Abstract
School-aged children and adolescents with autism demonstrate circumscribed attentional patterns to nonsocial aspects of complex visual arrays (Sasson et al.2008). The current study downward extended these findings to a sample of 2–5 year-olds with autism and 2–5 year-old typically developing children. Eye-tracking was used to quantify discrete aspects of visual attention to picture arrays containing combinations of social pictures, pictures of objects frequently involved in circumscribed interests in persons with autism (e.g., trains), and pictures of more commonplace objects (e.g., clothing). The children with autism exhibited greater exploration and perseverative attention on objects related to circumscribed interests than did typically developing children. Results suggest that circumscribed attention may be an early emerging characteristic of autism.
Keywords: Autism, Attention, Visual exploration, Toddlers, Perseveration
Atypical exploration of objects may be a specific behavioral marker of the autism phenotype in infancy (Morgan et al. 2008; Watt et al. 2008). This restricted and repetitive repertoire of exploratory behaviors may share cognitive mechanisms with the repetitive behaviors characteristic of autism in older children. Circumscribed interests are a factor within the repetitive behaviors domain and are orthogonal to social-communication deficits and cognitive functioning (Lam et al. 2008). We recently designed a passive viewing visual exploration task to quantify the effect of circumscribed interests on patterns of visual attention (Sasson et al. 2008). School-aged children with autism spectrum disorders (ASD) demonstrated reduced exploratory behavior, increased perseverative attention, and a more detail-oriented perceptual profile on this task, though these effects were driven primarily by the disproportionate restriction of attention to objects of “High Autism Interest” (HAI) that are frequent targets of circumscribed interests (South et al. 2005). In the absence of HAI objects, visual attention patterns did not differ between children with and without ASD, suggesting a critical role played by circumscribed interests in the restriction of visual attention.
Given that a substantial proportion of toddlers and preschoolers exhibit intense interests (DeLoache et al. 2007), it is unclear whether circumscribed interests manifest differently in young children with autism. The current study sought to determine whether 2–5 year olds with autism would demonstrate similar patterns of circumscribed attention found in older children with ASD using the same visual exploration task (Sasson et al. 2008). We hypothesized that the visual exploration task would elucidate circumscribed attentional patterns in a group of young children with autism similar to those elicited from schoolaged children with autism, namely through the disproportionate visual exploration and perseveration of HAI objects.
Methods
Participants
The participants included two groups of young children: 10 with diagnoses of autism (AUT) and 14 who were typically developing (TYP). After one AUT and one TYP child were excluded from analyses due to missing and low quality data derived from inaccurate eye tracking calibrations, the final sample consisted of 9 children with AUT (8 males, 1 female; mean age, 43.2 months (SD, 9.2), range, 32–56 months) and 13 TYP children (12 males, 1 female, mean age, 40.2 (SD, 14.3), range, 25–59 months). The AUT and TYP groups did not differ statistically in sex or chronological age. None of the participants had a seizure disorder, an acute medical or genetic condition, or any visual impairment uncorrectable with eyeglasses. The University of North Carolina (UNC) Autism Research Registry in conjunction with regional TEACCH (Treatment and Education of Autistic and related Communication-handicapped CHildren) clinics served as the recruitment sources for the children with autism. Each child in the AUT group had a DSM-IV diagnosis of Autistic Disorder made by a licensed clinician experienced in the assessment and diagnosis of autism. As per TEACCH protocols, if a clinical diagnosis was questioned, diagnosis was confirmed with the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000), which occurred in three cases in the present sample.
Children in the TYP group were recruited via an email sent to UNC faculty and staff. TYP children did not have a history of any psychiatric condition or developmental disorder as assessed through an unstructured interview, were not taking psychotropic medication, and did not have an immediate family member with ASD.
The UNC-Chapel Hill School of Medicine Biomedical Institutional Review Board approved the protocol for this study, and the legal guardians of each participating child gave informed written consent before the study began.
