Abstract
It was investigated whether a standard mouse diet (AIN-76A) supplemented with walnuts reduced the establishment and growth of LNCaP human prostate cancer cells in nude (nu/nu) mice. The walnut-enriched diet reduced the number of tumors and the growth of the LNCaP xenografts; 3 of 16 (18.7%) of the walnut-fed mice developed tumors; conversely, 14 of 32 mice (44.0%) of the control diet-fed animals developed tumors. Similarly, the xenografts in the walnut-fed animals grew more slowly than those in the control diet mice. The final average tumor size in the walnut-diet animals was roughly one-fourth the average size of the prostate tumors in the mice that ate the control diet.
Keywords: LNCaP cells, Prostate cancer, Walnuts, F2-isoprostanes
INTRODUCTION
Individual constituents in whole foods may be factors that contribute to a reduction in cancer risk (1–3). While the experimental data supporting the role of individual phytochemicals in cancer prevention are convincing (4–6), epidemiological studies that have attempted to identify the association between dietary ingredients and cancer have yielded less convincing results (7–10).
Yet, certain whole foods, consumed on a regular basis, may be instrumental in reducing cancer risk (11–14).
The walnut (Juglans regia L) is a whole food that has attracted considerable interest within the last decade because of its reported health benefits. Thus, studies in humans and in animals have documented that consuming walnuts daily reduces parameters of systemic inflammation, improves endothelial-dependent vasodilation, and reduces serum levels of omega-6 fats (15–19). While these clinical studies have been directed toward defining the benefits of walnuts in terms of cardiovascular health, recent experimental investigations also have focused on the ability of walnut consumption to alter cancer growth and development (20–24). Of special note is the work of Hardman and coworkers (21, 23). Hardman and Ion (21) found that feeding nude female mice a walnut-enriched diet slowed the growth of established MDA-MB 231 human breast cancer cells that had been injected subcutaneously into these animals. Likewise, in another study by Hardman and colleagues (23), a walnut diet reduced the incidence of spontaneous mammary tumors in female transgenic mice expressing large T antigen in their mammary tissue (25). In this report, the pregnant dams and their female offspring at weanling were fed either a walnut-enriched diet or a diet not including the walnut supplement. The mice eating the walnut diet had a reduced tumor incidence (fraction of mice with at least one tumor), reduced tumor multiplicity (more than one tumor per mouse), and a lower final tumor size (23). There are a number of constituents in walnuts, as summarized in the Discussion, that may account for their ability to reduce tumor growth.
Considering the findings of Hardman and coworkers (21, 23) documenting that a walnut-enriched diet inhibited the initiation and progression of two types of experimental mammary cancer, we deemed it important to test whether the same diet would influence the growth of prostate cancer xenografts growing in male nude mice. Previous studies had shown that consuming walnuts on a regular basis did not influence serum prostate-specific antigen (PSA) levels but it did improve biomarkers of prostate health in normal men (26, 27).
MATERIALS AND METHODS
Animals
Forty-eight male athymic immunocompromised nude mice (nu/nu), six weeks of age, were purchased from Harlan Labs (Houston, TX). Mice were housed four per cage and after a two-week period of acclimation they were given either a control diet or a walnut-enriched diet ad libitum; 32 mice received the control diet and 16 mice were given the walnut diet to eat. The animals were individually ear-marked and weighed weekly beginning when the cancer cells were inoculated. The animal room was maintained under a light:dark cycle of 12:12 hr and the temperature was controlled at 24°C. Fresh sterile cages, bedding, and water were provided twice weekly. All procedures related to the animals were approved by the Institutional Animal Care and Utilization Committee (IACUC) of the University of Texas Health Science Center, San Antonio, Texas.
LNCaP human prostate cells
LNCaP cells were a gift of Dr. LuZhe Sun (Department of Cellular and Structural Biology, UT Health Science Center, San Antonio, Texas). LNCaP cells are androgen-sensitive adenocarcinoma prostate cells derived from a metastatic site of a 50-year-old Caucasian male. This cell type has been extensively used in experimental oncology to examine growth characteristics of androgen-sensitive prostate cancers and to test the efficacy of various drugs/diets in terms of their ability to inhibit prostate tumor growth. LNCaP cells maintain their malignant properties when grown in nude mice and they are capable of developing tumors at the injection site. LNCaP cells express prostate-specific antigen (PSA) (28).
LNCaP cells were cultured under standard conditions. When a sufficient number of fresh cells became available, 1.5 × 106 cells were subcutaneously inoculated into each of the 48 unanesthetized mice. The inoculated cells were contained in a 0.1 ml 1:1 mixture of incubation medium and Matrigel. Matrigel is a gelatinous mixture that resembles the extracellular environment and is commonly used in studies such as described herein. Matrigel serves as a basement membrane matrix which allows cells to exhibit the complex cellular behavior of the cell type being studied.
Experimental design
Of the 48 mice inoculated with the cancer cells, 32 had been consuming the control diet and 16 had eaten the walnut-enriched diet for several weeks. Following the LNCaP cell inoculations, the animals continued on the same diets.
The reason the group sizes were not the same, i.e., 32 versus 16, is that the original experimental design differed from that of the final study because the percentage of mice on the control diet that developed palpable tumors was less than anticipated. Based on the published literature, it was expected that roughly 75% (24 of 32) of the mice receiving the control diet would eventually develop subcutaneous prostate tumors. The study was originally powered based on this assumption. Had that occurred, these 24 mice were then to be divided in two groups of 12 mice each, one of which would have been switched to the walnut-enriched diet with the other group continuing on the control (nonwalnut) diet. Since only 14 of the 32 mice on the control diet develop palpable tumors, it was the judgment of the investigators that there were an insufficient number of tumor-bearing mice to divide them into two groups and expect to get unambiguous data. One of the 14 mice that developed a particularly large tumor had already died (see below) and a second mouse, also with a large tumor, had begun to lose body weight indicative of pending death. Thus, at this point, all remaining animals were killed (at 126 days after tumor cell inoculation) and the measurements outlined below were performed.
