Abstract
Although circumscribed interests are a hallmark characteristic of autism spectrum disorders, providing a means for quantifying their functional impairment has proven difficult. We developed a passive viewing task to measure aspects of visual attention in children with autism spectrum disorders and typically developing controls. Task stimuli included picture arrays that were matched for social and nonsocial content. Nonsocial content was balanced to include items related to circumscribed interests (e.g., trains) as well as more commonplace items (e.g., furniture). Discrete aspects of gaze behavior were quantified using eye-tracking technology. Results indicate that visual attention in the autism group was more circumscribed (as indicated by the exploration of fewer images), more perseverative (as indicated by longer fixation times per image explored), and more detail oriented (as indicated by a greater number of discrete fixations on explored images). This pattern of results was similar for both social and object arrays. Within the autism group, overall severity of repetitive behavior symptoms correlated positively with exploration of object pictures and negatively with perseveration on social pictures. Results suggest that children with autism have a domain-general pattern of atypical visual attention that may represent an exaggeration of a typical attentional process and is related to a tendency to perseverate on images of interest and explore them in a more detail-oriented manner. Discrete measures of visual attention may therefore provide a reasonable means of quantifying aspects of the repetitive behavior phenotype in autism.
Keywords: autism, repetitive behavior, circumscribed interests, visual exploration, attention
Introduction
Circumscribed interests are a type of repetitive behavior occurring commonly in persons with autism [Baron-Cohen and Wheelwright, 1999; Bartak and Rutter, 1976; Kanner, 1943; Lewis and Bodfish, 1998; Ozonoff et al., 2000; South et al., 2005] that are characterized by intense interest in a narrow range of subjects and by rigid organization of activities exclusively around this interest (e.g., collecting, manipulating, reading, playing, conversing, etc.). Common examples include a fascination with Japanese animation (e.g., Pokemon), gadgets, devices, vehicles, electronics, dinosaurs, schedules, and numbers [South et al., 2005]. This is not limited to higherfunctioning individuals with autism, as similar levels of circumscribed interests are found in both low- and high-functioning autism [Bartak and Rutter, 1976; Freeman et al., 1981].
Circumscribed interests are clinically significant for several reasons. First, intense restricted interests may interfere with learning and the development of adaptive behavior [Koegel and Covert, 1972; Koegel et al., 1974; Lovaas et al., 1971; Varni et al., 1979]. Second, circumscribed interest symptoms do not improve with age as much as other core symptoms of autism [Fecteau et al., 2003; Piven et al., 1996; South et al., 2005]. Finally, parents report that circumscribed, rigid, and repetitive patterns of behavior are the most difficult aspect of autism to deal with on an everyday basis [Mercier et al., 2000; South et al., 2005].
Studies of repetitive behaviors that include circumscribed interests have primarily involved descriptive accounts by children and adults with autism [Bartak and Rutter, 1976; Bodfish et al., 2000; South et al., 2005; Turner, 1999]. These studies have been valuable in terms of establishing psychometrically sound methods for symptom measurement [Bodfish et al., 1999; Lam and Aman, 2007; Rutter et al., 2003b; Scahill et al., 2006; Turner, 1997] and in detailing phenomenological [Bodfish et al., 2000; Cuccaro et al., 2003; Lam and Aman, 2007; South et al., 2005; Szatmari et al., 2006] and developmental [Richler et al., 2007] profiles of repetitive behaviors in autism. An alternative to this descriptive symptom-based approach is identifying processes whose derailment could represent core deficits in autism and thus could underlie patterns of symptom expression in autism [Klin et al., 2002a].
Recently, Pierce and Courchesne [2001] provided a novel conceptual model for a process that may underlie the expression of repetitive behaviors in autism. They reasoned that rigid repetitive behaviors in general and circumscribed interests in particular should lead persons with autism to perform poorly on exploration tasks. This model posits that tasks measuring exploration may provide a means for quantifying functional impairment related to circumscribed interests. To test this model, they developed a room exploration task and found that children with autism displayed reduced exploration of nonsocial objects. Their rates of exploration were inversely related to the degree of repetitive behaviors displayed during the task.
Assessment of visual attention is another methodology that has been used to examine exploration of, or interest in, particular features of the environment in both typical development [Colombo et al., 1995; Gerhardstein and Rovee-Collier, 2002; Haith et al., 1979; Parkhurst and Niebur, 2003; Treisman, 1982] and autism [Courchesne et al., 1994; Dawson et al., 1998; Klin et al., 2002b; Sasson et al., 2007]. Gaze-tracking technology has been used to analyze visual scanning in autism, though much of this work has focused on attention to social information such as static pictures of faces or social scenes [Pelphrey et al., 2002; Sasson et al., 2007] or dynamic moving images of social interactions [Klin et al., 2002a,b]. These studies have consistently found that gaze to social information is diminished in persons with autism [Dawson et al., 1998; Sasson, 2006]. Klin et al. [2002b], however, did compare attention with social vs. nonsocial aspects of social scenes and found that persons with autism attended more to both noncritical social elements (e.g., mouths vs. eyes) and nonsocial elements of social scenes (e.g., objects vs. faces). Klin and colleagues hypothesized that increased saliency of nonsocial information may reduce attention to social information for persons with autism. Because their study did not control for the amount of presented social and nonsocial information (and indeed nonsocial information constituted only a small proportion of what was shown), they suggested that future studies could directly examine saliency effects of social vs. nonsocial sources of information by systematically controlling for them while also incorporating nonsocial features of common interest to persons with autism.
