Abstract
The incidence of lung cancers in 2012 is estimated to reach 226,160 new cases, with only a third of patients suitable surgical candidates. Tumor ablation has emerged as an important and efficacious treatment option for nonsurgical lung cancer patients. This localized minimally invasive therapy is best suited for small oligonodular lesions or favorably located metastatic tumors. Radiofrequency ablation has been in use for over a decade, and newer modalities including microwave ablation, cryoablation, and irreversible electroporation have emerged as additional treatment options for patients. Ablation therapies can offer patients and clinicians a repeatable and effective therapy for palliation and, in some cases, cure of thoracic malignancies. This article discusses the available technologies and techniques available for tumor ablation of thoracic malignancies including patient selection, basic aspects of procedure technique, imaging follow-up, treatment outcomes, and comparisons between various therapies.
Keywords: lung cancer, thermal ablation, radiofrequency ablation, microwave ablation, cryoablation, interventional radiology
Objectives: Upon completion of this article, the reader will be able to demonstrate the basics of tumor ablation for the treatment of thoracic malignancies including patient selection criteria, basic aspects of procedure technique, imaging follow-up, treatment outcomes, and the differences between various therapies.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Lung cancer is currently the second most commonly diagnosed cancer and the leading cause of cancer death in the United States. It is projected that 226,160 new cases of lung cancer will be diagnosed in 2012, and there will be an estimated 160,340 deaths related to lung cancer.1 Additionally, the lung is a frequent site for metastatic disease, with common primary malignancies including those of the breast, colon, prostate, kidney and bladder, as well as sarcoma.2 Surgical resection, consisting of a lobectomy and complete lymph node dissection, is the reference standard for the treatment of early stage lung cancer and offers patients the best chance of long-term disease-free survival.3 However, it is estimated that only a third of patients with non-small cell lung cancer (NSCLC) are candidates for curative resection.4 For patients who are not surgical candidates, minimally invasive treatment options including percutaneous thermal ablation therapies and high-dose radiation therapies have emerged as safe and effective treatment alternatives.5,6,7,8,9,10,11,12,13,14
Image-guided tumor ablation allows for the controlled and direct destruction of solid neoplasms using thermal or electrical energy. It is a cost-effective minimally invasive alternative to surgical resection that allows for greater preservation of normal lung parenchyma. Most ablation treatments can be performed under conscious sedation and in an outpatient setting, allowing for quicker recovery and discharge compared with surgery. In addition, tumor ablation can enhance the efficacy of chemotherapy and/or external-beam radiation, via cytoreduction induced by thermocoagulation.15 Since its emergence as a treatment for malignancies over a decade ago, tumor ablation has continued to gain momentum as an important tool in the treatment of primary and secondary lung neoplasms, offering patients and clinicians a repeatable and effective therapy for palliation and, in some cases, cure of thoracic malignancies. Most of the available data analyzing the ablation of thoracic malignancies evaluates radiofrequency ablation (RFA); however, additional thermal ablation modalities including microwave ablation (MWA) and cryoablation have emerged as viable treatment options. Additionally, one of the newest ablation technologies, irreversible electroporation (IRE), is discussed here. The goal of this article is to discuss the available technologies and techniques available for tumor ablation of thoracic malignancies including patient selection, basic aspects of procedure technique, imaging follow-up, treatment outcomes, and comparisons between various therapies.
Patient Selection
Image-guided lung ablation therapies are best suited for patients who are not surgical candidates as a result of comorbid cardiopulmonary disease (including severe chronic obstructive pulmonary disease), or who are unable to tolerate lung loss. In general, patients who are able to undergo a computed tomography (CT)-guided needle biopsy are appropriate candidates for tumor ablation. Patients referred for ablation treatment of early stage lung cancer usually have poor pulmonary function; however, there are generally no lower limits of forced expiratory volume in 1 second or diffusing capacity of lungs for carbon monoxide in ablation candidates.
Contraindications for treatment include underlying interstitial disease, such as pulmonary fibrosis, in which exacerbation of the underlying disease is likely to result in severe pulmonary failure and even death.16 Patients with severe underlying respiratory disease, an asymptomatic tumor, and a life expectancy estimated at <1 year, are not considered good candidates for treatment.17 Overall, patients best suited for ablation are those who are high-risk surgical candidates with early stage lung cancer, marked by a small oligonodular tumor or favorably located pulmonary metastases without any hilar or mediastinal nodal involvement or extrathoracic involvement.18 Also, patients seeking palliative measures for tumor-related symptoms or who have a recurrent thoracic malignancy within appropriate treatment fields should be considered candidates for ablation.
