Abstract
Anorexia nervosa (AN) is a life-threatening condition, with a significant risk for death, due to cardiovascular complications. It is characterized by abnormal eating behavior and has the highest mortality rate of all psychiatric disorders. It has been associated with bradycardia (a heart rate [HR] of less than 60 beats per minute) (up to 95%), hypotension, mitral valve prolapse, and heart failure. The diagnosis of AN can be elusive, and more than half of all cases are undetected. The purpose of this study was to raise and improve awareness to the possible diagnosis of AN in adolescent and young adult patients with weight loss displaying bradycardia and new cardiac disorders. Clinical characteristics, HR, and electrocardiographic data of 23 consecutive patients (20 females) with AN and of 10 young adults (8 females) without AN, between the years 2006 and 2009, were recorded and summarized. At presentation 16/23 (69.6%) showed HR < 50 bpm. The mean lowest HR of all patients was 44 ± 6 (range 26 to 68) bpm. No patient needed pacemaker therapy. Bradycardia in young adults, especially females with weight loss, should raise the possible diagnosis of AN, so it can be treated early in-time, and thus prevent premature death.
Keywords: anorexia nervosa, bradycardia, sudden death
One Friday evening, during routine work, I was called to the Children and Psychiatry Department to check a 17-year-old girl, with the diagnosis of anorexia nervosa (AN), because of bradycardia (heart rate (HR) of 38/min). A month later, a friend of mine asked me to check her 16-year-old daughter who has lost weight and has HR of 47/min. At that time, I realized that not all physicians (in hospitals, the community, or in the military service) are acquainted with the simple, easy to check physical sign of bradycardia in AN, and thus I became involved and decided to collect data concerning this issue.
Eating disorders, and particularly AN, confer a long-lasting increase in morbidity and mortality especially among young adults. It is a life-threatening disorder, with a significant risk for sudden death (5 to 20%) due to severe cardiovascular complications. It is a generalized disorder that affects multiple organ systems, such as skeletal bones, linear growth, brain development, and fertility functions. Previous reports pointed out conflicting evidence concerning its reversibility upon treatment.1,2,3,4,5,6,7,8
AN carries the highest mortality rate of all psychiatric disorders, mostly due to sudden unexpected cardiac arrhythmic death.9,10 Studies have disclosed a mortality rate of up to 20%.11 Cardiac arrest secondary to electrolytic disturbances, and QTc(QT corrected to HR) interval prolongation (secondary/primary) were recorded among AN patients.12,13
The mechanism of sudden death in AN is obscure and is attributed to sudden arrhythmic death. Terminal ventricular tachyarrhythmias (VT) were documented in AN patients, by Isner et al,14 with long QT interval prolongation on electrocardiography (ECG), such as sudden death in liquid-protein dietary, that may result from VT, related to QT interval prolongation.
The QT prolongation in Isner's patients was attributed to hypothalamic-pituitary disturbances and to alteration in sympathetic-parasympathetic tone. Eckardt et al15 described in detail the mechanism of lethal VT of torsade de pointes, which simulated conditions that are likely to exist in the clinical setting of torsade de pointes in animal models, such as bradycardia and hypokalemia.
In vivo, in canine and rabbit models, torsade-like polymorphic VT had been induced by administration of various drugs—to mimic the clinical situation.
It was apparent to them that the first beats of torsade occurred due to early afterdepolarization and triggered activity. They suggested re-entry, based on inhomogeneity of refractoriness, as the underlying mechanism.
Early identification of the disorder is therefore essential for reducing disabling complications and preventing premature death.
The objective of the present study was to summarize and identify cardiac rhythm disorders in a series of consecutive hospitalized adolescent patients with AN, and to verify the clinical significance of bradycardia in this entity. More broadly, we intended to raise or increase the awareness of the possible diagnosis of AN, in young patients with weight loss, displaying bradycardia or new cardiac disorders.
Patients and Methods
The clinical data of 23 consecutive patients (20 females) hospitalized with the diagnosis of AN during the years 2006 to 2009 were collected and summarized as a retrospective observational study. All patients were free of other illness and without medication on admission.
On admission, medical history, physical examination, a standard 12-lead ECG, and routine laboratory data (hematological, biochemistry tests), including endocrinology test (thyroid-stimulating hormone IU/mL) were performed. A transthoracic echocardiogram (two-dimensional Doppler) examination and Holter ECG recordings were also obtained. The study had been approved by the local hospital committee for human rights.
AN was defined according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV, 1995).16 The criteria for AN were as follows: (1) refusal to maintain body weight at or above a minimally normal weight for age, or weight loss leading to maintenance of body weight less than 85% of the expected; (2) intense fear of gaining weight or becoming fat; (3) postmonarchial female amenorrhea, that is, absence of at least three consecutive menstrual cycles.
