Abstract
Background
The Seattle Heart Failure Model (SHFM) is a well-validated prediction model of all-cause mortality in patients with heart failure, but its relationship with generic health status measures has not been evaluated. We sought to investigate relationships between SHFM scores and health utility weights, which are necessary to estimate quality-adjusted life-years in cost-effectiveness analyses.
Methods and Results
We applied mixed linear regression to examine relationships between baseline SHFM scores and EQ-5D–derived health utilities collected longitudinally in a large clinical trial. A 1-unit increase in SHFM score (higher predicted mortality) was associated with a 0.030 decrease in utility (P < .001) and an additional 0.006 decrease per year (P < .001). With SHFM score modeled as a categorical variable, EQ-5D utilities for patients with rounded SHFM scores of 1 or 2 were significantly lower (–0.041 and –0.053, respectively; both P < .001) and declined more rapidly over time (–0.011 and –0.020, respectively; both P ≤ .004) than for patients with scores of –1.
Conclusion
Patients with higher SHFM-predicted mortality had significantly lower health utilities at baseline and greater rates of decline over time, compared with patients with lower SHFM-predicted mortality. These relationships can be applied when examining the cost-effectiveness of heart failure interventions.
Keywords: Cost-Benefit Analysis, Disease Management, Heart Failure, Mortality, Quality of Life
Introduction
The development and implementation of disease management programs represent a major advance in the care of patients with heart failure. These programs are generally associated with better clinical outcomes, including lower readmission rates and higher survival rates,1,2 but the specific characteristics of disease management programs vary. As they are implemented more broadly, heart failure disease management programs are under increasing pressure to demonstrate their cost-effectiveness in comparison with other approaches to improving patient outcomes.3
To assist researchers in conducting economic evaluations, our group is developing a generalized probabilistic disease simulation model to evaluate the cost-effectiveness of heart failure disease management programs.4 The Tools for the Economic Analysis of Patient Management Interventions in Heart Failure (TEAM-HF) Cost-Effectiveness Model generates patient-level predictions of survival time using the Seattle Heart Failure Model (SHFM), an externally validated, multivariable risk model that estimates survival time in patients with heart failure on the basis of clinical, laboratory, and treatment characteristics.5 The SHFM survival estimates are then adjusted for between-patient differences in health-related quality of life over time using utility weights to derive estimates of quality-adjusted life-years (QALYs), the key measure of effectiveness in cost-effectiveness (or cost-utility) analyses.
To facilitate development of the TEAM-HF Cost-Effectiveness Model, we previously examined relationships between SHFM scores and medical resource use and costs and found that SHFM scores predicted medical resource use and costs among patients enrolled in Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION).6 In the present study, we explored baseline and longitudinal relationships between SHFM scores and health utilities, as measured with the EQ-5D, in the same population.
Methods
Study Population
HF-ACTION was a multicenter, randomized controlled trial designed to evaluate the efficacy and safety of aerobic exercise training plus usual care, compared with usual care alone, in patients with chronic heart failure.7 Eligible participants had New York Heart Association (NYHA) class II to IV heart failure with left ventricular ejection fraction of 35% or less at baseline. The trial enrolled 2331 patients between April 2003 and February 2007 with a mean follow-up of 2.5 years. The primary end point consisted of a composite of all-cause mortality or hospitalization and was observed in 65% of patients randomly assigned to receive exercise training plus usual care versus 68% of patients randomly assigned to receive usual care alone (hazard ratio [HR], 0.93; 95% CI, 0.84-1.02; P = .13).8
Health Utilities
The EQ-5D is a widely used generic, multidimensional measure of health status in 5 domains: mobility, self-care, usual activity, pain/discomfort, and anxiety/depression.9 It has been incorporated into numerous clinical trials to evaluate the impact of medical interventions and to generate estimates of QALYs for use in cost-effectiveness analyses with proven validity and reliability.10-12 The version of the EQ-5D administered to participants in HF-ACTION (ie, the EQ-5D-3L) allowed 3 possible response options in each domain, representing no problem, some/moderate problem, or severe/extreme problem.
