Abstract
Restricted and repetitive behaviors in autism spectrum disorders have been conceptualized to reflect impaired executive functions. In the present study, we investigated the performance of 6–17-year-old children with and without an autism spectrum disorder on a dimension-change card sort task that explicitly indicated sorting rules on every trial. Diagnostic groups did not differ in speed of responses after the first rule switch or in speed or accuracy on blocks with mixed versus single sort rules. However, performance of the ASD group was significantly slower and less accurate overall than the typically-developing group. Furthermore, within the ASD group, poorer DCCS task performance did not predict more severe autism symptoms. Implications for the executive dysfunction theory of autism are discussed.
Keywords: Autism, Set shifting, Dimension-change card sort task, Repetitive behaviors, Executive functioning, Children
Introduction
The executive dysfunction account of autism conceptualizes symptoms of restricted and repetitive behaviors, a core feature of autism, to reflect the impaired ability to adapt flexibly to changing environmental contingencies (Russell 1997; Turner 1999). Executive functions refer to a range of abilities, including behavioral inhibition, planning, working memory, set shifting, and mental flexibility (Baddeley 1986; Lezak 1995; Pennington 1994; Hill 2004). These abilities require the integration of a variety of basic skills (e.g., language and working memory) to achieve the higher-order processing of information, goal attainment, and appropriate emotional responses (Christ et al. 2007). Difficulties with cognitive flexibility are consistent with the clinical phenomenon of the repetitiveness and rigidity that characterizes autism: cognitive inflexibility is manifest as repetitive motor behaviors, perseverative responding, and difficulty with modulating ongoing cognitive and motor behavior (Lopez et al. 2005). Moreover, executive deficits may mediate, at least in part, poorly modulated social behaviors in autism (Happe et al. 2006).
Numerous studies have documented impaired executive function in autism spectrum disorders (ASDs). For example, a large-scale study of neuropsychological profiles of individuals with autism on The Cambridge Neuropsychological Test Automated Battery (CANTAB, Robbins et al. 1994) found significant group differences in planning efficiency and extradimensional shifting (Ozonoff et al. 2004), confirming previous reports of poor planning (Ozonoff 1998) and “stuck-in-set” (Hughes et al. 1994) deficits in autism. The largest effect sizes of executive function deficits have been found on the Wisconsin Card Sorting Test (WCST) and Tower of London and Tower of Hanoi tasks (Pennington and Ozonoff 1996; Sergeant et al. 2002), and an oft-cited review by Elizabeth Hill (2004) indicates that a majority of studies have found perseverative impairments on the WCST in autism (e.g., Ozonoff and Jensen 1999; Ozonoff et al. 1991).
However, clearly not all studies of executive function in autism indicate deficits (e.g., Minshew et al. 1992; Nyden 1999; for a review, see Geurts et al. 2009). These seemingly contradictory findings may reflect that executive function is not a unitary construct, but may be subdivided into more elemental components (see Kenworthy et al. 2005 for a review). For example, set-shifting tasks such as the WCST, the Trail-making task, and the Intradimensional/ Extradimensional (ID/ED) task from the CANTAB require not only flexible adaptation to changing rules, but also working memory of each new rule (e.g., Russell et al. 1996). This potential confounding factor is particularly noteworthy given that working memory has been reported to be impaired in ASD (Barnard et al. 2008; Belleville et al. 2006; Bennetto et al. 1996; Russell et al. 1996; Williams et al. 2005).
Another contributing factor to inconsistencies in the literature may be that individuals with autism perform better on tests of perseveration when administered by a computer rather than by an experimenter (cf. Ozonoff 1995), suggesting that the social-cognitive demands of responding to a person, relative to a computer, may spuriously inflate perseverative deficits in autism samples. Both potential confounding factors are mitigated by the use of the computerized version of the Dimensional-Change Card-Sort task (DCCS; Zelazo et al. 1996) that explicitly indicates the sort rule on every trial.
By minimizing working memory demands, the DCCS allows for a more narrowly-defined measure of set-shifting. The DCCS was originally developed to evaluate set shifting abilities in nonclinical samples, and typically developing 3–4-year-old children exhibit a particular pattern of responding: although they can report the correct sorting rules throughout the task, they nevertheless sort incorrectly when the sort rule changes (Diamond and Kirkham 2005). This behavior has been conceptualized to reflect a broader dissociation between action and explicit knowledge in children, and accounts for the ability of children to act appropriately despite an inability to describe the basis of such actions, and, conversely, to act inappropriately despite knowing what to do (Church and Goldin-Meadow 1986; Dempster 1992). This tendency to perseverate to previously- learned rules has been described as “attentional inertia,” and may be attenuated when children are encouraged to refocus their attention or strengthened when incorrect rules are made more salient (Kirkham et al. 2003). Furthermore, the dissociation between intent and appropriate action appears to decline with development, only to reappear later in life (Dempster 1992). An alternative explanation that has been proposed by Perner (2002) is the “redescription hypothesis,” which postulates that young children have difficulty understanding that an object may be labeled in multiple ways.
Despite evidence that by the age of 4–5, typicallydeveloping children “solve” the DCCS task by demonstrating adult-like accuracy performance (Zelazo et al. 1996), Diamond and Kirkham (2005) demonstrated that even typically-developing adults demonstrate delayed reactions times, despite near-perfect accuracy, on trials where the sorting rule is different from the initial rule. Thus, although DCCS accuracy is the more sensitive measure of attentional inertia in children (Cohen et al. 2001), reaction time appears to capture the phenomenon in adults, reflecting that adults are able to respond correctly, but only at the cost of response speed.
