Abstract
Standard treatment options in patients with lung cancer and pulmonary metastases are surgery, radiotherapy, chemotherapy, and immunotherapy. For reducing clinical complications of surgery and achieving a better local response, transpulmonary chemoembolization of the lungs is a possible interventional technique in which anticancer drugs are administered directly into a tumor through its feeding vessels followed by occlusive agents that are injected through the delivery catheter for blocking the vessel. This allows a longer contact period in the tumor with a higher cytostatic drug concentration. The technique is safe and results present promising local response rates, but the influence on survival is still questionable. This article describes the current role of intravascular therapies in the treatment of pulmonary malignancies.
Keywords: primary lung malignancy, secondary lung malignancy, transpulmonary chemoembolization (TPCE), isolated lung perfusion, bronchial artery infusion, lung suffusion, interventional radiology
Objectives: Upon completion of this article, the reader will be able to discuss the advantages and disadvantages of techniques providing regional chemotherapy to the lungs.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Lung cancer is the main cause of death among all kinds of cancers.1 It is responsible for ~23% of total cancer deaths. The main types of lung cancer are small cell lung cancer and non-small cell lung cancer. Long-term exposure to tobacco smoke causes 80 to 90% of lung cancers.2 Nonsmokers account for 10 to 15% of lung cancer cases, and these cases are often attributed to a combination of genetic factors or other exposures such as radon gas, asbestos, and pollution including secondhand smoke. Common treatments depend on the cancer's specific cell type (pathology), staging, and the patient's performance status. Traditional treatment options are surgery, chemotherapy, immunotherapy, radiation therapy, and palliative care. In patients with lung metastases from a nonpulmonary source, prognosis and outcome depend on the primary cancer, number of metastatic lesions, and degree of pulmonary involvement. Different treatment protocols are available, mainly for lung metastases from colorectal cancer.
Pulmonary Circulatory System
Pulmonary Artery
The lungs receive the entire cardiac output for the pulmonary circulation, with a volume of 5000 mL/min. The lungs represent a high-flow/low-pressure vascular system. Characteristics unique to the pulmonary arterial system include systolic, diastolic, and mean pressures of 25, 10, and 15 mm Hg, respectively, and thin vessel walls secondary to limited smooth muscle in the media. Rich capillary networks supplying this system hold ~45% of the total pulmonary blood volume.3 This system also has a very high compliance (30 mL/mm Hg), with decreases in the pulmonary vascular resistance with increasing intravascular pressure due to distension of capillaries and recruitment of unused ones.4
Bronchial Artery
Similar to other vascular distributions in the body, there are significant variations in the anatomy of the bronchial artery. A single artery on one side and two on the other is the most common, seen in >50% of people. Anastomosis between bronchial artery and pulmonary circulation occurs at the capillary level.5
Regional Lung Chemotherapy
Only a minority of patients with pulmonary malignancies are eligible for curative surgical treatment. Systemic chemotherapy, immunotherapy, and radiotherapy are established treatment options in palliative settings. Because drug concentration at the tumor site has been shown to be low after systemic chemotherapy, the concept of local drug delivery of such drugs is directed to transport high drug concentrations to the target site; a secondary benefit of such directed therapy is that side effects are reduced in comparison with systemic therapy.6,7 In pulmonary chemoembolization (TPCE), chemotherapeutic agents (such as mitomycin C, cisplatin, gemcitabine, and doxorubicin) as well as embolization agents (such as lipiodol or temporary embolization agents such as degradable microspheres) are used in combination and delivered directly to the lung vasculature.
Intravascular Techniques for Localized Delivery of Chemotherapeutic Agents
The main techniques of regional delivery of drugs for lung cancer therapy are arterial chemoembolization, bronchial artery infusion (BAI), isolated lung perfusion (ILP), and lung suffusion. Each method has specific advantages, disadvantages, and side effects. Contraindications for these procedures are generic, such as pregnancy, breast-feeding, and allergy to iodinated contrast media. Typical main side effects can be fever, chest pain, cough, vomiting, hemoptysis, mild and transient hemodynamic changes, and hematoma at the site of percutaneous puncture.
Transpulmonary Chemoembolization
Transarterial chemoembolization has been used successfully for many years as a method for treating primary and secondary hepatic malignancies,8 and it is now under evaluation as a less invasive choice for the treatment of lung cancer.9
On this basis TPCE was introduced, which does not require thoracotomy and can be repeatedly performed percutaneously through an endovascular catheter under fluoroscopic guidance. As a routine approach, a 5F catheter and 7F sheath are placed transfemorally into the pulmonary artery and advanced fluoroscopically over a guidewire into a segmental pulmonary artery. Commonly, a 7-mm balloon catheter is placed10 and chemotherapeutic agents are infused following balloon inflation. This selective catheterization results in obstruction of the arterial supply, with resultant regional ischemic necrosis of tumor while minimizing damage to the normal lung parenchyma (Fig. 1). Simultaneous blockage of the inflow and perfusion by embolization allows the use of higher chemotherapeutic doses for therapy. Permanent occlusion can be achieved by using material such as steel coils, polyvinyl alcohol, or agents like lipiodol. Degradable starch microspheres and gelatin sponges are temporary agents that may be used. New approaches using drug-eluting beads for controlled release of the therapeutic agent are currently under investigation.11
Figure 1.