For this study, we developed a visual analog of the exploration paradigm developed by Pierce and Courchesne [2001] and added eye-tracking technology to directly measure aspects of visual attention in typically developing children and children with autism. Following the suggestion of Klin et al. [2002a], we examined salience effects by using a variety of image types. Task stimuli included selections of both social (i.e., faces) and nonsocial (i.e., objects) pictures. Further, within the nonsocial category, we included selections of pictures of items that have been found to be involved in circumscribed interests in persons with autism (e.g., trains, electronic devices) [South et al., 2005] in addition to common everyday items (e.g., furniture, food). This selection of stimulus pictures allowed us to examine potential group differences in the overall amount of visual exploration and salience effects that could contribute to differential attention to social or nonsocial pictures. We extracted discrete quantitative indices of visual attention (exploration, perseveration, and detail orientation) and attempted to map these onto clinically relevant characteristics of restricted repetitive behaviors.
On the basis of the extant literature, we expected that participants with autism would display reduced overall visual exploration characterized by a relative bias toward nonsocial pictures at the expense of social pictures. Further, we expected that discrete measures of visual attention such as perseveration and detail orientation would be associated with symptom-based measures of repetitive behavior in participants with autism.
Methods
Participants
The participants included two groups of children: 29 children with autism spectrum disorders (ASD) and 24 children who were typically developing (TYP). Given that this is the first task to examine visual exploratory behavior in autism, a TYP group was selected as the basis of comparison. All participants met the following general inclusion criteria: age between 6 and 17 years; intelligence quotient (IQ) greater than 70; absence of seizure disorder, acute medical or genetic condition; and absence of any visual impairment uncorrectable with eyeglasses. Participants with ASD were recruited through the University of North Carolina (UNC) Autism Research Registry in conjunction with regional treatment and education of autistic and related communication-handicapped children clinics. Inclusion in the registry required a previous Diagnostic and Statistical Manual of Mental Disorders-IV diagnosis of ASD made by a licensed clinician experienced in the assessment and diagnosis of autism, and based on parent interview and direct observation for the completion of one or more standardized autism diagnostic assessment instruments (Autism Diagnostic Interview-Revised (ADI-R), Social Communication Questionnaire (SCQ), Autism Diagnostic Observation Schedule, Childhood Autism Rating Scale). Following referral from the registry, all ASD participants were evaluated by trained study personnel using (a) the ADI-R [Lord et al., 1994; Risi et al., 2006] to examine lifetime criteria for ASD, (b) the Social Responsiveness Scale (SRS)[Constantino and Gruber, 2002] to examine current severity of autism symptoms, and (c) the Leiter International Performance Scale-Revised (Leiter-R) [Roid and Miller, 2002] to examine general cognitive ability. All participants had previous comprehensive intelligence testing documented in their subject registry record that indicated high-functioning status. The Leiter was administered to confirm previous IQ testing results as it is a reasonable estimate of overall intellectual level that is relatively unbiased by verbal abilities. Children in the ASD group were required to have a Diagnostic and Statistical Manual of Mental Disorders-IV clinical diagnosis of ASD, meet lifetime criteria for autism or ASD on the ADI-R, and meet current criteria for ASD on the SRS. TYP children were recruited via an email sent to UNC faculty and staff. TYP children were excluded if they had a history of psychiatric or developmental disorder, if they were currently taking psychotropic medication, if an immediate family member had an ASD diagnosis, or if they received a score above the ASD cutoff on the SRS.
Sixty-two children were recruited to participate, 38 with ASD and 24 typically developing. Of 38 potential ASD participants, nine were excluded from analysis because they did not meet inclusion criteria for this study: five had SRS scores below the ASD cutoff and four had IQ scores below 70. No TYP children were excluded. The resulting sample included 29 children with ASD (autism (n = 17), Asperger syndrome (n = 8), pervasive developmental disorders not otherwise specified (n = 4)) and 24 TYP children (n = 24). There were no significant differences between groups for chronological age, t(53) = −0.01, race, χ2 (2, N = 53) = 1.6, or sex, χ2 (2, N = 53) = 0.04, all Ps>0.05. There was a significant difference between groups for IQ, t(53) = 3.1, P>0.01, with children in the TYP group scoring higher on the Leiter-R. See Table I for further description of the sample. Before participation all individuals and their legal guardians supplied written informed consent for study participation. The protocol for this study was approved by the UNC-Chapel Hill School of Medicine Biomedical Institutional Review Board.
Table I.
Demographic and Clinical Features of Participants
| Variable | ASD group (n=29) Mean (SD) |
TYP group (n=24) Mean (SD) |
|---|---|---|
| Age in months | 114.83 (29.28) | 114.71 (33.79)a |
| Leiter-R Brief IQ | 99.48 (16.56) | 112.75 (13.91)b |
| Gender (% male) | 93% | 2%a |
| Ethnicity (% Caucasian) | 79% | 92%a |
| SCQ social | 4.48 (1.75) | 0.63 (0.71)b |
| CCC | 61.24 (22.18) | 7.67 (1.75)b |
| RBS-R | 19.93 (13.24) | 1.58 (2.59)b |
| IRB | 44.43 (19.44) | — c |
| IS | 12.89 (3.08) | 8.96 (2.40)b |
P>0.05.
P<0.01.
Interview for repetitive behavior data were not collected for typical control participants, given low rates of occurrence of repetitive behaviors for this group.
SCQ, social communication questionnaire (subscale score); CCC, children’s communication checklist (total score); RBS-R, repetitive behavior scale (total frequency score); IRB–interview for repetitive behaviors (total severity score); IS–interests scale (total severity score).