In HF-ACTION, the EQ-5D was administered at baseline, every 3 months for the first year of follow-up, then annually and at the final study visit. We converted each set of 5 responses to a single summary index score representing a utility weight. The utility weights were based on valuation scores collected from a representative sample of the US population, with 1 denoting perfect health and 0 denoting death.13
SHFM Scores
For each patient, 20 variables representing clinical, treatment, and laboratory characteristics are required to derive the SHFM score.4 Three of the required variables (percent lymphocytes, uric acid, and allopurinol use) were not collected in HF-ACTION. Therefore, we generated predicted values for percent lymphocytes and uric acid at the patient level using multivariable linear regression models developed from the original SHFM cohort, and we assumed no allopurinol use. For patients with missing values for cholesterol (35%), hemoglobin (24%), or sodium (11%), we imputed the data using mean values. We limited the analysis to patients with rounded SHFM scores between –1 and 2.
Statistical Analysis
We report means and SDs for continuous variables and frequencies and percentages for categorical variables. To evaluate the relationships between SHFM scores and health utilities over time, we applied mixed linear regression modeling, a widely used approach for analyzing longitudinally collected data. We constructed 2 models. In the first, we analyzed SHFM scores as a continuous variable; in the second, we analyzed SHFM scores as a categorical variable using rounded values (–1, 0, 1, and 2). For both models, the dependent variable was the EQ-5D–derived health utility, and the independent variables included a variable representing the patient's baseline SHFM score, a variable corresponding to the timing of the utility assessment relative to baseline (in years), and interactions between SHFM scores and time to evaluate whether longitudinal changes in health utilities differed across baseline SHFM scores.
In the base-case analysis, we used utility scores available prior to death. To evaluate the influence of death on changes in utility scores, we conducted a sensitivity analysis in which we assigned a utility score of 0 at the time of death for patients who died and included those values in the regression analyses.
We used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses.
Results
Of the 2331 patients in HF-ACTION, SHFM scores were derived for 2293 (98%). Among these, 2282 patients had rounded SHFM scores between –1 and 2. Table 1 shows the baseline characteristics of the study population. Mean age was 59 years, 28% of the patients were women, 64% had NYHA class II heart failure, 36% had NYHA class III heart failure, and 1% had NYHA class IV heart failure.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | All Patients (n = 2288) | Rounded SHFM Score | |||
|---|---|---|---|---|---|
| –1 (n = 264) | 0 (n = 1313) | 1 (n = 621) | 2 (n = 90) | ||
| Age, mean (SD) | 59.1 (12.6) | 52.5 (12.3) | 58.1 (12.2) | 62.5 (12.0) | 67.7 (11.7) |
| Sex, No. (%) | |||||
| Female | 648 (28.3) | 87 (33.0) | 410 (31.2) | 143 (23.0) | 8 (8.9) |
| Male | 1640 (71.7) | 177 (67.0) | 903 (68.8) | 478 (77.0) | 82 (91.1) |