Procedure
Visual Exploration Task
The visual exploration task consisted of 12 static arrays with 24 color images each. Half of the arrays were “social + object arrays” (i.e., they contained pictures of people with clearly visible faces along with pictures of objects), and half were “object only arrays” (i.e., they contained object pictures only). Half of all object pictures were “High Autism Interest” (HAI). These objects were selected from nine categories which previous research has shown to be frequent targets of circumscribed interests in individuals with autism: trains, vehicles, planes, blocks, home electronics, computer equipment, road signs, and sporting equipment (South et al. 2005). The other half of object pictures were “Low Autism Interest” (LAI) and selected from nine categories that have not been reported to be of common circumscribed interest in autism: clothing, food, furniture, plants, school supplies, bathroom supplies, gloves, hats, and bags. The proportion of social, HAI and LAI images within each array was counterbalanced to create different levels of competition for visual attention across social and nonsocial images. For more detail concerning the construction of the task, please see Sasson et al. (2008).
Testing Conditions
All testing was conducted at UNC-Chapel Hill. Participants sat approximately 60 cm from a Tobii 1750 eye tracking system (Tobii Technology, Stockholm, Sweden). The eye tracking equipment is integrated within a 17 display (visual angle, ~32°) set at a resolution of 1,152 × 864 pixels with a sampling frequency of 50 Hz. The equipment does not interfere with task administration and allows for head motion within a cubic range of 30 × 15 × 20 cm.
A brief (~15 s) calibration procedure was administered before the task began. The participant was then instructed to view the forthcoming pictures. Arrays were presented one at a time for 10 s each in one of three pseudorandom orders. A centrally presented crosshair appeared between arrays to reorient the participant and ensure that all visual patterns began at the same point for each array. No array contained an image at this center location.
Data Reduction and Statistical Analysis
Fixation analysis served as the basis for examining eye tracking patterns. Consistent with Sasson et al. (2008), fixations were defined as gaze remaining within a radius of 30 pixels (visual angle, ~0.80°) for a minimum of 100 ms, a threshold that matches (e.g., Merin et al. 2007) or exceeds (e.g., Dalton et al. 2005) many other investigations of autism using eye tracking. Three discrete aspects of visual attention comprised the outcome measures: (a) exploration (the number of different images on which a fixation was recorded) (b) perseveration (the total fixation time per image explored) and (c) detail-orientation (the number of discrete fixations per image explored). These variables were selected for their relevance to attentional and perceptual characteristics of the autism phenotype and were intended to be conceptually distinct.
Separate repeated measures ANOVAs for the social + object arrays and the object only arrays on each primary variable were conducted to determine whether patterns of visual attention differed between groups for different types of stimuli. For the social + object arrays, object type (HAI vs. LAI) and item type (social vs. object) were the within-group variables and group (AUT vs. TYP) was the between-group variable. For the object only arrays, object type (HAI vs. LAI) was the within-group variable and group (AUT vs. TYP) was the between group variable.
The groups did not differ in amount of missing data (i.e., eye blinks and gaze time off the display) (F (1, 20) < .01, ns). Missing data also not differ by array type (F (1, 20) = .49, ns), nor was there a Group x Array Type interaction (F (1, 20) = .33, ns). However, to control for individual differences in missing data, exploration was analyzed as a ratio of on screen gaze time (i.e., the number images fixated per second on screen). Calculations of perseveration and detail-orientation were not affected by on screen gaze time.
Results
Examination of the three outcome variables confirmed that they were largely independent, though perhaps related in interesting ways. While perseveration and detail-orientation were significantly correlated (r = .52, p = .01), correlations were not significant between exploration and perseveration (r = − .18), and between exploration and detail-orientation (r = − .35). Shapiro–Wilk W tests indicated that each outcome variable was normally distributed (exploration, W = 0.98, = .95; perseveration, W = .94, p = .16; detail-orientation, W = .95, p = .29) with levels of skew and kurtosis in the normal range.