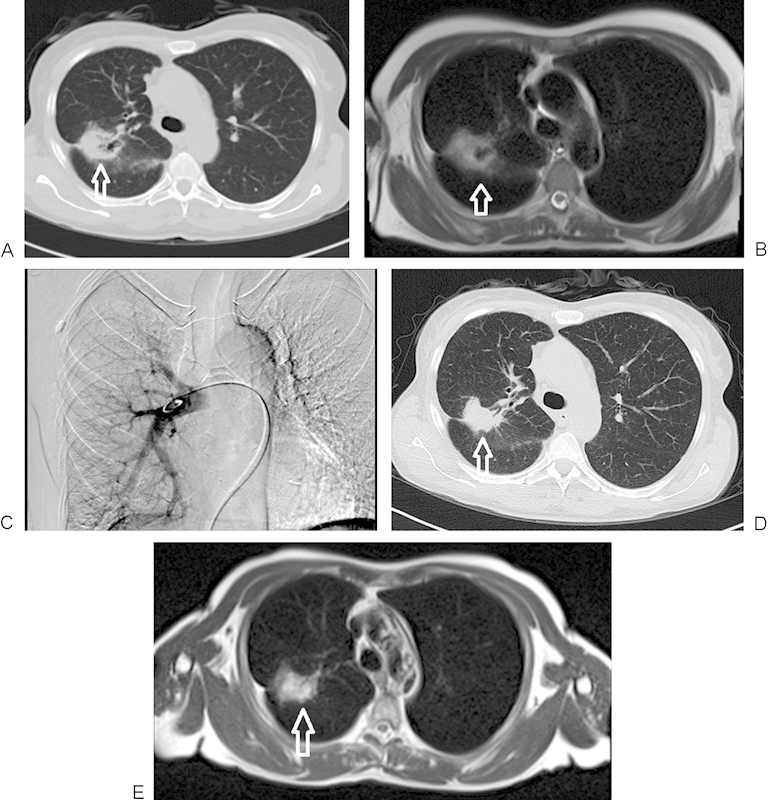
A 60-year-old woman with primary non-small cell lung carcinoma in the right lung, segment 6, undergoing treatment with transpulmonary chemoembolization. (A) Nonenhanced computed tomography (CT) image of lung before chemoembolization, demonstrating a 41 × 35 mm right lobe lesion (arrow). (B) T2-weighted nonenhanced magnetic resonance imaging (MRI) (2300/90 [TR/TE]) demonstrating the pretreatment tumor extension in the lung (arrow). (C) Angiographic verification of the catheter position in the main right pulmonary artery during the treatment. (D) CT image of the same patient during follow-up at 9 months, demonstrating significant downsizing of the tumor volume (36 × 25 mm) (arrow). (E) T2-weighted nonenhanced MRI of lung on 9-month follow-up, again demonstrating significant shrinkage of the tumor (arrow).
In 2005, our group reported treatment of 23 patients treated with TPCE. The study protocol was approved by the institutional review board, and patient consent was obtained. In these patients, 26 lung metastases of different origins were treated locally by a transpulmonary approach. Tumor-supplying pulmonary arteries were selectively identified, and 5 to 10 mg of mitomycin C and 5 to 10 mL of iodized oil and microsphere particles were delivered with balloon protection and flow arrest. Patients were followed at 3-month intervals by unenhanced and contrast-enhanced computed tomography. Treatment was well tolerated in all patients, with no major side effects or complications. By using morphological criteria, volume regression of embolized areas was obtained in eight patients (34.8%), stable disease was revealed at follow-up in six patients (26.1%), and progression of treated intrapulmonary metastases was detected in nine patients (39.1%) (Fig. 2) and (Fig. 3). TPCE was considered a well-tolerated palliative treatment option in patients with pulmonary metastases.12
Figure 2.