| Race, No. (%) | |||||
| Black or African American | 739 (32.8) | 92 (35.7) | 424 (32.9) | 196 (31.9) | 27 (30.0) |
| White | 1394 (61.9) | 156 (60.5) | 789 (61.2) | 390 (63.4) | 59 (65.6) |
| Other or missing | 120 (5.3) | 10 (3.9) | 77 (6.0) | 29 (4.7) | 4 (4.4) |
| NYHA class, No. (%) | |||||
| II | 1457 (63.7) | 249 (94.3) | 978 (74.5) | 219 (35.3) | 11 (12.2) |
| III | 811 (35.5) | 15 (5.7) | 335 (25.5) | 394 (63.4) | 67(74.4) |
| IV | 20 (0.9) | 0 (0.0) | 0 (0.0) | 8 (1.3) | 12 (13.3) |
| Etiology of heart failure, No. (%) | |||||
| Ischemic | 1176 (514) | 66 (25.0) | 634 (48.3) | 406 (65.4) | 70 (77.8) |
| Nonischemic | 1112 (48.6) | 198 (75.0) | 679 (51.7) | 215 (34.6) | 20 (22.2) |
| Ejection fraction, mean (SD) | 25.2 (7.5) | 26.7 (6.7) | 25.8 (7.6) | 23.5 (7.1) | 23.4 (7.5) |
| Systolic blood pressure, mean (SD), mm Hg | 114.2 (18.4) | 128.7 (19.3) | 115.4 (17.2) | 107.2 (16.2) | 104.3 (17.2) |
| Diastolic blood pressure, mean (SD), mm Hg | 70.5 (11.3) | 78.8 (12.4) | 71.1 (10.6) | 66.5 (10.2) | 63.8 (8.7) |
| Laboratory, mean (SD) | |||||
| Total cholesterol, mg/dL | 167.6 (45.2) | 184.9 (41.8) | 172.1 (44.4) | 154.7 (43.0) | 135.3 (43.1) |
| Hemoglobin, g/dL | 13.5 (1.6) | 14.4 (1.3) | 13.7 (1.5) | 12.9 (1.7) | 11.9 (1.7) |
| Medications, No. (%) | |||||
| ACE inhibitor | 1701(77.6) | 222 (84.1) | 996 (75.86) | 438 (70.5) | 45 (50.0) |
| Angiotensin receptor blocker | 537 (23.5) | 61 (23.1) | 308 (23.5) | 144 (23.2) | 24 (26.7) |
| β-Blocker | 2163 (94.5) | 264 (100.0) | 1269 (96.7) | 550 (88.6) | 80 (88.9) |
| Loop diuretic | 1776 (77.6) | 160 (60.6) | 971 (74.0) | 560 (90.2) | 85 (94.4) |
| Lipid-lowering agent | 1074(46.9) | 165 (62.5) | 664 (50.57) | 213 (34.3) | 32 (35.6) |
| Spironolactone/eplerenone | 1,030 (45.0) | 167 (63.3) | 594 (45.2) | 238 (38.3) | 31 (34.4) |
| Automatic ICD, No. (%) | 917 (40.1) | 129 (48.9) | 499 (38.0) | 251 (40.4) | 38 (42.2) |
Abbreviations: ACE, angiotensin-converting enzyme; ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association; SHFM, Seattle Heart Failure Model.
The mean utility derived from the EQ-5D administered at baseline was 0.808 (SD, 0.141; median, 0.82; interquartile range [IQR], 0.76 to 0.86). The mean baseline SHFM score was 0.24 (SD, 0.64; median, 0.18; IQR, –0.20 to 0.63), corresponding to a predicted 1-year survival rate of 95.0% and an observed 1-year survival rate of 95.2% in HF-ACTION. Table 2 shows mean utilities corresponding to rounded SHFM score groups. The mean utilities were 0.822, 0.820, 0.783, and 0.768 for the rounded SHFM scores of –1, 0, 1, and 2 (Table 2), corresponding to predicted 1-year survival of 98.5%, 96.0%, 89.6%, and 74.1% and predicted 3-year survival of 95.6%, 88.6%, 71.8%, and 40.7%.
Table 2.
Baseline EQ-5D Health Utilities and SHFM Scores
| EQ-5D Health Utilities | Rounded SHFM Score | ||||
|---|---|---|---|---|---|
| All Patients (N = 2288) | –1 (n = 264) | 0 (n = 1313) | 1 (n = 621) | 2 (n = 90) | |
| Mean (SD) | 0.808 (0.141) | 0.822 (0.149) | 0.820 (0.134) | 0.783 (0.148) | 0.768 (0.147) |
| Median (IQR) | 0.82 (0.76-0.86) | 0.83 (0.77-1.00) | 0.83 (0.77-0.86) | 0.80 (0.71-0.84) | 0.78 (0.71-0.84) |
Abbreviations: IQR, interquartile range; SHFM, Seattle Heart Failure Model.