Walnut and control diets
Given that Hardman and colleagues (21, 23) had previously shown that a standard mouse diet enriched with walnuts restrained the growth of MDA-MB 231 human breast cancer cells in nude mice, we opted to use the same diet in the current study. The diets were modified AIN 76A diet as originally described in 1977 by the American Institute of Nutrition (29) but the soybean oil was replaced with corn oil; corn oil contains omega-3 fats which are required by rodents. Corn oil also contains omega-6 fats in higher concentrations than omega-3. The composition of the diets is listed in Table 1. In brief, the diets were formulated to be isocaloric, iso nutrient, and to be relevant to the total amount of walnuts that could be reasonably eaten on a daily basis by humans; thus, the equivalent human diet would include two servings (2 ounces) of walnuts daily which would provide 370 calories or 18.5% of a 2,000 calorie/day diet (30). Walnut kernels were supplied gratis by the California Walnut Commission and 100% corn oil (Hill Country brand, preservative and additive-free) was purchased locally. Walnuts were stored at −20°C until ground and added to the diet.
Table 1 .
Characteristics of the Mouse Food AIN-76-A Diet Modified to 10% w/dry w Corn Oil or 2.63% Corn Oil and 18% of Calories from Walnut
| Walnut Diet (18% of Calories from Walnut)
|
|||||
|---|---|---|---|---|---|
| Control Diet |
Additional Nutrient | ||||
| Contained in 11.3 g | |||||
| Ingredient | % of Weight | Amount /100 g | Amount /100 g | Walnut/100 g diet | Calories /100 g |
| Casein (protein) | 20% | 20 g | 18.3 g | Protein—1.72 g | 80 |
| Sucrose | 45% | 45 g | 45 g | 180 | |
| Corn starch (carbo) | 15% | 15 g | 13.5 g | Carbo—1.55 g | 60 |
| Alpha-Cel (fiber) | 5% | 5 g | 4.8 g | Fiber—0.2 g | 0 |
| Choline bitartrate | 0.2% | 0.2 g | 0.2 g | 0 | |
| DL-methionine | 0.3% | 0.3 g | 0.3 g | 0 | |
| Mineral mix AIN-76 | 3.5% | 3.5 g | 3.5 g | 0 | |
| Vitamin mix AIN-76 | 1.0% | 1 g | 1.0 g | 0 | |
| Ground walnut | 0 | 11.1 g | 0 | ||
| Corn oil (fat) | 10% | 10 g | 2.63 g | Fat—7.37 g | 90 |
| Total | 100% | 100 g | 100.3 g | (0.46 g water in walnut) | 410 |
| n3/n6 | 0% n3, 50% fat is n6 | (1.02 g n3)/(1.3 g + 4.3 g n6) = 0.18 | |||
| Total fat | 10 g | 10.0 g | 90 | ||
| Total protein | 20 g | 20.0 g | 80 | ||
| Total carbohydrate | 60 g | 60.0 g | 240 | ||
Before being added to the diet, whole walnut kernels including the brown husk were ground into a fine powder in a food processor and added to the dry AIN 76A dietary ingredients. Pulverizing the walnuts into a powder prevented the mice from preferentially selecting walnut pieces for consumption. This diet provided an estimated 18% of the calories from walnuts. The control AIN-76A diet was modified to contain 10% wt/dry wt corn oil. Both diets were prepared fresh weekly and provided as a dry powder mixture served in specially designed food containers to reduce wastage (#91007 Feeding jars; #910024 Deluxe lids; #91006 Food follower; Dyets, Inc., Bethlehem, PA). When the mice were given fresh diets, which occurred three times weekly, the remaining food in the containers was removed and discarded. The animals had free access to the diets and water was provided ad libitum throughout the 126-day experimental period. Even though the food was provided in specially designed food containers, the mice managed to scatter some food onto the bottom of the cages so the quantity of food consumed per mouse could not be accurately calculated.
To prevent deterioration or the development of rancidity of the diets, bulk diets were always kept refrigerated and when fed to the animals the diets were changed frequently. We were continuously attentive to any discoloration or smell to the diets that would have suggested rancidity; neither was ever noticed.
Butylhydroxytoluene (BHT) was not added to the diets as a preservative since the intent was to duplicate the walnut diet used in previous studies where it successfully reduced tumor growth (21, 23). Also, since one of the potential mechanisms by which omega-3 fats alter cancer cell growth involves free radical mechanisms, BHT was not added since it may have interfered with these processes.
Monitoring tumor growth
After the subcutaneous inoculation of the cancer cells, the mice were examined daily for the development of a palpable tumor. When a tumor was first identified (usually when a tumor had a diameter of approximately 3 mm), the number of days after LNCaP cell injection was recorded. Following the appearance of a tumor, it was measured (maximal length and maximal width since the tumors were not always circular) no less than twice weekly using a digital display calipers. Tumor measurements required two individuals, one to restrain the mouse and one to make the measurements. The animals were not anesthetized for this procedure. The tumor measurements were done by the following coauthors in various combinations: DXT, LCM, AK, and LFB. Tumor size was calculated using the following formula: length × width2 in mm/2 = volume in mm3.
Animal sacrifice and tissue collection
The experiment ended (after 126 days) when no tumors had appeared in either the control or experimental groups of mice for several weeks and some tumors in the control mice had grown to the size that they were jeopardizing the survival of the host animal. Indeed, one control animal had died at 118 days after tumor cell inoculation. Since this animal had been dead for an estimated 12–18 hr before it was found, the tumor weight of this animal was estimated based on the last size measurement and in comparison with other tumors of known weights.
All remaining control and experimental mice were killed (by cervical dislocation) at 126 days (18 weeks) after tumor cell inoculation. Tumors were dissected and weighed to the nearest milligram. Tumors were fixed in 10% neutral buffered formalin. A portion of each liver was frozen on solid CO2, individually labeled, and stored at −80°C until analyzed for isoprostane levels. At the time of necropsy, the liver and lungs of each mouse were grossly examined for metastases.
F2-isoprostane measurements
F2-isoprostanes, a highly sensitive indicator of lipid peroxidation, were measured using gas chromatography/negative chemical ion mass spectrometry (GC/MS) as described by Morrow and Roberts (31). Briefly, thawed hepatic tissue samples, weighing 150–200 mg each, were homogenized in ice-cold Folch solution (chloroform/methanol; 2:1 ratio) that contained 5 mg/100 mL butylated hydroxytoluene. The lipids were extracted from the homogenate and hydrolyzed with 15% potassium hydroxide. Following acidification with HCl [2H4] 8-Iso-PGF2α, trimethylsilyl ether was added as an internal standard. The F2-isoprostanes were then extracted through a C18- Sep-Pak and a silica Sep-Pak column, converted to pentafluorobenzyl esters, and purified by thin layer chromatography. The F2-isoprostanes were subsequently derivatized to PGF2α derivatives and quantified by GC/MS as previously described (32, 33). The quantities of F2-isoprostanes are expressed as 8-Iso- PGF2α/g hepatic tissue.
