Keywords: aripiprazole, antidepressant therapy, major depressive disorder, older adults
Introduction
Depression in older individuals is common across many settings, and its association with impairments in physical and cognitive function, as well as mortality, makes it an important public health concern (Blazer, 2003). Although a recent meta-analysis has shown that antidepressants are more effective than placebo in older (>60 years) depressed patients, the effects were only modest, with only five out of every 100 older patients achieving remission from symptoms as the result of drug treatment not accounted for by placebo (Nelson et al., 2008). Thus, there remains a need for more effective treatments.
A number of treatment strategies can be used to increase the odds of achieving remission in patients who do not show an adequate response to antidepressant monotherapy. One approach is antidepressant augmentation with agents not routinely used as antidepressant monotherapy and atypical antipsychotics are increasingly being used since the first reports of their use in 1999 (Ostroff and Nelson, 1999). A recent meta-analysis identified 16 randomized, double-blind, placebo-controlled trials investigating their use, making atypical antipsychotic augmentation the most studied strategy (Nelson and Papakostas, 2009).
Aripiprazole, an atypical antipsychotic, has received an indication from the Food and Drug Administration as adjunctive therapy in patients with major depressive disorder (MDD). Adjunctive aripiprazole has been shown to be significantly more effective than antidepressants alone for the improvement of depression symptoms in patients with MDD who have had inadequate response to antidepressant therapy (ADT) (Berman et al., 2007; Marcus et al., 2008; Berman et al., 2009). Although only a few small studies have evaluated aripiprazole augmentation in older patients with depression, those that have been conducted have shown promising results (Rutherford et al., 2007; Sheffrin et al., 2009).
The objective of this current post hoc analysis was to provide further insight into the use of aripiprazole as an augmentation agent for the treatment of major depression in older patients aged 50–67 years. Data were pooled from three short-term studies of aripiprazole augmentation to evaluate the efficacy and tolerability of adjunctive aripiprazole for the treatment of major depression in this patient population and to compare the findings to those in younger patients (aged 18–49 years). At the outset, we recognized that 50–67 years is lower than that typically used to define late-life depression; however, given the lack of data pertaining to the use of adjunctive aripiprazole in older patients, we thought that use of this threshold was justified.
Methods
Study design and patients
The design of all three studies was essentially the same and has been reported in detail elsewhere (Berman et al., 2007; Marcus et al., 2008; Berman et al., 2009). Enrolled patients were 18–65 years old who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition—Text Revision (DSM-IV-TR) criteria for a major depressive episode (APA, 2000) of at least 8 weeks’ duration and who had reported inadequate response to previous ADT. Full details of inclusion and exclusion criteria have been reported previously (Berman et al., 2007). All patients provided written informed consent to participate.
All studies were multicenter, randomized, double-blind, and placebo-controlled, consisting of three phases: a 7–28-day screening phase; an 8-week single-blind, prospective treatment phase designed to establish inadequate response to standard ADT (escitalopram, fluoxetine, paroxetine controlled-release, sertraline or venlafaxine extended release, based on investigator clinical judgment and dosed according to current labeling); and a 6-week, randomized, double-blind phase in which participants were randomized (1:1) to continue antidepressant treatment (with no dose adjustment) plus either adjunctive placebo or aripiprazole (2–20 mg/day). Patients randomized to receive aripiprazole started with aripiprazole 5 mg/day. This could be decreased to 2 mg/day due to tolerability issues at any time or increased by 5 mg/day once per week (to a maximum of 20 mg/day) in the first 4 weeks based on efficacy.
Assessments
Patients were evaluated weekly for the 6-week double-blind treatment phase. The primary efficacy measure was the mean change in the Montgomery–Åsberg Depression Rating Scale (MADRS) total score (Montgomery and Asberg, 1979) from the end of the prospective treatment phase (Week 8) to the end of the randomized, double-blind phase (Week 14). MADRS response rate (defined as a reduction in MADRS total score of at least 50% relative to Week 8) and MADRS remission rate (defined by an absolute MADRS total score of ≤10 and at least 50% reduction in MADRS total score relative to Week 8) were also evaluated. A key secondary endpoint was the mean change in Sheehan Disability Scale (SDS) scores during the randomized, double-blind phase (Leon et al., 1992). The incidence of treatment-emergent adverse events (AEs) was assessed during the double-blind phase. Additional safety analyses included mean change in lipid, glucose, and prolactin levels, as well as mean change in the Massachusetts General Hospital Sexual Functioning Inventory (SFI) scores (Labbate and Lare, 2001) from Week 8 to Week 14.
