Abstract
The peptidomimetic LTX109 (arginine-tertbutyl tryptophan-arginine-phenylethan) was previously shown to have antibacterial properties. Here, we investigated the activity of this novel antimicrobial peptidomimetic on the yeast Saccharomyces cerevisiae. We found that LTX109 was an efficient fungicide that killed all viable cells in an exponentially growing population as well as a large proportion of cells in biofilm formed on an abiotic surface. LTX109 had similar killing kinetics to the membrane-permeabilizing fungicide amphotericin B, which led us to investigate the ability of LTX109 to disrupt plasma membrane integrity. S. cerevisiae cells exposed to a high concentration of LTX109 showed rapid release of potassium and amino acids, suggesting that LTX109 acted by destabilizing the plasma membrane. This was supported by the finding that cells were permeable to the fluorescent nucleic acid stain SYTOX Green after a few minutes of LTX109 treatment. We screened a haploid S. cerevisiae gene deletion library for mutants resistant to LTX109 to uncover potential molecular targets. Eight genes conferred LTX109 resistance when deleted and six were involved in the sphingolipid biosynthetic pathway (SUR1, SUR2, SKN1, IPT1, FEN1 and ORM2). The involvement of all of these genes in the biosynthetic pathway for the fungal-specific lipids mannosylinositol phosphorylceramide (MIPC) and mannosyl di-(inositol phosphoryl) ceramide (M(IP)2C) suggested that these lipids were essential for LTX109 sensitivity. Our observations are consistent with a model in which LTX109 kills S. cerevisiae by nonspecific destabilization of the plasma membrane through direct or indirect interaction with the sphingolipids.
Introduction
Infections caused by pathogenic yeast such as Candida spp. affect a large number of immunosuppressed patients and are an increasing medical problem [1], [2]. Fungal infections are currently treated with one of four major classes of antifungals. Azoles target ergosterol synthesis [3], polyenes bind to ergosterol in the cell membrane and form pores [4], [5], echinocandins inhibit cell wall synthesis [6], and 5-fluorocytosine interferes with protein and DNA synthesis [7].
Decreased susceptibility to the most frequently used antifungal, fluconazole, has recently been reported, and the number of nonsusceptible C. glabrata isolates from humans is increasing [8], [9]. Resistance towards 5-fluorocystosine is also rapidly developing [10]. Polyenes can be toxic [11] and echinocandins have a narrow spectrum of activity [12]. An additional complication in the treatment of nosocomial fungal infections is the frequent formation by fungi of sessile communities called biofilms in association with medical implants [13]. Limited nutrient access leads to slow-growing, antibiotic tolerant cells in biofilms that can serve as a reservoir for infection [14], [15]. Most systemic antifungals are fungistatic against yeasts, so they are primarily effective against actively growing cells and have poor activity against cells in biofilms.
The limited number of antifungal classes and drugs with fungicidal properties raises the need for novel drugs with activity against slow-growing and biofilm-forming pathogenic fungi [16], [17]. Antimicrobial peptides (AMPs) and modified forms of AMPs offer an attractive alternative to conventional antifungal drugs. AMPs are cationic and amphipathic peptides of 12–50 amino acids that are produced by species in almost every kingdom and phylum of life [18]. The amphipathic structure of AMPs suggests that they might have targets that are different from conventional antifungals [19], [20]. The high degradation rate of many natural AMPs can be circumvented by backbone and side chain alterations that create structural analogs that mimics natural peptides [21]. A number of synthesized peptidomimetics have in vitro antifungal activity, making these compounds attractive candidates for novel antifungal drugs [22]–[24].
We tested the antifungal activity of the short, antibacterial peptidomimetic LTX109 (arginine-tertbutyl tryptophan-arginine-phenylethan). LTX109 is based on an Arg-Trp-Arg sequence found in the AMP bovine lactoferricin and was originally developed as an antibacterial [25]–[27].
We used killing kinetics to describe the antimicrobial effect of LTX109 and investigated its mode of action by measuring transport of H+, K+, amino acids and a fluorescent dye across the cell membrane. To uncover potential molecular targets that would explain the fungicidal activity of LTX109, we screened a Saccharomyces cerevisiae gene deletion library for mutants resistant to LTX109. Most mutations that led to LTX109 resistance were in genes involved in the synthesis of the sphingolipids mannosylinositol phosphorylceramide, MIPC, and mannosyl di-(inositol phosphoryl) ceramide, M(IP)2C. These results indicate that M(IP)2C and/or MIPC in the plasma membrane are essential for the action of LTX109.
Materials and Methods
Strains, growth media and antifungal drugs
The S288c S. cerevisiae strain M3750 (MAT a ura3-52) [28] was used as the reference strain in all experiments unless otherwise indicated, while the barcode-tagged deletion-mutant library was from Johnston and coworkers [29]. Σ1278b (10560-2B; MAT a ura3-52 leu2:hisG his3:hisG) was used for biofilm susceptibility experiments [30]. Complex YPD medium [31] was used in all experiments except for amino acid release and biofilm where cells were grown in synthetic complete medium [31]. LTX109 (Lytixar; LytixBiopharmaAS, Oslo, Norway) and amphotericin B (Sigma) were dissolved in water and stock solutions were kept at −20°C.
Broth microdilution minimal inhibitory concentrations
Minimal inhibitory concentration (MIC) values were measured under static conditions in polystyrene microtiter plates. Two-fold dilution series of antifungal drug were prepared in fresh YPD medium and distributed to microtiter-plate wells. Overnight cultures of wild type (WT) S288c were diluted and added to antifungal-containing wells to a final concentration of 2×105 cells/ml. Growth inhibition was recorded with absorbance at 600 nm after 24 hours at 30°C. The lowest drug concentration resulting in 90% growth inhibition was the MIC. MIC values of LTX109 were determined three times with triplicate measurements, while MIC values of amphotericin B was determined once with triplicate measurements.
Killing kinetics
Overnight cultures of S. cerevisiae were diluted in fresh, preheated YPD to 4×105 cells/ml and incubated at 30°C with aeration. Exponential growth phase cells were challenged with LTX109 or amphotericin B at concentrations that were five times the MIC. Control samples were treated with water to ensure that cells applied in the time-kill experiment were in exponential growth phase. Samples were taken at the indicated time points, diluted 10-fold, and plated on YPD agar to determine colony forming units (CFUs). The time-kill experiment was conducted in triplicates.