Procedure
Visual exploration task
The visual exploration task was designed for this study and is composed of 12 static, high-quality color picture arrays (for examples, see Fig. 1). Six of the 12 arrays are “social1object arrays” (i.e., they contain pictures of people with clearly visible faces along with pictures of objects) and six are “object-only arrays” (i.e., they contain object pictures only). All pictures were public domain photographs obtained from the Internet selected for being relatively similar in complexity and size. All social pictures were of people displaying a happy expression. Half of all object pictures were selected from nine categories previously demonstrated to be a common focus of circumscribed interests to individuals with autism: trains, vehicles, planes, blocks, home electronics, computer equipment, road signs, and sporting equipment [South et al., 2005]. For the purposes of this study, these pictures are referred to as “high-autism-interest” (HAI) objects. The other half of object pictures was selected from nine categories that were not common circumscribed interest objects and were thus less likely to be compelling to individuals with autism: clothing, food, furniture, plants, school supplies, bathroom supplies, gloves, hats, and bags. These pictures are referred to as “low-autism-interest” (LAI) objects.
Figure 1.
Examples of social+object (top) and object (bottom) arrays.
Each of the 12 picture arrays contained 24 total images. The mixture of social, HAI, and LAI images within each array was determined by a set of image-type ratios (5:1, 1:1, 1:5) designed to counter-balance the image contents of the arrays. The contents of the 24 images on each of the six “social1object arrays” shown were as follows: (a) 20 social images and four HAI images, (b) 20 social images and four LAI images, (c) 12 social images and 12 HAI images, (d) 12 social images and 12 LAI images, (e) four social images and 20 HAI images, and (f) four social images and 20 LAI images. The contents of the 24 images on each of the six “object-only arrays” shown were as follows: (a) 20 HAI images and four LAI images (version 1), (b) 20 HAI images and four LAI images (version 2), (c) 12 HAI images and 12 LAI images (version 1), (d) 12 HAI images and 12 LAI images (version 2), (e) four HAI images and 20 LAI images (version 1), and (f) four HAI images and 20 LAI images (version 2). Two versions of each image-type ratio (5:1, 1:1, 1:5) were used for the “object-only arrays” to produce six total arrays to allow for counter-balancing with the six “social1object” arrays. This counter-balancing of ratios of image type (social, HAI, LAI) across arrays was designed to minimize expectancy effects and re-create varying degrees of “competition” for visual attention across a social vs. nonsocial dimension. As the study focused on circumscribed interests, we omitted arrays containing only social pictures to limit the task to an analog of situations where images related to circumscribed interests compete with either social or other nonsocial information.
Visual exploration testing procedure
Testing occurred in a research laboratory on the campus of the UNC-Chapel Hill. Subjects sat approximately 60 cm from a 1,024 horizontal ×768 vertical 17 in display and viewed stimuli subtending a visual angle of 16.1°. Before the task began, the participant was presented with a brief procedure to calibrate the eye-tracking system (described below) lasting approximately 15 sec. The participant was then told that he/she was going to be shown a series of pictures on the computer screen and was instructed to look at them however he/she wanted. Arrays were displayed one at a time for 10 sec each. Across all participants, arrays were presented in one of the three pseudo-randomly generated orders. In between the display of each array, a crosshair appeared at the center of the screen to reorient the participant’s gaze. This ensured that all visual patterns began at the same point for each array. No array contained an image at this location.
Eye tracking
Eye-movement data were recorded with a Tobii 1750 eye tracker (Tobii Technology, Stockholm, Sweden). The eye-tracking equipment is integrated within the frame of a 17 in thin-film transistor monitor, and thus does not interfere with task administration. The system tracks movement of the participant’s pupil at 50 Hz by using infrared light to produce reflection patterns on the corneas and monitors the movement of these reflections relative to the eye’s position. The spatial resolution of the system is 0.25° and average accuracy is ~0.5°, resulting in recordings of high precision. Additionally, because the system allows for head motion within a cubic space of 30×15×20 cm from a distance of 60 cm, the participant was free to view the stimuli in a naturalistic manner.
Eye-tracking patterns were analyzed by conducting fixation analyses. Fixations were defined as gaze remaining within a radius of 30 pixels for a minimum of 100 msec. Three discrete aspects of visual attention were extracted from the eye-tracking data stream: (a) exploration (how many different images were explored) was quantified by tabulating the total number of different images on which the participant recorded a fixation, (b) perseveration (how long individual images were explored) was quantified by tabulating the total fixation time per image explored, and (c) detail orientation (how many different times individual images were explored) was quantified by tabulating the number of discrete fixations per image explored. These variables were selected for their potential relevance to characteristics of the autism phenotype and were intended to be conceptually distinct.
Autism symptom measures
Although existing autism rating scales tend to measure severity of symptoms in a global manner, our goal in selecting severity measures was to arrive at orthogonal measures of each domain for the present sample. Social impairments were measured with the social subscale of the SCQ, a 40-item parent-completed questionnaire that screens for the presence of ASD. The content of the SCQ parallels the ADI-R, and the agreement between SCQ and ADI-R scores is high [Rutter et al., 2003a,b]. The social subscale of the SCQ has 15 items, has no items related to language impairment or repetitive behaviors, and a higher score indicates greater impairment (range 0–15). Communication impairments were measured with the Children’s Communication Checklist-2, a 70-item checklist that assesses possible deficits in structural and pragmatic language skills as well as autistic-type language [Bishop, 2003]. To obtain a measure of communication deficits independent of both social deficits and repetitive behaviors, a modified total was obtained by subtracting the 15 items related to repetitive behaviors, resulting in a score that could range from 0 to 165. A higher score indicates greater impairment.