Means and standard deviations for the AUT and TYP groups on exploration, perseveration and detail-orientation can be found for the social + object arrays in Table 1 and for the object only arrays in Table 2.
Table 1.
Visual attention on social + object arrays
| Variable | AUT Mean (SD) | TYP Mean (SD) |
|---|---|---|
| Social arrays w/HAI | ||
| Exploration | ||
| Social images explored | 7.22 (3.49) | 8.38 (4.50) |
| HAI images explored | 10.78 (4.21) | 10.15 (4.25) |
| Perseveration | ||
| Fixation time per social image explored (ms) | 384 (165) | 544 (321) |
| Fixation time per HAI image explored (ms) | 658 (191) | 524 (331) |
| Detail-orientation | ||
| Fixations per social image explored | 1.56 (.41) | 2.02 (.92) |
| Fixations per HAI image explored | 2.20 (.54) | 1.80 (.62) |
| Social arrays w/LAI | ||
| Exploration | ||
| Social images explored | 10.44 (4.39) | 11.54 (5.61) |
| LAI images explored | 9.56 (3.43) | 9.85 (4.49) |
| Perseveration | ||
| Fixation time per social image explored (ms) | 398 (147) | 458 (176) |
| Fixation time per LAI image explored (ms) | 506 (178) | 475 (233) |
| Detail-Orientation | ||
| Fixations per social image explored | 1.71 (.40) | 1.75 (.52) |
| Fixations per LAI image explored | 1.80 (.46) | 1.79 (.56) |
| Social arrays total | ||
| Exploration | ||
| Social images explored | 17.67 (6.80) | 19.92 (9.26) |
| Object images explored | 20.33 (6.96) | 20.00 (7.93) |
| Perseveration | ||
| Fixation time per social image explored (ms) | 405 (113) | 497 (176) |
| Fixation time per object image explored (ms) | 585 (178) | 495 (228) |
| Detail-Orientation | ||
| Fixations per social image explored | 1.67 (.29) | 1.85 (.41) |
| Fixations per object image explored | 2.01 (.47) | 1.79 (53) |
Abbreviations: AUT Autism, TYP Typically Developing, HAI High Autism Interest, LAI Low Autism Interest
Table 2.
Visual attention on object only arrays
| Variable | AUT Mean (SD) | TYP Mean (SD) |
|---|---|---|
| HAI images | ||
| Exploration | ||
| HAI images explored | 21.67 (7.66) 1 | 18.84 (8.91) |
| Perseveration | ||
| Fixation time per HAI image explored (ms) | 646 (253) | 534 (207) |
| Detail-Orientation | ||
| Fixations per HAI image explored | 2.33 (.55) | 2.08 (.63) |
| LAI images | ||
| Exploration | ||
| LAI images explored | 11.67 (6.48) | 16.92 (7.30) |
| Perseveration | ||
| Fixation time per LAI image explored (ms) | 370 (104) | 480 (185) |
| Detail-Orientation | ||
| Fixations per LAI image explored | 1.53 (.32) | 1.72 (.39) |
| HAI and LAI images (combined) | ||
| Exploration | ||
| All images explored | 33.33 (12.06) | 35.77 (14.87) |
| Perseveration | ||
| Fixation time per image explored (ms) | 549 (192) | 509 (156) |
| Detail-Orientation | ||
| Fixations per image explored | 2.07 (.49) | 1.91 (.36) |
Abbreviations: AUT Autism, TYP Typically Developing, HAI High Autism Interest, LAI Low Autism Interes
Exploration
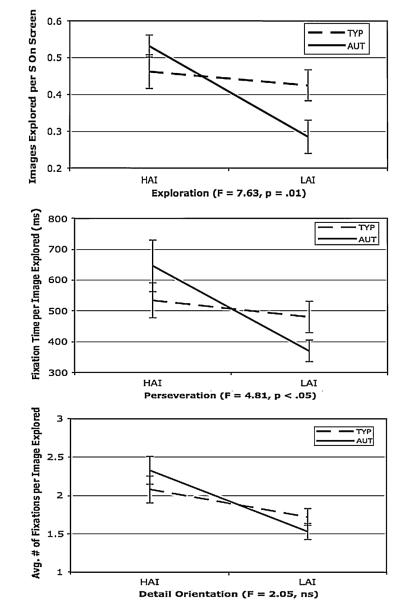
The AUT and TYP groups did not differ in visual exploration (i.e., the number of different images viewed) across all images on the social + object arrays (F (1, 20) = .04, p = .85, . A significant interaction emerged between object type and item type (F (1, 20) = 8.96, p < .01, . Post hoc analysis determined that participants explored fewer social images when the alternative was HAI compared to LAI objects (t (21) = 4.87, p < .01). On the object only arrays, a significant interaction occurred between group and object type (F (1, 20) = 7.63, p = .01, ; see Fig. 1). Follow up analyses indicated that this interaction was driven by the AUT children exploring significantly more HAI than LAI images (t (8) = 4.31, p < .01), while TYP children did not differ in their exploration of the two object types (t (12) = .76, p = .46).