Post hoc and statistical analyses
For the present analyses, data were pooled from patients who participated in the three aripiprazole studies. Two age groups were defined: younger patients aged 18–49 years and older patients aged 50–67 years (of note, three patients over 65 years were included). The older patient group was further divided into the following three subgroups: 50–55, 56–60, and 61–67 years. The safety sample included all randomized patients who received at least one dose of study medication (adjunctive aripiprazole or adjunctive placebo) during the 6-week, double-blind treatment phase. The efficacy sample included all patients in the safety sample who had at least one efficacy assessment during the 6-week, double-blind treatment phase. Efficacy analyses were conducted on the last observation carried forward (LOCF) data set and safety analyses were conducted using the observed cases (OC) data set.
Mean change in MADRS total score was evaluated using analysis of covariance (ANCOVA), with treatment as the main effect and the end of prospective treatment MADRS total score and age (18–49 and 50–67 years) as covariates. Age-group-by-treatment interaction in MADRS total scores was evaluated using ANCOVA, with treatment and age category (18–49 and 50–67 years) as the main effects, Week 8 MADRS total score as a covariate, and age category-by-treatment as an interaction effect. Cohen’s D Effect sizes were calculated for the older age group sub-populations as the difference between the least squares (LS) mean estimates for placebo and aripiprazole ((i.e., LS Mean placebo–LS Mean aripiprazole) divided by the estimated pooled standard deviation obtained from the square root of the mean square error of the ANCOVA model). An effect size of ≥0.8 is considered to be large, an effect size of ≥0.5 is considered to be moderate and an effect size of ≤0.2 is considered to be small (Cohen, 1988). For the older age group only, the mean change in MADRS total score was also evaluated for the single and recurrent episode populations. Additionally, at Week 14, an Interaction Test was performed to test the subgroup (single/recurrent episode)-by-treatment interaction effect in the regression model. This was evaluated using ANCOVA, with double-blind treatment and subgroup as the main effects and Week 8 MADRS total score and age as covariates, and the subgroup-by-treatment as an interaction effect. Number-needed-to-treat (NNT) for response and remission were also determined.
Mean change in total SDS scores, individual domains and SFI scores were evaluated using ANCOVA, with treatment as the main effect, study as a stratification effect and Week 8 scale score as a covariate. Age-group-by-treatment interaction in SDS scores and SFI overall improvement scores at Week 14 were evaluated using ANCOVA, with treatment as the main effect, study and age-group as stratification effects, Week 8 scale score as a covariate, and age-group-by-treatment as an interaction effect. In older patients, the gender-by-treatment interaction was evaluated using the ANCOVA model, with double-blind treatment, study and gender as the main effects, Week 8 score as a covariate, and gender-by-treatment as an interaction effect.
A predictive analysis using univariate logistic regression, with age as a continuous variable for response/remission rate assessments for each age group (18–49 and 50–67 years), was also performed. The association between treatment and response/remission/completion rates was evaluated using the Cochran–Mantel–Haenszel General Association Test.
Results
Patient disposition and characteristics
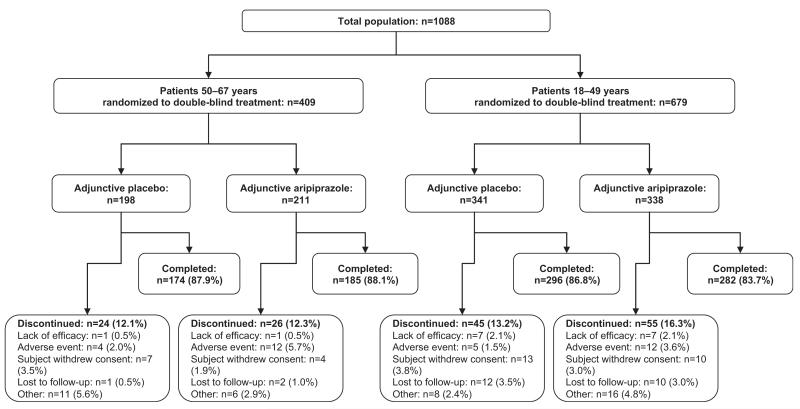
In total, 409 older patients (aged 50–67 years) (placebo, n = 198, aripiprazole, n = 211) and 679 younger patients (aged 18–49 years) (placebo, n = 341; aripiprazole, n = 338) were randomized to double-blind treatment. Patient disposition, stratified by age group, is shown in Figure 1.
Figure 1.
Patient disposition by age group (randomized sample). Other includes poor/noncompliance, patient no longer meets study criteria, other known causes. Note: one younger patient treated with aripiprazole was missing their disposition status.
Overall, 87.8% of the older patients (aged 50–67 years) completed the double-blind treatment phase, and completion rates were similar between aripiprazole and placebo treatment groups (88.1% vs. 87.9%) (Figure 1). The most common reasons for discontinuation in older patients were withdrawal of consent (3.5%) in the placebo group and AEs (5.7%) in the aripiprazole group. Completion rates in younger patients were also similar between treatment groups (aripiprazole, 83.7%; placebo, 86.8%).