Prostate-specific antigen immunohistochemistry
Portions of tumors fixed in 10% neutral buffered formalin were embedded in paraffin and cut on a rotating microtone at 6 microns. After the sections were placed on glass slides, they were stored at room temperature until dried. They were then deparaffinized and treated with antibody [PSA-(clone ER-PR-8) antibody, Dako, Carpinteria, CA] and counterstained (hematoxylin II, Ventana Medical, Tucson, AZ). These procedures were performed in the Department of Pathology, UT Health Science Center, San Antonio, Texas, using a Ventana Benchmark XT IHC stainer. The slides were evaluated by RJR.
Statistical analyses
Statistical analyses of the same data were performed independently by two statisticians, W. Elaine Hardman and William W. Morgan (Professor Emeritus, UT Heath Science Center). Since the analyses varied according to the data being evaluated, the statistics are described in the figure legends. The reader should see the section on Experimental Design to learn how the study was originally powered.
RESULTS
Body weights
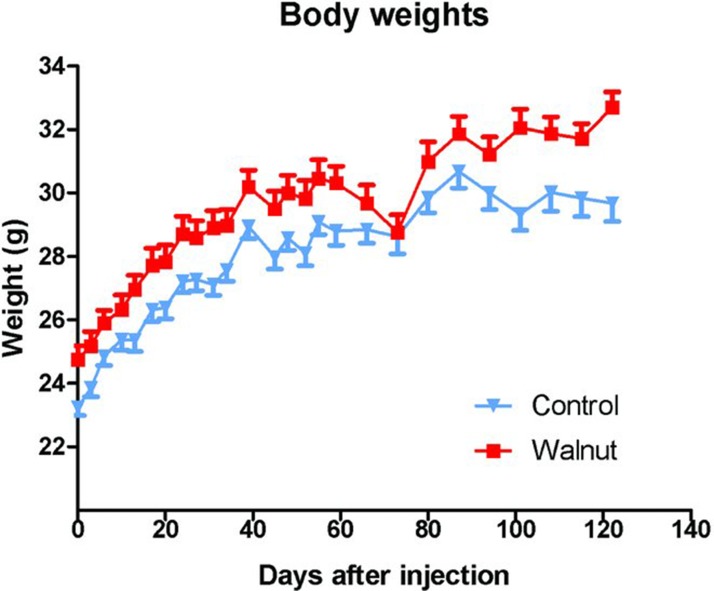
Although the animals were randomly segregated into the control and experimental groups shortly after the animals arrived in the laboratory, at the time of the first body weight measurement (when the tumor cells were inoculated), the mean body weights of the two groups differed (Figure 1); this difference persisted for essentially the duration of the study. In the control animals, the body weights plateaued during the last several weeks of the study, presumably related to the large tumors in some of these animals which compromised their health. Because of the difference in the mean body weights, the rates of change of body weight increases were also calculated each time the body weights were measured. These values were the same for the two groups with the exception of the last body weight measurement where, again, the body weights of the control animals had plateaued, presumably as a consequence of the large tumors in these mice.
Figure 1 .
Body weights (±SEM) of the control-fed and walnut-fed animals following the subcutaneous inoculation of tumor cells. The animals had been randomly divided before the specific diets were initiated and the difference in the body weights was not believed to be due to the diets per se but was coincidental. During the first 100 days of the study, the groups gained body weight at the same rate. We took this as evidence that the mice were consuming equivalent amounts of diet. Near the end of the experimental period (after 100 days), the curves begin to diverge due to the reduced body weights of the large tumor bearing mice. The initial body weights differed with a p < 0.05 (t-test); at most time points thereafter this difference persisted.
The fact that both the control-fed and walnut diet-fed mice gained weight at the same rate was taken as evidence that there was equivalent food intake by the groups. As mentioned, it was not possible to measure individual food consumption because the animals were group-housed. Also, despite the use of specially designed food containers to limit the dispersal of food in the cages, the mice always managed to displace large amounts (50% or more) of the diet from the food containers to the floor of the cages. This would presumably also have been a problem if the animals were individually housed.
Tumor onset, growth, and size
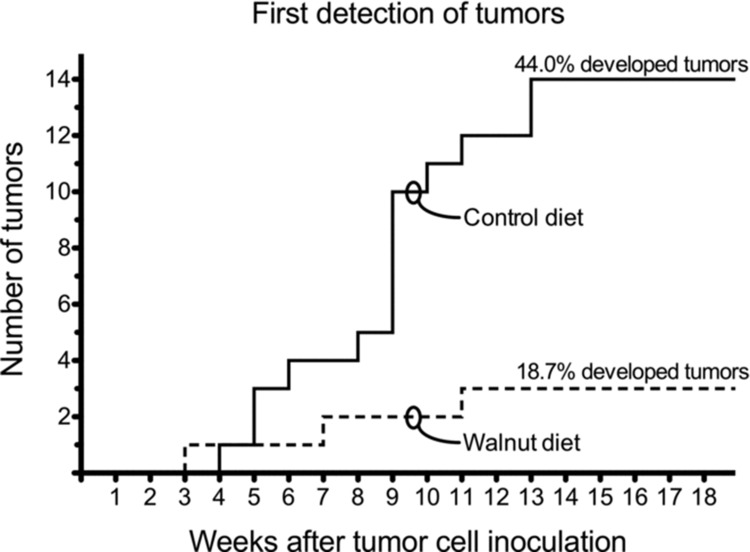
The first palpable tumors appeared three to four weeks after LNCaP tumor cell inoculation (Figure 3). The first recognizable tumor appeared in a walnut-consuming mouse. Thereafter, two additional mice from this group developed palpable tumors during the subsequent 18 weeks such that at the end of the experiment 3 of 16 mice (18.7%) had palpable tumors. In the control group of mice, a palpable tumor first became apparent at four weeks after tumor cell inoculation. After that, tumors began to appear in other animals at an accelerated rate (relative to walnut-diet mice) such that, by 18 weeks, 14 of 32 mice (44.0%) had obvious subcutaneous tumors (Figure 2).