Our criteria for hospitalization were as follows: (1) Body mass index (BMI) less than 17 kg/m2; or (2) bradycardia less than 50 bpm; or (3) failure to persist or respond to outpatient treatment.
Mitral valve prolapse (MVP) was defined clinically and echocardiographically, as the clinical findings of mid-systolic click or click and late systolic murmur do not always exist on physical examination.17
The diagnosis of MVP was based on parasternal long-axis view echocardiography and was defined as systolic displacement (2 mm) of one or both mitral leaflets into the left atrium, below the plane of the mitral annulus.18
QT interval and QTc were measured from ECG.
Our retrospective data collection did not include HR variable parameters and QT dispersion, at that time. Our retrospective data collection did not include data concerning myocardial tissue Doppler or strain data, nor data concerning diastolic heart function.
The clinical data of 10 consecutive young adults (8 females and 2 males) with the mean age of 15 years, hospitalized with other psychiatric disorders, and with no evidence of heart abnormalities and with no medication on admission, served as a control group, and their clinical data were collected and summarized.
The mean age was 16 ± 3 (range 11.5 to 20 years). Mean weight loss was 13.5 ± 4 kg (range 6 to 26 kg) and mean BMI was 15.4 ± 3.2 kg/m2 (range 10.9 to 20 kg/m2).
Bradycardia among patients with AN was defined as a HR < 50 bpm during day time and < 45 bpm during night time.19 Each patient had a clinical follow-up after 6 months, where ECG was undertaken, and a telephonic follow-up, a year after hospital discharge.
Statistical Analysis
Quantity data were described by mean, standard deviation, median, and range. Quality data presented as frequencies and percentages. Sensitivity and specificity were calculated.
For comparisons between groups of quantity data, independent sample t test or Wilcoxon rank-sum test were used, as appropriate.
For comparisons of qualitative data, Fisher exact test was performed.
Correlations examined by Pearson correlation coefficient test or Spearman correlation coefficient test, as appropriate.
Sensitivity and specificity measures were selected as the appropriate indices for this type of study.
Results
Descriptive demographic, auscultatory, ECG, and echocardiographic findings of 23 AN patients and 10 non-AN patients are displayed in Tables 12345678.
Table 1. Demographic and clinical parameters (AN patients) on admission.
| Patient no. | HR (bmp) | Diagnosis | BMI | Age (years) | Sex |
|---|---|---|---|---|---|
| 1 | 38 | AN | 16.5 | 13 | F |
| 2 | 34 | AN | 13 | 17 | F |
| 3 | 36 | AN | 19.5 | 14 | F |
| 4 | 40 | AN | 16.3 | 16 | F |
| 5 | 38 | AN | 10.9 | 17 | F |
| 6 | 26 | AN | 17 | 19a | F |
| 7 | 41 | AN | 17 | 14 | F |
| 8 | 28 | AN | 18.7 | 14 | F |
| 9 | 39 | AN | 17 | 20a | F |
| 10 | 45 | AN | 12.2 | 15 | F |
| 11 | 60 | AN | 15 | 14 | F |
| 12 | 68 | AN | 19 | 20a | F |
| 13 | 50 | AN | 18 | 15 | F |
| 14 | 43 | AN | 17 | 16 | F |
| 15 | 50 | AN | 14.8 | 16 | F |
| 16 | 34 | AN | 13.4 | 15 | F |
| 17 | 38 | AN | 14 | 11.5 | M |
| 18 | 65 | AN | 20 | 17.5 | F |
| 19 | 65 | AN | 14.7 | 16.5 | F |
| 20 | 57 | AN | 14 | 16 | F |
| 21 | 43 | AN | 15.5 | 13 | M |
| 22 | 44 | AN | 13 | 17 | M |
| 23 | 38 | AN | 14.13 | 15 | F |
Abbreviations: AN, anorexia nervosa; BMI, body mass index; F, female; HR, heart rate (bpm); M, male.
From the Department of Internal Medicine.
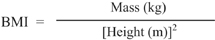
Table 2. Demographic and clinical parameters (non-AN patients) on admission.
| Patient no. | HR (bmp) | Diagnosis | BMI | Age (years) | Sex |
|---|---|---|---|---|---|
| 1 | 72 | Other (no AN) | 21.4 | 12 | F |
| 2 | 99 | Other (no AN) | 31.4 | 15 | F |
| 3 | 76 | Other (no AN) | 23.4 | 15 | M |
| 4 | 69 | Other (no AN) | 29.2 | 17 | M |
| 5 | 84 | Other (no AN) | 18.7 | 14 | F |
| 6 | 66 | Other (no AN) | 32.2 | 17 | F |
| 7 | 68 | Other (no AN) | 32.7 | 17 | F |
| 8 | 67 | Other (no AN) | 27.3 | 12 | F |
| 9 | 68 | Other (no AN) | 31.6 | 15 | F |
| 10 | 71 | Other (no AN) | 19.4 | 14 | F |
Abbreviations: AN, anorexia nervosa; BMI, body mass index; F, female; HR, heart rate (bpm); M, male.