Patient demographic characteristics by age group are shown in Table 1. No clinically relevant differences between treatment groups were observed.
Table 1.
Baseline demographic and disease characteristics of randomized patients by age group, randomized sample
| Characteristic | Older patients (50-67 years) |
Younger patients (18-49 years) |
||
|---|---|---|---|---|
| Adjunctive placebo |
Adjunctive aripiprazole |
Adjunctive placebo |
Adjunctive aripiprazole |
|
| Patients, n | 198 | 211 | 341 | 338 |
| Gender, n (%) | ||||
| Male | 79 (39.9) | 76 (36.0) | 101 (29.6) | 96 (28.4) |
| Female | 119 (60.1) | 135 (64.0) | 240 (70.4) | 242 (71.6) |
| Age, mean (SD), years | 55.8 (4.3) | 56.2 (4.2) | 38.2 (8.1) | 38.7 (7.8) |
| Weight, mean (SD), kga | 86.9 (20.7) | 86.1 (19.7) | 88.0 (23.2) | 84.4 (20.8) |
| Race, n (%) | ||||
| White | 178 (89.9) | 191 (90.5) | 304 (89.2) | 293 (86.7) |
| Black/African American | 15 (7.6) | 15(7.1) | 27 (7.9) | 27 (8.0) |
| American Indian/Alaskan native | 1 (0.5) | 1 (0.5) | 1 (0.3) | 1 (0.3) |
| Asian | 1 (0.5) | 1 (0.5) | 5(1.5) | 8 (2.4) |
| Other | 3(1.5) | 3(1.5) | 4(1.2) | 9 (2.7) |
| Duration of current episode, mean (SD), monthsb | 38.9 (69.5) | 30.5 (30.4) | 35.1 (53.5) | 40.0 (61.7) |
| Single depressive episode, n (%) | 35 (17.7) | 34 (16.1) | 74 (21.7) | 65 (19.2) |
| No. of adequate trials in current episode, n (%)c | ||||
| 0 | 1 (0.5) | 1 (0.5) | 4(1.2) | 2 (0.6) |
| 1 | 135 (68.2) | 148 (70.1) | 227 (66.8) | 234 (69.4) |
| 2 | 52 (26.3) | 51 (24.2) | 90 (26.5) | 81 (24.0) |
| 3 | 9 (4.6) | 11 (5.2) | 18 (5.3) | 19 (5.6) |
| >3 | 1 (0.5) | 0 | 1 (0.3) | 1 (0.3) |
| MADRS total scored: mean (SD) | 26.5 (6.1) | 25.9 (6.2) | 26.8 (5.8) | 25.8 (6.0) |
SD, standard deviation; MADRS, Montgomery-Åsberg Depression Rating Scale; LOCF, last observation carried forward; ADT, antidepressant therapy.
50-67 years: placebo n = 197; aripiprazole n = 210; 18-49 years: placebo n = 335, aripiprazole n = 334.
50-67 years: placebo n = 67, aripiprazole n = 80; 18-49 years: placebo n = 131, aripiprazole n = 123.
One patient in each treatment group had no previous ADT trials.
MADRS total scores (efficacy sample, LOCF; 50-67 years: placebo n = 195, aripiprazole n = 210; 18-49 years: placebo n = 330, aripiprazole n = 329); assessed at the end of the prospective treatment phase.
Treatment and dosing
In older patients, the distribution of each ADT at randomization to double-blind treatment was as follows: escitalopram, 32.6%; fluoxetine, 17.0%; paroxetine, 7.2%; sertraline, 18.0%; venlafaxine, 25.2%; and was similar between treatment groups. A similar distribution of each ADT was seen in younger patients. The mean ADT dosing was comparable between treatment and age groups.
In older patients, the mean dose of aripiprazole during the last week of the randomized, double-blind treatment phase was 9.9 mg/day. The mean dose of aripiprazole was 10.0, 10.9, and 7.9 mg/day for the age subgroups 50–55, 56–60, and 61–67 years, respectively.