To date there are few investigations of relations between measures of executive function and repetitive behaviors in ASDs (c.f. Dichter et al. 2009). In one such study,South et al. (2007) reported a positive correlation between perseverative responding on the WCST and stereotyped behaviors on the Autism Diagnostic Interview-Revised (Lord et al. 1994) and the Autism Diagnostic Observation Schedule (Lord et al. 2000). However, they did not find significant relations between perseverative responses and repetitive behaviors as assessed via the Repetitive Behavior Interview (Turner 1997) or the Yale Special Interests Interview (South et al. 1999). The authors highlighted the need to use symptom measures that encompass the full range of repetitive behaviors observed in ASD. They also suggested a focus on narrower neurocognitive constructs to further refine the boundary conditions of executive function deficits in autism. The present study addresses these two recommendations by using the Repetitive Behavior Scale-Revised (Bodfish et al. 1999; Lam and Aman 2007), a measure of repetitive behaviors that assesses five subscales of repetitive behaviors, and by using the DCCS to assess set shifting abilities independent of working memory demands.
In summary, the purpose of the present study was to evaluate set shifting abilities via the DCCS in a large sample of children with ASD and to relate performance to core autism symptoms. As outlined in Diamond and Kirkham (2005), the primary metric of set shifting abilities was RT differences in responses to single-task versus mixed-task blocks. Based on conceptual and empirical linkages between executive function and repetitive behaviors, we hypothesized that both neurotypical and ASD children would be characterized by increased reaction times when switching from the first to the second sorting rule and when performing the mixed-task blocks, relative to the single-task blocks, due to the unpredictability of the mixed-task block. We predicted that these increases would be more pronounced in the ASD group due to the greater set shifting impairments. Additionally, if such group differences were evident, we further hypothesized that increased reaction time on mixed- relative to single-task DCCS blocks in the ASD group would be associated with higher levels of symptoms of restricted repetitive behaviors, and in particular “higher-order” repetitive behaviors (e.g., compulsions, rituals/insistence on sameness, and circumscribed interests) rather than “lower-order” repetitive behaviors (e.g., motor stereotypies and self-injurious behaviors).
Methods
Participants
In an effort to form a sample of ASD cases who demonstrated a range of symptom severities, participants were not recruited based on the presence of specific symptoms (e.g. repetitive behaviors). Children with ASD were recruited through the University of North Carolina (UNC) Autism Research Registry in conjunction with regional TEACCH (Treatment and Education of Autistic and related Communication- handicapped CHildren) clinics. They were diagnosed according to DSM-IV criteria for autism (American Psychiatric Association 1994), met lifetime criteria for autism or ASD on the Autism Diagnostic Interview-Revised (ADI-R, Lord et al. 1994), and met current criteria for ASD on the Social Responsiveness Scale (SRS, Constantino et al. 2003).
Typically developing children were recruited via mass emails sent to UNC faculty and staff, verified during a phone screen that they did not have a history of any psychiatric or developmental disorders, were not taking psychotropic medications, did not have an immediate family member with an ASD diagnosis, and did not score above the ASD cutoff on the SRS. Inclusion criteria for both diagnostic groups included: (a) 6–17 years of age; (b) intelligence scores > 70 on the Leiter International Performance Scale-Revised (Leiter et al. 2002); and (c) the absence of seizure disorders, acute medical conditions, genetic conditions, or uncorrectable visual impairments.
A total of 65 children with ASD and 43 children who were typically developing were recruited, of whom 50 children with ASD and 42 typically developing children met inclusion criteria. Participants were drawn from a larger study and DCCS data were available from 32 children with ASD (1 female; 27 Caucasian) and 34 typically developing children (1 female; 26 Caucasian). All participants and their guardians supplied written informed consent, and the protocol was approved by the UNC-Chapel Hill School of Medicine Biomedical Institutional Review Board.
Measures
Dimensional Change Card Sort Task (DCCS; Zelazo et al. 1996)
Administration and scoring procedures of the computerized DCCS are described in Diamond and Kirkham (2005). Participants were told that they would be playing a sorting game and would have to sort by either shape or color. They were instructed to use their dominant hand and to keep going even if they made an incorrect response. Participants first completed 15 practice trials presented with performance feedback (i.e., “correct” or “incorrect”) prior to the test blocks.
During the seven blocks of test trials, participants did not receive feedback. Each block used one of two sorting criteria, either color or shape. Blocks 1, 3, and 6 were one criteria, blocks 2, 5, and 7 were the other criteria (counterbalanced across participants), and block 4 was a mixed task (i.e., color and shape). The mixed task block contained 13 nonswitch trials and 7 switch trials presented in pseudorandom order. Each block consisted of ten trials, except the mixed run contained 20 trials. There were no breaks between blocks or other indications to the participant that there were blocks of trials.