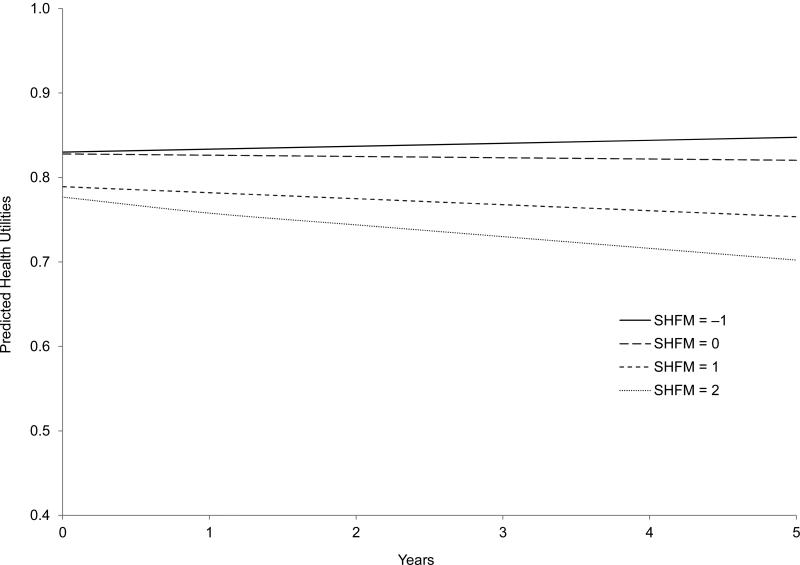
Table 3 shows the results of both the base-case and sensitivity analyses evaluating relationships between SHFM scores and EQ-5D health utilities over time. In the base-case analysis, when we modeled the SHFM score as a continuous variable, each 1-unit increase in the SHFM score was associated with a 0.030 decrease in EQ-5D utilities (P < .001). Although the main effect of time was not statistically significant (P = .16), the interaction term indicated that EQ-5D utilities decreased by an additional 0.006 points per year with each 1-unit increase in SHFM score (P < .001). When we modeled the SHFM score as a categorical variable, patients with rounded scores of 1 and 2 had significantly lower EQ-5D–derived utilities (–0.041 and –0.053 per year, respectively; P < .01 for both) and experienced accelerated declines in utilities over time (–0.011 and –0.020 per year, respectively; P < .004 for both) than patients with rounded scores of –1. EQ-5D–derived utilities for patients with rounded SHFM scores of 0 did not significantly differ from utilities for patients with rounded scores of –1 at baseline (–0.002; P = .81) and did not experience an accelerated decline in utilities over time (–0.005 per year; P = .12). Figure 1 displays the predicted trajectories of health utilities over the 5-year period for each of the rounded SHFM score groups for the base-case analysis.
Table 3.
Associations Between SHFM Scores and EQ-5D Health Utilities Over Time
| Base-Case Analysis | Sensitivity Analysis* | |||
|---|---|---|---|---|
| Effect (95% CI) | P Value | Effect (95% CI) | P Value | |
| Model 1: SHFM Score Modeled as Continuous Variable | ||||
| Intercept | 0.8227 (0.8170 to 0.8284) | < .001 | 0.8170 (0.8102 to 0.8238) | < .001 |
| Change in EQ-5D utilities per 1-unit increase in SHFM score | –0.0300 (–0.0382 to –0.0215) | < .001 | –0.0405 (–0.0505 to –0.0305) | < .001 |
| Change in EQ-5D utilities per year | –0.0015 (–0.0035 to 0.0006) | 0.16 | –0.0164 (–0.0195 to –0.0134) | < .001 |
| Additional change in EQ-5D utilities per year per 1-unit increase in SHFM score | –0.0062 (–0.0095 to –0.0030) | <.001 | –0.0223 (–0.0271 to –0.0176) | < .001 |
| Model 2: SHFM Score Modeled as Categorical Variable | ||||
| Intercept | 0.8300 (0.8143 to 0.8457) | < .001 | 0.8277 (0.8089 to 0.8466) | < .001 |
| SHFM score group | ||||
| ≤ –1 | [Reference] | — | [Reference] | — |
| 0 | –0.0021 (–0.0193 to 0.0151) | 0.81 | –0.0045 (–0.0251 to 0.0162) | 0.67 |
| 1 | –0.0409 (–0.0596 to –0.0222) | < .001 | –0.0528 (–0.0753 to –0.0303) | < .001 |
| ≥ 2 | –0.0532 (–0.0849 to –0.0217) | 0.001 | –0.0910 (–0.1287 to –0.0533) | < .001 |
| Change in EQ-5D utilities per year | 0.0035 (–0.0022 to 0.0092) | 0.23 | 0.0013 (–0.0072 to 0.0098)) | 0.77 |
| Additional Change in EQ-5D Utilities per Year by SHFM Score group | ||||
| SHFM score group | ||||
| –1 | [Reference] | — | [Reference] | — |
| 0 | –0.0050 (–0.0112 to 0.0013) | 0.12 | –0.0193 (–0.0285 to –0.0010) | < .001 |
| 1 | –0.0106 (–0.0175 to –0.0037) | 0.0025 | –0.0342 (–0.0444 to –0.0241) | < .001 |
| 2 | –0.0197 (–0.0329 to –0.0065) | 0.0035 | –0.0724 (–0.0909 to –0.0538) | < .001 |