Efficacy in older patients (50–67 years) and older patient subgroups
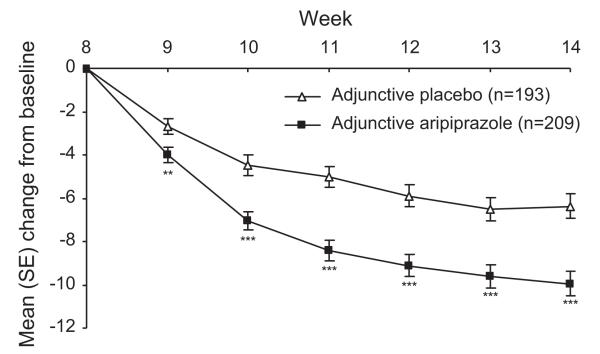
Significantly greater improvements in MADRS total score in the aripiprazole-treated patients compared with the placebo-treated patients were observed as early as the first week of double-blind treatment in older patients (−4.0 vs. −2.7; p = 0.009; LOCF) and continued to remain significant through to endpoint (−10.0 vs. −6.4; p < 0.001; LOCF) (Figure 2), giving an effect size of 0.44 (95% CI = 0.24–0.64; p < 0.001).
Figure 2.
Mean (SE) change in MADRS total score for older patients (LOCF data set), efficacy sample. **p < 0.01 vs. adjunctive placebo; ***p < 0.001 vs. adjunctive placebo. MADRS, Montgomery–Åsberg Depression Rating Scale; LOCF, last observation carried forward; SE, standard error.
Older patients with both single and recurrent episodes also experienced significantly greater improvements in MADRS total score with aripiprazole compared with placebo at Week 14 (LOCF); treatment difference (placebo–aripiprazole) of 6.7 (95% CI = 2.6–10.8; p = 0.002) and 3.0 (95% CI = 1.2–4.8; p = 0.001), respectively. At Week 14, the depressive episode-by-treatment interaction was not statistically significant (p = 0.157), suggesting that the treatment difference between aripiprazole and placebo did not differ significantly in single-episode compared to recurrent-episode patients.
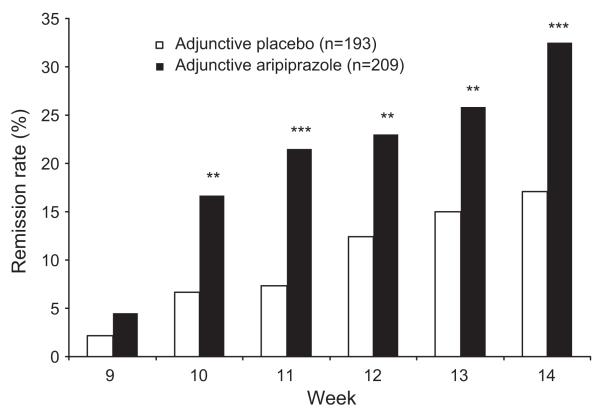
Remission rates in older patients treated with aripiprazole were significantly greater than those treated with placebo from Week 10 through to Week 14 (32.5% vs. 17.1%, p < 0.001) (Figure 3). At Week 14 (LOCF), aripiprazole also resulted in significantly greater response rates (39.7% vs. 24.4%, p = 0.001) than placebo in the older age group (aged 50–67 years). This produced an NNT for remission of 7 and an NNT for response of 7.
Figure 3.
Remission rates with adjunctive placebo or adjunctive aripiprazole during the double-blind treatment phase in older patients (LOCF). **p < 0.01 vs. adjunctive placebo; ***p < 0.001 vs. adjunctive placebo; LOCF, last observation carried forward.
For all older patient subgroups, patients receiving aripiprazole experienced significantly greater improvement in MADRS total score than those receiving placebo at Week 14 (all p < 0.05). The treatment effect size was 0.39 (95% CI = 0.11–0.67; p < 0.01) in the 50–55-year-old subgroup, 0.36 (95% CI 0.00–0.71; p < 0.05) in the 56–60-year-old subgroup, and 0.75 (95% CI = 0.27–1.23; p < 0.01) in the 61–67-year-old subgroup.
For the 56–60 and 61–67-year-old subgroups, significantly more patients achieved remission with aripiprazole compared with placebo at Week 14 (56–60 years: 29.4% vs. 14.0%, p < 0.05; 61–67 years 42.1% vs. 14.7%, p < 0.05). In the 50–55-year-old subgroup, aripiprazole also resulted in greater remission rates (31.1%) than placebo (19.6%) at Week 14, although the difference did not reach statistical significance (p = 0.06).
In older patients, age (continuous variable) did not predict remission (aripiprazole OR = 1.03; 95% CI = 0.96–1.10; placebo OR = 0.95; 95% CI = 0.87–1.04) or response (aripiprazole OR = 1.05; 95% CI = 0.98–1.12; placebo OR = 0.94; 95% CI = 0.87–1.01) in either treatment arm.