Previous studies have shown that a wide variety of repetitive behavior symptoms occurs in autism [Bodfish et al., 2000; Lam and Aman, 2007; Turner, 1999] and that existing autism instruments likely under-sample this domain [Lecavalier et al., 2006]. For this reason we used a multi-modal three-stage procedure for measuring repetitive behaviors. First, the Repetitive Behavior Scale-Revised (RBS-R) [Bodfish et al., 1999; Lam and Aman, 2007] was used to determine the presence or absence of specific repetitive behaviors. The RBS-R is an informantbased questionnaire that assesses 43 discrete types of repetitive behavior within five categories (motor stereotypy, repetitive self-injury, compulsions, routines/ sameness, restricted interests). Second, the Interview for Repetitive Behaviors (IRB) [Bodfish, 2003a] was used to confirm participant-specific forms of repetitive behavior endorsed on the RBS-R and assign a severity score (0–15; higher score indicates greater severity) to each using a structured clinical interview process. Third, detailed information on the presence and severity of circumscribed interests was obtained using the Interests Scale (IS) [Bodfish, 2003b]. The IS contains a checklist of current interests and additional lifetime interests and an intensity rating for the child’s strongest interest, which is a summary score of the flexibility, frequency, intensity, interference, and accommodation of that interest (range 0–23; higher score indicates greater severity). This battery of repetitive behavior measures allowed us to examine both global severity of repetitive behaviors (a composite score computed as the mean of the standardized scores on the RBS-R, IRB, and IS) and also the severity of discrete types of repetitive behavior (computed as the total severity scores on each of the subscales of the IRB for motor stereotypy, self-injury, compulsions, and routines/ sameness, and for circumscribed interests using the IS).
Statistical analysis
Because the social+object arrays and the object-only arrays differed in composition (i.e., social+object arrays contained either HAI or LAI objects, whereas object-only arrays contained both concurrently), an overall one-way multivariate analysis of variance was used to assess group differences (ASD vs. TYP) in the three visual behavior variables (exploration, perseveration, and detail orientation) across the entire task. A more exploratory examination of the attention patterns between the groups was then conducted by employing separate repeated measures analyses of variance for the social+object arrays and the object arrays only each primary variable. For the social+object arrays, object type (HAI vs. LAI) and item type (social vs. object) were the within-group variables and group (ASD vs. TYP) was the between-group variable. For the object arrays, object type (HAI vs. LAI) was the within-group variable and group (ASD vs. TYP) was the between-group variable. Correlational analysis was used to determine the degree of independence of our set of autism severity measures. Pearson correlations were used to examine the degree of relationship between the exploration task performance measures and measures of global severity of repetitive behaviors, social deficits, and communication deficits.
The groups differed in seconds of gaze time on screen across both array types (ASD social+object array, mean: 46.70; ASD object-only array, mean: 47.78; TYP social+object array, mean: 52.10; TYP object-only array, mean: 51.96; F(1, 51) = 5.24, P>0.05). There was no main effect of array type (F(1, 51) = 0.75, ns), nor an interaction between group and array type (F(1, 51) = 1.28, ns). To control for variability in missing data (i.e., eye blinks or gaze time off screen), each of these three eye-tracking variables was calculated as a ratio of gaze time on screen. Further, because IQ differed between groups, it was covaried in all analyses.
Results
Although the three outcome measures (exploration, perseveration, and detail orientation) were designed to be theoretically distinct, inter-correlations were conducted for statistical confirmation. Correlations between exploration and perseveration (r =−0.33) and between perseveration and detail orientation (r = 0.36) were not significant. The correlation between exploration and detail orientation (r = −0.62) reached significance at P>0.05. These results suggest that the outcome measures are largely independent, but may be related in interesting ways. Initial examination also revealed that each of the three outcome measures was normally distributed with acceptable levels of skewness and kurtosis.
Overall Task Performance
Multivariate analysis of overall (across all arrays) group differences in performance showed that the groups differed across all three task variables (Wilks’ λ = 0.797. F(3, 48) = 4.08, P = 0.01, = 0.20). Univariate analyses revealed that, compared with the TYP group, the ASD group explored fewer total images (F(1, 50) = 6.74, P = 0.01, = 0:12), perseverated longer on the images they did explore (F(1, 50) = 5.89, P>0.05, = 0:11), and demonstrated more detail orientation on images they did explore (F(1, 50) = 10.684, P>0.01, = 0:18). These overall results are displayed in Figure 2. Qualitative examples of visual scanpaths for representative participants from each group can be seen in Figure 3.
Figure 2.
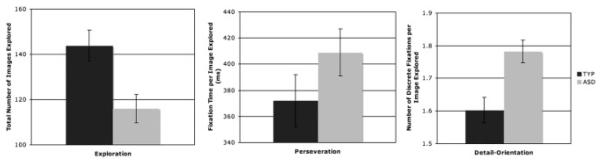
Overall group differences across all arrays on the three visual attention measures: exploration (left), perseveration (middle), and detail orientation (right).
Figure 3.
Examples of visual scanpaths from a TYP child (top) and an ASD child (bottom) on a single array presented for 10 sec. Blue circles indicate fixations and blue lines indicate saccades. Larger circles indicate longer fixations. The numbers within circles denote the order of fixations.
Exploration
For visual exploration (number of different images viewed), a main effect of group was found across all images on the social1object arrays (F(1, 50) = 5.57, P>0.05, = 0:10), indicating that the ASD group explored fewer images than the TYP group (see Table II). A main effect was also found for item type (F(1, 50) = 4.87, P>0.05, = 0:09), demonstrating that both ASD and TYP children explored more object images than social images. Lastly, a significant three-way interaction emerged between group, object type, and item type (F(1, 50) = 4.92, P>0.05, = 0:09), indicating that, relative to the TYP group, the ASD group explored fewer social images when the alternative was HAI compared with LAI objects. On the object arrays, the ASD group explored fewer object images than the TYP group (F(1, 50) = 4.81, P>0.05, = 0:09; see Table III). A trend-level main effect of object type (F(1, 50) = 2.75, P = 0.10, = 0:05) was found, suggesting that both ASD and TYP children may have preferred exploring HAI objects relative to LAI objects. The group × object type interaction did not approach significance.