Table 3. Clinical (auscultatory) findings in AN patients.
| Patient no. | Systolic murmur | Systolic click |
|---|---|---|
| 1 | No | No |
| 2 | No | No |
| 3 | No | No |
| 4 | No | No |
| 5 | No | No |
| 6 | No | No |
| 7 | No | No |
| 8 | No | No |
| 9 | No | Yes |
| 10 | Yes | Yes |
| 11 | No | No |
| 12 | No | Yes |
| 13 | No | No |
| 14 | No | No |
| 15 | No | No |
| 16 | No | Yes |
| 17 | No | Yes |
| 18 | Yes | Yes |
| 19 | Yes | Yes |
| 20 | No | Yes |
| 21 | No | Yes |
| 22 | Yes | No |
| 23 | No | No |
Abbreviation: AN, anorexia nervosa.
Table 4. Clinical (auscultatory) findings in non-AN patients.
| Patient no. | Systolic murmur | Systolic click |
|---|---|---|
| 1 | No | Yes |
| 2 | No | No |
| 3 | Yes | No |
| 4 | No | No |
| 5 | No | No |
| 6 | No | No |
| 7 | Yes | No |
| 8 | No | No |
| 9 | No | Yes |
| 10 | No | Yes |
Abbreviation: AN, anorexia nervosa.
Table 5. ECG and Holter ECG data in AN patients.
| Patient no. | Ventricular arrhythmias | QTc interval (s) |
|---|---|---|
| 1 | No | 0.44 |
| 2 | No | 0.40 |
| 3 | No | 0.36 |
| 4 | No | 0.40 |
| 5 | No | 0.38 |
| 6 | Few VPBs | 0.44 |
| 7 | No | 0.44 |
| 8 | No | 0.40 |
| 9 | No | 0.40 |
| 10 | No | 0.36 |
| 11 | 1 VPB | 0.39 |
| 12 | No | 0.36 |
| 13 | No | 0.40 |
| 14 | 1 VPB | 0.40 |
| 15 | No | 0.38 |
| 16 | No | 0.40 |
| 17 | No | 0.36 |
| 18 | Few VPBs and couplets | 0.40 |
| 19 | Few VPBs | 0.36 |
| 20 | No | 0.40 |
| 21 | No | 0.40 |
| 22 | No | 0.40 |
| 23 | No | 0.40 |
Abbreviations: AN, anorexia nervosa; ECG, electrocardiographic; VPBs, ventricular premature beats.
Table 6. ECG and Holter ECG data in non-AN patients.
| Patient no. | Ventricular arrhythmias | QTc interval (s) |
|---|---|---|
| 1 | No | 0.36 |
| 2 | Few VPBs | 0.32 |
| 3 | No | 0.32 |
| 4 | No | 0.34 |
| 5 | No | 0.30 |
| 6 | No | 0.32 |
| 7 | No | 0.32 |
| 8 | No | 0.36 |
| 9 | No | 0.36 |
| 10 | No | 0.34 |
Abbreviations: AN, anorexia nervosa; ECG, electrocardiographic; VPBs, ventricular premature beats.
Table 7. Echocardiographic data in AN patients.
| Patient no. | Ventricular (LV and RV) global systolic function | Valvular Doppler study | Pericardial effusion |
|---|---|---|---|
| 1 | N | N | No |
| 2 | N | N | No |
| 3 | N | N | No |
| 4 | N | N | No |
| 5 | N | N | No |
| 6 | N | Mild MR | No |
| 7 | N | N | No |
| 8 | N | N | No |
| 9 | N | MVP and mild MR | No |
| 10 | N | Mild MR | No |
| 11 | N | N | No |
| 12 | N | Mild MR | No |
| 13 | N | N | No |
| 14 | N | N | No |
| 15 | N | N | No |
| 16 | N | Mild MR | No |
| 17 | N | N | No |
| 18 | N | Mild AS and mild AR | No |
| 19 | N | MVP and mild MR | No |
| 20 | N | N | No |
| 21 | N | N | No |
| 22 | N | MVP and mild MR | No |
| 23 | N | N | Minimal |
Abbreviations: AN, anorexia nervosa; AR, aortic regurgitation; AS, aortic stenosis; LV, left ventricle; MR, mitral regurgitation; MVP, mitral valve prolapse; N, normal; RV, right ventricle.
