Abstract
Multiple myeloma (MM) is an incurable neoplasm caused by proliferation of malignant plasma cells in the bone marrow (BM). MM is characterized frequently by a complete or partial deletion of chromosome 13q14, seen in more than 50% of patients at diagnosis. Within this deleted region the tripartite motif containing 13 (TRIM13, also termed RFP2) gene product has been proposed to be a tumour suppressor gene (TSG). Here, we show that low expression levels of TRIM13 in MM are associated with chromosome 13q deletion and poor clinical outcome. We present a functional analysis of TRIM13 using a loss-of-function approach, and demonstrate that TRIM13 downregulation decreases tumour cell survival as well as cell cycle progression and proliferation of MM cells. In addition, we provide evidence for the involvement of TRIM13 downregulation in inhibiting the NF kappa B pathway and the activity of the 20S proteasome. Although this data does not support a role of TRIM13 as a TSG, it substantiates important roles of TRIM13 in MM tumour survival and proliferation, underscoring its potential role as a novel target for therapeutic intervention.
Keywords: TRIM13 (RFP2), 13q, Myeloma, Proliferation, Proteasome
Introduction
Multiple myeloma (MM) is a common haematological malignancy of mature B cells. It is typically preceded by Monoclonal Gammopathy of Undetermined Significance (MGUS), which is present in 1–10% of adults over the age of 25 years and may progress to MM at a rate of 0.5–3% annually (Kyle and Rajkumar 2004, Mitsiades, et al 2004). MM remains incurable, despite recent advances in treatment, highlighting the need to understand the molecular and genetic events of MM pathogenesis in order to develop novel targeted therapies.
MM is characterized by multiple chromosomal aberrations (Carrasco, et al 2006, Fonseca, et al 2004). One of the most common genetic changes is deletion of chromosome 13, especially band 13q14, present in more than 50% of patients at diagnosis, and also in some of MGUS patients. Interestingly, this region overlaps with a minimal common region (MCR) of deletion identified in chronic lymphocytic leukaemia (CLL), mantle cell lymphoma, Waldenstrom Macroglobulinaemia and other B-cell lymphoid malignancies (Carrasco, et al 2006, Fonseca, et al 2004, Kapanadze, et al 2000, Kohlhammer, et al 2004, Schop, et al 2002). Lost in MGUS and early MM, this locus is speculated to harbour tumour suppressor genes (TSGs). Using high-resolution analysis of recurrent DNA losses and gene expression profiling (GEP) (Carrasco, et al 2006, Elnenaei, et al 2003), we and others have identified a consistently deleted 10 MB MCR located at chromosome band 13q14 that, when lost in MM patients, confers high prognostic risk. It has been argued that this risk is more pronounced when seen by conventional cytogenetics and not by fluorescence in situ hybridization (FISH), and is commonly accompanied by the t(4:14) translocation, which, by itself, confers the worse outcome of these patients (Herve, et al 2011). In addition, the 13q14 deletion is associated with downregulation of resident genes such as RB1, TRIM13 (also termed RFP2, DLEU5), DLEU2 and MIR15A and MIR16-1. Although RB1 has been implicated in retinoblastoma, it is not a likely candidate in MM because mutations, biallelic deletions or inactivations of RB1 are very rare (Tonon 2007). In contrast, TRIM13has been proposed to be a TSG (Lerner, et al 2007, Mertens, et al 2002, Tonon 2007, van Everdink, et al 2003) for several reasons: i) by GEP studies, it is the only gene residing on chromosome 13q that is consistently downregulated and is associated with poor clinical outcome (Shaughnessy, et al 2007); ii) it is centred at the most commonly lost region within the MCR on chromosome 13q14, adjoining the MIR15A/16-1 cluster and DLEU2 (Carrasco, et al 2006); and iii) it shares homology to critical TSGs belonging to the large RING–B-box–coiled-coil (RBCC) protein family involved in the ubiquitination of various protein targets implicated in the regulation of cell cycle, transcription, apoptosis and DNA repair (Lerner, et al 2007, van Everdink, et al 2003). Although TRIM13 and associated proteins are found in the endoplasmic reticulum (ER) of cells, its downstream target/s remains to be identified.
The lack of information about the status and function of the genes downregulated as a consequence of 13q14 deletion in MM, coupled with the adverse associated clinical outcome, have provided the framework for our study of the functional role of TRIM13 in MM. Here, we demonstrate that TRIM13 downregulation, in contrast to its presumed function as a TSG, decreases MM cell survival and proliferation. We provide evidence that TRIM13 downregulation enhances nuclear levels of I-Kappa B alpha (IκBα), thereby inhibiting nuclear factor kappa B (NFκB) pathway activation, as well as inhibiting the activity of the 20S proteasome. These data indicate that TRIM13 has a central role in promoting MM tumour survival and proliferation, suggesting its potential as a novel therapeutic target in MM.
Materials and Methods
Patient samples and cell lines
MM patient and normal samples were obtained under the auspices of a Dana-Farber Cancer Institute Institutional Review Board-approved protocol. Survival data on MM patients were determined according to the Institutional Review Board of the University of Arkansas as previously described (Shaughnessy, et al 2007). Cultured MM cell lines were collected from different sources (Dutta-Simmons, et al 2009, Mani, et al 2009, Sukhdeo, et al 2007) and maintained as previously described in RPMI media supplemented with 10% heat inactivated fetal bovine serum.
Immunofluorescence (IF) and Immunoblot (IB) analysis
Cytospin samples of cultured MM lines were prepared as previously described (Dutta-Simmons, et al 2009, Mani, et al 2009, Sukhdeo, et al 2007) Images were obtained with a BioRad Radiance 2000 laser scanning phase contrast microscope. IB analysis was done as previously described (Hideshima, et al 2005, Sukhdeo, et al 2007). Primary antibodies are listed in the supplementary materials.
Gene expression profiling (GEP), fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), and bone marrow tissue micro arrays (BM-TMA) analysis
The array-comparative genomic hybridization (array-CGH) data for chromosome 13 of MM primary tumours as well as cell lines is available at (http://genomic.dfci.harvard.edu/array_cgh_db.htm) and (http://www.sanger.ac.uk/Teams/Team70/supplemental-data/) respectively. The data on TRIM13 expression profiling using Affymetrix GeneChip, of MM primary tumours, cell lines, and normal plasma cells was obtained from the National Center for Biotechnology Information Gene Expression Omnibus (GEO; http/www.ncbi.nlm.gov/geo). Gene expression levels were assessed as previously described (Shaughnessy, et al 2007). FISH analyses were performed as in prior studies (Protopopov, et al 1996). The RP11-240f24 BAC probe was used to detect TRIM13. TMAs were constructed manually using paraffin-embedded blocks from MM patient BM biopsies.
Lentiviral infections of MM cells
Lentiviral particles were generated as described in the supplementary methods and transduced, as previously described (Dutta-Simmons, et al 2009, Mani, et al 2009, Willis, et al 1998). Green fluorescent protein (GFP) positive cells were sorted by Flow cytometry 4–12 days after the last infection and used for the in vitro growth assays.
