Abstract
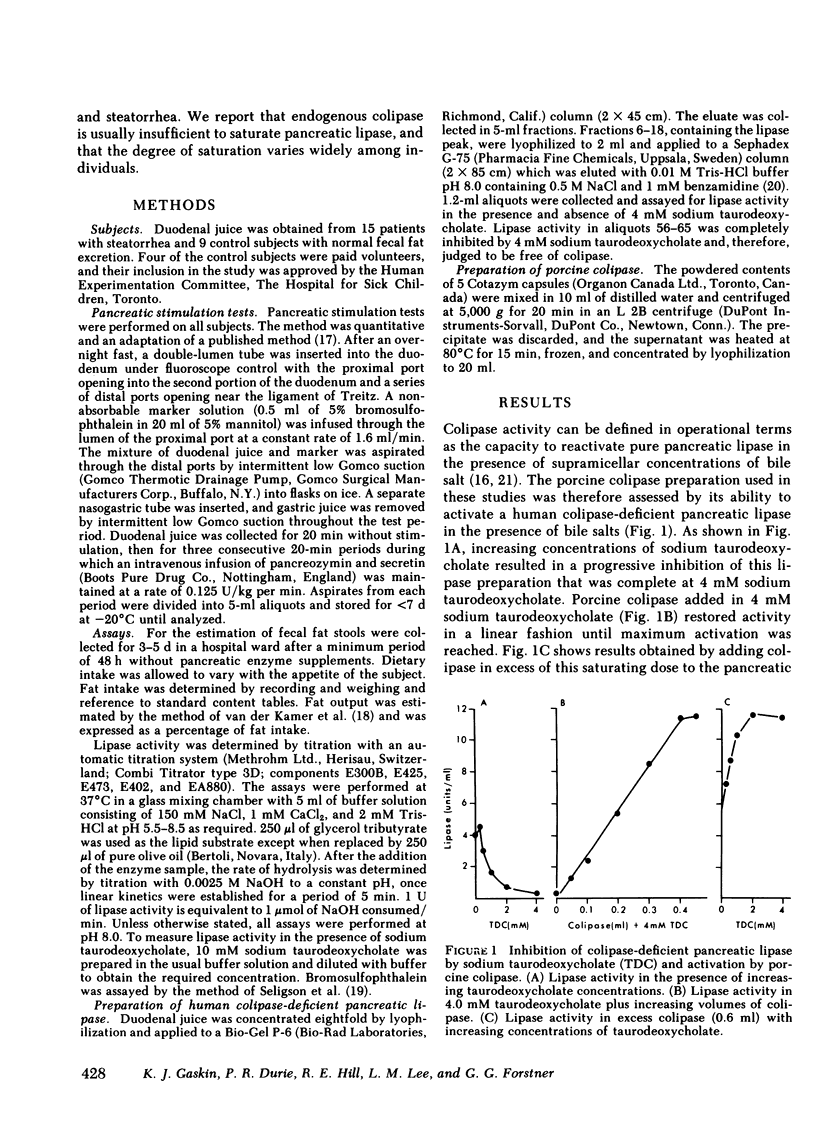
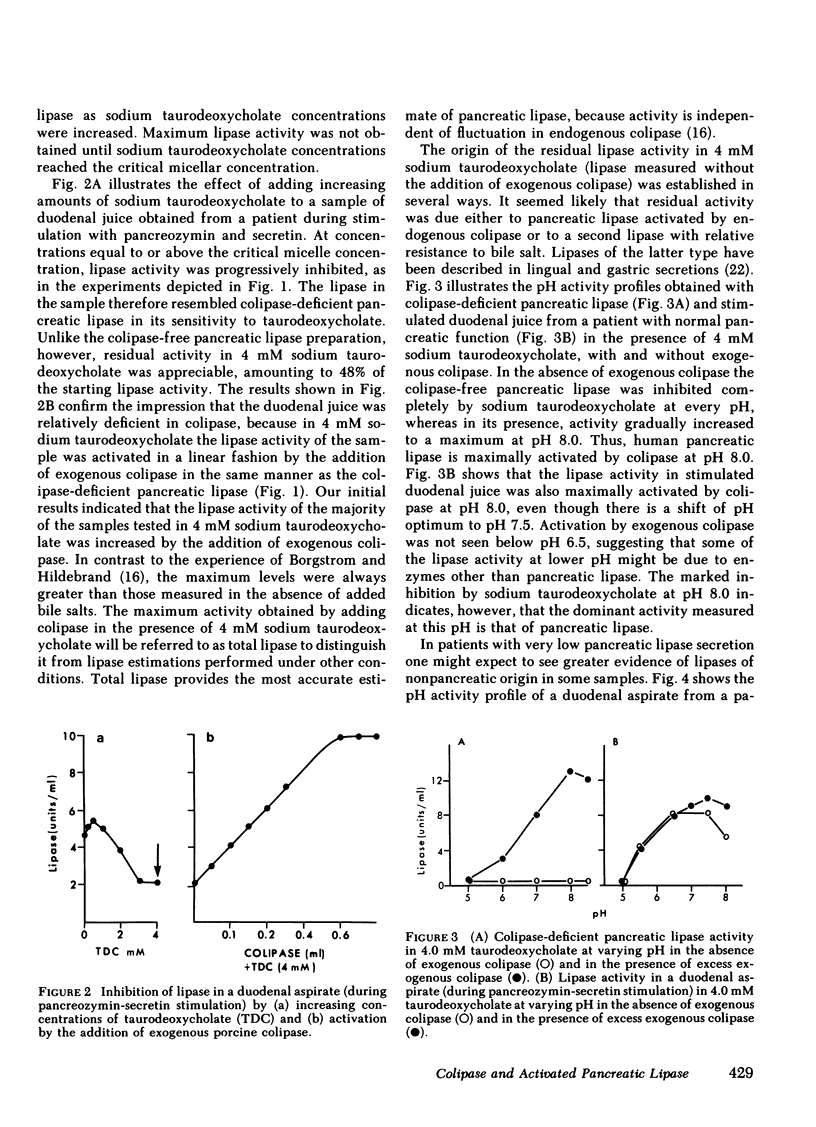
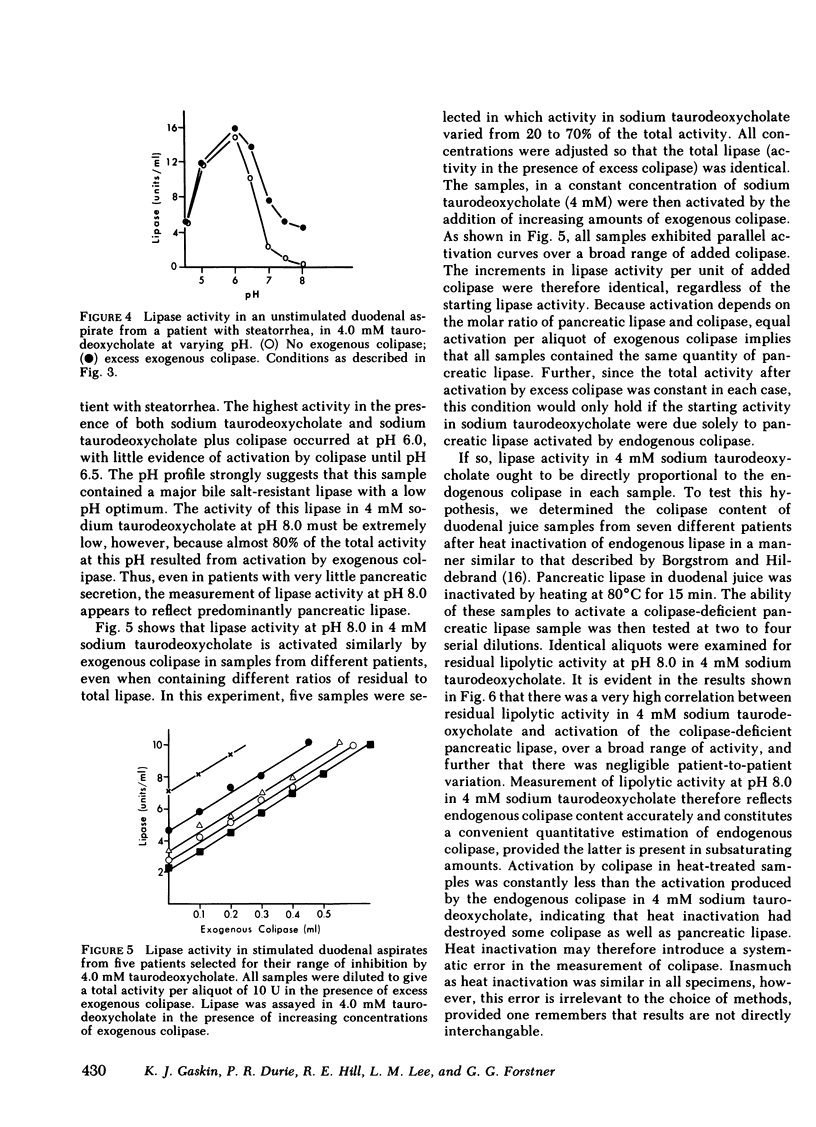
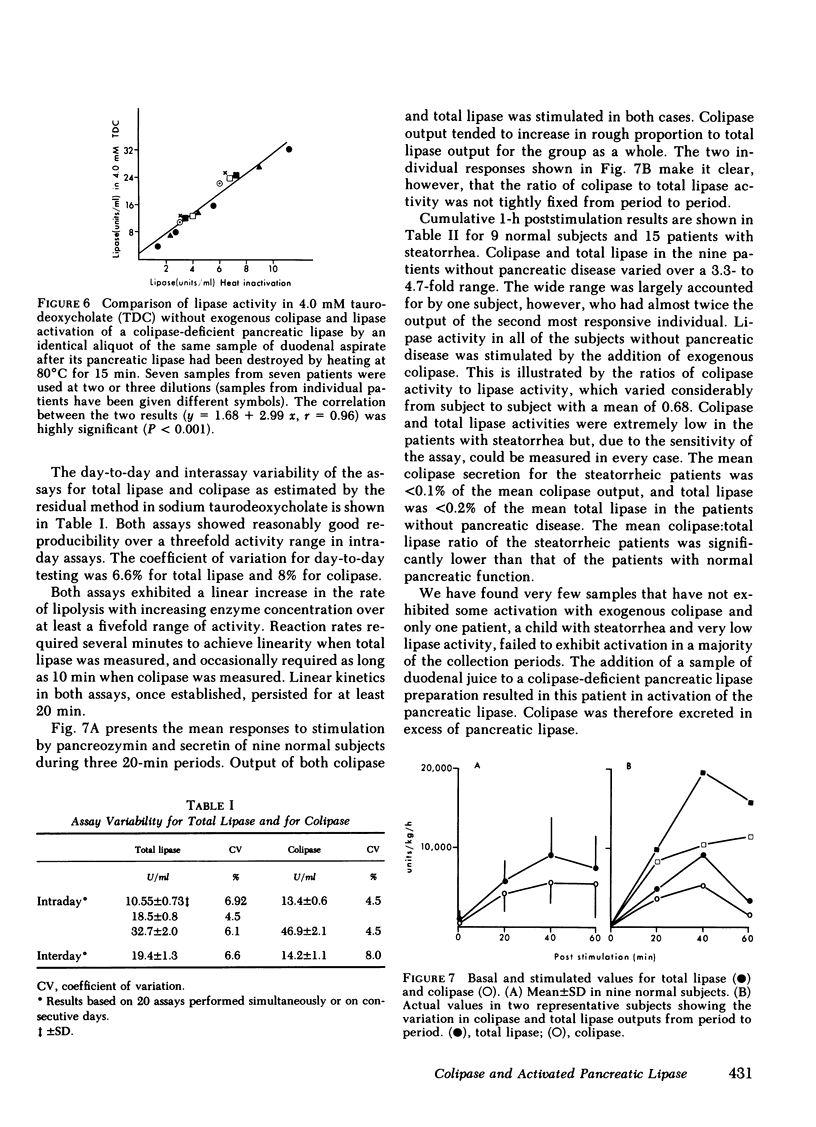
Human pancreatic lipase in duodenal secretions was studied under conditions of maximal activation by porcine colipase and maximal inhibition by sodium taurodeoxycholate. In almost all samples, total lipase activity in 4 mM sodium taurodeoxycholate was activated by the addition of porcine colipase. Activation was linear until saturation by cofactor was reached, and maximum activity was greater than that obtained in the absence of bile salts. At pH 8.0 in 4 mM sodium taurodeoxycholate, lipase activity was due to pancreatic lipase in samples from normal and steatorrheic individuals and was proportional to the concentration of endogenous colipase in samples that could be activated by exogenous colipase. In these samples, therefore, colipase activity could be conveniently assayed as the lipase activity at pH 0.8 in 4 mM sodium taurodeoxycholate. Colipase to total pancreatic lipase ratios varied widely from individual to individual and on average were significantly lower in steatorrheic patients. In individual samples, colipase secretion was stimulated by pancreozymin and secretin roughly in parallel with total pancreatic lipase, but some variation in the ratio of the two was often seen in