Results from the regression model including 0 utility weights after patients died.
Figure.
Predicted Heath Utilities by Rounded SHFM Scores From the Base-Case Analysis (Model 2)
Note: Predicted health utilities for the rounded SHFM score group of −1 increase non-significantly over time.
In the sensitivity analysis, for which we included utility scores of 0 to reflect mortality for patients who died, the longitudinal effects were magnified with an annual utility reduction of 0.0373 for every 1-unit increase in SHFM score (Table 3) when the SHFM score was modeled as a continuous variable (P <.001). When we modeled the SHFM score as a categorical variable (Table 3), patients with rounded scores of 1 and 2 reported significantly lower utilities than patients with rounded scores of –1 (–0.053 and –0.091, respectively; P < .001 for both) and experienced accelerated declines in utilities over time (–0.034 and –0.072 per year, respectively; P < .001 for both). Relative to patients with rounded SHFM scores of –1 at baseline, EQ-5D utilities were not significantly different for patients with rounded SHFM scores of 0 (–0.005; P = .67), but these patients experienced significantly accelerated rates of decline in utilities over time (–0.019 per year; P < .001).
Discussion
In both the base-case and sensitivity analyses of data from HF-ACTION, we found significant inverse relationships between SHFM scores and EQ-5D health utilities. Patients with greater risk of mortality according to SHFM scores had significantly lower health utilities overall and experienced greater rates of decline in health utilities over time, compared with patients at lower mortality risk. Although the SHFM has been used extensively as a prediction tool or risk-adjustment factor in investigating clinical outcomes in heart failure, ours is the first study to examine relationships between SHFM scores and health utilities over time.
In decision-analytic models evaluating the cost-effectiveness of various therapeutic and disease management programs in heart failure, physiologic measures and clinical outcomes such as NYHA class and number of hospitalizations have been widely used to define health states.14-17 Although NYHA class and readmissions generally represent progression of disease and are a convenient means of defining health states for decision-analytic models, health utilities may vary considerably across patients with lower and higher levels of functioning within a given NYHA class.15,16 In addition, NYHA class, as a subjective assessment of functional status, has been reported to have considerable variation, and the interobserver agreement was only 55%.18,19 In contrast, SHFM score, derived using a patient's demographic characteristics and clinical, treatment, and laboratory data, is less prone to subjective variability. At baseline in HF-ACTION, SHFM scores for patients with NYHA class II symptoms ranged from –1.435 to 2.476, which correspond to a predicted 3-year survival of 97.15% to 23.57%. With the wide application of the SHFM score in both clinical practice and research in heart failure, we sought to use SHFM scores to model changes in health utilities over time as an alternative to NYHA class.