Table 8. Echocardiographic data in non-AN patients.
| Patient no. | Ventricular (LV and RV) global systolic function | Valvular Doppler study | Pericardial effusion |
|---|---|---|---|
| 1 | N | N | No |
| 2 | N | N | No |
| 3 | N | N | No |
| 4 | N | N | No |
| 5 | N | N | No |
| 6 | N | N | No |
| 7 | N | N | No |
| 8 | N | N | No |
| 9 | N | N | No |
| 10 | N | N | No |
Abbreviations: AN, anorexia nervosa; AR, aortic regurgitation; AS, aortic stenosis; LV, left ventricle; MR, mitral regurgitation; MVP, mitral valve prolapse; N, normal; RV, right ventricle.
Sixteen out of 23 (69%) patients showed HR < 50 bpm at presentation.
During 24 hours of Holter monitoring, 22 out of 23 (95%) patients showed prolong period (more than 180 minutes) of bradycardia where the mean lowest HR of all patients was 44 ± 6 bpm (range 26 to 68 bpm) and the mean lowest HR of the control group was 74 bpm (range 66 to 99 bpm) (p < 0.001).
The leading ECG findings were sinus and nodal bradycardia (Figs. 123), with no evidence of other arrhythmias. Only few ventricular premature beats were recorded in four patients with AN and in one patient with non-AN. QT and QTc intervals were in the normal range in all patients (Tables 5 and 6).
Fig. 1.
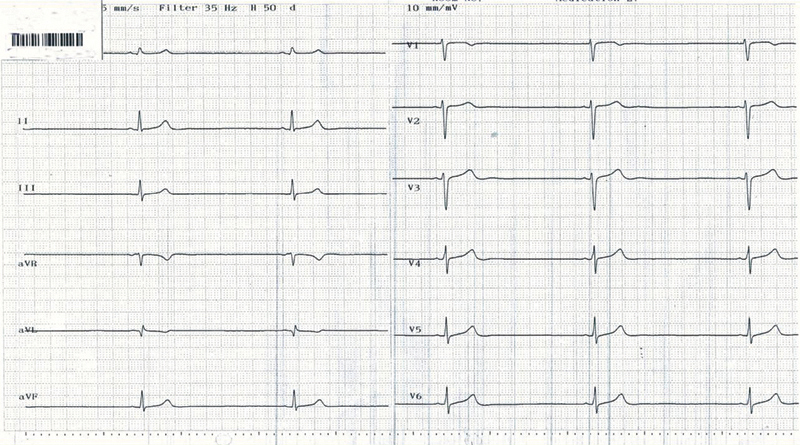
Bradycardia of 31/bpm in 19-year-old female with anorexia nervosa.
Fig. 2.
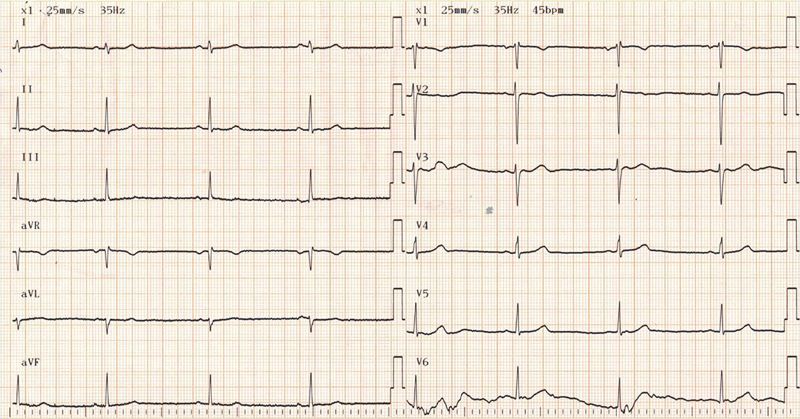
Bradycardia of 45/bpm in 16-year-old female with anorexia nervosa.
Fig. 3.
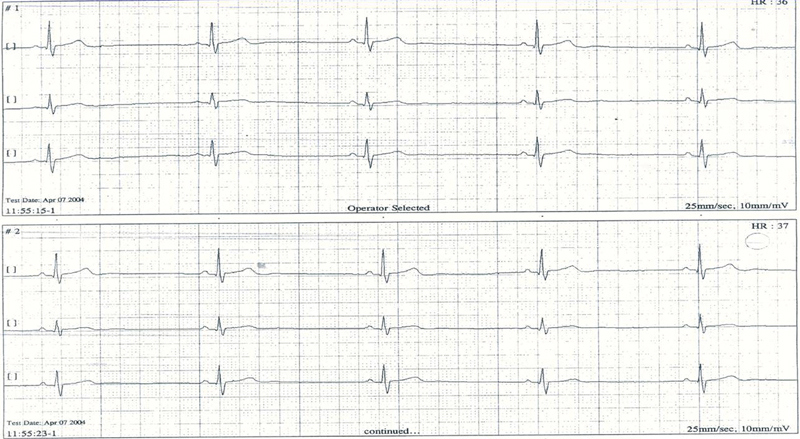
Bradycardia of 38/bpm in 17-year-old female with anorexia nervosa.