Cell proliferation, cell cycle, apoptosis, viability and reporter assays
DNA synthesis was measured by incorporation of [H]3-thymidine (Amersham, GE Healthcare, Little Chalfont, Buckinghamshire, UK), Soft agar colony assays and cell cycle analyses were performed as described previously (Dutta-Simmons, et al 2009, Mani, et al 2009, Sukhdeo, et al 2007). Cell counting by Trypan blue exclusion was done using equal numbers of control or shRNA cells plated for up to 15 days and counted in triplicate. Apoptosis was measured by annexin V staining and 7-aminoactinomycin D exclusion (BD Pharmingen, San Jose, CA, USA) using flow cytometric analysis. Data represents the average of at least three different experiments. Viability assays were performed by plating 10,000 cells/well in 96-well plates, and assessed with Cell-Titer-Glo (Promega, Madison, WI, USA), as previously described (Nelson, et al 2008). NFκB activity was measured with the NFkB reporter (Stratagene, Santa Clara, CA, USA) and PGL3 control assays using Dual Luciferase Reporter System (Promega), as previously described (Dutta-Simmons, et al 2009, Mani, et al 2009, Sukhdeo, et al 2007). Results were normalized to the control reporter and renilla measurements. Experiments were done in triplicate and repeated at least three times.
Proteasome inhibition assays
Proteasome activity was assessed in protein cell extracts using fluorogenic peptides according to the manufacturer's instructions (Chemicon, Billerica, MA, USA). Reactions were started by adding an aliquot of cellular extract, and the fluorescence of released 7-amino-4-methylcoumarin (AMC; excitation, 380 nm; emission, 460 nm) was monitored with a spectrofluorometer after 1 h at 37°C. Assays were standardized to free AMC curves, and the reactions were calibrated to the substrate curves.
Results
Expression of TRIM13 in MM
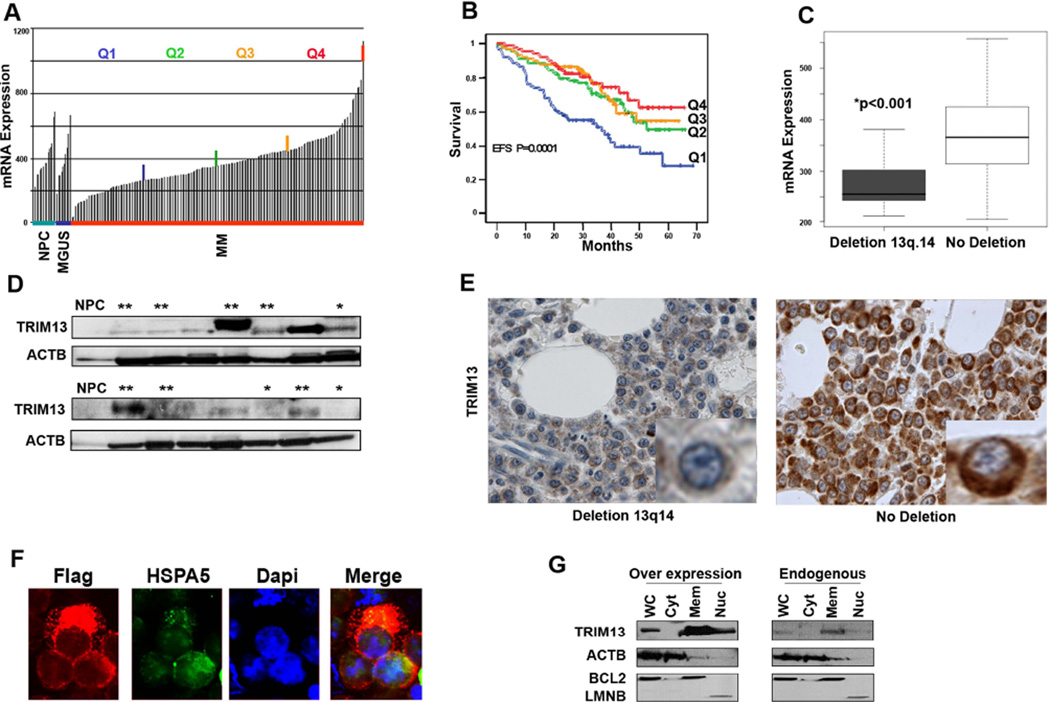
To gain insights into the clinical significance of the TRIM13 gene in MM pathogenesis, we first utilized reported data (Shaughnessy, et al 2007) and analyzed its expression levels in normal plasma cells and in 351 patients with MGUS or MM (adapted to the expression data regarding TRIM13), as well as its association with prognosis (Figures 1A and B). Lower levels of TRIM13 mRNA in MM cells were associated with a significantly worse prognosis. In addition, TRIM13 mRNA levels correlated with chromosome 13q14 deletion status (Figure 1C) in an independent cohort (Carrasco, et al 2006) of untreated MM patients for whom aCGH data was available (n=64). Evaluation of TRIM13 protein expression levels by IB (Figure 1D) and IHC (Figure 1E) revealed a correlation between TRIM13 protein levels and status of chromosome 13. In addition, IHC analysis revealed TRIM13 protein in a perinuclear pattern, suggesting that TRIM13 is localized to the ER in MM cells, and was further substantiated in MMS1 cells using IF (Figure 1F) and IB (Figure 1G) analysis. Overall, these results indicate that lower expression of TRIM13 in MM is associated with chromosome 13q14 deletion and decreased patient survival.
Figure 1. Reduced levels of TRIM13 gene expression in MM are correlated with chromosome 13q deletion and poor clinical outcome.
(A) TRIM13 mRNA expression levels in normal bone marrow (BM) plasma cells (NPC; n=22), monoclonal gammopathy of undetermined significance (MGUS; n=14), and multiple myeloma (MM) primary tumours as divided by quartiles (n=351). (B) Lowest levels (Q1) of TRIM13 expression impacts patient event-free survival (EFS). (C) Low TRIM13 mRNA expression levels correlate with the presence of chromosome 13q.14 deletion in MM patient samples (p<0.001). (D) Immunoblot analysis showing generally lower levels of TRIM13 protein expression in MM patient samples with chromosome 13q deletion (*) than without deletion (**). For samples with no asterisk the karyotype is unknown. Normal BM CD138 purified plasma cells (NPC). (E) Immunohistochemical analysis of decreased TRIM13 protein expression in a MM patient with chromosome 13q.14 deletion on BM- tissue microarrays. Two representative cases are shown. Note the cytoplasmic perinuclear localization of TRIM13 (insert, brown colour). (F) Immunofluorescence analysis, with anti FLAG antibodies in dsRED, showing the localization of FLAG-tagged TRIM13 to the ER in MMS1 cells. HSPA5 (Bip) protein stained with fluorescein isothiocyanate conjugated secondary antibodies is used as ER marker and 4',6-diamidino-2-phenylindole (Dapi) for nuclear staining. (G) Immunoblot analysis showing that endogenous and overexpressed TRIM13 is localized to the ER in MMS1 cells. Controls used are Actin (ACTB) for Whole cell extract (WC); Cytoplasmic (Cyt); BCL2 for Membranous (Mem); and Lamin B (LMNB) for Nuclear (Nuc).