Procedure
Prior to tumor ablation, patients are evaluated in a clinical setting, and a medical history, physical examination, and laboratory analysis are performed. Additionally, preprocedural imaging studies are ordered, and the risks and benefits of the procedure are discussed with the patient. Possible side effects are explained including the possibility of postablation syndrome, an inflammatory response caused by circulating tumor necrosis factor and associated with fever, malaise, and anorexia. Patients are also informed of possible mild to moderate pain (usually adequately controlled with analgesics), mild pyrexia (usually self-limited and lasting several weeks), pneumothorax (possibly requiring chest tube placement), hemorrhage, hemoptysis, bronchopleural fistulas (possibly requiring treatment with chest tube suction, Heimlich valves, chemical or mechanical pleurodesis, surgery, or endobronchial valves19), acute respiratory distress syndrome, reactive pleural effusion (usually self-limited), damage to surrounding structures, skin burns resulting from inappropriate grounding pad placement or tract heating, infection, and abscess formation. It should be stressed to patients that the likelihood of a severe complication occurring is very rare.
Patients are instructed to fast overnight prior to the ablation, to reduce the probability of sedation-induced nausea or aspiration of stomach contents. Those patients on extensive medication regiments including antihypertensives and cardiac medications are instructed to take their pills on the morning of the procedure with a small amount of water. Insulin-dependent diabetics are advised to take half of their normal morning dose of insulin. Caution must be used in patients with implantable cardiac devices, such as pacemakers or defibrillators, because they are susceptible to interference from the energy spectrums of certain ablation modalities. We advise that when treating patients with pacemakers and defibrillators that treatments be coordinated with a cardiac electrophysiologist, to interrogate and program the pacemakers to automatic pacing modes (by placing a magnet over the device) and to turn off defibrillators during the ablation. In such patients, an external pacing and/or defibrillator system should be in place for emergency use. Additionally, grounding pads should be placed to guide the flow of current away from the cardiac device, and, when possible, electrodes should be inserted >5 cm from pacemaker and defibrillator leads.
On the day of the procedure we perform an abridged physical examination and an intravenous (IV) line is placed. Patients are brought to the CT scanner, and technical staff attach a reference electrode or grounding pad to the patient's skin, generally on the opposite chest wall or thigh. Most thermal ablations, including RFA, MWA, and cryoablation, are performed with IV sedation and analgesia with midazolam and fentanyl. Continuous monitoring of vital signs, electrocardiography, and blood pressure is performed to provide adequate and safe sedation. General anesthesia is used in pediatric patients or patients who cannot tolerate thermal ablation with conscious sedation alone. Initial scout images are taken, from which the skin entry site(s) is determined. Horizontal and vertical laser lights emitted from the CT gantry correspond to x- and y-axes from a grid on a computer screen; from these lines we extrapolate the correct entry sites on the patient's skin.
The area is sterilely prepped and draped, followed by the injection of local buffered lidocaine anesthesia, both intradermally and to the level of the pleura. A total of 10 to15 mL of lidocaine is instilled in the extrapleural location to minimize pain when the electrode is inserted. CT fluoroscopy is initiated, and images are obtained with a spinal needle in place to determine the appropriate electrode trajectory. A scalpel blade is used to make a 1- to 2-cm-deep skin incision at the appropriate electrode entry site.
Several studies and articles have been published regarding methods to reduce pain and complications when ablating thoracic malignancies. VanSonnenberg et al used intercostal and paravertebral nerve blocks with a long-acting local anesthetic when treating patients with painful osseous lesions and/or chest wall invasion, and they successfully reduced postprocedure pain.20 Cryoablation is also purported to provide an analgesic effect to the intercostal nerves during treatment.21 For tumors abutting or near sensitive structures, such as the phrenic nerve, vascular structures, or cartilaginous structures, hydrodissection or an artificial pneumothorax can be used to prevent injury. Solomon and colleagues used a needle with a spring-loaded blunt-tipped obturator and introduced room air into the pleural space, inducing a protective pneumothorax to displace the malignancy from adjacent vulnerable mediastinal or chest wall structures.22
Radiofrequency Ablation
In RFA, an electrode is connected to a generator that produces a voltage between the active electrode (or applicator) and the reference electrode (or grounding pad). The oscillating electrical current (in the frequency of radiowaves, 460 to 480 kHz) created between the active and reference electrode causes electron collisions with the adjacent molecules closer to the applicator, resulting in frictional heating. The radiofrequency (RF)-active electrode is placed directly through the skin and pleura, with the intended goal of heating the targeted tissue to 60 to 100°C. The application of energy from the electrode is maximized to create a zone of tissue necrosis that encompasses both the tumor and a margin of normal parenchyma immediately surrounding it (Fig. 1). This can at times be limited by the propensity of normal lung parenchyma to act as an insulator, concentrating the RF energy within the lung mass, and the so-called heat-sink effect.23 The heat-sink effect is the phenomenon by which medium to large blood vessels and airways dissipate the thermal energy away from normal adjacent tissue and concentrate it within the solid component of the target lesion. This effect can hinder the ability to treat large lesions successfully.24
Figure 1.