Using echocardiography, MVP (Fig. 4) was found in three patients, mitral regurgitation in four patients, and mild aortic stenosis in one patient. All patients in the control group had a normal echocardiogram (Table 8). Laboratory data were in the normal range in both groups. Summary of leading clinical data and statistical analysis are presented in Table 9. There were no differences between the two groups concerning age, gender, and auscultatory findings. BMI, HR, and QTc were statistically different (p value < 0.001). Total number of patients in AN group with bradycardia less than 50/min was16/23 (69.6%) with sensitivity of 69.6% and specificity of 100% (Table 10).
Fig. 4.
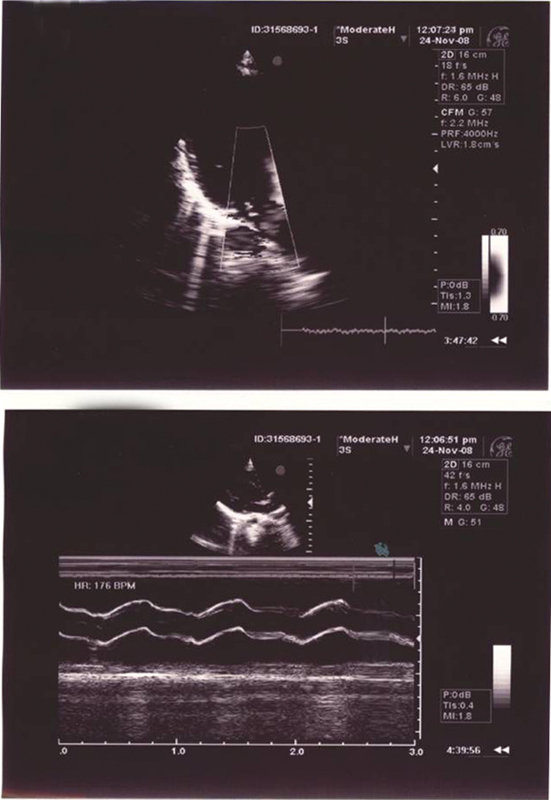
Echocardiogram in 12-year-old female with anorexia nervosa—demonstrating mitral valve prolapse and mitral regurgitation.
Table 9. Summary of demographic and clinical parameters in AN and non-AN patients.
| AN (n = 23) | Other diagnosis Non-AN (n = 10) |
p value | ||
|---|---|---|---|---|
| Age (Years) | Mean | 15.7 | 14.8 | 0.364a |
| Standard deviation | 2.2 | 1.9 | ||
| Median | 16 | 15 | ||
| Range | 11.5-20 | 12-17 | ||
| Gender | M | 3 13.0% |
2 20% |
0.727b |
| F | 20 87% |
8 80% |
||
| BMI (kg/m2) | Mean | 15.6 | 26.7 | p < 0.001c |
| Standard deviation | 2.4 | 5.5 | ||
| Median | 15.5 | 28.25 | ||
| Range | 10.9-20 | 18.7-33.5 | ||
| HR (bpm) |
Mean | 44.3 | 74 | p < 0.001c |
| Standard deviation | 11.6 | 10.3 | ||
| Median | 41 | 70 | ||
| Range | 26-68 | 66-99 | ||
| QTc (s) | Mean | 0.39 | 0.34 | p < 0.001a |
| Standard deviation | 0.024 | 0.018 | ||
| Median | 0.40 | 0.33 | ||
| Range | 0.36-0.44 | 0.32-0.36 | ||
| Ventricular arrhythmias (on Holter) |
No | 18 78.3% |
9 90% |
0.395d |
| Yes | 5 21.7% |
1 10% |
||
| Pericardial effusion (on echocardiogram) |
No | 22 95.7% |
10 100% |
0.697d |
| Yes | 1 4.3% |
0 0% |
||
| MVP on valvular Doppler study | No | 20 87% |
10 100% |
0.325d |
| MVP | 3 13% |
0 0% |
||
| Ventricular global systolic function (LV and RV) | Normal | 23 100% |
10 100% |
|
| Not normal | 0 0% |
0 0% |
||
| Systolic murmur | No | 19 82.6% |
8 80% |
1.000b 0.605d |
| Yes | 4 17.4% |
2 20% |
||
| Systolic click | No | 14 60.9% |
8 80% |
0.256d |
| Yes | 9 39.1% |
2 20% |
Abbreviations: AN, anorexia nervosa; BMI, body mass index; HR, heart rate; MVP, mitral valve prolapse; LV, left ventricle; RV, right ventricle.