Acidification assay
Glucose-induced acidification was measured as previously described [32] with modifications. Exponentially growing S. cerevisiae cells were washed and resuspended in sterile water to a final concentration of 108 cells/ml. Cells were subsequently incubated with LTX109 (100 µg/ml) or water (control) for 10 minutes before the assay was initiated by addition of glucose to a final concentration of 2% (w/v). The assay was conducted in triplicate at room temperature with continuous magnetic stirring. The assay was stopped by sampling at indicated time points, followed by immediate centrifugation (2000×g for two minutes). pH of the resulting supernatants was measured and changes in extracellular H+ concentration were calculated by applying the obtained values to the equation pH = −log [H+].
Potassium release
Exponentially growing S. cerevisiae were harvested and resuspended in sterile water as described above. The potassium release assay was initiated by addition of LTX109 to a final concentration of 10 times the MIC. Samples treated with water instead of LTX109 served as control. The assay was stopped by centrifugation of samples (13,000×g for 1 min) at indicated time points. Supernatants were transferred to sterile microtubes for spectrometric analysis. Potassium concentrations were measured with a FLM3 flame photometer (Radiometer). A standard concentration curve was generated from diluted S3336 urine flame standard (Radiometer). For spectrometric analysis, 5 µl of sample was added to 1000 µl of S3336 lithium solution (Radiometer). Experiments were carried out in triplicates at room temperature.
SYTOX Green uptake
SYTOX Green uptake was measured as previously described [33] with modifications. Exponentially growing S. cerevisiae cells were centrifuged, washed and suspended in 5 µM SYTOX Green (Life Technologies) to a final concentration of 108 cells/ml. LTX109 or water (control) was added to cell suspensions and SYTOX Green uptake was recorded microscopically after 4, 8, 16, 32, 64 and 128 minutes. Fluorescence was recorded with a Nikon Eclipse (Tokyo, Japan) fluorescence microscope equipped with a F36–525 EGFP HC-filter set (AHF Analysentechnik). Experiments were carried out at room temperature. SYTOX green uptake upon LTX109 treatment was observed in three independent experiments.
Amino acid release
Exponentially growing S. cerevisiae were harvested and suspended in sterile water or water with 10 times the MIC of LTX109 to a final concentration of 2×106 cells/ml. Loss of free amino acids from cells was recorded at room temperature after 16 minutes LTX109 exposure by instant centrifugation and subsequent HPLC of the cell free supernatant. Amino acids were detected and quantified by reverse-phase HPLC using an LKB-Alpha Plus amino-acid analyzer and a mixture of L-α-amino acids, 1 nmol each, as standards. The experiment was repeated three times.
Identification of LTX109-resistant mutants
Haploid knockout mutants of approximately 4000 nonessential genes in the S288c deletion mutant library [29] were pooled. About 106 cells from the mutant pool were transferred to YPD agar containing 10 times the MIC of LTX109. After 72 hours at 30°C, 17 colonies were picked from the LTX109 plates. LTX109-resistant clones were identified by PCR amplification and Sanger sequencing of the unique barcode tag of each mutant. PCR templates were DNA from clonal isolates of LTX109-resistant mutants. Primers were 5′-GATGTCCACGAGGTCTCT and 5′-CTGCAGCGAGGAGCCGTAAT. Gene deletions were identified using barcode sequences in the Stanford SGD deletion database.
Spot test
Exponentially growing S. cerevisiae cells were diluted to 107 cells/ml and 6 µl of a 10-fold dilution series was spotted on YPD agar and YPD agar containing 10 times the LTX109 MIC. Plates were incubated for 24 hours at 30°C and growth results were recorded.
Biofilm susceptibility
S. cerevisiae (Σ1278b) cells were grown in Lab-Tek™ Chamber Slide™ System; Permanox® (NUNC, Denmark) [34], [35] in 1 ml synthetic complete medium. Cells were initially allowed to form biofilm for 12 hours before LTX109 was added for 5 hour in a concentration of 0 or 70 µg/ml. The biofilm was subsequently stained 15 minutes with Syto 9 (Invitrogen, Irvine, CA) for life cell staining and propidium iodine for dead cell staining before confocal laser scanning microscopy (CLSM). Imaging was carried out using a 63x/0.95NA water immersion lens. CLSM was performed with a Zeiss LSM510 microscope. Staining of biofilm treated with LTX109 was repeated in four independent experiments.
Results
Fungicidal properties of LTX109
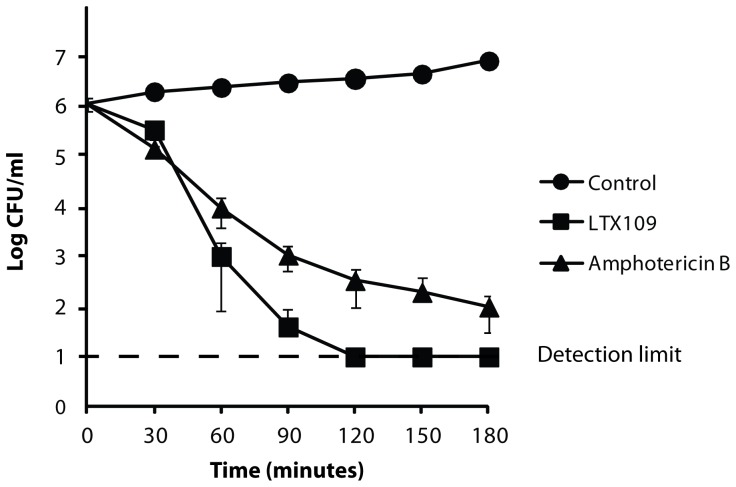
We tested the antifungal properties of the peptidomimetic LTX109 on the yeast S. cerevisiae using microdilution. LTX109 had antifungal activity against S. cerevisiae at 2×105 cells/ml with a MIC value of 8 µg/ml compared to 2 µg/ml amphotericin B. Assay to determine the killing kinetics of LTX109 against S. cerevisiae revealed rapid and efficient fungicidal properties resulting in a 3-log reduction in viable cells within one hour, while amphotericin B required 90 minutes to achieve a similar fungicidal effect when using drug concentrations in multiples of MIC (Fig. 1). Additionally, LTX109 reduced the yeast population to the detection limit within only 2 hours, an effect that was not achieved by amphotericin B in the first 3 hours of exposure.