successive collection periods. Because pancreatic lipase is usually unsaturated with respect to cofactor, lipolytic activity in duodenal secretions may be finely controlled by modulation of colipase secretion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASKYS B., KLEIN E., LEVER W. F. LIPASES OF BLOOD AND TISSUES. III. PURIFICATION AND PROPERTIES OF PANCREATIC LIPASE. Arch Biochem Biophys. 1963 Aug;102:201–209. doi: 10.1016/0003-9861(63)90171-1. [DOI] [PubMed] [Google Scholar]
- Benzonana G., Desnuelle P. Action of some effectors on the hydrolysis of long-chain triglycerides by pancreatic lipase. Biochim Biophys Acta. 1968 Sep 2;164(1):47–58. doi: 10.1016/0005-2760(68)90069-6. [DOI] [PubMed] [Google Scholar]
- Borgström B., Erlanson-Albertsson C., Wieloch T. Pancreatic colipase: chemistry and physiology. J Lipid Res. 1979 Sep;20(7):805–816. [PubMed] [Google Scholar]
- Borgström B., Erlanson C. Pancreatic juice co-lipase: physiological importance. Biochim Biophys Acta. 1971 Aug 20;242(2):509–513. [PubMed] [Google Scholar]
- Brogström B., Hildebrand H. Lipase and co-lipase activities of human small intestinal contents after a liquid test meal. Scand J Gastroenterol. 1975;10(6):585–591. [PubMed] [Google Scholar]
- Chapus C., Sari H., Sémériva M., Desnuelle P. Role of colipase in the interfacial adsorption of pancreatic lipase at hydrophilic interfaces. FEBS Lett. 1975 Oct 15;58(1):155–158. doi: 10.1016/0014-5793(75)80247-x. [DOI] [PubMed] [Google Scholar]