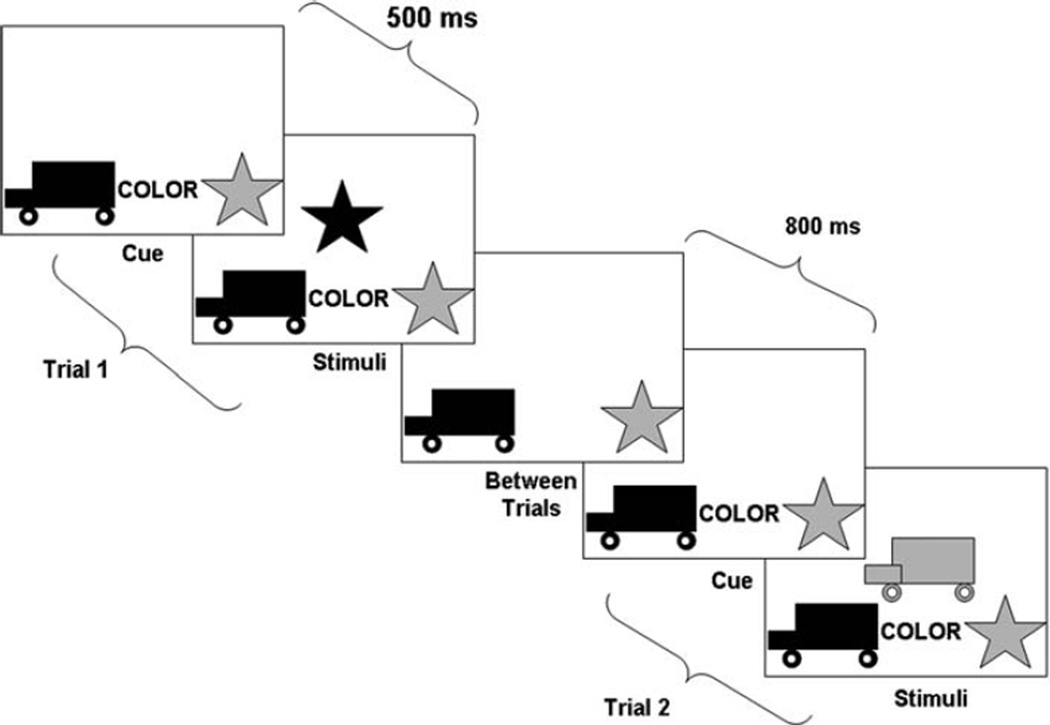
Each trial consisted of first a cue indicating the sorting rule, then a 500 ms delay followed by the test stimulus (see Fig. 1). The test stimulus was followed by an 800 ms intertrial interval. Throughout the trial, the response icons were presented on the bottom of the screen (i.e., a red truck on the left and a blue star on the right). Additionally, throughout a trial, the word “color” or “shape” was presented centrally in black bold font between the response icons, indicating the relevant sorting criterion for that trial. The participant indicated, as quickly as possible via button press, how to sort the centrally-presented stimulus. The stimulus never matched the response icons on both color and shape. Thus, the correct response when sorting by color was always the wrong response for sorting by shape, and visa-versa. Additionally, the sorting rule was presented throughout the trial, so correct performance was not contingent on recalling the correct sorting rule.
Fig. 1.
Schematic illustration of two sample trials from a singletask block of the Dimensional Change Card Sort Task (Zelazo et al. 1996)
The task was administered using a laptop computer with a 14” screen and responses were made on one of two 1.5” wooden squares with pictures of the response icons (i.e., a red truck and blue star). Accuracy and response times were recorded using E-Prime software v. 1.1 (Psychology Software Tools Inc., Pittsburgh, PA). In accordance with Diamond and Kirkham’s (2005) criteria, only correct responses were included in RT analyses. Additionally, trials where the RT was less than 200 ms or greater than 2.5 standard deviations above the mean were omitted. Two sample trials from a single-task block are presented in Fig. 1.
Cognitive Ability
Nonverbal intelligence was measured with the Leiter International Performance Scale-Revised (Roid and Miller 1997). A Brief IQ score was obtained based on four subtests of the Visualization and Reasoning Battery (i.e., Repeated Patterns, Sequential Order, Figure-Ground, and Form Completion).
General Autism Symptom Severity
General autism symptom severity was assessed via the total score of the 40-item Social Communication Questionnaire (SCQ, Rutter et al. 2003), a parental report measure of autism symptomatology. A higher score denotes greater impairment (range 0–40).
Communication Impairments
Communication impairments were measured with the Children’s Communication Checklist, 2nd edition (CCC; Bishop 1998). This 70-item checklist assesses language structure (e.g., speech, syntax, and semantics) and pragmatic use of language (e.g., initiation, context, and nonverbal communication). To obtain a measure of communication impairment independent of both social deficits and repetitive behaviors, a modified total was obtained by subtracting the 15 items related to social deficits and repetitive behaviors, resulting in a potential range from 0 to 165 (higher scores indicate greater impairment).
Repetitive Behaviors
Repetitive behaviors were assessed via the Repetitive Behavior Scale-Revised (RBS-R, Bodfish et al. 1999). The RBS-R is an informant-based questionnaire that assesses 43 discrete types of repetitive behaviors. The total RBS-R score was computed, as well as the “Ritualistic/Sameness Behavior,” “Stereotypic Behavior,” “Self-injurious Behavior,” “Compulsive Behavior,” and “Circumscribed Interests” subscales, as described in Lam and Aman (2007) (higher scores indicate more symptoms).