Table II.
Group Means and Standard Deviations for Exploration, Perseveration and Detail-Orientation on Social Arrays
| Variable | ASD Mean (SD) | TYP Mean (SD) |
|---|---|---|
| Social arrays w/HAI | ||
| Exploration | ||
| Social images explored | 14.00 (4.56) | 19.21 (5.08) |
| HAI images explored | 15.38 (5.54) | 18.08 (5.37) |
| Perseveration | ||
| Fixation time per social image explored xx2 (ms) |
364 (153) | 335 (103) |
| Fixation time per HAI image explored (ms) | 479 (227) | 423 (174) |
| Detail orientation | ||
| Fixations per social image explored | 1.57 (0.37) | 1.55 (0.31) |
| Fixations per HAI image explored | 2.01 (0.56) | 1.79 (0.42) |
| Social arrays w/LAI | ||
| Exploration | ||
| Social images explored | 16.72 (5.93) | 21.04 (5.57) |
| LAI images explored | 12.10 (4.69) | 15.71 (4.80) |
| Perseveration | ||
| Fixation time per social image explored (ms) |
361 (118) | 350 (116) |
| Fixation time per LAI image explored (ms) | 407 (177) | 383 (114) |
| Detail orientation | ||
| Fixations per social image explored | 1.64 (0.32) | 1.57 (0.32) |
| Fixations per LAI image explored | 1.74 (0.57) | 1.61 (0.32) |
| Social Arrays Total | ||
| Exploration | ||
| Social images explored | 30.72 (8.35) | 40.25 (9.89) |
| Object images explored | 27.48 (9.24) | 33.79 (9.36) |
| Perseveration | ||
| Fixation time per social image explored (ms) |
365 (117) | 345 (97) |
| Fixation time per object image explored (ms) |
443 (139) | 403 (119) |
| Detail orientation | ||
| Fixations per social image explored | 1.62 (0.29) | 1.56 (0.26) |
| Fixations per object image explored | 1.88 (0.38) | 1.71 (0.27) |
Abbreviations: ASD, autism spectrum disorders; TYP, typically developing; HAI, high autism interest; LAI, low autism interest.
Table III.
Group Means and Standard Deviations for Exploration, Perseveration and Detail-Orientation on Object Arrays
| Variable | ASD mean (SD) |
TYP mean (SD) |
|---|---|---|
| HAI images | ||
| Exploration | ||
| HAI images explored | 34.34 (7.80) | 40.54 (11.15) |
| Perseveration | ||
| Fixation time per HAI image explored (ms) | 439 (113) | 407 (123) |
| Detail orientation | ||
| Fixations per HAI image explored | 1.93 (0.33) | 1.75 (0.27) |
| LAI images | ||
| Exploration | ||
| LAI images explored | 23.14 (7.93) | 29.79 (10.12) |
| Perseveration | ||
| Fixation time per LAI image explored (ms) | 352 (107) | 350 (116) |
| Detail orientation | ||
| Fixations per LAI image explored | 1.54 (0.24) | 1.45 (0.25) |
| HAI and LAI images (combined) | ||
| Exploration | ||
| All images explored | 57.48 (13.75) | 70.33 (20.17) |
| Perseveration | ||
| Fixation time per image explored (ms) | 405 (89) | 382 (101) |
| Detail orientation | ||
| Fixations per image explored | 1.77 (0.22) | 1.63 (0.22) |
Abbreviations: ASD, autism spectrum disorders; TYP, typically developing; HAI, high autism interest; LAI, low autism interest.
Perseveration
The ASD group exhibited greater visual perseveration than the TYP group across all images on the social+object arrays (F(1, 50) = 3.91, P = 0.05, = 0:07). Both groups perseverated the object items more than the social items (F(1, 50) = 17.72, P>0.01, = 0:26; see Table II). The main effect of object type and all interactions did not approach significance. On object arrays, however, a trend-level main effect emerged for group (F(1, 50) = 3.07, P = 0.086, = 0.06), suggesting that the ASD group may perseverate longer than the TYP group on the object images they explored (see Table III). The main effect of object type and the group × object type interaction was not significant.
Detail Orientation
The ASD group averaged more fixations per image explored on the social+object arrays (F(1, 50) = 4.55, P>0.05, = 0.08; see Table II). Additionally, trend-level effects emerged for item type (F(1, 50) = 2.98, P = 0.09, = 0.06) and for the group × item interaction (F(1, 50) = 3.41, P = 0.07, = 0.06), suggesting that detail orientation may have been more pronounced for object items relative to social items, and that this pattern may be disproportionately exhibited by the ASD group. The groups also differed on the average number of fixations per object image explored on the object arrays (F(1, 50) = 6.74,P = 0.01, = 0.12; see Table III). Additionally, a trend-level group × object type interaction emerged on the object arrays (F(1, 50) = 2.98, P = 0.09, = 0.06), suggesting that relative to their TYP counterparts, ASD children may have averaged more fixations per HAI objects explored compared with LAI objects.