- Charles M., Astier M., Sauve P., Desnuelle P. Interactions of colipase with bile salt micelles. 1. Ultracentrifugation studies. Eur J Biochem. 1975 Oct 15;58(2):555–559. doi: 10.1111/j.1432-1033.1975.tb02405.x. [DOI] [PubMed] [Google Scholar]
- Erlanson C., Fernlund P., Borgström B. Purification and characterization of two proteins with co-lipase activity from porcine pancreas. Biochim Biophys Acta. 1973 Jun 15;310(2):437–445. doi: 10.1016/0005-2795(73)90127-x. [DOI] [PubMed] [Google Scholar]
- Figarella C., Negri G. A., Sarles H. Presence of colipase in a congenital pancreatic lipase deficiency. Biochim Biophys Acta. 1972 Sep 7;280(1):205–211. doi: 10.1016/0005-2760(72)90227-5. [DOI] [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Simultaneous measurements of total pancreatic, biliary, and gastric outputs in man using a perfusion technique. Gastroenterology. 1970 Mar;58(3):321–328. [PubMed] [Google Scholar]
- Hamosh M., Klaeveman H. L., Wolf R. O., Scow R. O. Pharyngeal lipase and digestion of dietary triglyceride in man. J Clin Invest. 1975 May;55(5):908–913. doi: 10.1172/JCI108019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien R., Rathelot J., Canioni P., Sarda L., Gregoire J., Rochat H. Horse pancreatic colipase: isolation by a detergent method and amino terminal sequence of the polypeptide chain. Biochimie. 1978;60(1):103–107. doi: 10.1016/s0300-9084(78)80207-7. [DOI] [PubMed] [Google Scholar]