Development of an in vitro system to functionally characterize TRIM13 in MM
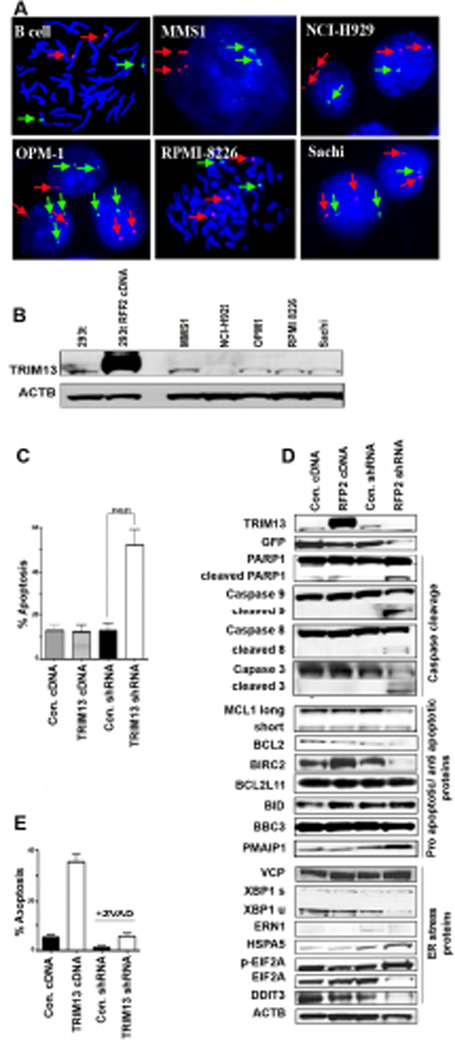
As patient MM cells are not amenable to functional studies, we next sought to identify which MM cell lines best mimic patient samples, with regard to the status of chromosome 13q14. FISH analyses for the TRIM13 gene was performed in a subset of MM cell lines (Figure 2A and data not shown). With the exception of MMS1, which had a bi-allelic homogenous population of cells, most cell lines had mono-allelic 13q14 deletion either in all cells or in a mixed population. In agreement with this observation, the highest levels of TRIM13 protein expression were documented in MMS1 cells (Figure 2B). Subsequently, we chose this cell line for in vitro assays to investigate the biological role of TRIM13.
Figure 2. TRIM13 downregulation induces apoptosis of MM cells through intrinsic and extrinsic pathways.
(A) FISH analysis showing chromosome 13q.14 status in various MM cell lines using a BAC probe for TRIM13-FITC (green), CEP3-Spectrum Orange (red). Some cell lines have a homogeneous population of cells having both (MMS1) or only one allele preserved (NCI-H929), whereas others have a mixed population of cells (OPM1, RPMI-8226, Sachi). (B) Immunoblot analysis of TRIM13 protein expression in MM cell lines. HEK 293T lentiviral transduced cells overexpressing TRIM13 protein showed as a positive control. (C) TRIM13 shRNA knockdown induces marked increase in apoptosis of MMS1 cells at day 6 post-transduction. A representative case of four independent assays for Annexin V and 7AAD staining is shown. (D) Immunoblot analysis showing that TRIM13 downregulation induces: i) caspase cleavage in both intrinsic and extrinsic apoptotic pathways (Different exposures of the full length and cleaved caspases IB are shown); ii) prominent upregulation of the pro-apoptotic protein PMAIP1 (Noxa) and downregulation of anti-apoptotic proteins (MCL1, BCl2, BIRC2 [cIAP1]) and no change in other proteins involved in apoptosis (BCL2L11 (Bim), BID, BBC3 (Puma); and iii) ER stress-induced apoptosis, as evidenced by upregulation of ER markers HSPA5 (Bip) and phosphorylated eIF2α (p-eIF2α), and other ER markers as DDIT3 (CHOP), and ERN1 (IRE1). (E) TRIM13 downregulation-induced apoptosis is abrogated when 20 µM pan-caspase inhibitor ZVAD-FMK is added daily on days 3–5 post-transduction. Apoptosis was measured in two separate experiments on day 6.
We first carried out gain and loss-of-function approaches by stably overexpressing FLAG tagged-TRIM13 (TRIM13 cDNA) or knocking down TRIM13 expression (TRIM13 shRNA), respectively. From a panel of seven TRIM13 shRNAs, two showed significant TRIM13 knockdown, designated shRNA#2 and #5 (Supplemental Figure 1A); the first was more effective and was therefore used for further studies. At day 6 post-transduction, almost 100% of cells were GFP-positive, as assessed by flow cytometry (Supplemental Figure 1B). Efficient overexpression and knockdown of TRIM13 in MM cell lines was verified by IB analysis (Supplemental Figure 1C).
TRIM13 downregulation causes profound apoptosis in MM cells
To determine the functional role of TRIM13, MMS1 cells were transduced using the above described lentiviral system. Cells with TRIM13 knockdown showed significantly increased apoptosis (60%) compared to control shRNA cells, as measured by flow cytometric annexin V staining (Figure 2C). The pro-apoptotic effect resulting from TRIM13 knockdown was confirmed in several MM cell lines (Supplemental Figure 2A). Given the proposed function of TRIM13 as a TSG, this finding was surprising and we therefore further validated knockdown-related apoptosis using another shRNA hairpin lentivirus (shRNA #5). This alternative construct yielded a similar pro-apoptotic phenotype (Supplemental Figure 2B).
In addition, IB analysis for caspase 8, 9 and 3 as well as PARP1 cleavage showed activation of both the intrinsic and the extrinsic apoptotic pathways upon TRIM13 down regulation (Figure 2D, top). Antiapoptotic proteins, such as BCL2 and MCL1, were down regulated, further supporting activation of apoptotic signalling. We demonstrated that the process is associated with activation of the pro-apoptotic protein PMAIP1 (Noxa), with little effect on other pro-apoptotic proteins (Figure 2D, middle). Given that TRIM13 is an ER-related protein in MM, we also investigated the impact on ER stress proteins and markers. As expected, TRIM13 downregulation in MMS1 cells significantly increased the levels of HSPA5 (Bip) and phosphorylated eIF2α (p-eIF2α), and a modest effect on XBP1 splicing (Figure 2D, bottom). Nevertheless, DDIT3 (CHOP) was still down regulated. Although TRIM13 has previously been linked to valosin-containing protein (VCP)(Lerner, et al 2007), another ER-related protein involved in apoptosis, we observed no change in VCP levels after transduction (Figure 2D, bottom). TRIM13 downregulation also significantly enhanced apoptosis induced by the ER stressor tunicamycin in HEK293 cells (Supplemental Figure 2C).
To further confirm that cell death is activated through apoptotic pathways, MMS1 cells were transduced with the shTRIM13 virus, and then grown in the presence or absence of ZVAD-FMK; this pan-caspase inhibitor abrogated the pro-apoptotic effects of TRIM13 downregulation (Figure 2E). Taken together, these results show that TRIM13 downregulation in various MM cell lines and model systems causes profound apoptosis, highlighting its role in promoting MM cell survival.
TRIM13 downregulation is associated with growth arrest in MM cells
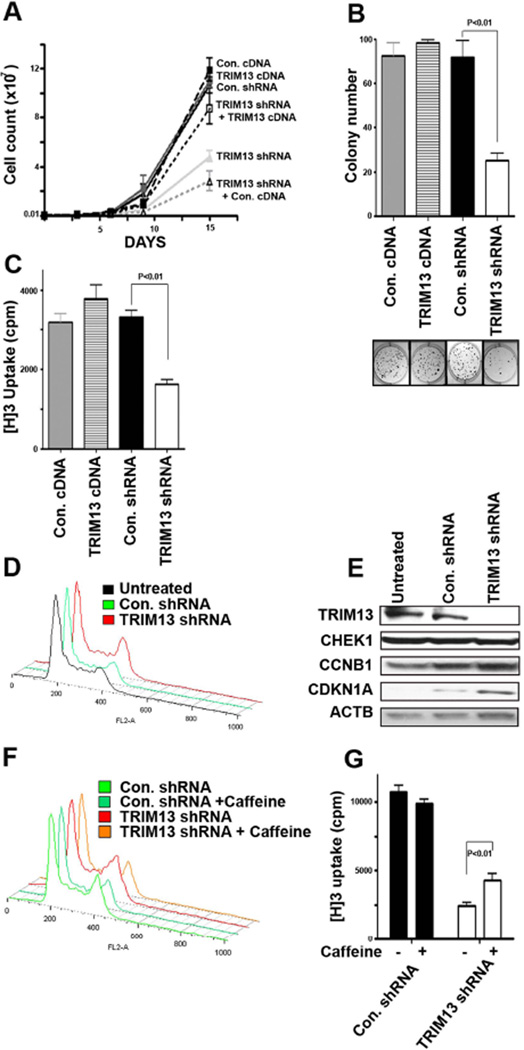
Sorted stably transduced cells were cultured over time (Figure 3A). As expected from its pro-apoptotic effect, TRIM13 downregulation was associated with a significant reduction in the number of MM cells. As a control for possible off-target RNAi effects, we showed that reconstitution of TRIM13 expression in knockdown cells partially rescued its growth inhibition.