Figure 1. Fungicidal properties of LTX109 and amphotericin B.
Time-kill kinetics of exponentially growing yeast cells exposed to water (circles) or five times the MIC of LTX109 (40 µg/ml) (squares) or amphotericin B (10 µg/ml) (triangles). Viability was examined every half hour as CFUs. Each data point is the average of three individual measurements ± standard deviation.
Exposure to LTX109 disrupts plasma membrane integrity
The speed with which LTX109 killed S. cerevisiae suggested that the compound was acting directly on the plasma membrane. To investigate the effect of LTX109 on plasma membrane integrity, we measured H+ efflux, ion loss, loss of amino acids and uptake of the fluorophore SYTOX Green across the plasma membrane.
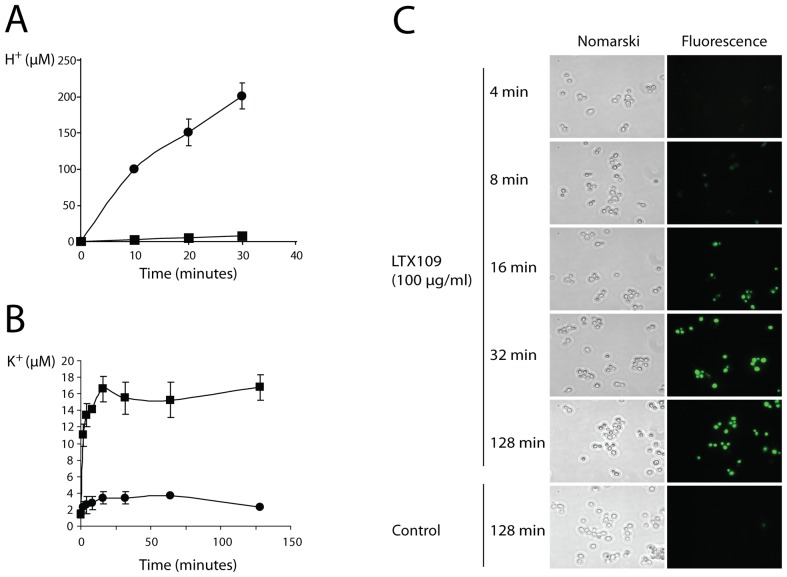
Yeast cells treated with glucose acidify their surroundings primarily by active transport of H+ by the plasma membrane H+-ATPase [36]. We found that glucose-induced acidification was completely absent when cells were treated with LTX109 for 10 minutes before glucose addition (Fig. 2A). These results suggested that LTX109 decoupled the plasma membrane potential directly or indirectly by inhibition of e.g. ATP synthesis.
Figure 2. Transport of H+, K+ and a fluorescent dye by cells treated with LTX109.
(A) Glucose-induced acidification of medium by yeast cells. Exponentially growing S. cerevisiae was washed and suspended in sterile water and exposed to 100 µg/ml LTX109 (squares) or water (circles) before glucose addition at time zero. Medium pH was measured and H+ concentration calculated from pH = −log [H+]. Each data point is the average of three individual measurements with standard deviations as error bars. (B) Potassium release from yeast cells. Exponentially growing yeast cells were washed, resuspended in water, and challenged with 100 µg/ml LTX109 (squares) or water (circles) at time zero. Potassium release was measured using flame atomic absorption spectrometry in binary increasing intervals. Each data point is the average of three individual measurements ± standard deviation. (C) Nomarski (left) and fluorescent (right) microscopy of SYTOX Green-stained yeast cells. Exponential growing cells were exposed to 100 µg/ml LTX109 and SYTOX Green uptake was monitored. Cells treated with SYTOX Green and 0 µg/ml LTX109 served as control. SYTOX green uptake upon LTX109 treatment was observed in three independent experiments.
We next tested the loss of potassium from cells treated with LTX109. Potassium release occurred immediately and increased during the first 16 minutes of exposure to LTX109, reaching a steady state that was more than four times higher than the maximum of the untreated control (Fig. 2B). Much of the K+ that was lost was detected within the first two minutes of challenge with a high LTX109 concentration. These results suggested that LTX109 acted by direct interaction with and disturbance of the plasma membrane rather than through indirect inhibition of metabolism or another intracellular pathway.
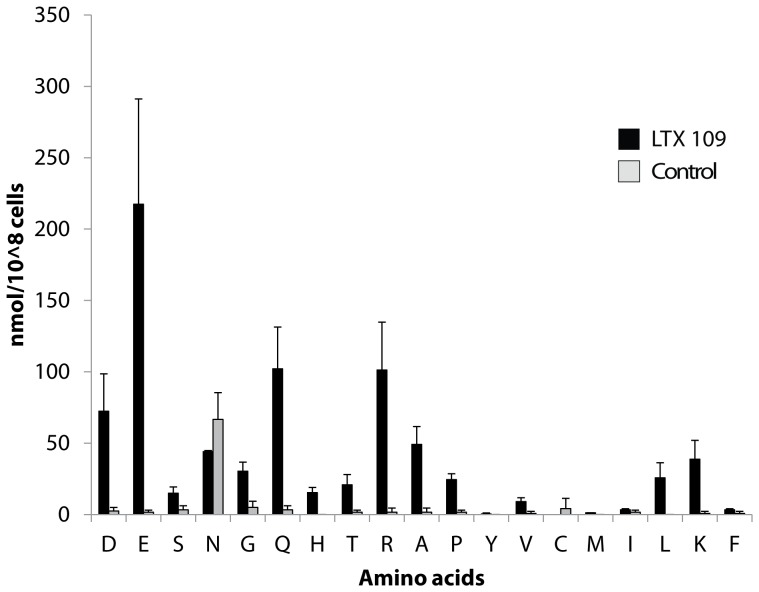
To investigate if LTX109 treatment also led to loss of other small molecules, cells were treated with LTX109 for 16 minutes and free amino acids measured in the extract. Yeast cells treated with LTX109 lost substantial amounts of at least 14 different amino acids whereas cells treated with water only leaked aspartate (Fig. 3). The loss corresponds well to the pool of intracellular amino acids found in other experiments [37], suggesting that most if not all free amino acids are depleted from cells treated with LTX109.