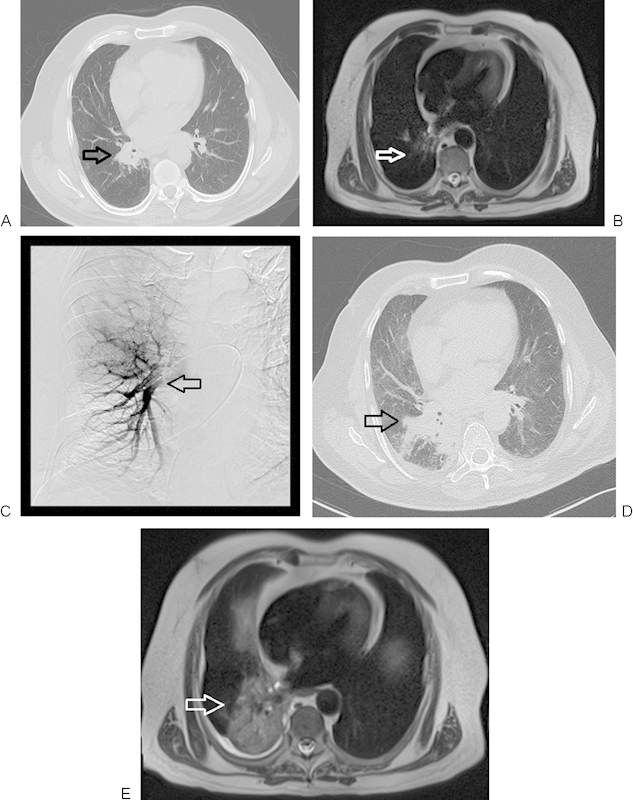
A 71-year-old man with primary non-small cell lung carcinoma in a right perihilar location, with progression following regional chemoembolization. (A) Nonenhanced computed tomography (CT) image of lung before chemoembolization. The initial lesion measured 39 × 32 mm (arrow). (B) Axial T2-weighted nonenhanced magnetic resonance imaging (2500/90 [TR/TE]) presenting primary lung cancer spreading before chemoembolization (arrow). (C) Transpulmonary chemoembolization with catheter placement in the right perihilar region (arrow). (D) Axial CT follow-up study 4 months after transpulmonary chemoembolization (TPCE) demonstrating tumor progression (59 × 58 mm) (arrow). (E) Axial T2-weighted nonenhanced MRI of the lung, 4 months post-TPCE with further tumor progression (arrow).
Figure 3.
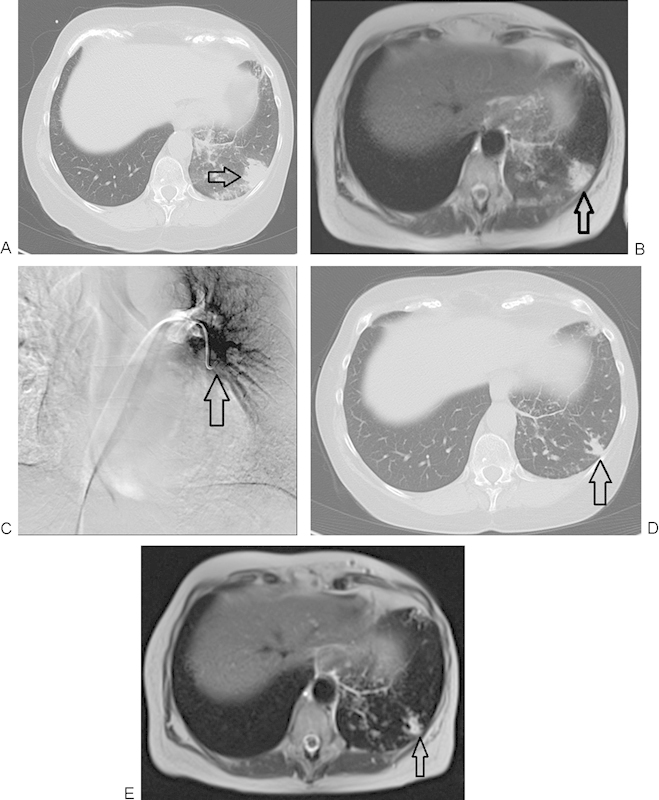
A 64-year-old woman with breast cancer therapy and resistant lung metastasis. (A) Nonenhanced computed tomography (CT) image of the lung demonstrating metastatic breast cancer before chemoembolization. Lesion initially measured 27 × 26 mm in diameter (arrow). (B) Axial T2-weighted nonenhanced magnetic resonance imaging (MRI) of the lung demonstrating metastasis of lung cancer before chemoembolization (arrow). (C) Transpulmonary chemoembolization (TPCE) with catheter positioned in the middle segment of the left lung (arrow). (D) Axial CT follow-up study 12 months post-TPCE with significant downsizing of the tumor, now measuring 15 × 10 mm (arrow). (E) Axial T2-weighted nonenhanced MRI of the lung in a follow-up study 12 months post-TPCE. Again demonstrated is the significant decrease in lesional diameter (arrow).