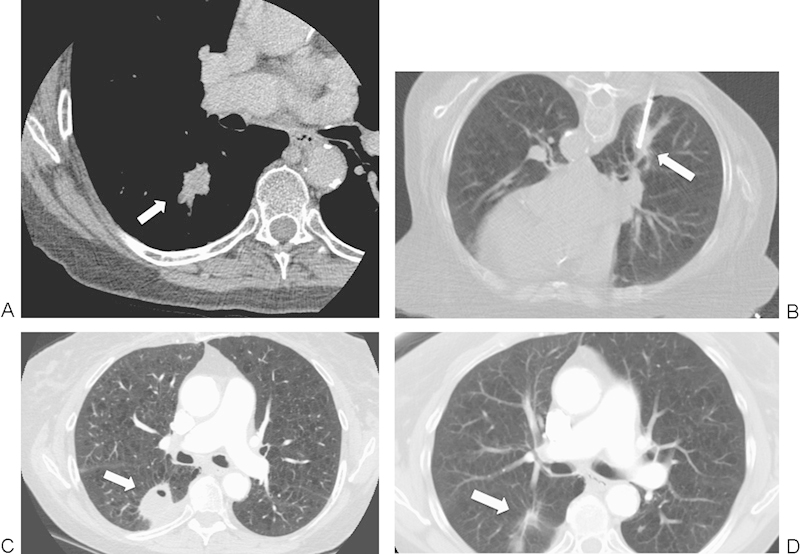
A 74-year-old woman with a non-small cell lung cancer in the right lower lobe referred for radiofrequency ablation (RFA). (A) Axial contrast-enhanced computed tomography (CT) image shows a 2.5-cm mass (arrow). (B) Axial CT image with patient prone during RFA shows a single RFA electrode (arrow) in the center of the mass. (C) Axial contrast-enhanced CT image at 3-month follow-up shows reactionary inflammation and enhancement (seen on other windows) along the adjacent pleura (arrow); this is a normal postablation finding and does not represent residual disease. (D) An axial contrast-enhanced CT image at 31-month follow-up shows near complete involution of the thermal scar (arrow).
CT fluoroscopy is used to evaluate the placement of the RF electrode angle and to correct it as necessary. The choice of electrode length, active tip length, and the number of electrodes used is determined by the size and location of the tumor. The targeted lesion should be entered along the tumor's longitudinal axis, allowing for sequential overlapping tandem ablations. Lesions >2 cm should either be treated with larger electrodes or with several overlapping ablation zones to ensure adequate thermocoagulation of the tumor. Decisions regarding temperature and/or impedance should be made according to the manufacturer specifications for the RF device used. RF electrodes have an internal thermocouple that measures the temperature of the heated tissue. Electrodes can also be coupled with infusion pumps that pump ice water or cold saline to internally cool the electrode tip, which minimizes charring and enhances heat conduction. Treatments generally range between 4 and 12 minutes in any given position; however, multiple applications can be performed to enhance coverage of larger tumors. Electrodes used in RFA may be single-tip applicators or cluster electrodes (three closely spaced electrodes that can create thermocoagulation diameters ranging from 3 to 5 cm).25
Three commercially available RF ablation systems are currently available in the United States used to treat lung neoplasms. Two of the systems (Boston Scientific/RadioTherapeutics, Watertown, MA; and RITA Medical Systems, Mountain View, CA) use a deployable array RF electrode consisting of 4 to 16 wire tines that are deployed through a 14- to 17-gauge needle. The third RF system (Cool-tip, Covidien, Boulder, CO) uses a single or cluster electrode that is perfused with small amounts of saline to distribute tissue heating. In the Covidien system, a switching controller can be used to allow for the simultaneous placement of up to three separate single electrodes spaced between 1.5 and 2.5 cm apart, allowing for a greater volume of tissue thermocoagulation in a single application.