Fig. 1.
Group × object type interactions on object only arrays. Abbreviations: AUT Autism, TYP Typically Developing, HAI High Autism Interest, LAI Low Autism Interest
Perseveration
On the social + object arrays, a significant interaction emerged between group and item type (F (1, 20) = 4.39, p < .05, . Follow up analyses indicated that, while ASD children perseverated significantly more on object items relative to social items (t (8) = 4.56, p < .01),TYP children did not differ in their perseveration on the two item types (t (12) = .03, p = .98). On object only arrays, a significant interaction occurred between group and object type (F (1, 20) = 4.81, p < .05, ; see Fig. 1). Follow up analyses determined that AUT children perseverated HAI objects significantly more than LAI objects (t (8) = 4.03, p < .01), while TYP children did not differ in their perseveration on the two item types (t (12) = .77, p = .46).
Detail-orientation
No significant main effects or interactions were found on the social + object arrays. On the object-only arrays, a main effect of object type emerged indicating that across both groups HAI objects were inspected in a more detailoriented fashion than LAI objects (F (1, 20) = 15.07, p < .01, . The Group × Object Type interaction approached but did not reach significance (see Fig. 1).
Discussion
This report demonstrates the feasibility of evaluating circumscribed attentional patterns in young children with autism using a passive viewing visual exploration task. By disaggregating attentional performance into exploration, perseveration, and detail-oriented processes that are operationally identical to those observed in school-aged children (Sasson et al. 2008), the current study provides evidence of heightened visual attention to high-autism interest (HAI) objects in 2–5 year olds with autism, and suggests that these stimuli may be disproportionately salient to young children with autism relative to their typically developing peers. Viewed in context of results using the same task with older children (Sasson et al. 2008), these findings suggest that 1. Age may be associated with an increase in the flexible allocation of attention, and 2. Restricted attentional patterns to objects of circumscribed interest may characterize ASD from a very young age.
The presence of circumscribed attentional patterns specifically related to HAI objects in young children with autism supplements data showing atypical motor movements and abnormal object exploration in infants and toddlers who go onto receive an autism diagnosis (Morgan et al. 2008; Ozonoff et al. 2008; Watt et al. 2008). Taken together, this evidence suggests that the early expression of repetitive behaviors may be phenomenologically similar to the variety of repetitive behaviors present in school-aged children and adults with autism (Bodfish et al. 2000), and posits that abnormal fixation, exploration and manipulation of object stimuli may be a distinctive characteristic of the early autism phenotype.