Correlation With Clinical Measures
Within the ASD group, the overall severity measures for each symptom domain (composite repetitive behavior measure, SCQ social subscale score, and Modified Children’s Communication Checklist communication score) were not significantly inter-correlated (all Ps>0.05), suggesting that they measure reasonably separate aspects of the clinical phenotype in this sample. Composite repetitive behavior scores were positively correlated with exploration of object images (r(27) = 0.38, P = 0.04), but were negatively correlated with perseveration on social images (r(27) =− 0.39, P = 0.04). Social deficits were positively correlated with exploration of object images (r(27) = 0.37, P = 0.04). Communication deficits were not correlated with attentional performance.
Discussion
This study offers evidence of abnormal patterns of visual attention in ASD. Compared with controls, visual attention in children with ASD was more circumscribed (indicated by the exploration of fewer images overall), more perseverative (indicated by longer fixation times per image explored), and more detail oriented (indicated by a greater number of discrete fixations on explored images). The overall reduction in visual exploration found in ASD can thus be explained by a tendency in these children to fixate longer on the items they explored. Visual perseveration and detail orientation may therefore act as mechanisms for reduced visual exploration in autism and suggests that salient items may disproportionately “capture” and “trap” attention in ASD. This over-focused, perseverative or detail-oriented style may differentiate attention abnormalities in ASD from other developmental disorders such as attention deficit hyperactivity disorder that are characterized by under-focused attention.
The findings here are consistent with previous studies of visual attention in autism [Courchesne et al., 1994; Dawson et al., 1998; Hermelin and O’Connor, 1967; Landry and Bryson, 2004; Swettenham et al., 1998; Wainwright-Sharp and Bryson, 1993, 1996]. These studies are driven by a conceptual model positing a network of interrelated attentional systems, including a subcortical “vigilance” system that maintains alertness and arousal, a posterior “selectivity” system responsible for attentional disengagement and shifting, and an anterior “executive” system that recruits attentional resources for goal-directed behavior [Posner and Dehaene, 1994]. Consistent with this model, our finding that children with ASD displayed increased perseveration and detail orientation parallels findings of previous studies indicating that attentional abnormalities in autism involve impairments in attentional disengagement [Landry and Bryson, 2004; Zwaigenbaum et al., 2005] and attentional shifting [Courchesne et al., 1994]. Additionally, this study extends these models by demonstrating that stimulus saliency is an important modulator of attentional performance in autism within a visual representation of everyday situations where social and nonsocial stimuli compete for attention. In the ASD group, we found that attention to social items tended to be diminished in the presence of HAI items. This suggests that social and nonsocial information differ in relative reward value in children with ASD and that increased reward value of nonsocial information may contribute to its relative saliency in ASD. In line with Dawson et al. [1998], this finding supports a model of diminished social reward/motivation in autism and extends this model to include the possibility that diminished social motivation may result in a compensatory increase in nonsocial motivation that can drive idiosyncratic patterns of attention and behavior.
Further, because reduced visual exploration in the ASD group extended to both social and object arrays, attentional abnormalities in autism do not appear restricted to social stimuli, but rather may reflect more generalized impairment. Within social arrays, however, the ASD group exhibited a pattern of visual attention not shared by the TYP group, in which the exploration of social images was modulated by the type of object images concurrently displayed. Children with ASD were less likely than their TYP counterparts to explore social images when presented simultaneously with HAI items, again suggesting that HAI images may “trap” attention in ASD to a greater degree than in TYP children. Furthermore, because ASD children were more likely to explore social stimuli when presented in tandem with LAI images, it appears unlikely that they actively avoided social images. Rather, it suggests that the presence of highly salient items of circumscribed interest, in this case HAI images, may unduly distract ASD children from attending to social stimuli by eliciting visual perseveration.
Although a trend-level main effect of object type on the object arrays suggests that HAI images indeed constitute especially salient stimuli for ASD children, the lack of a group by object type interaction implies that the pattern of selective attention in ASD to HAI images may represent an exaggeration of a typical attentional process rather than a qualitatively different one. The ASD group did not exhibit an impairment of differential attention—both groups showed a preference for HAI items—but rather demonstrated a problem with attentional control by displaying greater overall perseveration. Furthermore, the saliency of HAI items for ASD children is reflected by the fact that they average a greater number of fixations than TYP children on HAI images relative to LAI or social images. This finding suggests that ASD children may be more likely than TYP children to fixate on multiple parts of an object image, consistent with previous studies demonstrating that children and adults with autism demonstrate a bias toward detail-oriented processing [Happe and Frith, 2006]. Moreover, because this pattern appears restricted to HAI images, items of circumscribed interest may disproportionately elicit a detail-oriented attentional style in children with ASD [Mottron and Belleville, 1993; Mottron et al., 1999, 2007]. Future studies are needed to determine whether this attentional style is elicited more because of the content of HAI images or because of their greater perceptual complexity.
We also found a relationship between discrete measures of exploration and overall severity of repetitive behavior. Severity of repetitive behavior symptoms in the ASD group was associated with more attention to nonsocial images and was also associated with less attention to social images. This may seem counterintuitive as it might be assumed that increased time spent engaging in repetitive behaviors would correspond to less time spent exploring the environment. Indeed, Pierce and Courchesene [2001] in their examination of object exploration in autism found a significant negative association between time spent engaging in stereotyped movements and time spent exploring objects. However, greater attention to and interest in nonsocial as opposed to social events is a quintessential pattern of autistic behavior. Overall or global repetitive behavior severity may simply index a tendency to engage with nonsocial information or objects and neglect or avoid social information or events. This is perhaps most readily seen in certain types of repetitive behaviors characteristic of autism such as circumscribed interests and the preoccupation with parts of objects or with object actions. These actions do not typically involve social situations and instead involve excessive engagement with nonsocial aspects of the environment.