Wilcoxon rank-sum test, two-sided.
Fisher exact test, two-sided.
Wilcoxon rank-sum test, one-sided.
Fisher exact test, one-sided.
Table 10. No. of patients with bradycardia and anorexia nervosa.
| Other diagnosis | Anorexia | |
|---|---|---|
| HR < 50 | 0 0% |
16 69.6% |
| HR ≥ 50 | 10 100% |
7 30.4% |
| Total | 10 100% |
23 100% |
Abbreviation: HR, heart rate.
Note: Sensitivity = 69.6% and specificity = 100%.
A positive correlation was found between HR and BMI (Fig. 5A) and a negative one between QTc and BMI (Fig. 5B) and QTc and HR (Fig. 5C).
Fig. 5.
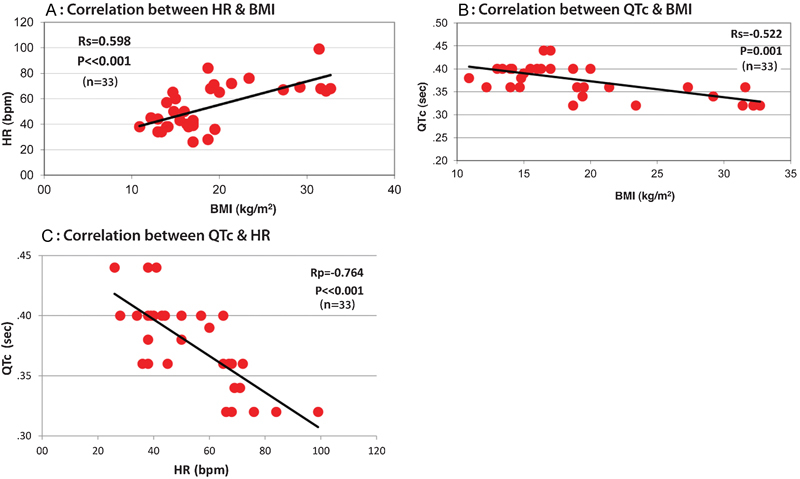
(A) Correlation between HR and BMI. (B) Correlation between QTc and BMI. (C) Correlation between QTc and HR. BMI, body mass index; HR, heart rate.
Clinical Course and Follow-Up
No patient needed positive chronotropic pharmacological intervention or pacemaker therapy. Under refeeding and psychological support, gradual increase in HR was observed. Six months after discharge, the mean HR increased from 48 ± 12 to 65 ± 6 bpm. No cardiac-related complications were recorded after 1 year of follow-up.
Discussion
Our study demonstrated the high prevalence of bradycardia among AN patients. This simple and “easy to get” clinical finding may be used as a marker for the possible presence of this entity, especially among hidden cases.
Similar to other reports,19,20,21 our article reconfirms the observation that bradycardia is a common finding among patients with AN.
Among psychosocial disturbances, eating disorders has the highest mortality rate.9 A 21-year follow-up of 34 patients with AN showed that 16% of them died of eating disorders-related complications.22 Most patients with AN are generally asymptomatic even in the presence of bradycardia, unless it is associated with hypotension or other arrhythmias. However, even in apparently asymptomatic patients, bradycardia can be a precursor of a potentially lethal arrhythmia.
The absence of lethal arrhythmia in our group is most probably related to either a short follow-up period and/or clinical improvement in their medical status.
Similar to our findings, it was postulated that refeeding should be gradual, thus preventing the refeeding syndrome, characterized by tachycardia, heart failure, and electrolytes abnormalities, that can lead to cardiac arrest.23
Cardiovascular complications are common in patients with AN, but sinus bradycardia has been described as the most common cardiovascular physical finding and the most common arrhythmia in this group of patients.19 It is important to appreciate its significance in this clinical setting, because it may be associated with sudden death, especially in the presence of other arrhythmias or ECG abnormalities, such as prolonged QTc interval secondary to electrolyte disturbances (such as hypokalemia, hypomagnesemia, hypophosphatemia), or due to congenital abnormalities such as long QT syndrome.20
The mechanism of bradycardia in AN is thought to be a physiological adaptation to increased vagal tone and decreased metabolism of energy utilization, due to the low caloric intake.21 It has been postulated that bradycardia results from structural changes in the heart, such as a decrease in the left ventricular muscle mass, secondary to malnutrition.24 It was suggested that in patients with atrophic hearts, bradycardia may be a compensatory mechanism to prevent heart failure. Vázquez et al described decreased glycogen content of myocardial cells and cellular atrophy that can contribute to the bradycardia.25
It was emphasized that the mechanism for bradycardia in patients with AN is different from bradycardia seen in athletes.26
There is evidence that the bradycardia of AN is reversible, and that following treatment, clinical improvement, and weight gain, the bradycardia is lessened and ameliorated at rest and during exercise.1,27 It is important to note that bradycardia among patients with weight loss could originate from conduction system disease that eventually needs permanent pacemaker therapy.28,29,30
It is well known that denial of being ill is common in patients with AN and therefore, symptoms associated with AN, such as fatigue, are not reported. The Society for Adolescent Medicine recommends referring these patients to hospital when day-time HR is < 50 bpm and night HR is < 45 bpm.29,31
Based on our data, it is recommended that physicians from different disciplines, family practitioners, pediatricians, gynecologists, primary care, endocrinologists, and military physicians who take care of adolescents, should be aware of and identify cardiovascular findings, particularly bradycardia (HR < 50 bpm), in adolescents who may have the diagnosis of AN. In other words, bradycardia may be the first sign of a possible eating disorder, both AN and bulimic nervosa.