Results
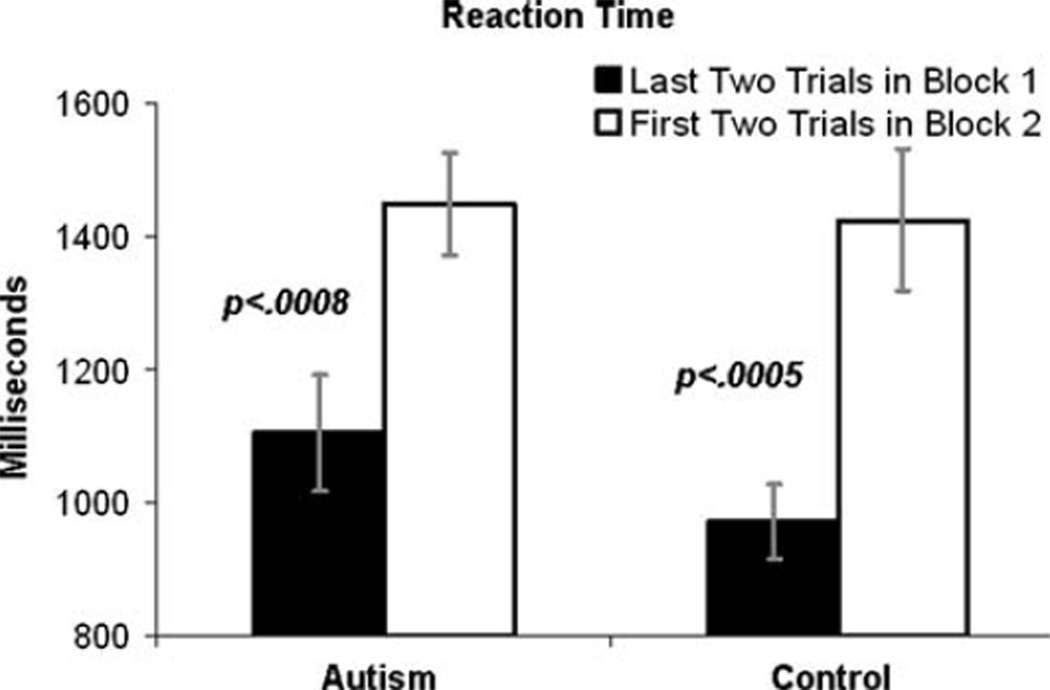
Table 1 illustrates the demographic and clinical characteristics of both diagnostic groups. There was a significant difference between groups on nonverbal intelligence (see Table 1). Consistent with the analysis strategy of Diamond and Kirkham (2005), the DCCS reaction time (RT) cost associated with switching from the first dimension (i.e., the first single-task block) to the second dimension (i.e., the next single-task block) was analyzed by examining the change in RT between the last two trials of block 1 and the first two trials in block 2 (see Fig. 2). A Group (Autism, Control)×Trial Type (last 2 trials of block 1, first 2 trials in block 2) rMANOVA indicated a main effect of Trial Type, multivariate F(1,68) = 29.13, p < .0001, reflecting that, across both diagnostic groups, RTs were quicker at the end of block 1 than at the beginning of block 2, but no main effect or interaction with Group, p’s > .40. An exploratory examination of trends within each group revealed that, in the control group, responses were quicker at the end of block 1 [mean (SD) = 971 ms (328)] than at the beginning of block 2, [mean (SD) = 1,386 ms (584)], t(32) = 3.92, p < .0005. The autism group revealed a highly similar pattern: RTs were quicker at the end of block 1 [mean (SD) = 1,105 ms (521)] than at the beginning of block 2, [mean (SD) = 1,396 ms (423)], t(30) = 3.69, p < .0008.
Table 1.
Mean (SD) demographic and clinical scores for the ASD and control groups
| Autism (n = 32) | Control (n = 34) | t Value (64) | p Value | |
|---|---|---|---|---|
| Age (years) | 9.99 (2.78) | 10.50 (3.31) | −0.70 | 0.49 |
| Leiter-R | 103.34 (18.69) | 112.09 (14.33) | −2.18 | 0.033 |
| SCQaa | 15.59 (4.34) | 3.32 (2.16) | 15.28 | <.0001 |
| CCC-2a | 60.34 (21.37) | 7.35 (8.42) | 13.10 | <.0001 |
| RBS-R Totala | 24.51 (15.85) | 1.32 (2.59) | 8.54 | <.0001 |
| RBS-R Stya | 4.71 (4.36) | 0.12 (0.41) | 6.21 | <.0001 |
| RBS-R SIBa | 2.40 (2.61) | 0.09 (0.29) | 5.20 | <.0001 |
| RBS-R COMPa | 2.94 (3.43) | 0.12 (0.41) | 4.84 | <.0001 |
| RBS-R RITSAMa | 7.86 (5.59) | 0.50 (1.19) | 7.61 | <.0001 |
| RBS-R CIa | 3.40 (2.30) | 0.26 (0.71) | 7.69 | <.0001 |
Significance values are two-tailed and not corrected for multiple comparisons
Note: Leiter-R: Leiter International Performance Scale-Revised (Leiter et al. 2002). SCQ: Social Communication Questionnaire (Rutter et al. 2003). CCC-2: Children’s Communication Checklist, 2nd edition (Bishop 1998), without the 15 items related to repetitive behaviors. RBS-R Total: Total score of the Repetitive Behavior Scale-Revised (Bodfish et al. 1999; Lam and Aman 2007). RBS-R Sty: Stereotypic behavior RBS-R factor. RBS-R Sib: Self-injurious behavior RBS-R factor. RBS-R Comp: Compulsive behavior RBS-R factor. RBS-R RitSam: Rituals/Sameness RBS-R factor. RBS-R CI: Circumscribed Interests RBS-R factor
Welch-Satterthwaite approximation reported due to heterogeneous group variances
Fig. 2.