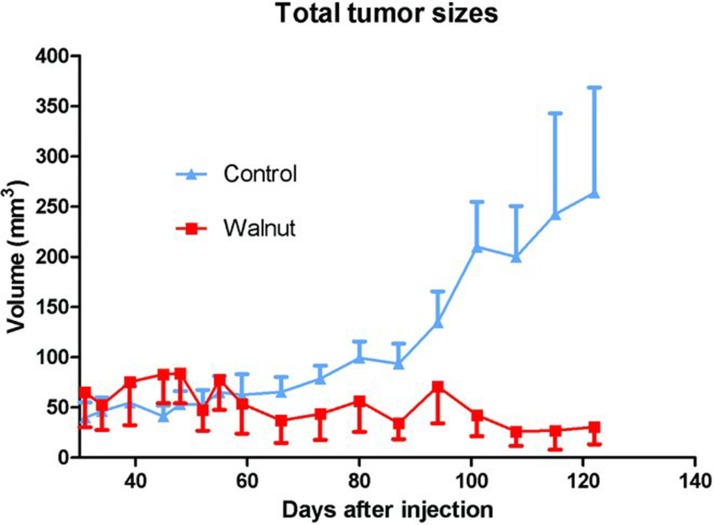
Figure 3 .
Summary of the calculated tumor sizes (±SEM) during the course of the study in the control-fed and walnut-fed mice. The calculated size of the three tumors in the walnut-fed mice remained small throughout the study while those in the control-fed animals continued to grow. The tumor volumes in the control animals near the end of the study varied widely among the animals. These data were analyzed independently by statisticians WEH and William W. Morgan using the Mann–Whitney U and the Kruskal–Wallis test, neither of which yielded statistical significance. This again was likely due to the small number of tumors in the walnut-fed animals and the large variation in tumor sizes.
Figure 2 .
Tumor onset and number of animals that developed tumors in the control and walnut diet-fed mice. The first tumor appeared in the control diet-fed animal during week 3. Thereafter, only two other mice developed overt tumors so 3 of 16 mice (18.7%) had a tumor at the conclusion of the study. Conversely, the first tumor in a walnut-fed mouse was detected during the fourth week. Thereafter, there was a steady increase in the number of mice that developed tumors until the 14th week of the study when 14 of 32 mice (44.0%) had a subcutaneous prostate cancer. These data were independently analyzed by WEH and William W. Morgan using the following procedures: Chi-square test with the Yates correction and two-tailed Fisher's exact test. Using these procedures yielded no statistically significant differences. There was agreement that the small number of tumors (three each) in the walnut-fed animals negated statistical differences.
Tumor growth rates also differed in the two experimental groups. The tumor size in the mice eating a walnut-enriched diet remained small and essentially stable throughout the experimental period (Figure 3). Conversely, the tumors in the mice consuming the control diet began to grow more rapidly; at 60 days after their appearance and thereafter, the tumors in the control-diet mice were always larger than the tumors in the walnut-diet mice. At the conclusion of the study, the calculated volume of the tumors in the control mice was much larger than those of tumors in animals consuming walnuts in their diet (Figure 3). Moreover, the variation in tumor size was much greater in the control mice than in the walnut-diet mice.
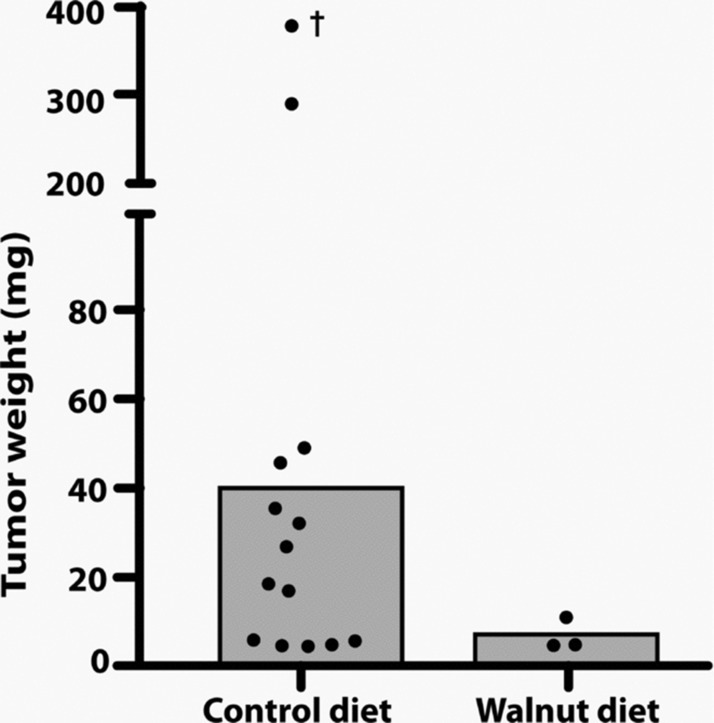
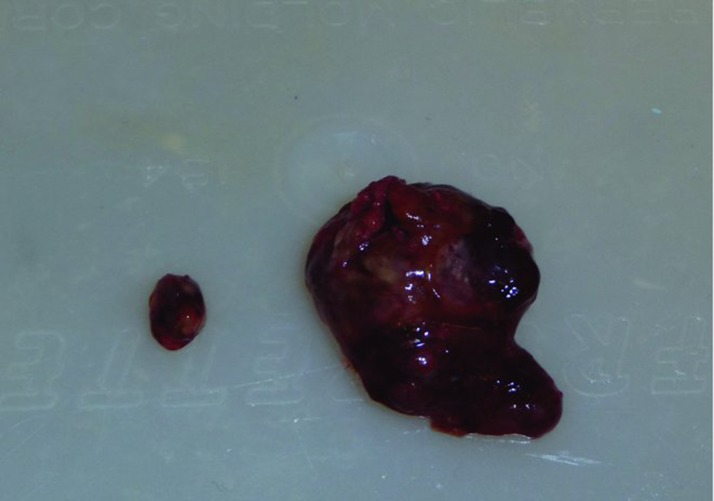
When the tumors were weighed, these values were consistent with the volume measurements as determined using the formula mentioned in the subsection “Monitoring tumor growth.” The mean tumor weight in the animals that did not consume the walnut-enriched diet was 38.5 mg while in those that had eaten walnuts, the average tumor size was 4.7 mg (Figure 4). Figure 5 shows the largest tumor in a nonwalnut consuming diet group in comparison to the average tumor size in a mouse that ate the walnut diet.