Bronchial Artery Infusion
The method of localized chemotherapy by using BAI first appeared in the medical literature in 1951. This transarterial method of drug delivery has shown good response rates, particularly in tumors in organs with dual blood supply such as liver tumor metastases (hepatic artery) and lung metastases (bronchial arteries).13 Kahn et al first demonstrated BAI chemotherapy in 1965 by inserting a transfemoral 5F catheter into the bronchial artery under angiographic guidance14
The use of coaxial microcatheters is essential for superselective arterial catheterization; by these means single or more anticancer agents can be delivered. This approach can be repeated for several weeks and used as a primary and palliative treatment option. Some groups have used a combination of this technique with radiotherapy.15 In the presence of several feeding arteries, multiarterial infusion chemotherapy can be performed.16 Here angiographic structures are needed for exact identification of tumor and feeding arteries.17 Although most complications are not significant, bronchial or esophageal ulceration, spinal cord injury, and formation of a bronchoesophageal fistula may rarely occur as very serious side effects of the technique.18
Isolated Lung Perfusion
ILP is performed by cannulation of the ipsilateral pulmonary artery and veins; the system is connected with an extracorporeal flow system, allowing an enclosed perfusion circuit. In some cases bilateral ILP has been used; in these instances, cardiopulmonary bypass by staged unilateral ILPs or by total lung perfusion with cannulation of the right atrium and the ascending aorta is performed. Selective delivery of agents to the lungs via the pulmonary artery combined with localized hyperthermia provides a better condition for more tissue absorption and the cytotoxicity effect of the delivered drugs.19
In this method, systemic anticoagulation therapy is necessary. Pulmonary ventilation should remain intact for spreading the agents. Perfusion techniques takes ~30 to 90 minutes. ILP is a complex method because safe cannulation of these vessels requires thoracotomy. Complications such as adverse systemic inflammatory responses occur frequently due to direct toxic effect as a consequence of leakage and/or release of cytokines.18
Lung Suffusion
The term suffusion has been used to provide slow, diffuse permeation of the tissues by an injection during occlusion of arteries or veins.20 This technique requires coordination between radiologic control of the pulmonary artery and thorascopic control of the pulmonary veins for isolating chemotherapy into the lung. It is assumed that distention of the pulmonary capillary pool as a result of the increase in pulmonary vascular resistance (a consequence of venous constriction) facilitates organ permeation by the chemotherapy agent. Another identical vascular network is in the heart, and for this reason clinical experience exists for similar suffusion techniques such as retrograde (coronary vein-perfused) cardioplegia.21 For regional lung chemotherapy techniques, important factors include rapid systemic clearance of the released drug, high uptake by the target tissue, and relatively brief exposure times.
It is conceivable to use this method as a debulking tool for primary or metastatic cancer as a neoadjuvant prior to lung resection. Micrometastatic disease in the lung or its lymphatic drainage system is another target for nonselective methods like ILP or suffusion.
Results
In reviewing the current literature for the previously discussed methods, we identified 20 articles with 422 documented patients with primary or secondary lung cancers. Ten of these articles with 318 cases were primary lung cancers; 8 studies with 96 cases represented secondary lung tumors.
In three articles with ~100 cases, TPCE was the method of choice for therapy. In all three articles, tumor response was reported as “good” and with “no major complications.”
In four articles with 162 cases, BAI was the method of choice for therapy. In this group, the outcome in one article was documented as “good” and with “no major complications.” In another study the outcome was “good” but with “minor complications.”
In five articles with 58 cases, ILP was the method of choice for therapy. In this group, three articles with 30 cases described the outcome as “good,” in one article with 20 cases the outcome was “relatively good” (with minor complications), and in one article with 8 cases outcome was “unsatisfactory” (with major complications).
In one article with four cases, lung suffusion was the method of choice for therapy. In this article the outcome was “good” without any major complication.
In six articles with 168 cases, a combination of regional and systemic therapies was the method of therapy. In all of these articles, the outcome was “good” without any major complications (Table 1).
Table 1. Regional chemotherapy of the lung: List of references.