Group-average reaction time cost in switching from sorting by one rule in Block 1 to the other rule in Block 2. Error bars represent standard errors of the mean
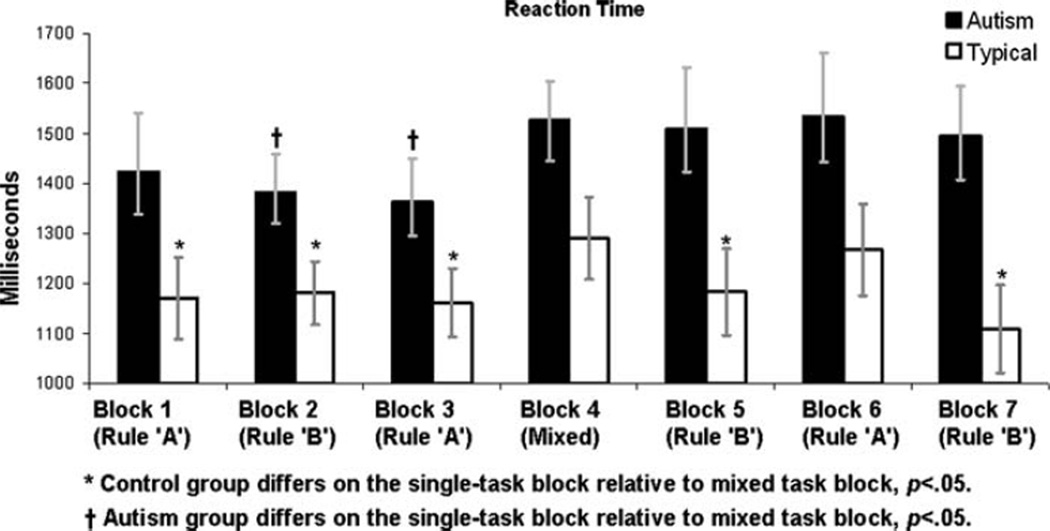
Figure 3 illustrates mean RTs for both groups for each block. A Group (Autism, Control) × Block Type (mixed-task, single-task) rMANOVA conducted on RTs indicated a main effect of Block Type, multivariate F(1,64) = 6.52, p < .018 reflecting that RTs were slower across groups on the mixed-task, relative to single-task, blocks, and a main effect of Group, F(1,64) = 6.31, p < .017, reflecting that the autism group was slower overall, but, contrary to predictions, no interaction of Group and Block Type, multivariate F(1,64) = .26, p > .60. An exploratory examination of trends within each group revealed that, in the control group, responses were slower during the mixed-task block, [mean (SD) RT = 1,290 ms (475)], than on the single task blocks [mean (SD) RT = 1,179 ms (397)], t(32) = 3.25, p < .003. This trend replicates the pattern of responses reported for adults in Diamond and Kirkham (2005). Within the autism group, however, this pattern was not evident: reaction times on the mixed-task blocks [mean (SD) RT = 1,526 ms (467)] were not significantly different from those on single task blocks [mean (SD) RT = 1,451 ms (453)], t (30) = 1.18, p > .24.
Fig. 3.
Group-average reaction times for each block of trials. Incorrect trials were removed prior to calculation of mean reaction times. Error bars represent standard errors of the mean
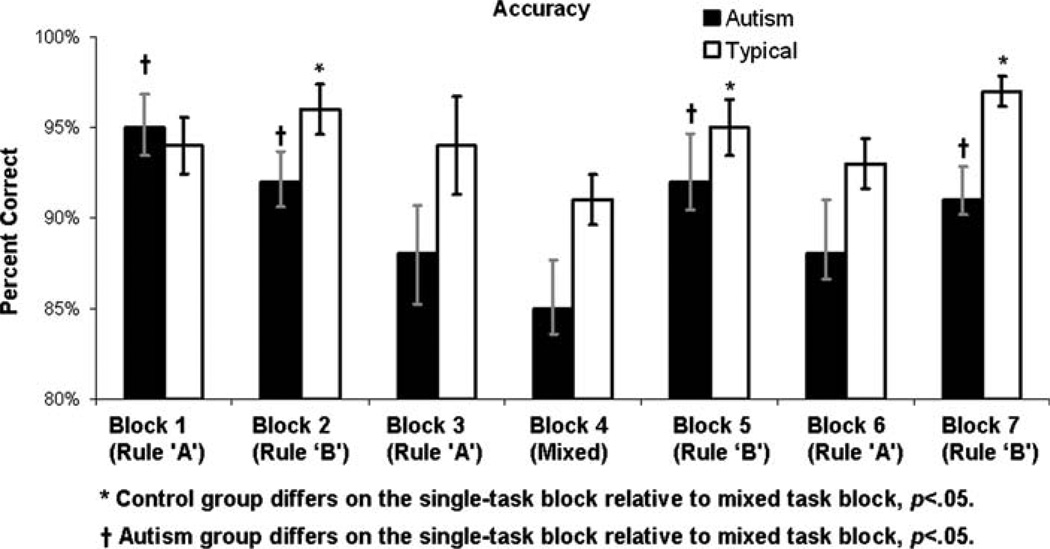
Figure 4 illustrates mean accuracy (i.e., percent correct) for both groups for each block. A Group (Autism, Control)×Block Type (mixed-task, single-task) rMANOVA conducted on accuracy revealed a main effect of Block Type, multivariate F(1,64) = 15.42, p < .0018, reflecting that accuracy was lower across groups on the mixed-task relative to single-task blocks, and a main effect of Group, F(1,64) = 5.92, p < .020, reflecting that the control group was more accurate overall, but, contrary to predictions, no interaction of Group and Block Type, multivariate F(1,64) = .75, p > .35. An exploratory examination of trends within each group revealed that, in the control group, responses were more accurate during the single-task block [mean (SD) percent correct = 95.0 (5.3)], than on mixed-task blocks [mean (SD) percent correct = 91.2 (8.3)], t(32) = 2.87, p < .007. Within the autism group, this pattern was evident as well: accuracy on the single-task blocks [mean (SD) percent correct = 91.8 (8.2)] was significantly higher than those on the mixed-task block [mean (SD) percent correct = 84.9 (16.2)], t (30) = 2.88, p < .007.