Figure 3. TRIM13 downregulation inhibits MM cell growth and proliferation through a G2/M cell cycle arrest.
(A) Decreased growth of MMS1 cells with TRIM13 downregulation (TRIM13-shRNA) as compared with control cells (Con-shRNA) and transduction of TRIM13 to the down regulated cells (TRIM13-shRNA+TRIM13-cDNA) partially abrogates this effect (B) Soft agar colony assay showing decreased colony size and numbers in MMS1 cells with TRIM13 downregulation. (C) TRIM13 downregulation directly inhibits proliferation of MMS1 cells, as measured by thymidine H3 incorporation. (D) A representative flow cytometric analysis by propidium iodide staining showing G2/M cell cycle arrest in the MMS1 TRIM13 downregulated cells. (E) Immunoblot analysis showing prominent upregulation of the proteins CDKN1A (P21) and CCNB1 (cyclin B1) in TRIM13 down regulated cells. (F) Caffeine was able to abrogate G2/M cell cycle arrest in TRIM13 down regulated cells. (G) Caffeine was able to overcome the functional growth arrest in TRIM13 down regulated cells, as measured by Thymidine incorporation.
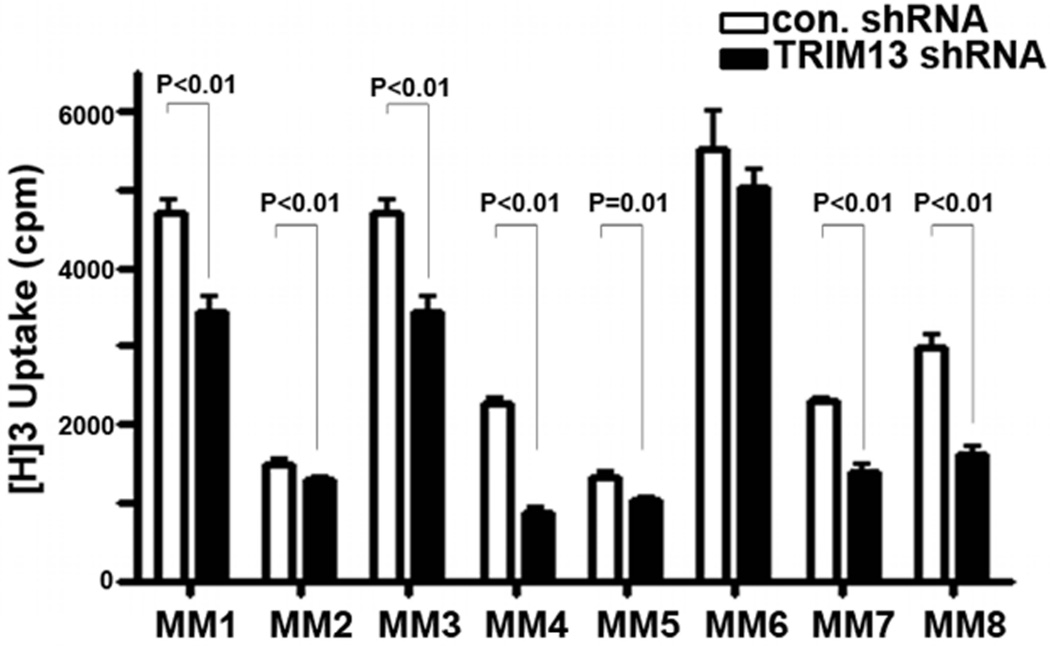
The reduced cell numbers following TRIM13 downregulation could be a result of decreased cell cycle progression, in addition to the observed increased apoptosis. We therefore next investigated the impact of TRIM13 levels on colony-forming activity (Figure 3B). Thymidine incorporation assays further showed that TRIM13 downregulation decreases proliferation (Figure 3C). The growth inhibition was mediated through a G2/M cell cycle arrest (Figure 3D), as assessed by flow cytometry and was associated with induction of CDKN1A (P21) and cyclin B1 protein expression (Figure 3E). These results were replicated using another shRNA to knockdown expression of TRIM13 (shRNA-TRIM13 #5), and in other two MM cell lines, RPMI-8226 and Sachi (not shown). To directly assess whether growth retardation occurs through accumulation of cells in the G2/M phase, cells were grown in the presence of caffeine as a G2/M promoting agent. As shown in Figure 3F, TRIM13 downregulation-mediated G2 arrest was abrogated in the presence of caffeine, as were its antiproliferative effects (Figure 3G). Finally, to confirm the relevance of our findings to MM, we performed proliferation assays using primary MM cells (Figure 4), showing similar effect. Overall, these results show that TRIM13 downregulation not only alters cell survival but also cell growth and proliferation, and highlight the potential of TRIM13 as a novel therapeutic target in MM.
Figure 4. TRIM13 downregulation inhibits primary MM cell growth.
Decreased growth of CD138+ selected primary MM cells with TRIM13 downregulation (TRIM13-shRNA) compared with control cells (Con. shRNA), as measured by thymidine H3 incorporation. Each experiment was performed in quadruplicate.