Additionally, attention biases to nonsocial aspects of the environment may have downstream effects on the development of social information processing. Flexibly attending to salient aspects of the environment supports experience-dependent learning during times of complex brain development, particularly during the first 3 years of life. Abnormalities in the distribution of attention, such as biases toward nonsocial information, may affect the development of neural specialization (Johnson 2000), including neural circuitry supporting abilities related to social information processing (Sasson 2006; Schultz 2005). Future research is needed to examine whether abnormalities in the allocation and prioritization of visual attention during early development directly relate to the emergence of phenotypic social impairments in ASD. Alternatively, attenuated social salience from very early life may prompt increased object fixation. Deconstructing the directionality of these interacting mechanisms will be critical for understanding the etiology of social information processing abnormalities in autism.
This study has several limitations. Challenges relating to recruitment in this age range resulted in a smaller sample size of children relative to similar studies (Sasson et al.2008). Nevertheless, the sample was sufficient for generating adequate power to detect strong group differences in visual attention patterns, though a larger sample may have enabled the detection of more sensitive differences. The lack of standardized diagnostic characterization in some of the children with ASD should also be considered when interpreting results reported here. Interpretation of visual attention patterns may have benefited from the inclusion of clinical measures of repetitive behaviors and circumscribed interests. Finally, although a similar pattern of heightened attention to HAI objects was found in older children with ASD even after controlling for I.Q. (Sasson et al. 2008), the lack of cognitive evaluation of the current sample is noteworthy. These limitations notwithstanding, the current study offers a quantitative measure of circumscribed attention in young children with autism that may prove useful for examining early risk and developmental factors related to the disorder.
Acknowledgments
This research was supported by R01 MH073402 (Bodfish). L.M. Turner-Brown was supported by NICHD T32-HD40127. G Dichter was supported by K23 MH081285. Assistance for this study was provided by Kristin S. L. Lam, Tia Holtzclaw, and the Subject Registry Core of the UNC Neurodevelopmental Disorders Research Center (P30 HD03110).
Contributor Information
Noah J. Sasson, School of Behavioral and Brain Sciences, University of Texas at Dallas, GR41 800 West Campbell Road, Richardson, TX 75080, USA
Jed T. Elison, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA; Department of Psychology, University of North Carolina at Chapel Hill School of Arts and Sciences, CB# 3270, Chapel Hill, NC 27599-3270, USA
Lauren M. Turner-Brown, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA
Gabriel S. Dichter, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA; Duke-UNC Brain Imaging and Analysis Center, Duke University Medical Center, Durham, NC 27710, USA; Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3026, Durham, NC 27710, USA
James W. Bodfish, Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA; Center for Development and Learning, University of North Carolina at Chapel Hill, CB # 7255, Chapel Hill, NC 27599-7255, USA
References
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoache JS, Simcock G, Macari S. Planes, trains, automobiles—and tea sets: extremely intense interests in very young children. Developmental Psychology. 2007;43:1579–1586. doi: 10.1037/0012-1649.43.6.1579. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Cortical specialization for higher cognitive functions: beyond the maturational model. Brain and Cognition. 2000;42:124–127. doi: 10.1006/brcg.1999.1180. [DOI] [PubMed] [Google Scholar]
- Lam KS, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behaviors in autism that differ in familiarity and association with other symptoms. Journal of Child Psychology and Psychiatry. 2008;49:1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interactions distinguish a subgroup of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Morgan L, Wetherby AM, Barber A. Repetitive and stereotyped movements in children with autism spectrum disorders late in the second year of life. Journal of Child Psychology and Psychiatry. 2008;49:826–837. doi: 10.1111/j.1469-7610.2008.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12:457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ. The development of face processing in Autism. Journal of Autism and Developmental Disorders. 2006;36(3):381–394. doi: 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KSL, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35:145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Watt N, Wetherby AM, Barber A, Morgan L. Repetitive and stereotyped behaviors in children with autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders. 2008;38:1518–1533. doi: 10.1007/s10803-007-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]