In older patients, numerically greater improvements in mean SDS total (−1.1 vs. −0.8), work (−0.7 vs. −0.6), social life (−1.2 vs. −0.9), and family life (−1.2 vs. −0.8) scores were observed with aripiprazole compared to placebo at Week 14; none reached statistical significance
Efficacy in younger patients (aged 18–49 years)
Symptom improvement in younger patients was similar to that seen in older patients. Younger patients receiving aripiprazole experienced a significantly greater improvement in MADRS total score than those receiving placebo from Week 9 to Week 14 (−9.0 vs. −6.1; p < 0.001; LOCF).
In younger patients, aripiprazole resulted in significantly greater remission (26.9% vs. 16.4%, p < 0.001) and response (35.8 vs. 21.5,p < 0.001) rates than placebo at Week 14 (LOCF). This produced an NNT for remission of 10 and an NNT for response of 7.
In younger patients, age (continuous variable) did not predict response (aripiprazole OR=1.01; 95% CI=0.98–1.04; placebo OR=1.00; 95% CI=0.96– 1.02) or remission (aripiprazole OR=1.01; 95% CI=0.98–1.04; placebo OR=1.00; 95% CI=0.97– 1.04) in either treatment arm.
For younger patients, mean SDS total (−1.3 vs. −0.6), social life (−1.4 vs. −0.6), and family life (−1.5 vs. −0.7) scores improved significantly more in the aripiprazole group compared with placebo at Week 14 (LOCF, all p < 0.001).
Comparisons between older and younger patients
There were no significant differences between age groups on the treatment difference (aripiprazole–placebo), as assessed by the mean change in MADRS total scores at Week 14 (ANCOVA interaction test: p = 0.576).
Comparison of remission and response rates between older and younger patients treated with aripiprazole revealed no statistically significant differences (all Fisher’s exact test, p > 0.05).
The interaction test at Week 14 revealed no significant interaction between treatment and age group for SDS total (p = 0.153) or individual domain scores (work: p = 0.851; social: p = 0.141; family: p = 0.204).
Tolerability
Adverse events at an incidence ≥5% in any aripiprazole-treated age group (or subgroup) and twice the placebo rate are shown in Table 2. Akathisia was the most common AE in both the older (17.1%) and younger (26.0%) patient groups. For the 36 older patients who experienced akathisia, the interventions permitted and chosen by study investigators included no intervention (n = 9; 25.0%), dose reduction only (n = 10; 27.8%), use of concomitant medications (benztropine only, n = 6 (16.7%); propranolol only, n = 2 (5.6%); benztropine and propranolol, n = 1 (2.8%)), a combination of dose reduction and concomitant medications (n = 4; 11.1%) or other (n = 4; 8.3%).
Table 2.
Incidence (%) of treatment-emergent adverse events (≥5% of aripiprazole-treated patients and twice the placebo rate in any group– safety sample) in (A) older patients, (B) younger patients, and (C) older age subgroups
| (A) Older patients (50-67 years) | ||
|---|---|---|
| Adverse events, n (%) | Pla (n = 195) | Ari (n = 210) |
| Akathisia | 7 (3.9) | 36 (17.1) |
| Restlessness | 9 (4.6) | 28 (13.3) |
| Somnolence | 3(1.5) | 18 (8.6) |
| Insomnia | 5 (2.6) | 17(8.1) |
| Vision blurred | 2(1.0) | 14 (6.7) |
| Fatigue | 6 (3.1) | 13 (6.2) |
|
| ||
|
(B) Younger patients (18-49 years)
| ||
| Adverse events, n (%) | Pla (n = 339) | Ari (n = 334) |
|
| ||
| Akathisia | 15 (4.4) | 87 (26.0) |
| Restlessness | 4(1.2) | 38 (11.4) |
| Fatigue | 15 (4.4) | 32 (9.6) |
| Insomnia | 11 (3.2) | 26 (7.8) |
| Vision blurred | 5(1.5) | 20 (6.0) |
| Sedation | 6(1.8) | 18 (5.4) |
|
| ||
|
(C) Older patient subgroups
| ||
| 50-55 years | ||
| Adverse events, n (%) | Pla (n = 104) | Ari (n = 103) |
|
| ||
| Akathisia | 6 (5.8) | 19 (18.4) |
| Restlessness | 5 (4.8) | 11 (10.7) |
| Somnolence | 1 (1.0) | 11 (10.7) |
| Insomnia | 3 (2.9) | 10 (9.7) |
| Nausea | 3 (2.9) | 8 (7.8) |
| Fatigue | 2(1.9) | 6 (5.8) |
| 56-60 years | ||
|
| ||
| Adverse events, n (%) | Pla (n = 57) | Ari (n = 69) |
|
| ||
| Restlessness | 3 (5.3) | 14 (20.3) |
| Akathisia | 1 (1.8) | 12 (17.4) |
| Somnolence | 2 (3.5) | 7(10.1) |
| Vision blurred | 1 (1.8) | 7(10.1) |
| 61-67 years | ||
|
| ||
| Adverse events, n (%) | Pla (n = 34) | Ari (n = 38) |
|
| ||
| Akathisia | 0 | 5 (13.2) |
| Dizziness | 0 | 5 (13.2) |
| Insomnia | 0 | 3 (7.9) |
| Restlessness | 1 (2.9) | 3 (7.9) |
| Back pain | 0 | 2 (5.3) |
| Balance disorder | 0 | 2 (5.3) |
| Hypertension | 0 | 2 (5.3) |
| Myalgia | 0 | 2 (5.3) |
| Peripheral edema | 0 | 2 (5.3) |
Pla, adjunctive placebo; Ari, adjunctive aripiprazole.