TRIM13 downregulation inhibits 20S proteasome activity in MM cells
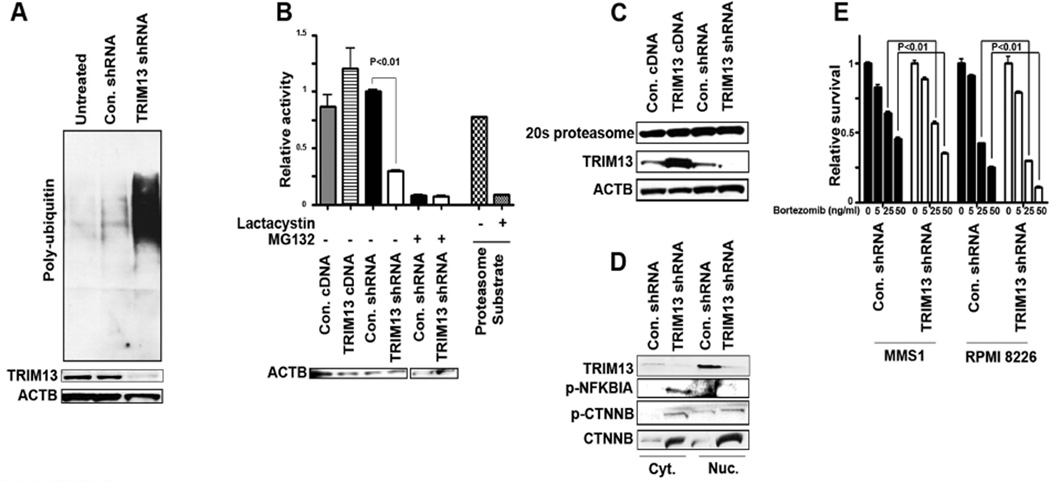
The above-described presence of ER stress, combined with the previous reports that TRIM13 has E3 ubiquitin ligase activity (Lerner, et al 2007, van Everdink, et al 2003), prompted us to assess total protein ubiquitination. Concordant with its effects on ER stress, TRIM13 downregulation was associated with significantly higher levels of poly-ubiquitinated proteins (Figure 5A, Supplemental Figure 3C). This feature, also observed with proteasome inhibitors, (Bianchi, et al 2009) lead us to hypothesize that manipulating TRIM13 levels might influence the activity of the 20S proteasome in general. Indeed, we were able to document a significant (p<0.01) reduction (60% inhibition) in 20S proteasome activity in TRIM13 down regulated cells (Figure 5B). IB analysis demonstrated that this inhibitory effect was not due to changes in protein levels of the 20S alpha subunit (Figure 5C). In addition, increased phosphorylated-NFKBIA (IκBα) levels were found in the cytoplasmic portion of the TRIM13 down regulated cells (Figure 5D) as well as an accumulation of total and phosphorylated-XTNNB (β-catenin), another protein dependent on the proteasome for degradation. Proteasome inhibition by TRIM13 down regulation was confirmed in other MM cell lines and was partially abrogated by restoring TRIM13 levels by overexpression (Supplemental Figure 3A and B). Importantly, TRIM13 down regulated cells were more sensitive to Bortezomib (Figure 5E); indeed proteasome inhibition was synergistic with TRIM13 downregulation in MM cells (Supplemental Figure 3D)
Figure 5. TRIM13 downregulation causes inhibition of proteasome 20S activity.
(A) Immunoblot showing TRIM13 downregulation is associated with accumulation of poly-ubiquinated proteins. (B) 20S proteasome activity was measured using 5 µg of MMS1 cellular protein extracts. Data are mean of three representative independent experiments, each performed in duplicate. For 20S proteasome activity, control substrate (10 µg) was incubated with or without lactacystin inhibition (25 µM) for 15 min. As another control, shRNA or control-shRNA MMS1 cells were incubated for 24 h with the pan-proteasome inhibitor MG132 (10 µM). Immunoblot with anti-actin (ACTB) antibody served as loading control. (C) Immunoblot of treated cells for levels of proteasome 20S alpha subunit shows no significant changes in protein levels. (D) Immunoblot in MMS1 TRIM13 downregulated cells shows accumulation of phosphorylated (p-)NFKBIA (IKBα) and p-CTNNB (β-catenin), as well as upregulation of CTNNB. (E) A viability assay showing that TRIM13 downregulated cells are more susceptible to treatment with the proteasome inhibitor Bortezomib. The 4 bars show 4 increasing concentrations (0, 5, 25 and 50 ng/ml). Experiments were done in triplicate and repeated three times.
TRIM13 downregulation inhibits the NFκB pathway by causing nuclear retention of IκBα in MM cells
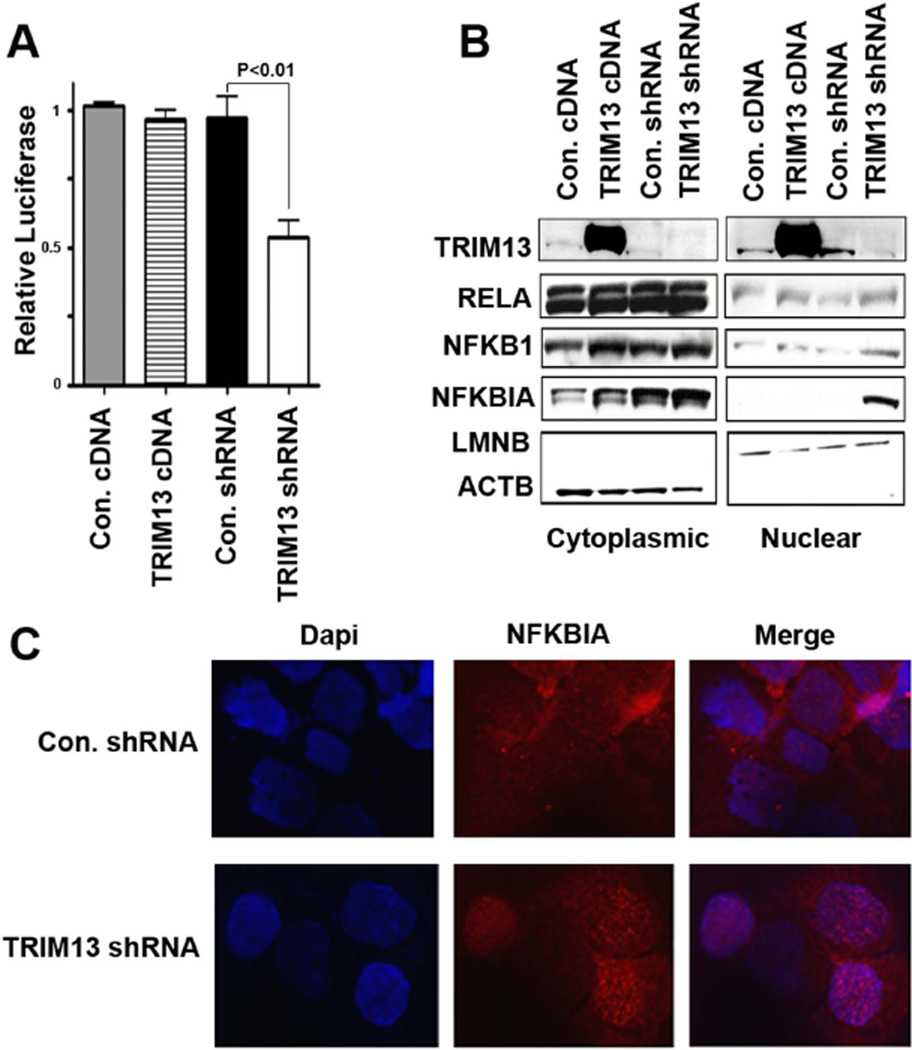
Inhibition of the NFκB pathway is a hallmark of proteasome-related growth retardation and apoptosis (Hideshima, et al 2005, Mitsiades, et al 2004). Furthermore, previously reported in a high throughput luciferase screening, TRIM13 was found to be an upstream activator of NFκB (Matsuda, et al 2003). We therefore performed NFκB luciferase reporter assays to confirm these results in MM cells. A significant (p<0.01) reduction was associated with TRIM13 downregulation (Figure 6A). To define the mechanism of this process, we examined the level of NFκB related proteins in nuclear and cytoplasmic fractions (Figure 6B). Interestingly, the most prominent effect observed in TRIM13 down regulated cells was increased levels of IκBα in the nucleus. This finding was corroborated with IF studies using a different primary antibody against IκBα (Figure 6C). In addition, upon re-introduction of TRIM13 by overexpression, nuclear IκBα localization was diminished (Supplemental Figure 4A), suggesting that this effect is specifically due to altered TRIM13 expression. This effect was also observed in other two MM cell lines, RPMI-8226 and MM1S.
Figure 6. TRIM13 downregulation is associated with inhibition of NFκB pathway activity.