We have nearly completed the development of a Web-based TEAM-HF cost-effectiveness analysis model to help clinicians and researchers conduct high-quality economic evaluations of disease management programs in heart failure.4 The TEAM-HF cost-effectiveness analysis model was designed to handle various study designs, including parallel clinical trial cohorts, pre-post study designs, and hypothetical cases. The model SHFM scores are generated for hypothetical patients according to input variables specified by the user and generates predicted estimates of medical resource use, costs, and utility weights across estimated survival time based on SHFM scores.
Our analysis has several limitations. First, although data on 17 of the 20 variables needed to compute SHFM scores were collected in HF-ACTION, we did not have patient-level information on lymphocytes, serum uric acid level, and allopurinol use, and a small proportion of patients were missing laboratory values. We do not expect that any significant bias was introduced by imputing percent lymphocytes or uric acid levels using patient-level characteristics based on externally generated prediction models derived from the original SHFM cohort, but the assumption that no patients received allopurinol likely biased the findings toward the null hypothesis of no relationship between SHFM scores and health utilities. The extent of actual allopurinol use could be expected to range from 4% to 18%, as reported in the external data sets used to validate the SHFM.5 To evaluate the impact of missing data imputation using the prediction models, we applied alternative imputation strategies and reevaluated the relationships between SHFM scores and health utilities. Among the external data sets used to validate the SHFM, patients in HF-ACTION were most similar to patients in Val-HeFT based on mean NYHA class (2.4 for both).5 In Val-HeFT, the reported mean value for uric acid was 7.5 mg/dL, and the mean value for percent lymphocytes was 25. We applied these mean values to the HF-ACTION cohort, assumed no use of allopurinol, and recalculated the SHFM scores. When the SHFM score was modeled as either a continuous variable or a categorical variable, we obtained consistent results with similar magnitude of effects as in the base-case analysis. The only exception was when the SHFM score was modeled as a categorical variable in which the decrease of health utilities over time for patients with a rounded SHFM score of 2 was not statistically significantly different from that for patients with a rounded SHFM score of –1 (P = .12). However, we believe this was due to lack of statistical power, given that the number of patients with a rounded SHFM score of 2 decreased from 90 to 48.
Second, health utilities were derived in HF-ACTION from the 3-level version of the EQ-5D. Despite all of the participants having a diagnosis of heart failure, approximately 21% at baseline and 30% at some point during the follow-up period provided responses indicating no problems or limitations in each of the 5 domains (mobility, anxiety/depression, self-care, usual activities, pain/discomfort) represented in the EQ-5D. Although the EQ-5D has been used extensively and has demonstrated validity and reliability, its ability to discriminate between small to moderate differences in health status between individuals or over time for individuals has been identified as a limitation.21 The 5-level version of the EQ-5D was developed to address this issue,20 but that version was not available at the time HF-ACTION began enrollment.
Third, HF-ACTION selectively enrolled heart failure patients with the capacity to perform exercise, so the study population consisted of relatively healthy patients. At baseline, 63% of the 2331 randomized patients had NYHA class II symptoms. However, 831 (36%) had NYHA class III symptoms. Also, some patients experienced disease progression over 2.5 years of follow-up. Thus, our findings are primarily representative of the decline in health utilities that would be expected among NYHA class II and class III heart failure patients and underrepresent what would be expected in patients with more advanced disease. We propose future research examining the relationships between SHFM scores and health utilities include more heterogeneous heart failure populations, especially NYHA class IV patients, and in various clinical settings to evaluate whether our findings can be corroborated and whether similar relationships exist in patients with more advanced disease.
In conclusion, our findings of significant relationships between SHFM scores and health utilities at baseline and over time suggest that SHFM scores could be used in the development of decision analytic models to evaluate the cost effectiveness of various treatments or disease management programs in heart failure.