For older patients, discontinuations due to AEs occurred in 4 (2.0%) patients in the placebo group and 12 (5.7%) patients in the aripiprazole group (randomized sample). Three older patients discontinued aripiprazole due to akathisia. Discontinuations due to AEs were similar in the younger patient group (Figure 1), with two younger patients discontinuing aripiprazole due to akathisia.
No deaths were reported in these studies. For older patients, treatment-related suicidality-related AEs (all suicidal ideation) occurred in 1 (0.5%) placebo patient and 1 (0.5%) aripiprazole patient. In younger patients, suicidal ideation occurred in 2 patients (0.6%) and 1 patient (0.3%), respectively. There were no reports of cerebrovascular accidents or tardive dyskinesia during these short-term studies in any age group.
The overall improvement rating on the SFI since medication change showed no significant improvement with aripiprazole compared with placebo in older patients (−0.1 vs. −0.1; p = 0.844), although aripiprazole demonstrated greater overall improvement than placebo in younger patients (−0.4 vs. −0.2; p < 0.01). Interaction tests at Week 14 revealed no significant interaction between treatment and age group for any of the SFI domains. In older patients, analysis of SFI ratings by gender revealed no significant improvements with aripiprazole compared with placebo, and no significant treatment-by-gender interactions were observed.
No clinically meaningful changes from baseline to Week 14 in mean fasting lipid, fasting glucose, and prolactin levels stratified by age group were observed (Table 3). At Week 14 (OC), there was a statistically significant increase in mean (±SE) body weight in the aripiprazole group compared with the placebo group in both the older (+1.4 ± 0.2 vs. +0.3 ± 0.2 kg; p < 0.001) and younger patient groups (+1.7 ± 0.1 vs. +0.8 ± 0.1 kg; p < 0.001).
Table 3.
Mean change from baseline to Week 14 in metabolic measures (OC, safety sample)
| Characteristic | Older patients (50-67 years) |
Younger patients (18-49 years) |
||
|---|---|---|---|---|
| Adjunctive placebo (n = 111) |
Adjunctive aripiprazole (n = 126) |
Adjunctive placebo (n = 185) |
Adjunctive aripiprazole (n = 164) |
|
| Fasting total cholesterol (mg/dl) | ||||
| Baseline, mean | 220.1 | 226.1 | 204.9 | 205.0 |
| Mean change at Week 14,mean (SE) | −3.1 (3.2) | 1.4 (3.0) | 6.1 (1.9) | 1.4 (2.1) |
| Fasting HDL-C (mg/dl) | ||||
| Baseline, mean | 57.2 | 59.2 | 54.4 | 57 |
| Mean change at Week 14,mean (SE) | 1.0 (0.9) | 2.0 (0.9) | 0.8 (0.5) | 1.7 (0.6) |
| Fasting LDL-C (mg/dl)a | ||||
| Baseline, mean | 127.6 | 137.2 | 120.7 | 120.4 |
| Mean change at Week 14,mean (SE) | −3.0 (2.6) | −3.0 (2.4) | 2.9 (1.7) | −1.7(1.8) |
| Fasting triglycerides (mg/dl)a | ||||
| Baseline, mean | 180.6 | 150.5 | 149.9 | 139.3 |
| Mean change at Week 14,mean (SE) | −8.4 (8.4) | 12.4 (7.8) | 12.3 (5.5) | 5.9 (5.8) |
| Fasting glucose (mg/dl)a | ||||
| Baseline, mean | 95.0 | 94.7 | 90.1 | 90.7 |
| Mean change at Week 14,mean (SE) | 0.8 (1.6) | 1.7 (1.5) | −0.1 (0.7) | −0.6 (0.8) |
| Prolactin (ng/ml)b | ||||
| Baseline, mean | 12.3 | 13.1 | 14.6 | 14.0 |
| Mean change at Week 14,mean (SE) | 0.2 (0.7) | −1.0 (0.6) | 0.2 (0.5) | −0.4 (0.5) |
18-49 years: adjunctive placebo n = 184.