It was recommended that patients with AN who are admitted to the hospital with a HR of less than 35 bpm should have continuous monitoring. Medication such as inotropic drugs should be avoided and are contraindicated because of the possibility of additional potential ventricular arrhythmias.19,32
Summary and Conclusions
Bradycardia is a common finding in AN. Its existence in young adults, especially young females with weight loss, represents an ominous sign for cardiac sudden death.
The presence of bradycardia among adolescents raises the possible diagnosis of an eating disorder, that is not always obvious, and that can be treated early and promptly in time. Thus it may prevent premature sudden death or disability in the future lives of these young patients.
References
- 1.American Heart Association Bradycardia Available at: http://www.heart.org/HEARTORG/Conditions/Arrhythmia/AboutArrhythmia/Bradycardia_UCM_302016_Article.jsp. Accessed September 6, 2012
- 2.Ulger Z, Gürses D, Ozyurek A R, Arikan C, Levent E, Aydoğdu S. Follow-up of cardiac abnormalities in female adolescents with anorexia nervosa after refeeding. Acta Cardiol. 2006;61(1):43–49. doi: 10.2143/AC.61.1.2005139. [DOI] [PubMed] [Google Scholar]
- 3.Ravaldi C, Vannacci A, Ricca V. Complicanze cardiache dell'anoressia nervosa. Recenti Prog Med. 2003;94(6):267–270. [PubMed] [Google Scholar]
- 4.McCallum K, Bermudez O, Ohlemeyer C, Tyson E, Portilla M, Ferdman B. How should the clinician evaluate and manage the cardiovascular complications of anorexia nervosa? Eat Disord. 2006;14(1):73–80. doi: 10.1080/10640260500403915. [DOI] [PubMed] [Google Scholar]
- 5.Steinhausen H C. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159(8):1284–1293. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan P F. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 7.Katzman D K Medical complications in adolescents with anorexia nervosa: a review of the literature Int J Eat Disord 200537(Suppl):S52–S59., discussion S87-S89 [DOI] [PubMed] [Google Scholar]
- 8.Mont L, Castro J, Herreros B. et al. Reversibility of cardiac abnormalities in adolescents with anorexia nervosa after weight recovery. J Am Acad Child Adolesc Psychiatry. 2003;42(7):808–813. doi: 10.1097/01.CHI.0000046867.56865.EB. [DOI] [PubMed] [Google Scholar]
- 9.Casiero D, Frishman W H. Cardiovascular complications of eating disorders. Cardiol Rev. 2006;14(5):227–231. doi: 10.1097/01.crd.0000216745.96062.7c. [DOI] [PubMed] [Google Scholar]
- 10.Golden N H, Meyer W. Nutritional rehabilitation of anorexia nervosa. Goals and dangers. Int J Adolesc Med Health. 2004;16(2):131–144. doi: 10.1515/ijamh.2004.16.2.131. [DOI] [PubMed] [Google Scholar]
- 11.Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. J Adolesc Health. 2003;32(6):416–421. doi: 10.1016/s1054-139x(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 12.Facchini M, Sala L, Malfatto G, Bragato R, Redaelli G, Invitti C. Low-K+ dependent QT prolongation and risk for ventricular arrhythmia in anorexia nervosa. Int J Cardiol. 2006;106(2):170–176. doi: 10.1016/j.ijcard.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Swenne I, Larsson P T. Heart risk associated with weight loss in anorexia nervosa and eating disorders: risk factors for QTc interval prolongation and dispersion. Acta Paediatr. 1999;88(3):304–309. doi: 10.1080/08035259950170079. [DOI] [PubMed] [Google Scholar]
- 14.Isner J M, Roberts W C, Heymsfield S B, Yager J. Anorexia nervosa and sudden death. Ann Intern Med. 1985;102(1):49–52. doi: 10.7326/0003-4819-102-1-49. [DOI] [PubMed] [Google Scholar]