Figure 4 .
This figure depicts the final tumor weights in the control-fed and walnut-fed animals at the conclusion of the study. The value identified with a † was estimated and was not used to calculate the mean tumor weight; it was found in a mouse that had been dead for a number of hours. The histograms indicate the mean tumor weights. As with the tumor volume, because of the small number of tumors that grew in the walnut fed mice (three each) and due to the large variation in tumor size, the median tumor weights did not differ between the groups when tested using either the Mann–Whitney U or Kruskal–Wallis tests.
Figure 5 .
Average sized tumor (0.12% of final body weight) from a walnut-fed mouse and the largest tumor (1.24%) from a control mouse at the conclusion of the study. In comparison, the maximal diameter of the small tumor in this figure is 6 mm.
No tumor metastases were grossly apparent in either the liver or lungs of the tumor bearing mice from either group.
F2-isoprostane levels
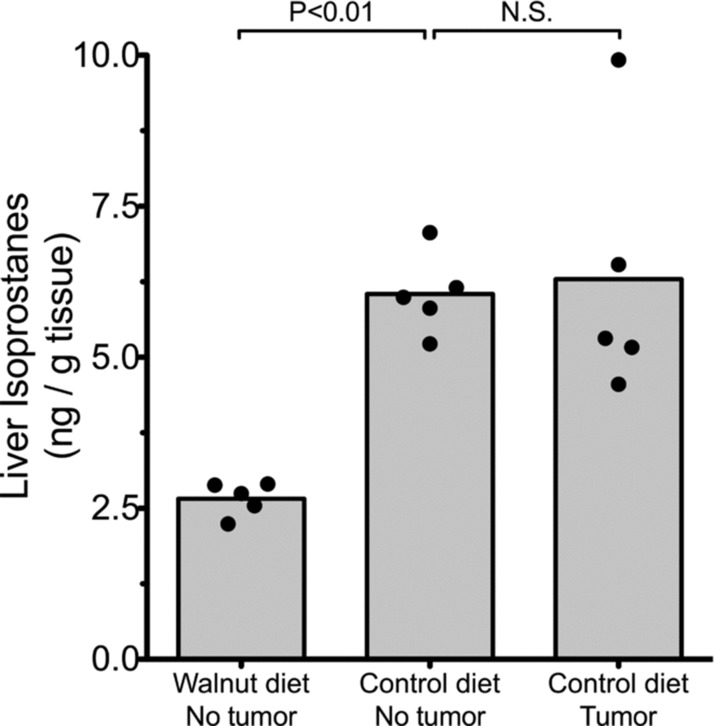
Liver samples of five walnut-fed mice without tumors and 10 liver samples (five without tumors and five with tumors) from control-fed mice were randomly selected for the measurement of F2-isoprostane levels. F2-isoprostanes are a sensitive index of the total oxidative load in animals. These constituents can be measured in any cell as well as in the urine. In the present study, we examined hepatic isoprostane levels as an index of the level of oxidative stress each mouse was experiencing. In the walnut-consuming mice that did not develop tumors, the mean F2-isoprostane levels were about 2.0 ng/g hepatic tissue (Figure 6). These values more than doubled in the mice that consumed the control diet that lacked walnuts; these higher levels of lipid peroxidation products in hepatocytes were independent of whether or not the mice had detectable subcutaneous prostate tumors (Figure 6).
Figure 6 .
Levels of F2-isoprostanes, a measure of lipid peroxidation, in the liver of control-fed and walnut-fed mice. The presence of the tumor in the control-fed mice did not impact the level of hepatic lipid peroxidation products. The walnut diet reduced basal levels of lipid peroxidation (p < 0.05; Kruskal–Wallis test) in the liver.
Protein-specific antigen immunocytochemistry
Immunocytochemically, PSA was apparent at highly different intensities in tumors from both groups of mice. There was no apparent correlation between tumor size and the intensity of immunocytochemically detected PSA. Because there were only three prostate tumors in the mice that consumed walnuts and due to the large variations in PSA levels (which also varied in different areas of any given tumor), it was not possible to reliably determine whether PSA measurements varied between the tumors of different animal groups.
DISCUSSION
Whole foods and dietary ingredients have produced inconsistent results in terms of their impact on the frequency of prostate cancer incidence and/or growth (34–38). Epidemiological studies designed to identify whole foods or dietary supplements that may inhibit prostate cancer also have not always yielded uniform data (39–41). Nevertheless, because of the success of some well-controlled studies in the laboratory setting (42, 43), interest in the use of optimal diets to influence prostate cancer in humans continues (44, 45).
The rationale for the current study was prompted by the observations that supplementing the diet of older men (45–75 years of age) with walnuts improved biomarkers of prostate and vascular health (26). In addition to this report, two studies from the laboratory of Hardman (21, 23) showed that a walnut-enriched diet markedly reduced the growth of subcutaneously-inoculated breast cancer cells and spontaneous tumor development in mice. The obvious implication of these studies is that walnuts contain a specific phytochemical or a group of ingredients that act synergistically to suppress tumor initiation and growth.
The results of the present study document that the consumption of a walnut-enriched diet influences the establishment and growth of LNCaP androgen-sensitive prostate adenocarcinoma xenografts after their subcutaneous inoculation into nude immune-compromised mice. LNCaP tumor cells are human-derived and are widely used in research to identify treatments that modify their growth (28, 46, 47). While the walnut diet did not defer the onset of tumor initiation, it limited the number of mice that developed overt tumors as well as the growth rate and final size of the tumors.
Walnuts have a number of ingredients that could account for their ability to suppress prostate tumor growth (48, 49). Most notably, walnuts contain high levels of omega-3 fatty acids (21); the elevated intake of long-chain [20c eicosapentaenoic acid (EPA) and 22c docosahexaenoic acid (DHA)] fats, in particular, slows cancer growth (50, 51). The walnut diet used in the current study, however, contained essentially no EPA or DHA. Hardman and Ion (21) have documented, however, that the mouse liver has the capability of elongating and desaturating alpha-linolenic acid (ALA) to EPA and DHA, probably in small amounts. On the basis of their most recent investigation in mice, Hardman and colleagues (23) concluded that omega-3 fats by themselves probably did not account for the inhibition of mammary cancer due to a walnut-supplemented diet. The association of fat intake to human prostate cancer remains essentially unknown.