18-49 years: adjunctive placebo n = 282, adjunctive aripiprazole n = 272; 50-67 years: adjunctive placebo n = 167, adjunctive aripiprazole = 181.
Discussion
The results of this post hoc analysis indicate that addition of aripiprazole to standard ADT is more effective than addition of placebo for the improvement of depressive symptoms in older patients (50–67 years) with an inadequate response to standard ADT. Significantly greater improvement in depressive symptoms with aripiprazole than placebo was observed in all older age subgroups at study endpoint. The MADRS effect sizes were moderate (0.4) in the 50–55 and 56–60-year-old subgroups, comparable to the effect size observed in younger patients, and large (0.8) in the oldest age subgroup (61–67 years). Although this difference was not statistically significant, it suggests that the efficacy of aripiprazole does not diminish with increasing age.
Importantly, given that remission is the goal of treatment for MDD, aripiprazole was also associated with significantly higher remission rates than placebo in older patients. Results from analysis of data from older patients with depression are generally in agreement with findings from the overall study populations (Berman et al., 2007; Marcus et al., 2008; Berman et al., 2009) and with those in younger patients reported here.
Adjunctive aripiprazole also resulted in a non-significant trend towards greater improvement in functional impairment compared with placebo in older patients, as measured using the SDS total and individual items. In comparison, younger patients experienced significant improvement in overall functioning, as well as in family and social life items, as was observed in the parent studies (Berman et al., 2007; Marcus et al., 2008; Berman et al., 2009).
In this study, adjunctive aripiprazole was relatively well tolerated in older patients with MDD, as seen by the high rate of study completion and the low rate of discontinuation due to AEs (5.7% with adjunctive aripiprazole). Importantly, the rate of akathisia was not increased in older MDD patients receiving treatment with aripiprazole and was lower than seen in younger patients. This finding is in agreement with a previous analysis demonstrating that older age is not a predictor of akathisia (Nelson et al., 2009). In this analysis of data from two of the studies included herein, the 18–40-year-old age group was associated with an increased risk of akathisia compared to the >50-year-old group, suggesting that younger patients would warrant a more gradual introduction (or initiation) of aripiprazole to their antidepressant regimen. Rates of treatment-emergent akathisia in older versus younger patients with MDD also appear consistent with findings in bipolar mania patients receiving aripiprazole monotherapy, as no akathisia events were reported with aripiprazole monotherapy in patients >55 years of age compared to a rate of 18.2% in younger patients (18–40 years of age) (Suppes et al., 2008).
Older individuals may be more sensitive to the common adverse effects of many antidepressant drugs, particularly the anticholinergic side effects. Thus, pharmacotherapy for depression in this population requires careful consideration of medication side-effect profiles (Salzman, 1990). With this in mind, dizziness was one of the most frequently reported AEs in 61–67-year-old patients, suggesting that older patients may be more susceptible to this AE and should be closely monitored. In addition, older patients may be more sensitive to the somnolence-related effects of neuroleptic agents, although the rates of somnolence did not appear to increase with age. There has also been concern surrounding potentially serious AEs associated with the use of atypical antipsychotics in older individuals, such as cardiovascular-related AEs and death. None of these events were reported over the 6 weeks of adjunctive aripiprazole treatment. Regarding cardiovascular-related AEs, including worsening glucose control or dyslipidemia, aripiprazole treatment produced no unusual changes in either population, consistent with earlier reports (Fava et al., 2009). This is important, given that older patients may already have cardiovascular risk factors and medical comorbidity. Tardive dyskinesia, another potentially serious AE, was also not reported in this study. Furthermore, in a 12-month open-label study of adjunctive aripiprazole in the treatment of MDD (Berman et al., 2008), four cases of tardive dyskinesia were observed—three cases in patients <50 years and one in a 68-year-old female. It is also important to note that all cases resolved; interventions to manage tardive dyskinesia included dose reduction (n = 2) and drug discontinuation (n = 2).
There are several limitations to this study. This was a post hoc analysis and the benefits of aripiprazole augmentation in older patients with MDD should be considered preliminary. This study used an age cut-off of ≥50years of age todefine depression in older individuals and patients older than 65 years of age were excluded from study participation. However, the relatively large numbers of older patients with MDD included in this analysis are helpful, given the paucity of research in this population. In addition, the numbers of patients in the 61–67-year-old subgroup are smaller and any suggested differences between these groups should be viewed with caution. Several other limitations also warrant consideration. The studies included in this analysis all excluded patients with a history, or evidence, of any medical conditions that would expose them to an undue risk of significant AEs. This limits the generalizability of these findings, especially as older individuals are more likely to suffer from comorbid medical conditions. Finally, the longer-term benefits of this treatment strategy are, as yet, not fully studied.