After the target tumor is treated, the RF electrode is removed and CT fluoroscopic images are obtained to evaluate technical success and the possible presence of a pneumothorax. All patients are observed for 2 to 4 hours postprocedure, and a repeat chest radiograph is obtained to confirm the absence of a pneumothorax prior to discharge. Most pneumothoraces are small and asymptomatic; they are simply monitored with chest radiography. For large and/or symptomatic pneumothoraces, a chest catheter attached to wall suction can be used to evacuate the air leak. After the placement of a chest catheter, subsequent chest radiographs are obtained to ensure resolution of the air leak prior to the patient being discharged. For those patients with a persistent air leak, a Heimlich valve can be placed at the end of the catheter prior to discharge, and the patient returns in 24 hours for an additional chest X-ray. Patients reporting pain, who have a persistent air leak requiring wall suction or who are unable to manage their chest catheter as an outpatient, are admitted.
Microwave Ablation
MWA uses electromagnetic waves in the microwave energy spectrum (300 MHz to 300 GHz) to produce tissue-heating effects. Similar to RFA, MWA can be performed percutaneously, laparoscopically, or via open surgical access; however, unlike RFA the energy is not distributed through an electrical current, thereby eliminating the need for grounding pads. Electromagnetic microwaves traveling at 9.2 × 108 Hz produce friction and heat by inducing kinetic energy in the surrounding water molecules; the spinning of water molecules interact with the surrounding tissues and transfer some of their kinetic energy, causing tissue heating (Fig. 2). Temperature serves as a proxy measurement of how fast the water molecules oscillate within the target lesion.
Figure 2.
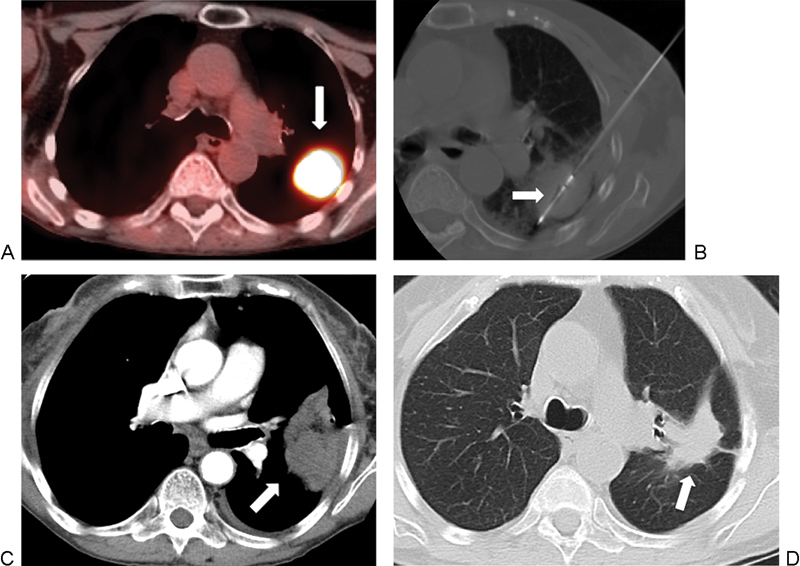
A 71-year-old woman with metastatic transitional cell carcinoma to the lung, referred for microwave ablation (MWA). (A) Axial positron emission tomography-computed tomography (PET-CT) image shows intense avidity in the left lower lobe, with a mass measuring 3.5 cm (arrow). (B) Axial CT image shows the ablation of the tumor using a 4-cm active tip MW antennae (arrow). (C) Axial contrast-enhanced CT image, mediastinal windows, 15 days after ablation shows lesion consolidation with peripheral and pleural reactive enhancement, which is a normal postprocedure imaging finding. (D) Axial CT 18 months after treatment shows a contracting thermal scar (arrow).
The preprocedural workup for MWA is similar to RFA, with ablations generally being performed under sedation and analgesia with IV midazolam and fentanyl. MW ablations can be performed using a single MW antenna, or a configuration of three antennae can be used to create a greater ablation volume. For tumors >3 cm, we recommend using 3-MW antennae to create a large area of thermocoagulation. Intratumoral temperature can be measured with a separately placed thermocouple.