Figure 3. Efflux of amino acids from cells treated with LTX109.
Exponentially growing yeast cells were washed, resuspended in water, and challenged with 70 µg/ml LTX109 (black bars) or water (grey bars) for 16 minutes. Amino acids (one letter code) in the extracellular medium were subsequently measured by HPLC. Each data point is the average of three individual measurements ± standard deviation.
We finally investigated if the membrane potential decoupling was a consequence of plasma membrane destabilization by monitoring the uptake of the 600-Dalton nucleic acid stain SYTOX Green. SYTOX is an inorganic compound that fluoresces upon DNA binding and SYTOX Green can only enter a cell and fluoresce if the plasma membrane is compromised [38]. We found that untreated cells were impermeable to SYTOX Green, while cells treated with LTX109 became permeable. The dye was visible in the nucleus of LTX109-treated cells after only eight minutes (Fig. 2C) and fluorescence increased with LTX109 exposure time.
Defects in sphingolipid synthesis lead to LTX109 resistance
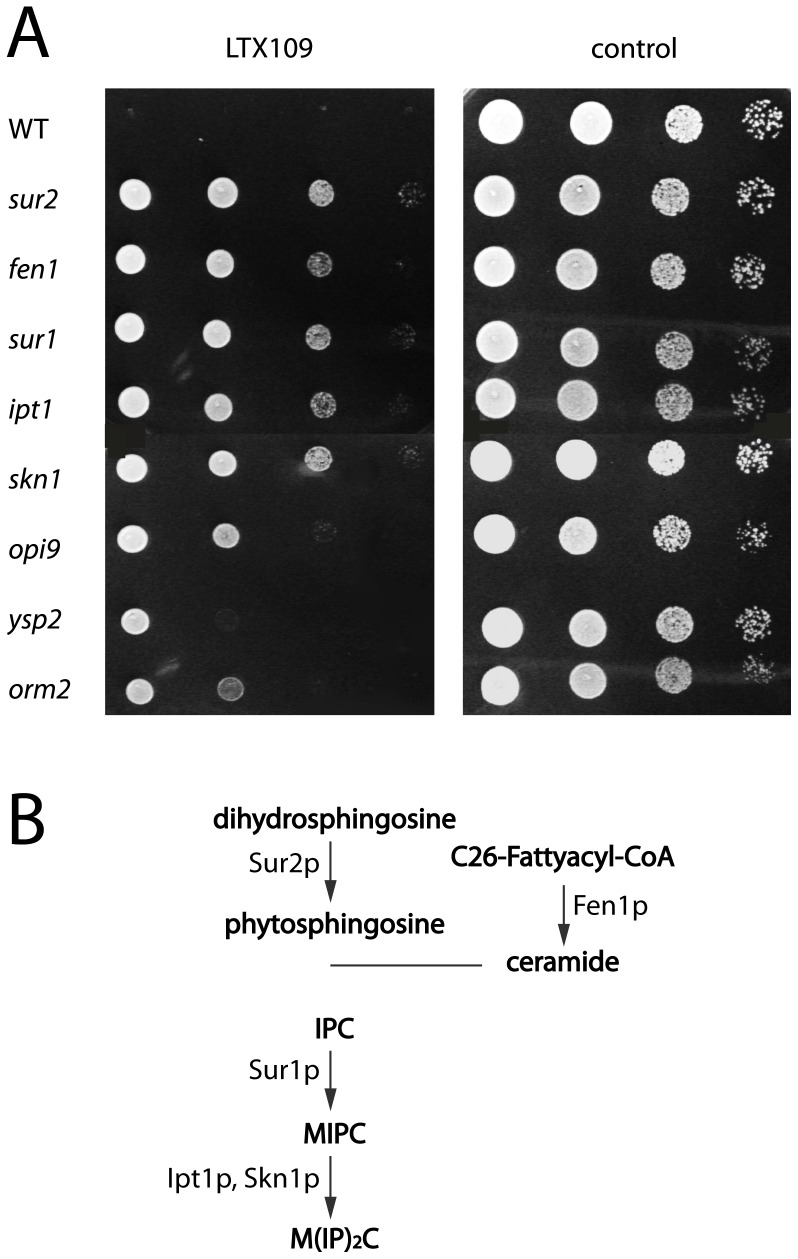
To gain further insight into the LTX109 mode of action, we screened a collection of haploid S. cerevisiae knockout mutants for LTX109 resistance. We isolated 17 mutants that were resistant to LTX109 at 10 times the MIC on YPD agar. Eight genes conferred LTX109 resistance when deleted (Table 1; Fig. 4A). Six of the identified genes (SUR1, FEN1, SUR2, IPT1, SKN1, ORM2) were involved in the biosynthesis of sphingolipids, which are a major plasma membrane component. Fen1p and Sur2p are involved in synthesis of ceramides, which are precursors for inositol phosphoceramide (IPC), the first complex sphingolipid in the synthesis pathway [39] (Fig 4B). Fen1p elongates long-chain fatty acids that are linked to a sphingoid base to form ceramides [40] and Sur2p hydroxylates dihydrosphingosine (DHS) to form phytosphingosine (PHS) [41], which is the most abundant sphingoid base in yeast ceramides [39]. Sur1p mannosylates IPC to form the intermediate sphingolipid mannose inositol phosphoceramide (MIPC) [42] and Skn1p and Ipt1p have similar functions in the biosynthesis of the terminal sphingolipid mannosyl di-inositol phosphorylceramide (M(IP)2C) [43]. Orm2p is a regulator of the sphingolipid biosynthesis that links the biosynthesis to the regulatory Target Of Rapamycin pathway [44]. Mutants that fail to activate Orm2p have reduced levels of sphingolipids as do fen1, sur1, ipt1 and skn1 mutants [40], [43], [45], [46], suggesting a role of sphinolipids in sensitivity to LTX109.