| Study | N | Pathology | Technique | Agents | Outcome | Complications. | Comment | |
|---|---|---|---|---|---|---|---|---|
| 1 | Vogl et al12 | 23 | 26 lung metastases of different origins | TPCE | Mitomycin C, iodized oil, microsphere particles | Volume regression in 8 patients; stable disease in 6 patients; progression in 9 patients | No major side effects or complication | Well tolerated |
| 2 | Lindemayr et al36 | Unresectable lung tumors | TPCE | Cytotoxic agents, microspheres, ethiodized oil | Well tolerated | |||
| 3 | Watanabe et al37 | 106 | Cases of stage III hilar tumor | BAI | Carboquone, mitomycin C, and/or nimustine | Tumor volume reduced in 41% and 84% with single and triple drug therapy, respectively | Transient hemiplegia | |
| 4 | Shi et al38 | 10 | with pulmonary artery infusion | Carboplatin and etoposide | 80% response | |||
| 5 | Demmy et al20 | 4 | Stage IV NSCLC | Cisplatin with systemic chemotherapy | 14-96% reduction in tumor volume | |||
| 7 | Ratto et al39 | 6 | Metastatic sarcoma | Cisplatin | Interstitial and alveolar edema | |||
| 8 | Nakanishi et al16 | 32 | NSCLC | Multiarterial infusion | Cisplatin, doxorubicin, and/or gemcitabine | 3% complete response; 50% partial response | Interstitial pneumonia, hepatic failure, respiratory failure | |
| 9 | So et al40 | 3 | Locally advanced NSCLC | Chemotherapy and carinal resection | Cisplatin | 100% complete response | ||
| 10 | Schneider et al41 | 12 | Temporary unilateral microembolization of the lung | Group 1: microspheres (DSMs) group 2: combined with carboplatin |
||||
| 11 | Osaki et al42 | 7 | Central early stage squamous carcinoma | BAI | Camptothecin 11 and cisplatin | 86% complete response | Bronchial ulcer, pulmonary hemorrhage, and death after 3 mo | |
| 12 | Burt et al43 | 8 | Metastatic sarcoma | Doxorubicin | Pulmonary fibrosis; reduced pulmonary function | |||
| 13 | Schröder et al44 | 4 | Metastatic sarcoma | Chemotherapy following metastectomy | Cisplatin | 75% disease free after 1 y | Localized pulmonary edema; reduced pulmonary function at 3 wk | |
| 14 | Karakousis et al45 | 7 | Soft tissue sarcomas with lung metastases | Regional chemotherapy via Swan-Ganz catheter | Adriamycin | |||
| 15 | Liu et al46 | 76 | Moderate or advanced NSCLC | CHM combined with BAI | Cisplatin, doxorubicin, and/or mitomycin C | 51% and 24% 2-y survival rate with and without Chinese herbal medication, respectively | Effect of BAIC could be enhanced by combining it with CHM | |
| 16 | Hendriks et al47 | 16 | Resectable pulmonary metastases | Melphan (MN) | ||||
| 17 | Koshiishi et al48 | 17 | Advanced cancer | BAI | Camptothecin 11, cisplatin, and/or etoposide | 12% partial response | Severe chest symptoms | |
| 18 | Johnston et al49 | 8 | Metastatic sarcoma or diffuse bronchioloalveolar carcinoma | Total lung perfusion | Doxorubicin or cisplatin | Pneumonia; respiratory failure | ||
| 19 | Pass et al50 | 20 | Pulmonary metastases | TNF-α and interferon-γ | 15% partial response | Lung abscess, TNF-α leakage and systemic toxicity | ||
| 20 | Wang et al51 | 63 | Cases of locally advanced bronchogenic cancer | Combined hyperfractionated radiotherapy and bronchial arterial infusion | Adriamycin, cisplatin, etoposide, and/or mitomycin C, with radiotherapy | 44% complete response; 40% partial response | Hematoma at site of percutaneous puncture |
Abbreviations: TPCE, transpulmonary chemoembolization; BAI, bronchial artery chemoembolization; NSCLC, Non-small cell lung cancer; Pao2, partial pressure of oxygen, arterial; DSMs, degradable starch microspheres; CHM, Chinese herbal medicine; TNF, tumor necrosis factor.
Discussion
Because only 46% of treated patients demonstrate long-term survival, treatment of pulmonary metastases is still a major challenge.22,23,24,25,26,27,28,29 Systemic chemotherapy also demonstrates marginal results. For this reason, multimodality therapy regimes have been postulated.30 A retrospective study comparing multimodality therapy (including modified pharmacokinetic-modulating chemotherapy, radiation, and RFA) versus single chemotherapy, published in 2005, showed a significant increase in survival rates with a multimodality regime therapy: the 3-year survival rate of patients in the multimodality group was 87.5% versus 33.3% in the chemotherapy group.31
In animal studies it has been shown that isolated lung perfusion was more effective than systemic chemotherapy,32,33 but the former requires thoracotomy, which is stressful for the patient and cannot be repeated extensively. Moreover, extracorporal circulation is required.34 TPCE of the lung offers the advantages of ILP over systemic chemotherapy,32,33 without the disadvantages just cited. A report published in 2007 showed TPCE to be even superior to ILP in terms of lipiodol uptake in a rat model. This showed that TPCE of the lung is a promising component in a multimodality therapy concept.