Visual attention task performance correlated not only with severity of repetitive behavior symptoms but also with severity of social deficits in the ASD group. Exploration of object images, but not social images, correlated with severity of social deficits. This, combined with the significant association with severity of repetitive behaviors, suggests that while features of autism can be separated at the symptom level for diagnostic purposes, they may be related at the level of underlying attentional or neurcognitive processes. Conceivably, increased salience of nonsocial information could lead to both (a) repetitive behavior symptoms (e.g., circumscribed interests) that develop as a result of increased experience and expertise with a particular area of focus, and also (b) social deficits as a result of diminished exploration of and expertise with the social world. However, because our resulting correlational findings were exploratory in number and modest in magnitude, additional work is needed to more rigorously test the relation of repetitive behavior and social symptoms at the level of underlying attentional processes.
This study has several weaknesses. Because our subject sample included only a limited range of ages and functional levels, we do not know whether our findings would apply to infants or adults with autism or to persons with autism and comorbid mental retardation. Previous studies have shown that repetitive behaviors tend to remain stable in persons with autism [Piven et al., 1996] and that circumscribed interests are present in both lowand high-functioning persons with autism [Bartak and Rutter, 1976]. Thus, although we anticipate that our findings would apply more generally across the autism spectrum, systematic replication with other ages and with less cognitively able subjects is needed. We intentionally sampled a variety of ASD subtypes to obtain a wide range of repetitive behavior severity. Although post hoc inspection revealed no autism subtype effects, future studies with larger samples of specific subtypes are required to better address potential subtype effects. As we did not employ either a neurodevelopmental (e.g., mental retardation) or a neuropsychiatric (e.g., attention deficit hyperactivity disorder or obsessive–compulsive disorder) comparison group, we do not know whether the obtained findings are specific to persons with autism. Finally, we employed only static images. Previous studies of visual behavior in autism have demonstrated that using dynamic images may more validly measure saliency effects in autism [Klin et al., 2002a,b]. Further, given anecdotal accounts of ASD children’s fascination with mechanical or moving objects, it is reasonable to expect that use of dynamic stimuli in the present paradigm would help establish key saliency effects in autism.
Our findings also have clinical relevance. Increased salience of nonsocial aspects of the environment may lead children with autism to explore, become familiar with, and become skillful with only a circumscribed portion of the totality of available experience. If true, it would be important to develop intervention methods specifically targeting both (a) diminishing habitual and rigid patterns of circumscribed attention and exploration of the environment and (b) increasing more varied and flexible attention and exploration of the environment. The task used here may also offer an effective method for empirically quantifying key aspects of the repetitive behavior phenotype in autism such as perseveration and detail orientation. To date, most studies of repetitive behavior in autism have relied on parental report measures owing to a lack of practical and valid observational measures for this domain. The quantitative measures of circumscribed interest derived from this task may provide useful for observational and dimensional markers of repetitive behavior in autism that can be used in genetic, neurobiological, and intervention studies. Given that passive viewing tasks are amenable to testing the early development of attention and cognition in infants, these measures may also be useful for studies of the early risk for and development of autism.
Acknowledgment
Assistance for this study was provided by Division TEACCH and also by the Subject Registry and Behavioral Measurement Research Cores of the UNC Neurodevelopmental Disorders Research Center. We thank Sharon Weeks for her help with data collection and analysis, and the participants and their families for their participation in this study.
Grant Sponsors: NIHR01 MH73402T32 HD40127U54 MH66418, NIMH Research Project GrantMH73402, NICHD Post-Doctoral Research Training GrantHD40127, NICHD Research Center GrantHD03110
References
- Baron-Cohen S, Wheelwright S. ‘Obsessions’ in children with autism or Asperger syndrome. Content analysis in terms of core domains of cognition. British Journal of Psychiatry. 1999;175:484–490. doi: 10.1192/bjp.175.5.484. [DOI] [PubMed] [Google Scholar]
- Bartak L, Rutter M. Differences between mentally retarded and normally intelligent children. Journal of Autism and Childhood Schizophrenia. 1976;6:109–120. doi: 10.1007/BF01538054. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. The children’s communication checklist second edition manual. Harcourt Assessment; London: 2003. [Google Scholar]
- Bodfish JW. Interests scale. University of North Carolina-Chapel Hill; 2003a. Unpublished rating scale. [Google Scholar]
- Bodfish JW. Interview for repetitive behaviors (IRB) University of North Carolina-Chapel Hill; 2003b. Unpublished rating scale. [Google Scholar]
- Bodfish JW, Symons FW, Lewis MH. The Repetitive Behavior Scale. Western Carolina Center; 1999. Research Reports. [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Colombo J, Ryther JS, Frick JE, Gifford JJ. Visual pop-out in infants: evidence for preattentive search in 3- and 4-month-olds. Psychonomic Bulletin and Review. 1995;2:266–268. doi: 10.3758/BF03210968. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) manual. Western Psychological Services; Los Angeles: 2002. [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, et al. Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert, et al. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview—Revised. Child Psychiatry and Human Development. 2003;34:3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Mottron L, Berthiaume C, Burack JA. Developmental changes of autistic symptoms. Autism. 2003;7:255–268. doi: 10.1177/1362361303007003003. [DOI] [PubMed] [Google Scholar]
- Freeman BJ, Ritvo ER, Schroth PC, Tonick I, Guthrie D, Wake L. Behavioral characteristics of high- and low-IQ autistic children. American Journal of Psychiatry. 1981;138:25–29. doi: 10.1176/ajp.138.1.25. [DOI] [PubMed] [Google Scholar]