(A) NFkB activity measured by Luciferase expression was decreased in cells with TRIM13 downregulation. (B) Immunoblot analysis showing that TRIM13 downregulation is associated with accumulation of nuclear NFKBIA (IκBα) in MMS1 cells. A modest accumulation of RELA (P50) and NFKB1 (P65) subunits of NFκB was also noted. (C) Immunofluorescence analysis shows nuclear localization of NFKBIA in TRIM13 down regulated cells.
Altogether, these results support our theory that the effects of TRIM13 downregulation are mediated through an inhibition of the NFκB pathway that is associated with increased nuclear IκBα as well as a decrease in 20S proteasome activity.
Discussion
Deletion of chromosome 13q14 is one of the most frequent copy number alterations, seen in MM and other lymphoproliferative diseases (Carrasco, et al 2006, Elnenaei, et al 2003, Fonseca, et al 2004, Kapanadze, et al 2000, Kohlhammer, et al 2004, Kyle and Rajkumar 2004, Mitsiades, et al 2004, Schop, et al 2002). Within this chromosomal band, a MCR of deletion has been identified which harbours the TSG candidate TRIM13 (Carrasco, et al 2006, Elnenaei, et al 2003). While TRIM13 has been described as an ER E3 ligase (Lerner, et al 2007), its function is not clear. Here, using a loss-of function approach, we have investigated the functional role of TRIM13 in MM.
Expression of TRIM13 in MM
Our studies indicate that the TRIM13 gene is a target of chromosome 13 deletion in MM. Using GEP analysis, we were able to show that lowest levels of TRIM13 mRNA expression are correlated with chromosome 13q14 deletion and worse MM patient survival. It is difficult to draw a firm conclusion when correlating protein levels and FISH data in MM patient samples. Although we saw a trend for lower TRIM13 protein expression in patient samples with chromosome 13 deletion, there were cases that did not follow this pattern. This can be explained by the existence of post-transcriptional mechanisms controlling TRIM13 protein levels including ubiquitination.
TRIM13 function in MM cells
Using a lentiviral-based system to stably knock down TRIM13 expression, we were able to show a prominent pro-apoptotic effect associated with caspase activation and an ER stress response, consistent with a previous report that TRIM13 is localized to the ER (Lerner, et al 2007). MM cells produce and secrete large amounts of immunoglobulins and are therefore under constant ER stress, (Davenport, et al 2007, Nakamura, et al 2006). Upon TRIM13 downregulation, we speculate that misfolded protein accumulation further increases the already high ER load in MM cells. This effect has previously been described in ER stressed cells (Shen, et al 2007), and is consistent with our observation of elevated levels of poly-ubiquitinated proteins and inhibition of 20S chymotryptic proteasome subunit activity in TRIM13 down regulated cells.
We were able to show a significant reduction in NFκB activity upon TRIM13 knockdown, as has been previously speculated in HEK 293t cells (Matsuda, et al 2003). This effect was associated with accumulation of IκBα in the nucleus, and is consistent with reports in other cellular systems (Aguilera, et al 2006, Castro-Alcaraz, et al 2002, Huang and Miyamoto 2001, Sorriento, et al 2008, Vu, et al 2008), a phenomenon associated with increased apoptosis and inhibition of NFκB activity. Taken together, these data suggest that NFκB inactivation involves nuclear retention of RELA (p50) and NFKB1 (p65) NFκB subunits by IκBα, thereby inhibiting their export to the cytoplasm, which is required for reactivation of the pathway (Huang and Miyamoto 2001), via 14-3-3 family of proteins and G-protein-coupled receptor Kinase 5 (GRK5). Although we examined 14-3-3 and GRP5 levels, as well as performing immunoprecipitated and probing for a direct interaction between TRIM13 and these proteins or IκBα (not shown), we found no such interaction. Based on our results, one speculative possibility is that the proteasome inhibitory effect of TRIM13 downregulation is mediated through qualitative changes in the 20S subunit. Last, we also demonstrated upregulation of Noxa, a pro-apoptotic protein induced by proteasome inhibitors (Rizzatti, et al 2008) and during ER stress-related apoptosis (Wang, et al 2009), further linking these findings together. Recently, over-expression of TRIM13 combined with ionizing radiation was shown to enhance apoptosis via the degradation of AKT and MDM2 (Joo, et al 2011). Although we did not combine TRIM13 over-expression with other modalities causing apoptosis, nor observed these findings, it should be noted that our system utilizes stable and long term expression, and our main focus for investigation was by loss of function analysis. We did not observe growth promoting effects by over-expressing TRIM13, suggesting that the basal levels of TRIM13 protein are sufficient for cellular maintenance. Importantly, our results showing decreased survival and proliferation of MM cells secondary to TRIM13 downregulation confirm that MM cells depend on their basal TRIM13 levels and are destined to apoptosis when these levels are further down regulated.
Linkage of the in vitro results with clinical data
Our loss-of-function studies showing inhibition of MM cell growth with TRIM13 downregulation are not consistent with the observation that low expression levels of TRIM13 are an adverse prognostic marker in MM, or with the hypothesis that TRIM13 is a TSG (Lerner, et al 2007, Mertens, et al 2002, Tonon 2007, van Everdink, et al 2003) and the higher tumour cell proliferation rates correlating with chromosome 13q deletion (Rajkumar, et al 1999, Shaughnessy, et al 2003). This might be attributed to another TSG residing on chromosome 13q14 in close proximity to the TRIM13 gene, such as MIR15A and MIR16-1 (Bonci, et al 2008). The latter are not represented by GEP probes, and the adjacent TRIM13 gene could therefore be regarded as a reporter for the concurrent loss of these miRs when chromosome 13q14 is deleted. On the other hand, it has been shown by several groups (Jagannath, et al 2007, Sagaster, et al 2007) that bortezomib, a proteasome inhibitor, overcomes the bad prognosis associated with chromosome 13q deletion in MM. This effect could be due, at least in part, to decreased proteasome activity in TRIM13-downregulated MM cells, rendering them more sensitive to bortezomib. Moreover, in CLL mice, when the genomic region deleted was enlarged to include a much wider common region, the phenotype was varied revealing a more aggressive phenotype (Lia, et al 2012) This finding implies the importance of the interplay among the different genes present at 13q14 as a cluster and not in a solitary manner (Mertens and Stilgenbauer 2012). In contrast to MM, chromosome 13q deletion in CLL confers a better prognosis compared with other chromosomal aberrations or normal karyotype. Therefore, it is possible that TRIM13 deletions causing slower growth rates may have a more significant impact in CLL, which is less aggressive than MM and employs different apoptotic pathways (Jahrsdorfer, et al 2005, Zent, et al 2003). It should also be noted that 13q deletion is commonly accompanied by other adverse prognostic translocation, such as t (4:14), which are the basis for the poor prognosis, rather than the 13q deletion by itself (Herve, et al 2011). Thus, the fact that TRIM13 acts in opposition to other genes (i.e. MIR15A and MIR16-1 as the presumed tumour suppressors) residing within the same region or another, is intriguing and not a contradiction. It could be additionally argued that, similar to that has been described for IRF4 (Shaffer, et al 2008), though not genetically altered in most myelomas, they are nonetheless addicted to an aberrant IRF4 regulatory network that fuses the gene expression programmes of normal plasma cells and activated B cells.