In conclusion, adjunctive aripiprazole was effective in improving depressive symptoms in older patients, 50–67 years of age, with MDD who have had an inadequate response to standard antidepressant medication. Aripiprazole was well tolerated and the frequency of the most common AE—akathisia—was reduced in older patients compared with younger patients. Further prospective studies are warranted to fully evaluate the use of adjunctive aripiprazole in this patient population, including patients over the age of 65 years.
Key Points.
Adjunctive aripiprazole was effective in improving depressive symptoms in older patients with MDD who have had an inadequate response to standard antidepressant medication.
Aripiprazole was well tolerated.
Acknowledgments
Conflicts of interest
David C. Steffens is a consultant to Transform Pharmaceuticals, Inc (Johnson & Johnson), is on the Speaker’s Bureau for Forest Pharmaceuticals and Wyeth Pharmaceuticals, received travel support to present at a scientific meeting from Bristol-Myers Squibb, and receives grant support from the National Institute of Mental Health.
J. Craig Nelson, during the past 3 years, has had the following relationships: Lecture Honoraria: Eli Lilly, Schering Plough/Merck, Otsuka; Speakers Bureau: none; Consultation/Advisory Board: Biovail, Bristol-Myers Squibb, Corcept, Covidien, Eli Lilly, Glaxo-SmithKline, Labopharm, Lundbeck, Medtronics, Merck, Novartis, Orexigen, Organon, Otsuka, Pfizer, Sanofi-Aventis, Sepracor, Shire, Sierra Neuropharmaceuticals; Research Support: NIMH, HRSA; Stock Ownership: Atossa.
James Eudicone, Candace Andersson, Berit X. Carlson, and Robert M. Berman are employees of Bristol-Myers Squibb.
Huyuan Yang is a former consultant to Bristol-Myers Squibb.
Robert A. Forbes is an employee of Otsuka and Quynh-Van Tran is a former employee of Otsuka.
Footnotes
Clinical trials registration: registered at http://clinicaltrials.gov/, numbers NCT00095823, NCT00095758 and NCT00105196.
References
- APA . Text Revision. 4th edn Washington, DC; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197–206. doi: 10.1017/s1092852900020216. [DOI] [PubMed] [Google Scholar]
- Berman RM, Hazel J, Swanink R, et al. Long-term safety and tolerability of open-label aripiprazole augmentation of antidepressant therapy in major depressive disorder (Study CNI38-164); American Psychiatric Association 161st Annual Meeting; Washington DC, USA. 3–8 May 2008.2008. [Google Scholar]
- Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatr. 2007;68:843–853. doi: 10.4088/jcp.v68n0604. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Erlbaum; Hillsdale: 1988. [Google Scholar]
- Fava M, Wisniewski SR, Thase ME, et al. Metabolic assessment of aripiprazole as adjunctive therapy in major depressive disorder: a pooled analysis of 2 studies. J Clin Psychopharmacol. 2009;29:362–367. doi: 10.1097/JCP.0b013e3181ac9b0b. [DOI] [PubMed] [Google Scholar]
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221–225. doi: 10.1159/000056257. [DOI] [PubMed] [Google Scholar]
- Leon AC, Shear MK, Portera L, Klerman GL. Assessing impairment in patients with panic disorder: the Sheehan Disability Scale. Soc Psychiatry Psychiatr Epidemiol. 1992;27:78–82. doi: 10.1007/BF00788510. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatr. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatr. 2008;16:558–567. doi: 10.1097/JGP.0b013e3181693288. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatr. 2009;166:980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Thase ME, Trivedi MH, et al. Safety and tolerability of adjunctive aripiprazole in major depressive disorder: a pooled analysis (Studies CN138-139 and CN138-163) Prim. Care Comp. J. Clin. Psychiatr. 2009;11:344–352. doi: 10.4088/PCC.08m00744gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatr. 1999;60:256–259. doi: 10.4088/jcp.v60n0410. [DOI] [PubMed] [Google Scholar]
- Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatr. 2007;22:986–991. doi: 10.1002/gps.1775. [DOI] [PubMed] [Google Scholar]
- Salzman C. Practical considerations in the pharmacologic treatment of depression and anxiety in the elderly. J Clin Psychiatr. 1990;51(Suppl):40–43. [PubMed] [Google Scholar]
- Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatr. 2009;70:208–213. doi: 10.4088/jcp.07m03805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppes T, Eudicone J, McQuade R, Pikalov A, 3rd, Carlson B. Efficacy and safety of aripiprazole in subpopulations with acute manic or mixed episodes of bipolar I disorder. J Affect Disord. 2008;107:145–154. doi: 10.1016/j.jad.2007.08.015. [DOI] [PubMed] [Google Scholar]