Fig. 4.
Group-average accuracy (i.e., percent correct) for each block of trials. Error bars represent standard errors of the mean
To assess potential relations between DCCS scores and autism symptoms, we conducted Pearson partial bivariate correlations between DCCS and symptom scores within the autism sample while controlling for variance due to intelligence. As is evident from Table 2, DCCS scores did not predict greater autism symptoms in any domain. The only significant correlations observed were positive correlations between accuracy on single-task blocks and RBS-R Rituals/ Sameness subscale scores, RBS-R total scores, and SCQ total scores, as well as a positive correlation between mixed-task block accuracy and RBS-R Circumscribed Interests subscale scores. In other words, on two measures of task accuracy, greater accuracy actually predicted worse symptoms.
Table 2.
Pearson bivariate partial correlations (p-values in parentheses) between DCCS variables and measures of autism symptoms among the 32 participants with autism spectrum disorders
| SCQ | CCC-2 | RBS-R Total |
RBS-R Sty |
RBS-R Sib |
RBS-R Comp |
RBS-R RitSam |
RBS-R CI |
|
|---|---|---|---|---|---|---|---|---|
| Accuracy | ||||||||
| Single- task blocks | 0.42 (0.018) | 0.059 (0.75) | 0.36 (0.049) | 0.30 (0.10) | 0.054 (0.77) | 0.20 (0.28) | 0.39 (0.030) | 0.27 (0.14) |
| Mixed-task blocks | 0.12 (0.52) | 0.21(0.26) | 0.25 (0.17) | 0.18 (0.34) | 0.17 (0.36) | 0.081 (0.66) | 0.26 (0.15) | 0.41 (0.020) |
| Reaction time | ||||||||
| Single- task blocks | −0.12 (0.50) | 0.059 (0.75) | 0.0098 (0.96) | 0.014 (0.94) | −0.0046 (0.98) | 0.021 (0.91) | 0.0245 (0.90) | 0.18 (0.33) |
| Mixed- task blocks | −0.0084 (0.96) | −0.13 (0.50) | 0.030 (0.87) | 0.092 (0.62) | −0.097 (0.60) | 0.035 (0.85) | −0.016 (0.93) | 0.16 (0.40) |
| Trial switch Δ RT | 0.17 (0.36) | −0.049 (0.79) | 0.030 (0.87) | 0.033 (0.86) | 0.12 (0.51) | −0.091 (0.63) | 0.017 (0.93) | 0.29 (0.12) |
Significance values are two-tailed and not corrected for multiple comparisons
Note: Trial Switch D RT, Last two trials of block 1 minus the first two trials in block 2; SCQ, Social Communication Questionnaire (Rutter et al. 2003); CCC-2, Children’s Communication Checklist, 2nd edition (Bishop 1998), without the 15 items related to repetitive behaviors; RBS-R Total, Total score of the Repetitive Behavior Scale-Revised (Bodfish et al. 1999; Lam and Aman 2007); RBS-R Sty, Stereotypic behavior RBS-R factor; RBS-R Sib, Self-injurious behavior RBS-R factor; RBS-R Comp, Compulsive behavior RBS-R factor; RBS-R RitSam, Rituals/Sameness RBS-R factor; RBS-R CI, Circumscribed Interests RBS-R factor
Discussion
The purpose of the present study was to assess set shifting abilities as measured by the Dimensional Change Card Sort Task (DCCS; Zelazo et al. 1996) in a sample of children with ASDs and to relate these abilities to core autism symptoms, particularly symptoms of “higher-order” repetitive behaviors. Although the ASD group was generally slower and less accurate overall, both groups demonstrated a comparable reaction time cost associated with switching from the first to the second block. Furthermore, both groups demonstrated less accuracy and slower responding on the mixed-task block relative to the singletask blocks. These results are not consistent with numerous reports of set shifting deficits in autism (e.g., Pennington and Ozonoff 1996; see Hill 2004 for a review; but see Geurts et al. 2009 for inconsistencies in this literature).
One potential explanation for these nonsignificant group differences in response cost due to mixed- versus singletask blocks is that the DCCS task minimizes working memory demands. This interpretation suggests that the presence of working memory demands on other common set shifting tasks (e.g., the WCST) may exacerbate perseverative tendencies in ASD. Although tasks in everyday life that require cognitive flexibility typically have working memory demands, the purpose of the present study was to assess basic cognitive abilities that may affect the expression of ASD symptomatology (Geurts et al. 2009). Future studies may further evaluate the effects of working memory on set shifting abilities in ASD by incorporating a systematic, graded manipulation of working memory demands to evaluate the influence of this factor on performance of individuals with ASD on tests of set shifting. Additionally, the DCCS is computer-administered, thereby minimizing social cognitive demands of the set shifting task (Ozonoff 1995).