- 15.Eckardt L, Haverkamp W, Borggrefe M, Breithardt G. Experimental models of torsade de pointes. Cardiovasc Res. 1998;39(1):178–193. doi: 10.1016/s0008-6363(98)00043-1. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association . Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. [Google Scholar]
- 17.Otto C M, Bonow R O. Philadelphia: Saunders, Elsevier Inc.; 2009. Valvular heart disease; p. 1512. [Google Scholar]
- 18.Connolly H M, Oh J K. Philadelphia: Saunders, Elsevier Inc.; 2009. Echocardiography; pp. 200–270. [Google Scholar]
- 19.Portilla M G. Bradycardia: an important physical finding in anorexia nervosa. J Ark Med Soc. 2011;107(10):206–208. [PubMed] [Google Scholar]
- 20.Vanderdonckt O, Lambert M, Montero M C, Boland B, Brohet C. The 12-lead electrocardiogram in anorexia nervosa: A report of 2 cases followed by a retrospective study. J Electrocardiol. 2001;34(3):233–242. doi: 10.1054/jelc.2001.25134. [DOI] [PubMed] [Google Scholar]
- 21.Kollai M, Bonyhay I, Jokkel G, Szonyi L. Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur Heart J. 1994;15(8):1113–1118. doi: 10.1093/oxfordjournals.eurheartj.a060636. [DOI] [PubMed] [Google Scholar]
- 22.Löwe B, Zipfel S, Buchholz C, Dupont Y, Reas D L, Herzog W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychol Med. 2001;31(5):881–890. doi: 10.1017/s003329170100407x. [DOI] [PubMed] [Google Scholar]
- 23.Kohn M R, Golden N H, Shenker I R. Cardiac arrest and delirium: presentations of the refeeding syndrome in severely malnourished adolescents with anorexia nervosa. J Adolesc Health. 1998;22(3):239–243. doi: 10.1016/S1054-139X(97)00163-8. [DOI] [PubMed] [Google Scholar]
- 24.Gottdiener J S, Gross H A, Henry W L, Borer J S, Ebert M H. Effects of self-induced starvation on cardiac size and function in anorexia nervosa. Circulation. 1978;58(3 Pt 1):425–433. doi: 10.1161/01.cir.58.3.425. [DOI] [PubMed] [Google Scholar]
- 25.Vázquez M, Olivares J L, Fleta J, Lacambra I, González M. Alteraciones cardiologicas en mujeres adolescentes con anorexia nerviosa. Rev Esp Cardiol. 2003;56(7):669–673. doi: 10.1016/s0300-8932(03)76937-1. [DOI] [PubMed] [Google Scholar]
- 26.Bjørnstad H, Storstein L, Meen H D, Hals O. Electrocardiographic findings in athletic students and sedentary controls. Cardiology. 1991;79(4):290–305. doi: 10.1159/000174893. [DOI] [PubMed] [Google Scholar]
- 27.Olivares J L, Vázquez M, Fleta J, Moreno L A, Pérez-González J M, Bueno M. Cardiac findings in adolescents with anorexia nervosa at diagnosis and after weight restoration. Eur J Pediatr. 2005;164(6):383–386. doi: 10.1007/s00431-005-1647-6. [DOI] [PubMed] [Google Scholar]
- 28.Tarlet J M, Boccara G, Foltzer E. et al. [Intrinsic sinus node dysfunction in adolescence during anorexia nervosa] Arch Mal Coeur Vaiss. 1997;90(11):1545–1548. [PubMed] [Google Scholar]
- 29.López-Guzmán A, Taboada F, Alvarez Escolá C. [Sinus bradycardia in anorexia nervosa] Nutr Hosp. 2002;17(1):46–47. [PubMed] [Google Scholar]
- 30.Kanbur NÖ, Goldberg E, Pinhas L, Hamilton R M, Clegg R, Katzman D K. Second-degree atrioventricular block (Mobitz Type I) in an adolescent with anorexia nervosa: intrinsic or acquired conduction abnormality. Int J Eat Disord. 2009;42(6):575–578. doi: 10.1002/eat.20647. [DOI] [PubMed] [Google Scholar]
- 31.Golden N H, Katzman D K, Kreipe R E. et al. Society For Adolescent Medicine . Eating disorders in adolescents: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33(6):496–503. doi: 10.1016/s1054-139x(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 32.Harris J P, Kreipe R E, Rossbach C N. QT prolongation by isoproterenol in anorexia nervosa. J Adolesc Health. 1993;14(5):390–393. doi: 10.1016/s1054-139x(08)80013-4. [DOI] [PubMed] [Google Scholar]