Other walnut ingredients that, theoretically at least, could explain the inhibitory effect of the walnut diet on LNCaP prostate cancer cells inoculated into nude mice include phytosterols (52, 53), gamma-tocopherol (54, 55), carotenoids (56, 57), polyphenolics (58, 59), ellagic acid and its derivatives (60, 61), and melatonin (62, 63). The bulk of these phytochemicals function as antioxidants, which generally are beneficial in terms of cancer suppression (64). While each of these ingredients would likely be absorbed, at least in part, after being consumed in a walnut-enriched diet, this has not been specifically tested with the exception of melatonin. Thus, when rats were fasted for 24 hr and then given exclusively walnuts to eat for 4 hr, serum levels of melatonin rose by roughly fourfold while the total antioxidant status [estimated using the trolox equivalent antioxidant capacity (TEAC) and the ferric-reducing antioxidant power (FRAP)] of the blood doubled (65). In the mice of the present study, however, the experimental design was different in that mice consumed a walnut-enriched diet (not exclusively walnuts) and the animals were not fasted. Thus, whether the walnut-supplemented diet was sufficient to elevate circulating melatonin levels is not known so no claims can be made that the melatonin content of the walnut had any impact on tumor growth. Because the total antioxidant capacity of the blood (the TEAC and FRAP values) rose as a consequence of walnut consumption by rats in the initial study (65) does not mean these increases were exclusively a result of the intake of melatonin, a potent antioxidant (66, 67), but could have been a consequence of any or all of the antioxidants mentioned earlier.
The seemingly most likely explanation for the finding that a walnut-enriched diet forestalled the growth of human prostate cancer cells growing in immune-compromised mice is that the inhibitory effect was a consequence of the combined actions of several phytochemicals in this nut which have been shown individually to inhibit experimental prostate cancer (51–62). Which of these functioned synergistically to suppress prostate cancer cell growth could not be determined from this study.
Cachexia in the two animals that lost weight in the final week of the experiment could theoretically have contributed to the outcome of the study. On the other hand, since the majority of mice were killed before they were at the stage that resulted in the loss of body weight, cachexia probably was not a major confounding factor in this study.
When the current paper was in review, another publication appeared in which a similar walnut-enriched diet, like the one used here, also inhibited prostate cancer growth in mice. In the study in question, Davis et al. (68) found that compared to a control diet lacking walnut, the experimental diet reduced the development of prostate adenocarcinoma in the TRAMP cancer model. Like the present study, this group also closely followed the protocol of Hardman and colleagues (21, 23) in developing the diet that proved successful in inhibiting prostate cancer.
The measurement of hepatic F2-isoprostanes, a sensitive index of oxidative damage to cell membrane lipids (31) also provided important information relative to the antioxidant capacity of walnuts. The lower levels of lipid peroxidation products in the walnut-consuming mice indicated that the basal levels of lipid peroxidation were depressed by the walnut diet. Clearly, the presence of a prostate tumor did not exaggerate the breakdown of hepatic lipids since the mice that consumed the control diet (lacking walnuts) all had elevated lipid peroxidation products in the hepatocytes whether or not the animal had developed a prostate tumor.
The intensity of the PSA immunoreactivity in the prostate cancers did not differ between the control and walnut diet-fed mice. This judgment may not have been totally reliable, however, since only three tumors were available from the walnut-fed mice and within any given tumor, individual cells exhibited widely different levels of PSA immunoreactive product. The failure of PSA levels to be noticeably influenced by walnut consumption is consistent with two previous studies in humans in which circulating levels of PSA were not changed as a result of walnut consumption (26, 27).
ACKNOWLEDGMENTS
The authors are grateful to Dr. LuZhe Sun for the gift of LNCaP cells. RJR was the principal investigator (PI) on the grant. DXT worked closely with the PI on the design and execution of the project. Tumor measurements, recording data, and preparing the diet were performed by DXT, LCM, AK, and LFB. WEH was consulted on various aspects of the experimental design and diet. LCM, AK, and SRC fed and weighed the animals as needed. WQ performed the F2-isonprostane measurements. All individuals participated in the discussion related to the study and all have read and approved the manuscript. WEH and Dr. William W. Morgan performed statistical analyses of data.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by a grant from the American Institute for Cancer Research (grant #09A073).