Although the ASD group showed comparable accuracy and reaction time costs associated with a rule shift, relative to the neurotypical comparison group, their performance was slower and less accurate overall than the control group, even in the presence of explicit rules. A global attentional inertia description of the attentional style of individuals with ASDs describes the data in the present study (i.e., overall slower and less accurate responding, rather than a differential deficit on runs requiring set shifting) and is consistent with multiple studies documenting difficulties with sustained attention in ASD (Rumsey and Hamburger 1988; Bogte et al. 2009). The attentional inertia demonstrated by individuals with ASDs may be, in fact, broad and general, rather than constrained to situations requiring set shifting. An interpretation of global attentional inertia is consistent with evidence of “sticky” attention on tasks requiring disengaging attentional focus in a variety of contexts (Sasson et al. 2008; Landry and Bryson 2004; van der Geest et al. 2001; O’Riordan and Plaisted 2001; O’Riordan et al. 2001). Alternatively, group differences in overall RT may be due to non-specific reaction time delays in a variety of contexts (South et al. 2008).
We note that although both groups had nonverbal intelligence scores in the average range, the ASD group had significantly lower nonverbal intelligence scores, which may have affected their DCCS performance. Additionally, DCCS performance may be verbally mediated (i.e., participants may mentally rehearse the rules as they complete the task), and future studies should evaluate whether task performance is mediated by verbal intelligence, which was not assessed in the present study. We also note that the DCCS was developed to assess set shifting in young children (typically-developing children as young as five demonstrate adult-like DCCS accuracy; Zelazo et al. 1996). The non-significant group differences in the present study may have been due to the use of older samples of children, thereby effectively allowing the ASD sample to “catch up” to performance levels of typicallydeveloping group. Subsequent research should use the DCCS to assess the development of set shifting strategies in younger children with ASD.
Previous studies have reported an association between repetitive behaviors and executive function abilities (Turner 1997), including perseverative responding (South et al. 2007) cognitive flexibility, working memory, and response inhibition (Lopez et al. 2005). In the present investigation, set shifting deficits were not found to be associated with increased frequency or severity of “lower-order” or “higher-order” repetitive behaviors. The only significant associations between DCCS metrics and severity of autism symptoms were contrary to predictions, findings that warrant replication but that may be due to the relatively moderate phenotypic expression of repetitive behaviors in this high functioning sample. Furthermore, future studies with lower functioning individuals may reveal larger group differences on DCCS performance.
Acknowledgments
This research was supported by R01 MH073402 (Bodfish). G. Dichter was supported by NIH/NCRR K12 RR023248 and by NIMH K23 MH081285. K. S. L. Lam and L. M. Turner-Brown were supported by NICHD T32-HD40127. Assistance for this study was provided by the Subject Registry Core of the UNC Carolina Institute for Developmental Disabilities (P30 HD03110).
Contributor Information
Gabriel S. Dichter, Email: dichter@med.unc.edu, Neurodevelopmental Disorders Research Center, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, USA; Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA; Duke-UNC Brain Imaging and Analysis Center, Duke University Medical Center, Durham, NC 27710, USA; Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Box 3026, Durham, NC 27710, USA.
Krestin J. Radonovich, Neurodevelopmental Disorders Research Center, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, USA Department of Psychiatry, University of Florida College of Medicine, PO Box 100234, Gainesville, FL 32610-0234, USA.
Lauren M. Turner-Brown, Neurodevelopmental Disorders Research Center, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, USA Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA.
Kristen S. L. Lam, Neurodevelopmental Disorders Research Center, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, USA Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA.
Tia N. Holtzclaw, Neurodevelopmental Disorders Research Center, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, USA Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA.
James W. Bodfish, Neurodevelopmental Disorders Research Center, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, USA Carolina Institute for Developmental Disabilities, University of North Carolina at Chapel Hill School of Medicine, CB# 3366, 101 Manning Drive, Chapel Hill, NC 27599-7160, USA; Department of Psychiatry, University of North Carolina at Chapel Hill School of Medicine, CB# 7160, Chapel Hill, NC 27599-7160, USA; Center for Development and Learning, University of North Carolina at Chapel Hill, CB # 7255, Chapel Hill, NC 27599-7255, USA.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. Washington. DC: American Psychiatric Association; 1994. [Google Scholar]
- Baddeley A. Working memory. New York, NY: Clarendon Press/Oxford University Press; 1986. [Google Scholar]
- Barnard L, Muldoon K, Hasan R, O’Brien G, Stewart M. Profiling executive dysfunction in adults with autism and comorbid learning disability. Autism. 2008;12(2):125–141. doi: 10.1177/1362361307088486. [DOI] [PubMed] [Google Scholar]
- Belleville S, Ménard É, Mottron L, Ménard M. Working memory in autism. In: Alloway TP, Gathercole SE, editors. Working memory and neurodevelopmental disorders. New York: NY Psychology Press; 2006. pp. 213–238. [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Development. 1996;67(4):1816–1835. [PubMed] [Google Scholar]
- Bishop DV. Development of the children’s communication checklist (CCC): A method for assessing qualitative aspects of communicative impairment in children. Journal of Child Psychology and Psychiatry. 1998;39(6):879–891. [PubMed] [Google Scholar]
- Bodfish JW, Symons FW, Lewis MH. The Repetitive Behavior Scale-Revised. Western Carolina Center Research Reports. 1999 [Google Scholar]
- Bogte H, Flamma B, Van Der Meere J, Van Engeland H. Divided attention capacity in adults with autism spectrum disorders and without intellectual disability. Autism. 2009;13(3):229–243. doi: 10.1177/1362361309103793. [DOI] [PubMed] [Google Scholar]