TPCE could be an additional tool for multimodality therapy, and in combination with thermal ablation even better results might be achieved.28,35 The macroscopic parts can be resected while the residual microscopic components can be treated by regional and systemic chemotherapy.28 Regional chemotherapy has potential advantages because it potentially confers a lower side-effect profile. Data show that TPCE using mitomycin followed by embolization agents such as lipiodol and microspheres is a well-tolerated treatment method for patients with unresectable lung metastases. The results obtained in other anatomical regions with hypervascular cancers such as thyroid, renal cell, or hepatocellular carcinoma prove the hypothesis of the major impact of the lipiodol uptake and its tumoricidal influence on the tumor matrix. With this method even a larger number of lesions can be treated in combination with other ablating procedures such as radiofrequency ablation (RFA) or microwave ablation (MWA). It is still too early to say whether RFA or MWA could be combined with TPCE in the future. However, the additional advantage of TPCE over ablating procedures with no risk of pneumothorax is obvious. Furthermore, healthy lung tissue is preserved due to the fact that a safety margin for adjacent structures, necessary for ablation procedures, is not required for TPCE.
TPCE is a well-tolerated palliative treatment option in patients with lung metastases and primary lung cancer. Early use of this method has resulted in a reduction in tumor volume and patient symptoms, and current data suggest that this approach is well tolerated.
References
- 1.Parkin D M, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Thun M J, Henley S J, Calle E E. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene. 2002;21(48):7307–7325. doi: 10.1038/sj.onc.1205807. [DOI] [PubMed] [Google Scholar]
- 3.Hughes J MB Morrell N W Pulmonary Circulation: From Basic Mechanisms to Clinical Practice London, United Kingdom: Imperial College Press; 2001:xix, 356 [Google Scholar]
- 4.Carden D L Matthay M A George R B Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. 5th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2005:xvii, 652 [Google Scholar]
- 5.Riquet M Bronchial arteries and lymphatics of the lung Thorac Surg Clin 2007174619–638., viii [DOI] [PubMed] [Google Scholar]
- 6.Schiller J H, Harrington D, Belani C P. et al. Eastern Cooperative Oncology Group . Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 7.Minchinton A I, Tannock I F. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 8.Gonsalves C F, Brown D B. Chemoembolization of hepatic malignancy. Abdom Imaging. 2009;34(5):557–565. doi: 10.1007/s00261-008-9446-y. [DOI] [PubMed] [Google Scholar]
- 9.Golder W A. Chemoembolization of the lungs: basic anatomical elements, experimental results, clinical experience. Onkologe. 2008;14:934–939. [Google Scholar]
- 10.Lindemayr S, Lehnert T, Korkusuz H, Hammerstingl R, Vogl T J. Transpulmonary chemoembolization: a novel approach for the treatment of unresectable lung tumors. Tech Vasc Interv Radiol. 2007;10(2):114–119. doi: 10.1053/j.tvir.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Lewis A L, Gonzalez M V, Lloyd A W. et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17(2 Pt 1):335–342. doi: 10.1097/01.RVI.0000195323.46152.B3. [DOI] [PubMed] [Google Scholar]
- 12.Vogl T J, Wetter A, Lindemayr S, Zangos S. Treatment of unresectable lung metastases with transpulmonary chemoembolization: preliminary experience. Radiology. 2005;234(3):917–922. doi: 10.1148/radiol.2343032091. [DOI] [PubMed] [Google Scholar]
- 13.Jiang G M, Zhao J W, Chen Y X, Tian F. Blood supply of pulmonary metastases and its clinical significance [in Chinese] Ai Zheng. 2006;25(7):885–887. [PubMed] [Google Scholar]
- 14.Kahn P C, Paul R E, Rheinlander H F. Selective bronchial arteriography and intra-arterial chemotherapy in carcinoma of the lung. J Thorac Cardiovasc Surg. 1965;50(5):640–645. [PubMed] [Google Scholar]