We have functionally linked TRIM13 activity with biological process essential to MM pathogenesis such as the ER stress response (Davenport, et al 2007, Nakamura, et al 2006), NFkB transcriptional activity (Chng, et al 2007), proteasome function and proteasome inhibitors (Bianchi, et al 2009) which have become an important part of the therapeutic arsenal in MM. Thus our data adds to the complexity of the expanding knowledge of accumulating high throughput genomic data, and underscores the need to validate it functionally. We challenge the dogma that a genomic area of deletion harbours primarily TSGs, and show that there are dual aspects to this concept. Our data not only highlights the above-mentioned complexity, but also provides both the framework for further investigation of the specific mechanisms involved in this process, and the rationale for development of novel therapeutic strategies to target TRIM13 in MM.
Supplementary Material
Acknowledgments
We thank Dr. William Hahn (DFCI, RNAi Consortium (TRC) at the Broad Institute) for lentiviral shRNA vectors. Financial support: M.E.G is supported by the American Physician Foundation for Israel. D.R.C is supported by MMRF, Senior Research Award and Claudia Barr Award; M.L, O.S and D.G are supported by the Swedish Research Council, the Swedish Cancer Society, the Cancer Research Funds of Radiumhemmet and King Gustaf V Jubilee Fund.; K.C.A is supported by NIH grants RO CA127435, PO CA78378.
Footnotes
Conflict of Interest Disclosures: All authors declare no conflict of interest.
Author contributions
M.E.G., K.T., M.M., M.L., M.P., T.H., D.E.C., A.P., E.I., O.S., D.G. performed the research. M.E.G, D.R.C. designed the research study. M.L., O.S., D.G., J.D.C., B.B. contributed essential reagents or tools. M.E.G., D.R.C. analysed the data. M.E.G., M.L., K.C.A., D.R.C. wrote the paper.
References
- Aguilera C, Fernandez-Majada V, Ingles-Esteve J, Rodilla V, Bigas A, Espinosa L. Efficient nuclear export of p65-IkappaBalpha complexes requires 14-3-3 proteins. J Cell Sci. 2006;119:3695–3704. doi: 10.1242/jcs.03086. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Oliva L, Cascio P, Pengo N, Fontana F, Cerruti F, Orsi A, Pasqualetto E, Mezghrani A, Calbi V, Palladini G, Giuliani N, Anderson KC, Sitia R, Cenci S. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113:3040–3049. doi: 10.1182/blood-2008-08-172734. [DOI] [PubMed] [Google Scholar]
- Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C, Bartucci M, Muto G, Peschle C, De Maria R. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, Stewart JP, Zhan F, Khatry D, Protopopova M, Protopopov A, Sukhdeo K, Hanamura I, Stephens O, Barlogie B, Anderson KC, Chin L, Shaughnessy JD, Jr, Brennan C, Depinho RA. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9:313–325. doi: 10.1016/j.ccr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Castro-Alcaraz S, Miskolci V, Kalasapudi B, Davidson D, Vancurova I. NF-kappa B regulation in human neutrophils by nuclear I kappa B alpha: correlation to apoptosis. J Immunol. 2002;169:3947–3953. doi: 10.4049/jimmunol.169.7.3947. [DOI] [PubMed] [Google Scholar]
- Chng WJ, Glebov O, Bergsagel PL, Kuehl WM. Genetic events in the pathogenesis of multiple myeloma. Best Pract Res Clin Haematol. 2007;20:571–596. doi: 10.1016/j.beha.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, Davies FE. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
- Dutta-Simmons J, Zhang Y, Gorgun G, Gatt M, Mani M, Hideshima T, Takada K, Carlson NE, Carrasco DE, Tai YT, Raje N, Letai AG, Anderson KC, Carrasco DR. Aurora kinase A is a target of Wnt/beta-catenin involved in multiple myeloma disease progression. Blood. 2009;114:2699–2708. doi: 10.1182/blood-2008-12-194290. [DOI] [PubMed] [Google Scholar]
- Elnenaei MO, Hamoudi RA, Swansbury J, Gruszka-Westwood AM, Brito-Babapulle V, Matutes E, Catovsky D. Delineation of the minimal region of loss at 13q14 in multiple myeloma. Genes Chromosomes Cancer. 2003;36:99–106. doi: 10.1002/gcc.10140. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, Davies FE, Drach J, Greipp PR, Kirsch IR, Kuehl WM, Hernandez JM, Minvielle S, Pilarski LM, Shaughnessy JD, Jr, Stewart AK, Avet-Loiseau H. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- Herve AL, Florence M, Philippe M, Michel A, Thierry F, Kenneth A, Jean-Luc H, Nikhil M, Stephane M. Molecular heterogeneity of multiple myeloma: pathogenesis, prognosis, and therapeutic implications. J Clin Oncol. 2011;29:1893–1897. doi: 10.1200/JCO.2010.32.8435. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Bradner JE, Wong J, Chauhan D, Richardson P, Schreiber SL, Anderson KC. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Miyamoto S. Postrepression activation of NF-kappaB requires the amino-terminal nuclear export signal specific to IkappaBalpha. Mol Cell Biol. 2001;21:4737–4747. doi: 10.1128/MCB.21.14.4737-4747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath S, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Cowan JM, Anderson KC. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21:151–157. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- Jahrsdorfer B, Wooldridge JE, Blackwell SE, Taylor CM, Link BK, Weiner GJ. Good prognosis cytogenetics in B-cell chronic lymphocytic leukemia is associated in vitro with low susceptibility to apoptosis and enhanced immunogenicity. Leukemia. 2005;19:759–766. doi: 10.1038/sj.leu.2403694. [DOI] [PubMed] [Google Scholar]
- Joo HM, Kim JY, Jeong JB, Seong KM, Nam SY, Yang KH, Kim CS, Kim HS, Jeong M, An S, Jin YW. Ret finger protein 2 enhances ionizing radiation-induced apoptosis via degradation of AKT and MDM2. Eur J Cell Biol. 2011;90:420–431. doi: 10.1016/j.ejcb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Kapanadze B, Makeeva N, Corcoran M, Jareborg N, Hammarsund M, Baranova A, Zabarovsky E, Vorontsova O, Merup M, Gahrton G, Jansson M, Yankovsky N, Einhorn S, Oscier D, Grander D, Sangfelt O. Comparative sequence analysis of a region on human chromosome 13q14, frequently deleted in B-cell chronic lymphocytic leukemia, and its homologous region on mouse chromosome 14. Genomics. 2000;70:327–334. doi: 10.1006/geno.2000.6386. [DOI] [PubMed] [Google Scholar]
- Kohlhammer H, Schwaenen C, Wessendorf S, Holzmann K, Kestler HA, Kienle D, Barth TF, Moller P, Ott G, Kalla J, Radlwimmer B, Pscherer A, Stilgenbauer S, Dohner H, Lichter P, Bentz M. Genomic DNA-chip hybridization in t(11;14)-positive mantle cell lymphomas shows a high frequency of aberrations and allows a refined characterization of consensus regions. Blood. 2004;104:795–801. doi: 10.1182/blood-2003-12-4175. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- Lerner M, Corcoran M, Cepeda D, Nielsen ML, Zubarev R, Ponten F, Uhlen M, Hober S, Grander D, Sangfelt O. The RBCC gene TRIM13 (Leu5) encodes a novel transmembrane E3 ubiquitin ligase involved in ERAD. Mol Biol Cell. 2007;18:1670–1682. doi: 10.1091/mbc.E06-03-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia M, Carette A, Tang H, Shen Q, Mo T, Bhagat G, Dalla-Favera R, Klein U. Functional dissection of the chromosome 13q14 tumor-suppressor locus using transgenic mouse lines. Blood. 2012;119:2981–2990. doi: 10.1182/blood-2011-09-381814. [DOI] [PubMed] [Google Scholar]