REFERENCES
- 1.Nath RG, Wu MY, Emami A, Chung FL. Effects of epigallocatechin gallate, L-ascorbic acid, alpha-tocopherol and dihydrolipoic acid on the formation of deoxyguanosine adducts derived from lipid peroxidation. Nutr Cancer. 2010;62:622–629. doi: 10.1080/01635580903532424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skrovankova S. Seaweed vitamins as neutraceuticals. Adv Food Nutr Res. 2011;64:357–369. doi: 10.1016/B978-0-12-387669-0.00028-4. [DOI] [PubMed] [Google Scholar]
- 3.Chahar M, Sharma N, Dobhal MP, Joshi YC. Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev. 2011;5:1–12. doi: 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabi T, Bishoyee A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res Treat. 2009;115:223–229. doi: 10.1007/s10549-008-0118-y. [DOI] [PubMed] [Google Scholar]
- 5.Bautros C, Somasundar P, Pozzak A, Helton S, Espat NJ. Omega-3 fatty acids: investigations from cytokine regulation of pancreatic cancer gene suppression. Arch Surg. 2010;145:515–520. doi: 10.1001/archsurg.2010.91. [DOI] [PubMed] [Google Scholar]
- 6.McNeil C. Vitamin E and prostate cancer: research focus turns to biologic mechanisms. J Natl Cancer Inst. 2011;103:1731–1734. doi: 10.1093/jnci/djr504. [DOI] [PubMed] [Google Scholar]
- 7.Pan SY, Zhou J, Gibbons L, Morrison H, Wen SW. Antioxidants and breast cancer risk—a population-based case-controlled study in Canada. BMC Cancer. 2011;11:372–381. doi: 10.1186/1471-2407-11-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murff HJ, Shu XO, Li H, Yang G, Wu X, Cai H, Wen W, Gao YT, Zheng W. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women; a prospective cohort study. Int J Cancer. 2011;128:1434–1441. doi: 10.1002/ijc.25703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nechuta S, Lu W, Chen Z, Zheng Y, Gu K, Cai H, Zheng W, Shu XO. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:262–271. doi: 10.1158/1055-9965.EPI-10-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Laing J, Zhang L, Zhu X, Liu X, Miao D. Fish consumption and the risk of gastric cancer: systematic review and meta-analysis. BMC Cancer. 2011;11:26–35. doi: 10.1186/1471-2407-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Hacker KD, Bonanome A, Coval SM, Binkowski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in food: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;9B(Suppl):715–885. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 12.Simopaulos AP. The traditional diet of Greece and cancer. Eur J Cancer. 2004;13:219–230. doi: 10.1097/01.cej.0000130011.99148.07. [DOI] [PubMed] [Google Scholar]
- 13.Djuric Z. The Mediterranean diet: effects on proteins that mediate fatty acid metabolism in the colon. Nutr Rev. 2011;69:730–744. doi: 10.1111/j.1753-4887.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontou N, Psaltoponlou T, Panagiotakos D, Diomopoulos MA, Linos A. The Mediterranean diet in cancer prevention: a review. J Med Food. 2011;14:1065–1078. doi: 10.1089/jmf.2010.0244. [DOI] [PubMed] [Google Scholar]
- 15.Kris-Etherton PM, Yu-Path S, Sabate J, Ratcliffe HE, Zhao G, Etherton TD. Nuts and their bioactive constituents: effects on serum lipids and other factors that affect disease risk. Am J Clin Nutr. 1999;70:504S–511S. doi: 10.1093/ajcn/70.3.504s. [DOI] [PubMed] [Google Scholar]
- 16.Ros E, Nunez I, Perez-Heras A. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Etherton TD, Martin KR, West SG, Gillis PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2291–2297. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 18.Davis P, Valachi G, Pagnin E, Shao Q, Gross HB, Calo L, Yokoyama W. Walnuts reduce aortic ET-1 mRNA levels in hamsters fed a high-fat atherogenic diet. J Nutr. 2006;136:428–432. doi: 10.1093/jn/136.2.428. [DOI] [PubMed] [Google Scholar]
- 19.Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E, Deolofeo R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 20.Kaur K, Michael H, Arora S, Harkonen PL, Kumar S. Studies on correlation of antimutagenic and antiproliferative activities of Juglans regia L. J Environ Pathol Toxicol Oncol. 2003;22:59–67. [PubMed] [Google Scholar]
- 21.Hardman WE, Ion G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer. 2008;60:666–674. doi: 10.1080/01635580802065302. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jeronimo C, Silva BM. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48:441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Hardman WE, Ion G, Akinsete JA, Witte TR. Dietary walnut suppressed mammary gland tumorigenesis in the C(3)1 TAg mouse. Nutr Cancer. 2011;63:960–970. doi: 10.1080/01635581.2011.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negi AS, Luqman S, Srivastava S, Krishna V, Gupta N, Darokar MP. Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharm Biol. 2011;49:669–673. doi: 10.3109/13880209.2010.537666. [DOI] [PubMed] [Google Scholar]
- 25.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci USA. 2001;91:11236–11240. doi: 10.1073/pnas.91.23.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaccarotella KJ, Kris-Etherton PM, Stone WL, Bagshaw DM, Fishell VK, West SG, Lawrence FR, Hartman TJ. The effects of walnut intake on factors related to prostate and vascular health in older men. Nutr J. 2008;1:13–22. doi: 10.1186/1475-2891-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon JA, Tanzman JS, Sabate J. Lack of effect of walnuts on serum levels of prostate specific antigen: a brief report. J Am Coll Nutr. 2007;26:317–320. doi: 10.1080/07315724.2007.10719617. [DOI] [PubMed] [Google Scholar]
- 28.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 29.American Institute of Nutrition Report of the American Institute of Nutrition Ad Hoc Committee in Standards for Nutritional Studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 30.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 31.Morrow JD, Roberts LJ., II Mass spectrometric quantitation of F2-isoprostanes in biological fluids and tissues as a measure of oxidative stress. Methods Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Wei W, Zu J, Min F, Wang L, Wang X, Cao S, Tan DX, Qi W, Reiter RJ. Inhibitory effect of melatonin on diquat-induced lipid peroxidation in vivo as assessed by the measurement of F2-isoprotanes. J Pineal Res. 2006;40:326–331. doi: 10.1111/j.1600-079X.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Sun S, Wei W, Fu J, Qi W, Manchester LC, Tan DX, Reiter RJ. Melatonin reduces mortality and oxidatively-mediated hepatic and renal damage due to diquat treatment. J Pineal Res. 2007;42:166–171. doi: 10.1111/j.1600-079X.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. Gamma-tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci USA. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Leary KA, de Pascual-Teresa S, Needs PW, Bao YB, O'Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004;551:245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Tamini RM, Hankinson SE, Campos H, Spiegelman D, Zhang S, Colditz GA, Willett WC, Hunter DJ. Plasma carotenoids, retinal, and tocopherols and risk of breast cancer. Am J Epidemiol. 2005;161:153–160. doi: 10.1093/aje/kwi030. [DOI] [PubMed] [Google Scholar]