Table 1. S. cerevisiae genes that confer LTX109 resistance upon deletion.
| Deleted gene and function | ORF | Gene product | n |
| Sphingolipid biosynthesis | |||
| SUR1 | YPL057C | Mannosylinositol phosphorylceramide (MIPC) synthase catalytic subunit | 2 |
| SUR2 | YDR297W | Sphinganine C4-hydroxylase | 8 |
| ORM2 | YLR350W | Sphingolipid homeostasis. Interacts with serine palmitoyl transferase (SPT) | 1 |
| IPT1 | YDR072C | Inositolphosphotransferase, involved in synthesis of mannose-(inositol-P)2-ceramide (M(IP)2C) | 1 |
| FEN1 | YCR034W | Involved in membrane-bound fatty acid elongation up to 24 C (ceramide precursor) | 1 |
| SKN1 | YGR143W | Involved in the terminal M(IP)C →M(IP)2C process | 2 |
| Apoptosis | |||
| YSP2 | YDR326C | Mitochondrial protein in programmed cell death. | 1 |
| Unknown function | |||
| OPI9 | YLR338W | Dubious ORF unlikely to encode a protein. Partly overlaps VRP1 | 1 |
n, number of mutants identified.
Figure 4. Mutants in sphingolipid biosynthesis are resistant to LTX109.
(A) Spot test of wild type (WT) S. cerevisiae and eight deletion mutants identified by screening a deletion collection for LTX109 resistance. Exponentially growing yeast was resuspended in YPD to 107 cells/ml and serially diluted 1∶10. Aliquots (6 µl) were spotted on solid YPD plates containing 70 µg/ml LTX109 (left panel), and without LTX109 (right panel). (B) S. cerevisiae sphingolipid biosynthetic pathway.
One LTX109-resistant mutant was affected in the YSP2 gene, which is involved in apoptosis, and another was affected in OPI9. OPI9 has an unknown function but partly overlaps with VRP1, which encodes an actin-associated protein with a role in actin filament organization. The opi9 mutant therefore also has a partial deletion of VRP1, so the LTX109-resistance phenotype could be caused by loss of Vrp1p activity. Resistance of each mutant was confirmed by spot-testing diluted yeast suspensions on YPD agar containing LTX109 (Fig. 4A). Five of the mutants affected in sphingolipid biosynthesis showed similar, high resistance towards LTX109 (sur1, fen1, sur2, ipt1 and skn1).
LTX109 efficiently kill S. cerevisiae growing as biofilm
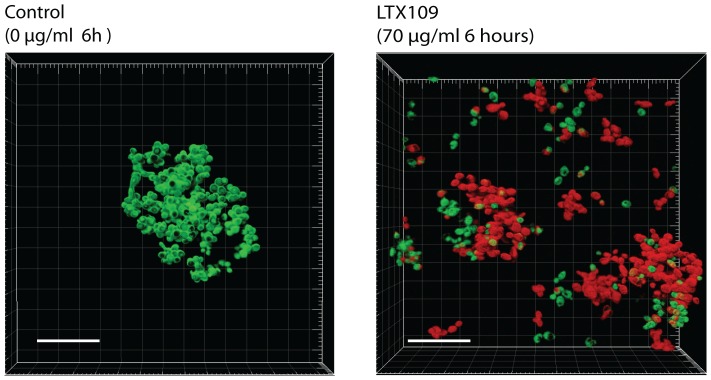
Because S288c is incompetent of biofilm growth [47], [48] we used the Σ1278b strain background to test the antifungal activity of LTX109 against S. cerevisiae biofilm. To visualize the antifungal properties of LTX109, we used CLSM in combination with Syto 9 DNA viability stain and propidium iodide that only penetrates damaged cell membranes. Intermediate (12 h) S. cerevisiae biofilm grown in batch culture slides were treated with 10 times MIC LTX109 for 5 hours before LIVE/DEAD staining and CLSM (Fig. 5). The LTX109 treatment killed the majority of the biofilm population as indicated by uptake and staining of dead cells with propidium iodide (Fig. 5), suggesting that LTX109 is also an efficient anti-biofilm agent in addition to its fungicidal activity against planktonic cells in exponential growth phase.
Figure 5. Activity of LTX109 against yeast biofilm.
Confocal Laser Scanning Microscopy of S. cerevisiae (Σ1278b) biofilm. Cells were grown in Lab-Tek™ Chamber Slide™ System; Permanox - (NUNC, Denmark) in 1 ml synthetic complete medium After 12 hours, the cells were exposed to 0 µg/ml LTX109 (control) or 70 µg/ml LTX109 for another 5 hours. The biofilm cells were then stained with Syto9 (green) and propidium iodide (red) LIVE/DEAD stain before confocal laser scanning microscopy. Images are 3D reconstructions of biofilm made from 2 µm thick images in stacks of 20 individual images. CLSM was perform with a Zeiss LSM510 microscope using a 63x/0.95NA a water immersion lens. Life dead staining of biofilm treated with LTX109 was repeated in four independent experiments. White bar is 30 µm.
Discussion
This study demonstrated the fungicidal activity of the peptidomimetic LTX109. Antimicrobial peptidomimetics are peptide-like compounds, of which most are bactericides [49]–[53]. LTX109 was previously shown to have bactericidal activity [27]. The arginine–tertbutyl tryptophan–arginine sequence of LTX109 makes it amphipathic, with two bulky side groups and two cationic side groups that are proposed to interact with negatively charged bacterial cell membranes [26].
We found similar killing kinetic for LTX109 and the membrane permeabilizing drug amphotericin B, suggesting that the two compounds could have a related mode of action. The rapid uptake of the fluorescent dye SYTOX Green, potassium and amino acid efflux from cells exposed to a high concentration of LTX109 suggest that this drug disturbs the plasma membrane by direct interaction with one or several components in the plasma membrane. Furthermore, inability of LTX109 treated cells to acidify their surrounding media support an effect on the cell membrane.
These results are similar to results with amphotericin B, which also causes yeast cells to inhibit glucose-induced acidification [32] and to release potassium as a consequence of general membrane disorganization [54], [55].
The high concentration of drug could have obscured other toxic effects of LTX109 on S. cerevisiae, so we cannot exclude that LTX109 has other effects in addition to membrane disruption as previously discussed for peptide drugs [56].