- Mani M, Carrasco DE, Zhang Y, Takada K, Gatt ME, Dutta-Simmons J, Ikeda H, Diaz-Griffero F, Pena-Cruz V, Bertagnolli M, Myeroff LL, Markowitz SD, Anderson KC, Carrasco DR. BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res. 2009;69:7577–7586. doi: 10.1158/0008-5472.CAN-09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, Hayashi H, Sugano S. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22:3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- Mertens D, Stilgenbauer S. CLL and deletion 13q14: merely the miRs? Blood. 2012;119:2974–2975. doi: 10.1182/blood-2012-01-400747. [DOI] [PubMed] [Google Scholar]
- Mertens D, Wolf S, Schroeter P, Schaffner C, Dohner H, Stilgenbauer S, Lichter P. Down-regulation of candidate tumor suppressor genes within chromosome band 13q14.3 is independent of the DNA methylation pattern in B-cell chronic lymphocytic leukemia. Blood. 2002;99:4116–4121. doi: 10.1182/blood.v99.11.4116. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades N, Munshi NC, Anderson KC. Focus on multiple myeloma. Cancer Cell. 2004;6:439–444. doi: 10.1016/j.ccr.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Gotoh T, Okuno Y, Tatetsu H, Sonoki T, Uneda S, Mori M, Mitsuya H, Hata H. Activation of the endoplasmic reticulum stress pathway is associated with survival of myeloma cells. Leuk Lymphoma. 2006;47:531–539. doi: 10.1080/10428190500312196. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Walker SR, Kepich A, Gashin LB, Hideshima T, Ikeda H, Chauhan D, Anderson KC, Frank DA. Nifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3. Blood. 2008;112:5095–5102. doi: 10.1182/blood-2007-12-129718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopov AI, Gizatullin RZ, Vorobieva NV, Protopopova MV, Kiss C, Kashuba VI, Klein G, Kisselev LL, Graphodatsky AS, Zabarovsky ER. Human chromosome 3: high-resolution fluorescence in situ hybridization mapping of 40 unique NotI linking clones homologous to genes and cDNAs. Chromosome Res. 1996;4:443–447. doi: 10.1007/BF02265051. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Fonseca R, Dewald GW, Therneau TM, Lacy MQ, Kyle RA, Greipp PR, Gertz MA. Cytogenetic abnormalities correlate with the plasma cell labeling index and extent of bone marrow involvement in myeloma. Cancer Genet Cytogenet. 1999;113:73–77. doi: 10.1016/s0165-4608(99)00009-6. [DOI] [PubMed] [Google Scholar]
- Rizzatti EG, Mora-Jensen H, Weniger MA, Gibellini F, Lee E, Daibata M, Lai R, Wiestner A. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-kappaB pathways. Leuk Lymphoma. 2008;49:798–808. doi: 10.1080/10428190801910912. [DOI] [PubMed] [Google Scholar]
- Sagaster V, Ludwig H, Kaufmann H, Odelga V, Zojer N, Ackermann J, Kuenburg E, Wieser R, Zielinski C, Drach J. Bortezomib in relapsed multiple myeloma: response rates and duration of response are independent of a chromosome 13q-deletion. Leukemia. 2007;21:164–168. doi: 10.1038/sj.leu.2404459. [DOI] [PubMed] [Google Scholar]
- Schop RF, Jalal SM, Van Wier SA, Ahmann GJ, Bailey RJ, Kyle RA, Greipp PR, Rajkumar SV, Gertz MA, Lust JA, Lacy MQ, Dispenzieri A, Witzig TE, Fonseca R. Deletions of 17p13.1 and 13q14 are uncommon in Waldenstrom macroglobulinemia clonal cells and mostly seen at the time of disease progression. Cancer Genet Cytogenet. 2002;132:55–60. doi: 10.1016/s0165-4608(01)00526-x. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W, Powell J, Dave S, Yu X, Zhao H, Zeng Y, Chen B, Epstein J, Staudt LM. IRF4 addiction in multiple myeloma. Nature. 2008;454:226–231. doi: 10.1038/nature07064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy J, Jr, Tian E, Sawyer J, McCoy J, Tricot G, Jacobson J, Anaissie E, Zangari M, Fassas A, Muwalla F, Morris C, Barlogie B. Prognostic impact of cytogenetic and interphase fluorescence in situ hybridization-defined chromosome 13 deletion in multiple myeloma: early results of total therapy II. Br J Haematol. 2003;120:44–52. doi: 10.1046/j.1365-2141.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- Shaughnessy JD, Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, Stewart JP, Kordsmeier B, Randolph C, Williams DR, Xiao Y, Xu H, Epstein J, Anaissie E, Krishna SG, Cottler-Fox M, Hollmig K, Mohiuddin A, Pineda-Roman M, Tricot G, van Rhee F, Sawyer J, Alsayed Y, Walker R, Zangari M, Crowley J, Barlogie B. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- Shen Y, Ballar P, Apostolou A, Doong H, Fang S. ER stress differentially regulates the stabilities of ERAD ubiquitin ligases and their substrates. Biochem Biophys Res Commun. 2007;352:919–924. doi: 10.1016/j.bbrc.2006.11.121. [DOI] [PubMed] [Google Scholar]
- Sorriento D, Ciccarelli M, Santulli G, Campanile A, Altobelli GG, Cimini V, Galasso G, Astone D, Piscione F, Pastore L, Trimarco B, Iaccarino G. The G-protein-coupled receptor kinase 5 inhibits NFkappaB transcriptional activity by inducing nuclear accumulation of IkappaB alpha. Proc Natl Acad Sci U S A. 2008;105:17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, Carrasco DE, Zheng M, He H, Tai YT, Mitsiades C, Anderson KC, Carrasco DR. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon G. Molecular pathogenesis of multiple myeloma. Hematol Oncol Clin North Am. 2007;21:985–1006. vii. doi: 10.1016/j.hoc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- van Everdink WJ, Baranova A, Lummen C, Tyazhelova T, Looman MW, Ivanov D, Verlind E, Pestova A, Faber H, van der Veen AY, Yankovsky N, Vellenga E, Buys CH. TRIM13, c13ORF1, and FAM10A4 are the most likely tumor suppressor gene candidates for B-cell chronic lymphocytic leukemia. Cancer Genet Cytogenet. 2003;146:48–57. doi: 10.1016/s0165-4608(03)00126-2. [DOI] [PubMed] [Google Scholar]
- Vu HY, Juvekar A, Ghosh C, Ramaswami S, Le DH, Vancurova I. Proteasome inhibitors induce apoptosis of prostate cancer cells by inducing nuclear translocation of IkappaBalpha. Arch Biochem Biophys. 2008;475:156–163. doi: 10.1016/j.abb.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci U S A. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis TG, Zalcberg IR, Coignet LJ, Wlodarska I, Stul M, Jadayel DM, Bastard C, Treleaven JG, Catovsky D, Silva ML, Dyer MJ. Molecular cloning of translocation t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21. Blood. 1998;91:1873–1881. [PubMed] [Google Scholar]
- Zent CS, Zhan F, Schichman SA, Bumm KH, Lin P, Chen JB, Shaughnessy JD. The distinct gene expression profiles of chronic lymphocytic leukemia and multiple myeloma suggest different anti-apoptotic mechanisms but predict only some differences in phenotype. Leuk Res. 2003;27:765–774. doi: 10.1016/s0145-2126(03)00015-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.