Acknowledgments
Damon M. Seils, MA, Duke University, assisted with manuscript preparation. Mr Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Funding/Support: This study was supported by grant 5R01NR011873-02 from the National Institute of Nursing Research. HF-ACTION was funded by grants 5U01HL063747, 5U01HL066461, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494, 5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, and 5U01HL064264 from the National Heart, Lung, and Blood Institute; and grants R37AG018915 and P60AG010484 from the National Institute on Aging.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, the National Heart, Lung, and Blood Institute, the National Institute on Aging, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, the National Heart, Lung, and Blood Institute, the National Institute on Aging, or the National Institutes of Health.
Disclosures: The University of Washington Center for Commercialization holds the copyright to the Seattle Heart Failure Model. Drs Schulman and Reed have made available online detailed listings of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp).
References
- 1.Gonseth J, Guallar-Castillón P, Banegas JR, Rodríguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J. 2004;25:1570–95. doi: 10.1016/j.ehj.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Lambrinou E, Kalogirou F, Lamnisos D, Sourtzi P. Effectiveness of heart failure management programmes with nurse-led discharge planning in reducing re-admissions: a systematic review and meta-analysis. Int J Nurs Stud. 2012;49:610–24. doi: 10.1016/j.ijnurstu.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Turner DA, Paul S, Stone MA, Juarez-Garcia A, Squire I, Khunti K. Cost-effectiveness of a disease management programme for secondary prevention of coronary heart disease and heart failure in primary care. Heart. 2008;94:1601–6. doi: 10.1136/hrt.2007.125708. [DOI] [PubMed] [Google Scholar]
- 4. [February 12, 2013];TEAM-HF: Tools for the Economic Analysis of Patient Management Interventions in Heart Failure. http://team-hf.org/.
- 5.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 6.Reed SD, Li Y, Ellis SJ, Whellan DJ, Schulman KA, O'Connor CM, et al. Seattle Heart Failure Model scores significantly predict medical resource use and costs in HF-ACTION.. Presented at: 2011 Annual Scientific Meeting of the Heart Failure Society of America; Boston, Massachusetts. [Google Scholar]
- 7.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 10.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. Oxford University Press; Oxford, United Kingdom: 1996. [Google Scholar]
- 11.Reed SD, Anstrom KJ, Ludmer JA, Glendenning GA, Schulman KA. Cost-effectiveness of imatinib versus interferon-alpha plus low-dose cytarabine for patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. 2004;101:2574–83. doi: 10.1002/cncr.20694. [DOI] [PubMed] [Google Scholar]
- 12.Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–84. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Yao G, Freemantle N, Flather M, Tharmanathan P, Coats A, Poole-Wilson PA, et al. Long-term cost-effectiveness analysis of nebivolol compared with standard care in elderly patients with heart failure: an individual patient-based simulation model. Pharmacoeconomics. 2008;26:879–89. doi: 10.2165/00019053-200826100-00007. [DOI] [PubMed] [Google Scholar]
- 15.Yao G, Freemantle N, Calvert MJ, Bryan S, Daubert JC, Cleland JG. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2007;28:42–51. doi: 10.1093/eurheartj/ehl382. [DOI] [PubMed] [Google Scholar]
- 16.Göhler A, Conrads-Frank A, Worrell SS, Geisler BP, Halpern EF, Dietz R, et al. Decision-analytic evaluation of the clinical effectiveness and cost-effectiveness of management programmes in chronic heart failure. Eur J Heart Fail. 2008;10:1026–32. doi: 10.1016/j.ejheart.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Chan DC, Heidenreich PA, Weinstein MC, Fonarow GC. Heart failure disease management programs: a cost-effectiveness analysis. Am Heart J. 2008;155:332–8. doi: 10.1016/j.ahj.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227–34. doi: 10.1161/01.cir.64.6.1227. [DOI] [PubMed] [Google Scholar]
- 19.Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007;93:476–82. doi: 10.1136/hrt.2006.089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macran S, Weatherly H, Kind P. Measuring population health: a comparison of three generic health status measures. Med Care. 2003;41:218–31. doi: 10.1097/01.MLR.0000044901.57067.19. [DOI] [PubMed] [Google Scholar]
- 21.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]