- 15.Hellekant C, Jonsson K. Double blood supply of bronchogenic carcinoma from multiple arteries. Acta Radiol Diagn (Stockh) 1981;22(4):403–406. doi: 10.1177/028418518102200403. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi M, Umeda Y, Demura Y. et al. Effective use of multi-arterial infusion chemotherapy for advanced non-small cell lung cancer patients: four clinical specified cases. Lung Cancer. 2007;55(2):241–247. doi: 10.1016/j.lungcan.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi M, Demura Y, Umeda Y. et al. Multi-arterial infusion chemotherapy for non-small cell lung carcinoma—significance of detecting feeding arteries and tumor staining. Lung Cancer. 2008;61(2):227–234. doi: 10.1016/j.lungcan.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Mallick R, Demmy T. Regional lung chemotherapy techniques. Innovations (Phila) 2011;6(1):1–9. doi: 10.1097/IMI.0b013e31820b1e63. [DOI] [PubMed] [Google Scholar]
- 19.Urano M, Kuroda M, Nishimura Y. For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia. 1999;15(2):79–107. doi: 10.1080/026567399285765. [DOI] [PubMed] [Google Scholar]
- 20.Demmy T L Tomaszewski G Dy G K et al. Thoracoscopic organ suffusion for regional lung chemotherapy (preliminary results) Ann Thorac Surg 2009882385–390.; discussion 390-391 [DOI] [PubMed] [Google Scholar]
- 21.Carpenter A J, Follette D M, Sheppard B, Yoshikawa R, Sam J. Simultaneous antegrade and retrograde reperfusion after cardioplegic arrest for coronary artery bypass. J Card Surg. 1999;14(5):354–358. doi: 10.1111/j.1540-8191.1999.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 22.Vogt-Moykopf I, Bulzebruck H, Kryza S, Kruschinski H, Branscheid D, Schirren J. Results in surgery of pulmonary metastases. Chirurgie. 1992;118(5):263–271. [PubMed] [Google Scholar]
- 23.Friedel G, Pastorino U, Buyse M. et al. [Resection of lung metastases: long-term results and prognostic analysis based on 5206 cases—the International Registry of Lung Metastases [in German] Zentralbl Chir. 1999;124(2):96–103. [PubMed] [Google Scholar]
- 24.Hendriks J M, Romijn S, Van Putte B. et al. Long-term results of surgical resection of lung metastases. Acta Chir Belg. 2001;101(6):267–272. [PubMed] [Google Scholar]
- 25.Abecasis N, Cortez F, Bettencourt A, Costa C S, Orvalho F, de Almeida J M. Surgical treatment of lung metastases: prognostic factors for long-term survival. J Surg Oncol. 1999;72(4):193–198. doi: 10.1002/(sici)1096-9098(199912)72:4<193::aid-jso3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Lanza L A, Putnam J B Jr, Benjamin R S, Roth J A. Response to chemotherapy does not predict survival after resection of sarcomatous pulmonary metastases. Ann Thorac Surg. 1991;51(2):219–224. doi: 10.1016/0003-4975(91)90789-s. [DOI] [PubMed] [Google Scholar]
- 27.Casson A G, Putnam J B, Natarajan G. et al. Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer. 1992;69(3):662–668. doi: 10.1002/1097-0142(19920201)69:3<662::aid-cncr2820690311>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 28.Ueda T, Uchida A, Kodama K. et al. Aggressive pulmonary metastasectomy for soft tissue sarcomas. Cancer. 1993;72(6):1919–1925. doi: 10.1002/1097-0142(19930915)72:6<1919::aid-cncr2820720621>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Weksler B, Ng B, Lenert J T, Burt M E. Isolated single-lung perfusion with doxorubicin is pharmacokinetically superior to intravenous injection. Ann Thorac Surg. 1993;56(2):209–214. doi: 10.1016/0003-4975(93)91149-h. [DOI] [PubMed] [Google Scholar]
- 30.Mountain C F, Khalil K G, Hermes K E, Frazier O H. The contribution of surgery to the management of carcinomatous pulmonary metastases. Cancer. 1978;41(3):833–840. doi: 10.1002/1097-0142(197803)41:3<833::aid-cncr2820410309>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Inoue Y, Miki C, Hiro J. et al. Improved survival using multi-modality therapy in patients with lung metastases from colorectal cancer: a preliminary study. Oncol Rep. 2005;14(6):1571–1576. [PubMed] [Google Scholar]
- 32.Van Putte B P Hendriks J M Romijn S et al. Single-pass isolated lung perfusion versus recirculating isolated lung perfusion with melphalan in a rat model Ann Thorac Surg 2002743893–898.; discussion 898 [DOI] [PubMed] [Google Scholar]
- 33.Romijn S, Hendriks J M, Van Putte B P. et al. Regional differences of melphalan lung levels after isolated lung perfusion in the rat. J Surg Res. 2005;125(2):157–160. doi: 10.1016/j.jss.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Schneider P, Kampfer S, Loddenkemper C, Foitzik T, Buhr H J. Chemoembolization of the lung improves tumor control in a rat model. Clin Cancer Res. 2002;8(7):2463–2468. [PubMed] [Google Scholar]