To gain further insight into the mode of function of LTX109, we screened for resistant mutants. Six of eight resistance mutants were affected in sphingolipid biosynthesis, and five of these showed similar, high resistance towards LTX109 (Fig. 4). fen1, sur1, ipt1 and skn1 mutants all have reduced amount of sphingolipids [40], [43], [45], [46] as do mutants that reduce Orm2p activity [44], suggesting an essential role of complex sphingolipids in sensitivity to LTX109. Lack of Sur2p lead to decreased sphinganine hydroxylation, but does not prevent formation of MIPC [57]. Furthermore, the fen1 mutant produce reduced amount of sphingolipids containing the C26 acyl group [40]. The resistance phenotype of the sur2 and fen1 mutants therefore suggested that it is not only the quantity, but also the structural modifications that occur during sphingolipid synthesis that is required for optimal LTX109 activity. The terminal steps of sphingolipid biosynthesis in yeasts are MIPC and M(IP)2C. The fact that these lipids are reduced in the resistant mutants suggests that MIPC and M(IP)2C are essential for the fungicidal activity of LTX109, either by direct interaction with LTX109 or by interaction with another membrane components that is the target for LTX109. It does however seem less likely that a component other than sphingolipids is the target for LTX109 for two reasons, (i) mutants depleted of the target would be expected to appear in the screen for mutants resistant to LTX109. (ii) Alternatively, the target could depend on sphingolipids for optimal activity, be essential for growth and thus not appear in the screen, but then fen1, sur1, ipt1, skn1 and orm2 mutants would be expected to have reduced growth rates which they do not (Fig. 4).
Sphingolipids are located primarily in the plasma membrane [58] and are often clustered together with ergosterol in lipid rafts [59]. Sphingolipids are not only a structural component of the cell membrane, but serve vital functions in the heat-shock response, cell cycle arrest, signaling pathways, endocytosis and protein trafficking [60], [61]. Fungal sphingolipids are highly similar to each other [62], [63], and the biosynthesis of complex fungal sphingolipids is distinctly different from mammals [64]. This makes the fungal sphingolipids attractive antifungal drug targets and several natural compounds with anti-IPC synthase activity have been identified [65]–[67].
The terminal M(IP)2C is the major sphingolipid in the fungal plasma membrane [46] and has previously been suggested as a target for the plant defensin Dahlia merckii antimicrobial peptide 1 (DmAMP1) [43], [68], [69]. DmAMP1 is a 50 amino acid amtimicrobial peptide that leads to nonselective passage of potassium, calcium [70] and SYTOX Green [33]. Hence, DmAMP1 and LTX109 could have similar modes of action, although DmAMP1 does not contain the Arg-Trp-Arg sequence that serves as basis for LTX109.
Amphotericin B is currently the last in line treatment option for severe fungal infections [71]. Alternative drug candidates might therefore be developed for treatment in cases where use of amphotericin B becomes limited due to resistance. Biofilm formation on medical devices is a major nosocomial problem and causes multidrug resistance [13]. Only a few of the current systemic antifungals have activity against yeast biofilms [72], [73], but often it requires removal of the implant for effective treatment [74]. Peptide antibiotics including LTX109 analogues have been shown to be efficient drugs to kill bacterial biofilm cells [25], [50]. This study shows for the first time a peptidomimetic with activity against yeast biofilm. This observation suggests antifungal peptidomimetics with rapid killing kinetics and membrane permeabilizing activities are attractive drugs for yeast biofilm treatment.
In conclusion, we have shown the efficient fungicidal properties of a synthetic peptidomimetic, LTX109, that killed the yeast S. cerevisiae with fast killing kinetics and complete eradication of viable cells in exponential growth phase. We found that yeast cells treated with a high concentration of LTX109 became permeable to free amino acids, potassium and SYTOX Green and prevented proton extrusion in response to a pulse of glucose. Fungal susceptibility to LTX109 depended on biosynthesis of sphingolipids. The sphingolipids M(IP)2C and its precursor MIPC are found in fungal, but not human membranes, making LTX109 and derivatives attractive drug candidates for fungal infection treatment as alternatives to amphotericin B.
Acknowledgments
LTX109 was kindly provided by LytixBiopharma AS, Tromsø, Norway.
Funding Statement
Funding provided by the Danish Agency for Science Technology and Innovation (FTP 10-084027). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, et al. (2004) Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 23: 317–322. [DOI] [PubMed] [Google Scholar]
- 2. Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20: 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vanden Bossche H, Koymans L, Moereels H (1995) P450 inhibitors of use in medical treatment: focus on mechanisms of action. Pharmacol Ther 67: 79–100. [DOI] [PubMed] [Google Scholar]
- 4. Teerlink T, de Kruijff B, Demel RA (1980) The action of pimaricin, etruscomycin and amphotericin B on liposomes with varying sterol content. Biochim Biophys Acta 599: 484–492. [DOI] [PubMed] [Google Scholar]
- 5. Ermishkin LN, Kasumov KM, Potzeluyev VM (1976) Single ionic channels induced in lipid bilayers by polyene antibiotics amphotericin B and nystatine. Nature 262: 698–699. [DOI] [PubMed] [Google Scholar]
- 6. Deresinski SC, Stevens DA (2003) Caspofungin. Clin Infect Dis 36: 1445–1457. [DOI] [PubMed] [Google Scholar]
- 7. Waldorf AR, Polak A (1983) Mechanisms of action of 5-fluorocytosine. Antimicrob Agents Chemother 23: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arendrup MC, Bruun B, Christensen JJ, Fuursted K, Johansen HK, et al. (2011) National surveillance of fungemia in Denmark (2004 to 2009). J Clin Microbiol 49: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oxman DA, Chow JK, Frendl G, Hadley S, Hershkovitz S, et al. (2010) Candidaemia associated with decreased in vitro fluconazole susceptibility: is Candida speciation predictive of the susceptibility pattern? J Antimicrob Chemother 65: 1460–1465. [DOI] [PubMed] [Google Scholar]