- Maylié M. F., Charles M., Gache C., Desnuelle P. Isolation and partial identification of a pancreatic colipase. Biochim Biophys Acta. 1971 Jan 19;229(1):286–289. doi: 10.1016/0005-2795(71)90347-3. [DOI] [PubMed] [Google Scholar]
- Morgan R. G., Barrowman J., Borgström B. The effect of sodium taurodesoxycholate and pH on the gel filtration behavior of rat pancreatic protein and lipases. Biochim Biophys Acta. 1969 Feb 4;175(1):65–75. doi: 10.1016/0005-2795(69)90146-9. [DOI] [PubMed] [Google Scholar]
- Morgan R. G., Hoffman N. E. The interaction of lipase, lipase cofactor and bile salts in triglyceride hydrolysis. Biochim Biophys Acta. 1971 Oct 5;248(1):143–148. doi: 10.1016/0005-2760(71)90086-5. [DOI] [PubMed] [Google Scholar]
- Patton J. S., Albertsson P. A., Erlanson C., Borgström B. Binding of porcine pancreatic lipase and colipase in the absence of substrate studies by two-phase partition and affinity chromatography. J Biol Chem. 1978 Jun 25;253(12):4195–4202. [PubMed] [Google Scholar]
- Rathelot J., Julien R., Canioni P., Coeroli C., Sarda L. Studies on the effect of bile salt and colipase on enzymatic lipolysis. Improved method for the determination of pancreatic lipase and colipase. Biochimie. 1975;57(10):1117–1122. doi: 10.1016/s0300-9084(76)80572-x. [DOI] [PubMed] [Google Scholar]