- 35.Wagner W, von Eiff M, Klinke F, Micke O, Rübe C, Willich N. [Neoadjuvant radiochemotherapy in locally advanced non-small cell bronchial carcinoma. Initial results of a prospective multicenter study [in German] Strahlenther Onkol. 1995;171(7):390–397. [PubMed] [Google Scholar]
- 36.Lindemayr S, Lehnert T, Korkusuz H, Hammerstingl R, Vogl T J. Transpulmonary chemoembolization: a novel approach for the treatment of unresectable lung tumors. Tech Vasc Interv Radiol. 2007;10(2):114–119. doi: 10.1053/j.tvir.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe Y, Shimizu J, Murakami S. et al. Reappraisal of bronchial arterial infusion therapy for advanced lung cancer. Jpn J Surg. 1990;20(1):27–35. doi: 10.1007/BF02470710. [DOI] [PubMed] [Google Scholar]
- 38.Shi W, Zhang S, Zhang X. Treatment of non-small-cell lung cancer by dual (bronchial and pulmonary) arterial drug infusion [in Chinese] Zhonghua Zhong Liu Za Zhi. 1995;17(2):146–148. [PubMed] [Google Scholar]
- 39.Ratto G B, Toma S, Civalleri D. et al. Isolated lung perfusion with platinum in the treatment of pulmonary metastases from soft tissue sarcomas. J Thorac Cardiovasc Surg. 1996;112(3):614–622. doi: 10.1016/S0022-5223(96)70043-0. [DOI] [PubMed] [Google Scholar]
- 40.So T, Osaki T, Nakata S, Hanagiri T, Sugio K, Yasumoto K. Carinal resection after induction bronchial arterial infusion for locally advanced non-small cell lung cancer. Jpn J Thorac Cardiovasc Surg. 2004;52(3):143–147. doi: 10.1007/s11748-004-0131-y. [DOI] [PubMed] [Google Scholar]
- 41.Schneider P, Foitzik T, Pohlen U, Golder W, Buhr H J. Temporary unilateral microembolization of the lung—a new approach to regional chemotherapy for pulmonary metastases. J Surg Res. 2002;107(2):159–166. doi: 10.1006/jsre.2002.6511. [DOI] [PubMed] [Google Scholar]
- 42.Osaki T, Hanagiri T, Nakanishi R, Yoshino I, Taga S, Yasumoto K. Bronchial arterial infusion is an effective therapeutic modality for centrally located early-stage lung cancer: results of a pilot study. Chest. 1999;115(5):1424–1428. doi: 10.1378/chest.115.5.1424. [DOI] [PubMed] [Google Scholar]
- 43.Burt M E, Liu D, Abolhoda A. et al. Isolated lung perfusion for patients with unresectable metastases from sarcoma: a phase I trial. Ann Thorac Surg. 2000;69(5):1542–1549. doi: 10.1016/s0003-4975(00)01131-0. [DOI] [PubMed] [Google Scholar]
- 44.Schröder C, Fisher S, Pieck A C. et al. Technique and results of hyperthermic (41 ° C) isolated lung perfusion with high-doses of cisplatin for the treatment of surgically relapsing or unresectable lung sarcoma metastasis. Eur J Cardiothorac Surg. 2002;22(1):41–46. doi: 10.1016/s1010-7940(02)00216-6. [DOI] [PubMed] [Google Scholar]
- 45.Karakousis C P, Park H C, Sharma S D, Kanter P. Regional chemotherapy via the pulmonary artery for pulmonary metastases. J Surg Oncol. 1981;18(3):249–255. doi: 10.1002/jso.2930180305. [DOI] [PubMed] [Google Scholar]
- 46.Liu C L, Wang Y D, Jin X J. Clinical observation on treatment of non-small cell lung cancer with Chinese herbal medicine combined with bronchial arterial infusion chemotherapy [in Chinese] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21(8):579–581. [PubMed] [Google Scholar]
- 47.Hendriks J M Grootenboers M J Schramel F M et al. Isolated lung perfusion with melphalan for resectable lung metastases: a phase I clinical trial Ann Thorac Surg 20047861919–1926.; discussion 1926-1927 [DOI] [PubMed] [Google Scholar]
- 48.Koshiishi H, Utsumi K, Tamamoto F, Takahashi E. Evaluation of bronchial arterial infusion (BAI) for high risk lung cancer [in Japanese] Gan To Kagaku Ryoho. 2000;27(12):1907–1910. [PubMed] [Google Scholar]
- 49.Johnston M R, Minchen R F, Dawson C A. Lung perfusion with chemotherapy in patients with unresectable metastatic sarcoma to the lung or diffuse bronchioloalveolar carcinoma. J Thorac Cardiovasc Surg. 1995;110(2):368–373. doi: 10.1016/S0022-5223(95)70232-6. [DOI] [PubMed] [Google Scholar]
- 50.Pass H I, Mew D J, Kranda K C, Temeck B K, Donington J S, Rosenberg S A. Isolated lung perfusion with tumor necrosis factor for pulmonary metastases. Ann Thorac Surg. 1996;61(6):1609–1617. doi: 10.1016/0003-4975(96)00166-X. [DOI] [PubMed] [Google Scholar]
- 51.Wang G, Song M, Xu H, Fang Y. Prospective trial of combined hyperfractionated radiotherapy and bronchial arterial infusion of chemotherapy for locally advanced nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys. 1996;34(2):309–313. doi: 10.1016/0360-3016(95)02111-6. [DOI] [PubMed] [Google Scholar]