- Christ SE, Holt DD, White DA, Green L. Inhibitory control in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2007;37(6):1155–1165. doi: 10.1007/s10803-006-0259-y. [DOI] [PubMed] [Google Scholar]
- Church BR, Goldin-Meadow S. The mismatch between gesture and speech as an index of transitional knowledge. Cognition. 1986;23:43–71. doi: 10.1016/0010-0277(86)90053-3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Bixenman M, Meiran N, Diamond A. Task switching in children. Paper presented at the South Carolina Bicentennial Symposium on Attention; University of South Carolina, Columbia, SC. 2001. [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Dempster N. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Diamond A, Kirkham N. Not quite as grown-up as we like to think: Parallels between cognition in childhood and adulthood. Psychological Science. 2005;16(4):291–297. doi: 10.1111/j.0956-7976.2005.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Lam K, Turner-Brown L, Holtzclaw T, Bodfish J. Generativity abilities predict communication deficits but not repetitive behaviors in autism spectrum disorders. J Autism Dev Disord. 2009 doi: 10.1007/s10803-009-0742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends in cognitive sciences. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: Examining profiles across domains and ages. Brain and Cognition. 2006;61(1):25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Science. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32(4):477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Kenworthy LE, Black DO, Wallace GL, Ahluvalia T, Wagner AE, Sirian LM. Disorganization: The forgotten executive dysfunction in high-functioning autism (HFA) spectrum disorders. Developmental neuropsychology. 2005;28(3):809–827. doi: 10.1207/s15326942dn2803_4. [DOI] [PubMed] [Google Scholar]
- Kirkham N, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6(5):449–476. [Google Scholar]
- Lam KS, Aman MG. The repetitive behavior scale-revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Muenz LR, Payton JB. Neuropsychological functioning in nonmentally retarded autistic individuals. Journal of Clinical and Experimental Neuropsychology. 1992;14(5):749–761. doi: 10.1080/01688639208402860. [DOI] [PubMed] [Google Scholar]
- Nyden A. Executive/attention deficits in boys with Asperger syndrome, attention disorder and reading/writing disorder. Autism. 1999;3:213–228. [Google Scholar]
- O’Riordan M, Plaisted K. Enhanced discrimination in autism. Quarterly Journal of Experimental Psychology. A, Human Experimental Psychology. 2001;54(4):961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Reliability and validity of the Wisconsin card sorting test in studies of autism. Neuropsychology. 1995;9(4):491–500. [Google Scholar]
- Ozonoff S. Assessment and remediation of executive dysfunction in autism and Asperger syndrome. In: Schopler E, Mesibov GB, Kunce LJ, editors. Asperger syndrome or high-functioning autism? New York: Plenum; 1998. pp. 263–292. [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, et al. Performance on Cambridge neuropsychological test automated battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the collaborative programs of excellence in autism network. Journal of Autism and Developmental Disorders. 2004;34(2):139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders. 1999;29(2):171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: Relationship to theory of mind. Journal of Child Psychology and Psychiatry. 1991;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Pennington B. The development of future-oriented processes. Chicago, IL: University of Chicago Press; 1994. The working memory function of the prefrontal cortices: Implications for developmental and individual differences in cognition; pp. 243–289. [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Perner J. Implicit versus explicit representation and intra-versus inter-modular processing. Computational Intelligence. 2002;18(1):55–58. [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge neuropsychological test automated battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale-Revised. Woodale, IL: Stoelting Co; 1997. [Google Scholar]
- Rumsey JM, Hamburger SD. Neuropsychological findings in high-functioning men with infantile autism, residual state. Journal of Clinical and Experimental Neuropsychology. 1988;10(2):201–221. doi: 10.1080/01688638808408236. [DOI] [PubMed] [Google Scholar]
- Russell J. Autism as an executive disorder. Oxford; New York: Oxford University Press; 1997. [Google Scholar]
- Russell J, Jarrold C, Henry L. Working memory in children with autism and with moderate learning difficulties. Journal of Child Psychology and Psychiatry. 1996;37(6):673–686. doi: 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire (SCQ): Western Psychological Services. 2003 [Google Scholar]
- Sasson N, Turner-Brown L, Holtzclaw T, Lam KS, Bodfish J. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behavioural Brain Research. 2002;130(1–2):3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- South M, Klin A, Ozonoff S. The Yale special interests interview. 1999 Unpublished measure. [Google Scholar]
- South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. 2007;11(5):437–451. doi: 10.1177/1362361307079606. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, Suchy Y, Kesner RP, McMahon WM, Lainhart JE. Intact emotion facilitation for nonsocial stimuli in autism: Is amygdala impairment in autism specific for social information? Journal of the International Neuropsychological Society. 2008;14(1):42–54. doi: 10.1017/S1355617708080107. [DOI] [PubMed] [Google Scholar]
- Turner MA. Towards an executive dysfunction account of repetitive behaviour in autism. In: Russell J, editor. Autism as an executive disorder. Oxford; New York: Oxford University Press; 1997. pp. 57–100. [Google Scholar]
- Turner MA. Annotation: Repetitive behaviour in autism: A review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–849. [PubMed] [Google Scholar]
- van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Eye movements, visual attention, and autism: A saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry. 2001;50(8):614–619. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. Journal of Autism and Developmental Disorders. 2005;35(6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11(1):37–63. [Google Scholar]