- Gerhardstein P, Rovee-Collier C. The development of visual search in infants and very young children. Journal of Experimental Child Psychology. 2002;81:194–215. doi: 10.1006/jecp.2001.2649. [DOI] [PubMed] [Google Scholar]
- Haith MM, Bergman T, Moore MJ. Eye contact and face scanning in early infancy. Science. 1979;198:853–855. doi: 10.1126/science.918670. [DOI] [PubMed] [Google Scholar]
- Happe F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hermelin B, O’Connor N. Perceptual and motor discrimination in psychotic and normal children. Journal of Genetic Psychology. 1967;110:117–125. doi: 10.1080/00221325.1967.10533723. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. The Nervous Child: Quarterly Journal of Psychopathology, Psychotherapy, Mental Hygiene, and Guidance of the Child. 1943;2:217–250. [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. American Journal of Psychiatry. 2002a;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002b;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Covert A. The relationship of selfstimulation to learning in autistic children. Journal of Applied Behavior and Analysis. 1972;5:381–387. doi: 10.1901/jaba.1972.5-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel RL, Firestone PB, Kramme KW, Dunlap G. Increasing spontaneous play by suppressing self-stimulation in autistic children. Journal of Applied Behavior and Analysis. 1974;7:521–528. doi: 10.1901/jaba.1974.7-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KSL, Aman MG. The Repetitive Behavior Scale—Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;27:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45:1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Aman MG, Scahill L, McDougle CJ, McCracken JT, et al. Validity of the Autism Diagnostic Interview—Revised. American Journal of Mental Retardation. 2006;111:199–215. doi: 10.1352/0895-8017(2006)111[199:VOTADI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Mental Retardation and Developmental Disabilities Research Reviews. 1998;4:80–89. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lovaas OI, Litrownik A, Mann R. Response latencies to auditory stimuli in autistic children engaged in self-stimulatory behavior. Behavioral Research Therapy. 1971;9:39–49. doi: 10.1016/0005-7967(71)90035-0. [DOI] [PubMed] [Google Scholar]
- Mercier C, Mottron L, Belleville S. A psychosocial study on restricted interests in high-functioning persons with pervasive developmental disorders. Autism. 2000;4:406–425. [Google Scholar]
- Mottron L, Belleville S. A study of perceptual analysis in a high-level autistic subject with exceptional graphic abilities. Brain and Cognition. 1993;23:279–309. doi: 10.1006/brcg.1993.1060. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA, Stauder JE, Robaey P. Perceptual processing among high-functioning persons with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40:203–211. [PubMed] [Google Scholar]
- Mottron L, Mineau S, Martel G, Bernier CS, Berthiaume C, et al. Lateral glances toward moving stimuli among young children with autism: Early regulation of locally oriented perception? Developmental Psychopathology. 2007;19:23–36. doi: 10.1017/S0954579407070022. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, South M, Miller JN. DSM-IV-defined Asperger syndrome: Cognitive, behavioral and early history differentiation from high-functioning autism. Autism. 2000;4:29–46. [Google Scholar]
- Parkhurst DJ, Niebur E. Scene content selected by active vision. Spatial Vision. 2003;16:125–154. doi: 10.1163/15685680360511645. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, et al. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: A retrospective study of high-IQ adolescents and adults. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:523–529. doi: 10.1097/00004583-199604000-00019. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends in Neurosciences. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Richler J, Bishop SL, Kleinke JR, Lord C. Restricted and repetitive behaviors in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:299–316. doi: 10.1007/s10803-006-0332-6. [DOI] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale—Revised. Stoelting; Wood Dale, IL: 2002. [Google Scholar]
- Rutter M, Bailey A, Lord C. Manual for the SCQ. Western Psychological Services; Los Angeles, CA: 2003a. SCQ: Social Communication Questionnaire. [Google Scholar]
- Rutter M, Le Couteur A, Lord C. ADI-R: The Autism Diagnostic Interview—Revised. Western Psychological Services; Los Angeles, CA: 2003b. [Google Scholar]
- Sasson NJ. The development of face processing in autism. Journal of Autism and Developmental Disorders. 2006;36:381–394. doi: 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn D,L, et al. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45:2580–2588. doi: 10.1016/j.neuropsychologia.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, McDougle CJ, Williams SK, Dimitropoulos A, Aman MG, et al. Children’s Yale-Brown Obsessive Compulsive Scale modified for pervasive developmental disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1114–1123. doi: 10.1097/01.chi.0000220854.79144.e7. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon W. Repetitive behavior profiles in Asperger Syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35:145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, et al. The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39:747–753. [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, et al. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Treisman A. Perceptual grouping and attention in visual search for features and for objects. Journal of Experimental Psychology Human Perception and Performance. 1982;8:194–214. doi: 10.1037//0096-1523.8.2.194. [DOI] [PubMed] [Google Scholar]
- Turner M. Annotation: repetitive behaviour in autism: A review of psychological research. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40:839–849. [PubMed] [Google Scholar]
- Turner MA. Towards an executive dysfunction account of repetitive behaviour in autism. In: Russell J, editor. Autism as an executive disorder. Oxford University Press; Oxford: 1997. pp. 57–100. [Google Scholar]
- Varni JW, Lovaas OI, Koegel RL, Everett NL. An analysis of observational learning in autistic and normal children. Journal of Abnormal Child Psychology. 1979;7:31–43. doi: 10.1007/BF00924508. [DOI] [PubMed] [Google Scholar]
- Wainwright JA, Bryson SE. Visual-spatial orienting in autism. Journal of Autism and Developmental Disorders. 1996;26:423–438. doi: 10.1007/BF02172827. [DOI] [PubMed] [Google Scholar]
- Wainwright-Sharp JA, Bryson SE. Visual orienting deficits in high-functioning people with autism. Journal of Autism and Developmental Disorders. 1993;23:1–13. doi: 10.1007/BF01066415. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]