- Rathelot J., Julien R., Canioni P., Sarda L. Isolation and partial characterization of bovine pancreatic colipase. Biochimie. 1975;57(10):1123–1130. doi: 10.1016/s0300-9084(76)80573-1. [DOI] [PubMed] [Google Scholar]
- SELIGSON D., MARINO J., DODSON E. Determination of sulfobromophthalein in serum. Clin Chem. 1957 Oct;3(5):638–645. [PubMed] [Google Scholar]
- Sternby B., Borgström B. Purification and characterization of human pancreatic colipase. Biochim Biophys Acta. 1979 Feb 26;572(2):235–243. doi: 10.1016/0005-2760(79)90039-0. [DOI] [PubMed] [Google Scholar]
- Sémériva M., Desnuelle P. Pancreatic lipase and colipase. An example of heterogeneous biocatalysis. Adv Enzymol Relat Areas Mol Biol. 1979;48:319–370. doi: 10.1002/9780470122938.ch7. [DOI] [PubMed] [Google Scholar]
- Vandermeers A., Vandermeers-Piret M. C., Rathe J., Christophe J. A simple automated method for the assay of pancreatic lipase. Clin Chim Acta. 1974 May 17;52(3):257–269. doi: 10.1016/0009-8981(74)90110-7. [DOI] [PubMed] [Google Scholar]
- Vandermeers A., Vandermeers-Piret M. C., Rathé J., Christophe J. On human pancreatic triacylglycerol lipase: isolation and some properties. Biochim Biophys Acta. 1974 Nov 25;370(1):257–268. doi: 10.1016/0005-2744(74)90050-3. [DOI] [PubMed] [Google Scholar]