- 37.Christy SM, Masher CE, Sloane R, Snyder R, Snyder DC, Lobach DF, Demark-Wahnefried W. Long-term dietary outcomes of the REST START intervention for breast and prostate cancer survivors. J Am Diet Assoc. 2011;111:1844–1851. doi: 10.1016/j.jada.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu J, La Vecchia G, De Groh M, Negri E, Morrison H, Mery L. Dietary cholesterol intake and cancer. Ann Oncol. 2012;23:491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 39.Correa P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981;41(9 Pt 2):3685–3690. [PubMed] [Google Scholar]
- 40.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostate cancer: a case-controlled study in Hawaii. Am J Epidemiol. 1988;127:999–1012. doi: 10.1093/oxfordjournals.aje.a114903. [DOI] [PubMed] [Google Scholar]
- 41.Helzlsauer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, Morris JS, Comstak GW. Association between alpha-tocopherol, gamma-tocopherol, selenium and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 42.Xue L, Lipkin N, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. J Natl Cancer Inst. 1999;91:176–181. doi: 10.1093/jnci/91.2.176. [DOI] [PubMed] [Google Scholar]
- 43.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 44.Ozten-Kandas N, Bosland MC. Chemoprevention of prostate cancer: natural compounds, antiandrogens and antioxidants—in vivo evidence. J Carcinog. 2011;10:27. doi: 10.4103/1477-3163.90438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heber D, Freedland SJ, Jones LW, Nelson WG. Santa Monica, CA: Prostate Cancer Foundation; 2009. Nutrition, Exercise and Prostate Cancer. [Google Scholar]
- 46.Chieu CP, Kokontis JH, Hiipakka RA, Fukuchi J, Lin HP. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J Biomed Sci. 2011;18:63–74. doi: 10.1186/1423-0127-18-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedrichs W, Ruparel SB, Marciniak RA, de Graffenried L. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr Cancer. 2011;63:771–777. doi: 10.1080/01635581.2011.570892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardman WE. (n-3) Fatty acids and cancer therapy. J Nutr. 2004;134:3427S–3430S. doi: 10.1093/jn/134.12.3427S. [DOI] [PubMed] [Google Scholar]
- 49.Connolly JM, Coleman M, Rose DP. Effects of dietary fatty acids on DU145 human prostate cell growth in athymic nude mice. Nutr Cancer. 1997;29:114–119. doi: 10.1080/01635589709514611. [DOI] [PubMed] [Google Scholar]
- 50.Huang YC, Jessup JM, Forse RA, Flickner S, Pleskow D, Anastopoulos HT, Ritter V, Blackburn GL. n-3 fatty acids decrease colonic epithelial cell proliferation in high-risk bowel mucosa. Lipids. 1996;31:S313–S317. doi: 10.1007/BF02637099. [DOI] [PubMed] [Google Scholar]
- 51.Hardman WE, Barnes CJ, Knight CW, Cameron IL. Effects of iron supplementation and ET-18-OCH3 on MDA-MB 231 breast carcinomas in nude mice consuming a fish oil diet. Br J Cancer. 1997;76:347–354. doi: 10.1038/bjc.1997.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradford PG, Awad AB. Phytosterols as anticancer compounds. Mol Nutr Food Res. 2007;51:339–350. doi: 10.1002/mnfr.200600164. [DOI] [PubMed] [Google Scholar]
- 53.Awad AB, Fink CS, Williams H, Kim U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur J Cancer. 2001;10:507–513. doi: 10.1097/00008469-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Campbell SE, Musich RR, Whaley SG, Stimmel JB, Leesnitzer LM, Dussus-Babus S, Duffourc M, Stone W, Newman RA, Yang P, Kirshnan K. Gamma-tocopherol upregulates the expression of 15-S-HETE and induces growth arrest through a PPAR gamma-dependent mechanism in PC-3 prostate cancer cells. Nutr Cancer. 2009;61:649–662. doi: 10.1080/01635580902825654. [DOI] [PubMed] [Google Scholar]
- 55.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong KN, Yang CS. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31:533–542. doi: 10.1093/carcin/bgp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang CM, Yen YT, Huang CS, Hu ML. Growth inhibitory efficacy of lycopene and β-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol Nutr Food Res. 2011;55:606–612. doi: 10.1002/mnfr.201000308. [DOI] [PubMed] [Google Scholar]
- 57.Ford NA, Elsen AC, Zuniga K, Lindshield BL, Erdman JW. Jr. Lycopene and apo-12′-lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. Nutr Cancer. 2011;63:256–263. doi: 10.1080/01635581.2011.523494. [DOI] [PubMed] [Google Scholar]
- 58.Malik A, Azam S, Hadi N, Hadi SM. DNA degradation by water extract of green tea in the presence of copper ions: implications for anticancer properties. Phytother Res. 2003;17:358–363. doi: 10.1002/ptr.1149. [DOI] [PubMed] [Google Scholar]
- 59.Chen KC, Peng CC, Peng RY, Su CH, Chiang HS, Yan JH, Hseih-Li HM. Unique Formosan mushroom Antrodia camphorata differentially inhibits androgen-responsive LNCaP and –independent PC-3 prostate cancer cells. Nutr Cancer. 2007;57:111–121. doi: 10.1080/01635580701268360. [DOI] [PubMed] [Google Scholar]
- 60.Malik A, Afaq S, Shahid M, Akhtar K, Assiri A. Influence of ellagic acid on prostate cancer cell proliferation: a caspase-dependent pathway. Asian Pac J Trop Med. 2011;8:550–555. doi: 10.1016/S1995-7645(11)60144-2. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez-Sarrias A, Gimenez-Bastida JA, Garcia-Conesa MT, Gomez-Sanchez MB, Garcia-Talavera NV, Gil-Izquierdo A, Sanchez-Alvarez E, Fontana-Compiano LO, Morga-Egea JP, Pastor-Quiranta FA, Martinez-Diaz F, Tomas-Barberan FA, Espin JC. Occurrence of urolithins and gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res. 2010;54:311–322. doi: 10.1002/mnfr.200900152. [DOI] [PubMed] [Google Scholar]
- 62.Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Siddiqui IA, Mukhtar H, Ahmad M. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res. 2011;50:140–149. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tam CW, Shiu SYW. Functional interplay between melatonin receptor-mediated antiproliferative signaling and androgen receptor signaling in human prostate epithelial cells: potential implications for therapeutic strategies against prostate cancer. J Pineal Res. 2011;51:297–312. doi: 10.1111/j.1600-079X.2011.00890.x. [DOI] [PubMed] [Google Scholar]
- 64.Khondrika L, Kumer B, Koul S, Maroni P, Kaul HK. Oxidative stress and prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reiter RJ, Manchester LC, Tan DX. Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition. 2005;21:920–924. doi: 10.1016/j.nut.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 67.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physiocochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 68.Davis PA, Vasu VT, Gohil K, Kim H, Khan IH, Cross CE, Yokoyama W. A high-fat diet containing whole walnuts (Juglans regia) reduces tumor size and growth along with plasma insulin-like growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. Br J Nutr. 2013;108:1764–1772. doi: 10.1017/S0007114511007288. [DOI] [PMC free article] [PubMed] [Google Scholar]