- 10. Vermes A, Guchelaar HJ, Dankert J (2000) Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother 46: 171–179. [DOI] [PubMed] [Google Scholar]
- 11. Bates DW, Su L, Yu DT, Chertow GM, Seger DL, et al. (2001) Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis 32: 686–693. [DOI] [PubMed] [Google Scholar]
- 12. Kauffman CA (2006) Fungal infections. Proc Am Thorac Soc 3: 35–40. [DOI] [PubMed] [Google Scholar]
- 13. Ramage G, Martinez JP, Lopez-Ribot JL (2006) Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6: 979–986. [DOI] [PubMed] [Google Scholar]
- 14. Brown MR, Allison DG, Gilbert P (1988) Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother 22: 777–780. [DOI] [PubMed] [Google Scholar]
- 15. Baillie GS, Douglas LJ (1998) Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother 42: 1900–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butts A, Krysan DJ (2012) Antifungal drug discovery: something old and something new. PLoS Pathog 8: e1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Weerden NL, Bleackley MR, Anderson MA (2013) Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol Life Sci. [DOI] [PMC free article] [PubMed]
- 18. Hancock RE, Scott MG (2000) The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A 97: 8856–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55: 27–55. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Xiang Q, Zhang Q, Huang Y, Su Z (2012) Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 37: 207–215. [DOI] [PubMed] [Google Scholar]
- 21. Marr AK, Gooderham WJ, Hancock RE (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6: 468–472. [DOI] [PubMed] [Google Scholar]
- 22. Maurya IK, Pathak S, Sharma M, Sanwal H, Chaudhary P, et al. (2011) Antifungal activity of novel synthetic peptides by accumulation of reactive oxygen species (ROS) and disruption of cell wall against Candida albicans. Peptides 32: 1732–1740. [DOI] [PubMed] [Google Scholar]
- 23. Trabocchi A, Mannino C, Machetti F, De Bernardis F, Arancia S, et al. (2010) Identification of inhibitors of drug-resistant Candida albicans strains from a library of bicyclic peptidomimetic compounds. J Med Chem 53: 2502–2509. [DOI] [PubMed] [Google Scholar]
- 24. Murillo LA, Lan CY, Agabian NM, Larios S, Lomonte B (2007) Fungicidal activity of a phospholipase-A2-derived synthetic peptide variant against Candida albicans. Rev Esp Quimioter 20: 330–333. [PubMed] [Google Scholar]
- 25. Flemming K, Klingenberg C, Cavanagh JP, Sletteng M, Stensen W, et al. (2009) High in vitro antimicrobial activity of synthetic antimicrobial peptidomimetics against staphylococcal biofilms. J Antimicrob Chemother 63: 136–145. [DOI] [PubMed] [Google Scholar]
- 26. Isaksson J, Brandsdal BO, Engqvist M, Flaten GE, Svendsen JS, et al. (2011) A synthetic antimicrobial peptidomimetic (LTX 109): stereochemical impact on membrane disruption. J Med Chem 54: 5786–5795. [DOI] [PubMed] [Google Scholar]
- 27. Saravolatz LD, Pawlak J, Johnson L, Bonilla H, Saravolatz LD 2nd, et al. (2012) In vitro activities of LTX-109, a synthetic antimicrobial peptide, against methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, daptomycin-nonsusceptible, and linezolid-nonsusceptible Staphylococcus aureus. Antimicrob Agents Chemother 56: 4478–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jorgensen MU, Gjermansen C, Andersen HA, Kielland-Brandt MC (1997) STP1, a gene involved in pre-tRNA processing in yeast, is important for amino-acid uptake and transcription of the permease gene BAP2. Curr Genet 31: 241–247. [DOI] [PubMed] [Google Scholar]
- 29. Giaever G, Chu AM, Ni L, Connelly C, Riles L, et al. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. [DOI] [PubMed] [Google Scholar]
- 30. Rupp S, Summers E, Lo HJ, Madhani H, Fink G (1999) MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J 18: 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sherman F (1991) Getting started with yeast. Methods Enzymol 194: 3–21. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka T, Nakayama K, Machida K, Taniguchi M (2000) Long-chain alkyl ester of AMP acts as an antagonist of glucose-induced signal transduction that mediates activation of plasma membrane proton pump in Saccharomyces cerevisiae. Microbiology 146 (Pt 2): 377–384. [DOI] [PubMed] [Google Scholar]
- 33. Thevissen K, Terras FR, Broekaert WF (1999) Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol 65: 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bojsen RK, Andersen KS, Regenberg B (2012) Saccharomyces cerevisiae--a model to uncover molecular mechanisms for yeast biofilm biology. FEMS Immunol Med Microbiol 65: 169–182. [DOI] [PubMed] [Google Scholar]
- 35. Haagensen JA, Regenberg B, Sternberg C (2011) Advanced microscopy of microbial cells. Adv Biochem Eng Biotechnol 124: 21–54. [DOI] [PubMed] [Google Scholar]
- 36. Serrano R (1980) Effect of ATPase inhibitors on the proton pump of respiratory-deficient yeast. Eur J Biochem 105: 419–424. [DOI] [PubMed] [Google Scholar]
- 37. Torbensen R, Moller HD, Gresham D, Alizadeh S, Ochmann D, et al. (2012) Amino acid transporter genes are essential for FLO11-dependent and FLO11-independent biofilm formation and invasive growth in Saccharomyces cerevisiae. PLoS One 7: e41272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roth BL, Poot M, Yue ST, Millard PJ (1997) Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol 63: 2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Funato K, Vallee B, Riezman H (2002) Biosynthesis and trafficking of sphingolipids in the yeast Saccharomyces cerevisiae. Biochemistry 41: 15105–15114. [DOI] [PubMed] [Google Scholar]
- 40. Oh CS, Toke DA, Mandala S, Martin CE (1997) ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. J Biol Chem 272: 17376–17384. [DOI] [PubMed] [Google Scholar]
- 41. Grilley MM, Stock SD, Dickson RC, Lester RL, Takemoto JY (1998) Syringomycin action gene SYR2 is essential for sphingolipid 4-hydroxylation in Saccharomyces cerevisiae. J Biol Chem 273: 11062–11068. [DOI] [PubMed] [Google Scholar]
- 42. Beeler TJ, Fu D, Rivera J, Monaghan E, Gable K, et al. (1997) SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37 degrees C, is required for mannosylation of inositolphosphorylceramide. Mol Gen Genet 255: 570–579. [DOI] [PubMed] [Google Scholar]
- 43. Thevissen K, Idkowiak-Baldys J, Im YJ, Takemoto J, Francois IE, et al. (2005) SKN1, a novel plant defensin-sensitivity gene in Saccharomyces cerevisiae, is implicated in sphingolipid biosynthesis. FEBS Lett 579: 1973–1977. [DOI] [PubMed] [Google Scholar]
- 44. Shimobayashi M, Oppliger W, Moes S, Jeno P, Hall MN (2013) TORC1-regulated protein kinase Npr1 phosphorylates Orm to stimulate complex sphingolipid synthesis. Mol Biol Cell 24: 870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stock SD, Hama H, Radding JA, Young DA, Takemoto JY (2000) Syringomycin E inhibition of Saccharomyces cerevisiae: requirement for biosynthesis of sphingolipids with very-long-chain fatty acids and mannose- and phosphoinositol-containing head groups. Antimicrob Agents Chemother 44: 1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dickson RC, Nagiec EE, Wells GB, Nagiec MM, Lester RL (1997) Synthesis of mannose-(inositol-P)2-ceramide, the major sphingolipid in Saccharomyces cerevisiae, requires the IPT1 (YDR072c) gene. J Biol Chem 272: 29620–29625. [DOI] [PubMed] [Google Scholar]
- 47. Reynolds TB, Fink GR (2001) Bakers' yeast, a model for fungal biofilm formation. Science 291: 878–881. [DOI] [PubMed] [Google Scholar]
- 48. Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR (1992) Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68: 1077–1090. [DOI] [PubMed] [Google Scholar]
- 49. Violette A, Fournel S, Lamour K, Chaloin O, Frisch B, et al. (2006) Mimicking helical antibacterial peptides with nonpeptidic folding oligomers. Chem Biol 13: 531–538. [DOI] [PubMed] [Google Scholar]
- 50. Liu Y, Knapp KM, Yang L, Molin S, Franzyk H, et al. (2013) High in vitro antimicrobial activity of beta-peptoid-peptide hybrid oligomers against planktonic and biofilm cultures of Staphylococcus epidermidis. Int J Antimicrob Agents 41: 20–27. [DOI] [PubMed] [Google Scholar]
- 51. Niu Y, Padhee S, Wu H, Bai G, Qiao Q, et al. (2012) Lipo-gamma-AApeptides as a new class of potent and broad-spectrum antimicrobial agents. J Med Chem 55: 4003–4009. [DOI] [PubMed] [Google Scholar]
- 52. Makobongo MO, Kovachi T, Gancz H, Mor A, Merrell DS (2009) In vitro antibacterial activity of acyl-lysyl oligomers against Helicobacter pylori. Antimicrob Agents Chemother 53: 4231–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Niu Y, Wang RE, Wu H, Cai J (2012) Recent development of small antimicrobial peptidomimetics. Future Med Chem 4: 1853–1862. [DOI] [PubMed] [Google Scholar]
- 54. Beggs WH (1994) Physicochemical cell damage in relation to lethal amphotericin B action. Antimicrob Agents Chemother 38: 363–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen WC, Chou DL, Feingold DS (1978) Dissociation between ion permeability and the lethal action of polyene antibiotics on Candida albicans. Antimicrob Agents Chemother 13: 914–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Theis T, Stahl U (2004) Antifungal proteins: targets, mechanisms and prospective applications. Cell Mol Life Sci 61: 437–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Haak D, Gable K, Beeler T, Dunn T (1997) Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J Biol Chem 272: 29704–29710. [DOI] [PubMed] [Google Scholar]
- 58. Patton JL, Lester RL (1991) The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J Bacteriol 173: 3101–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bagnat M, Keranen S, Shevchenko A, Simons K (2000) Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci U S A 97: 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cowart LA, Obeid LM (2007) Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim Biophys Acta 1771: 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dickson RC, Lester RL (2002) Sphingolipid functions in Saccharomyces cerevisiae. Biochim Biophys Acta 1583: 13–25. [DOI] [PubMed] [Google Scholar]
- 62. Vincent VL, Klig LS (1995) Unusual effect of myo-inositol on phospholipid biosynthesis in Cryptococcus neoformans. Microbiology 141 (Pt 8): 1829–1837. [DOI] [PubMed] [Google Scholar]
- 63. Wells GB, Dickson RC, Lester RL (1996) Isolation and composition of inositolphosphorylceramide-type sphingolipids of hyphal forms of Candida albicans. J Bacteriol 178: 6223–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dickson RC (1998) Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annu Rev Biochem 67: 27–48. [DOI] [PubMed] [Google Scholar]
- 65. Nagiec MM, Nagiec EE, Baltisberger JA, Wells GB, Lester RL, et al. (1997) Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J Biol Chem 272: 9809–9817. [DOI] [PubMed] [Google Scholar]
- 66. Mandala SM, Thornton RA, Milligan J, Rosenbach M, Garcia-Calvo M, et al. (1998) Rustmicin, a potent antifungal agent, inhibits sphingolipid synthesis at inositol phosphoceramide synthase. J Biol Chem 273: 14942–14949. [DOI] [PubMed] [Google Scholar]
- 67. Mandala SM, Thornton RA, Rosenbach M, Milligan J, Garcia-Calvo M, et al. (1997) Khafrefungin, a novel inhibitor of sphingolipid synthesis. J Biol Chem 272: 32709–32714. [DOI] [PubMed] [Google Scholar]
- 68. Thevissen K, Cammue BP, Lemaire K, Winderickx J, Dickson RC, et al. (2000) A gene encoding a sphingolipid biosynthesis enzyme determines the sensitivity of Saccharomyces cerevisiae to an antifungal plant defensin from dahlia (Dahlia merckii). Proc Natl Acad Sci U S A 97: 9531–9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aerts AM, Francois IE, Bammens L, Cammue BP, Smets B, et al. (2006) Level of M(IP)2C sphingolipid affects plant defensin sensitivity, oxidative stress resistance and chronological life-span in yeast. FEBS Lett 580: 1903–1907. [DOI] [PubMed] [Google Scholar]
- 70. Thevissen K, Ghazi A, De Samblanx GW, Brownlee C, Osborn RW, et al. (1996) Fungal membrane responses induced by plant defensins and thionins. J Biol Chem 271: 15018–15025. [DOI] [PubMed] [Google Scholar]
- 71. Chandrasekar P (2011) Management of invasive fungal infections: a role for polyenes. J Antimicrob Chemother 66: 457–465. [DOI] [PubMed] [Google Scholar]
- 72. Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, et al. (2002) In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother 46: 3591–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA (2002) Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46: 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lynch AS, Robertson GT (2008) Bacterial and fungal biofilm infections. Annu Rev Med 59: 415–428. [DOI] [PubMed] [Google Scholar]