In the past few years several reports have been published supporting the safety and efficacy of MWA for the treatment of pulmonary malignancies.26,27,28,29 There are currently six MW systems commercially available in the United States. The systems use either a 915-MHz generator (Evident, Covidien, Mansfield, MA; MicrothermX, BSD Medical, Salt Lake City, UT; Avecure, Medwaves, San Diego, CA) or a 2450-MHz generator (Certus 140, Neuwave, Madison, WI; Amica, Hospital Service, Rome, Italy; Acculis MTA, Microsulis, Hampshire, UK). The MW antennae used are straight applicators with active tips ranging in lengths from 0.6 to 4.0 cm. Five of the six available systems require that the antennae are internally cooled to reduce conductive heating and to prevent possible skin damage.
Cryoablation
Percutaneous cryoablation uses pressurized argon gas and distributes it to an area of lower pressure, allowing the gas to expand and reach ultracold temperatures, as low as −140°C (according to the Joule-Thomson effect). When living tissue reaches temperatures below −40°C, cryogenic destruction occurs as a result of protein denaturation, cell rupture (caused by osmotic shifts between intracellular and extracellular water), and tissue ischemia (caused by microvascular thrombosis).30
Percutaneous cryoablation lends itself to being performed under CT guidance due to the visible “ice ball” and edematous changes in the surrounding lung parenchyma that can serve as an estimation for the ablation zone margin (Fig. 3). Each cryoablation involves a 10-minute freeze of the tumor target, followed by an 8-minute helium thaw and another 10-minute freeze. This dual freeze cycle is a result of early animal studies suggesting that air leaks and bleeding could be reduced with this clinical protocol.31 Faster ablation schemes have the proposed benefit of an improved zone of ablation; these consist of a 3-minute freeze, 3-minute thaw, 7-minute freeze, 7-minute thaw, and a final 5-minute freeze. Ultimately, treatment parameters should be based on specific manufacturer guidelines.
Figure 3.
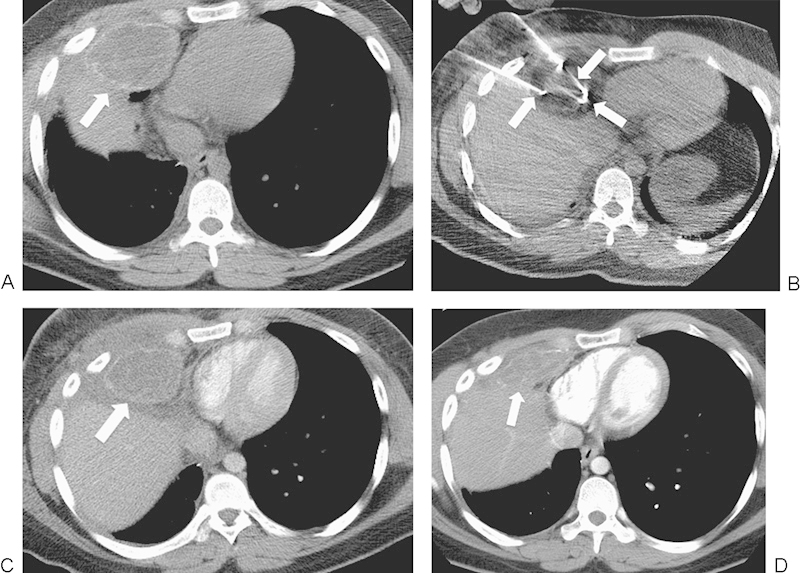
A 22-year-old man with widely metastatic osteosarcoma presented for palliative cryoablation treatment of an osteosarcoma metastasis in the right middle lobe, invading the chest wall and abutting the right atrium of the heart and the liver. (A) Axial computed tomography (CT) image shows a 6.9-cm mass (arrow). (B) Axial CT image shows three of the six cryoprobes (arrows) in the treated mass. There is a low attenuation ice ball surrounding the lesion. (C) Axial contrast-enhanced CT image 10 days after ablation shows a hypodense response consistent with cell death at the treatment site. Also, mineralization related to the original osteosarcoma is present at the margin of the tumor (arrow) and does not represent residual disease. (D) Axial contrast-enhanced CT image 1 year after ablation shows interval contraction of the ablation zone (arrow).
CT images are obtained after the first and second freeze. The low-density changes within the target tumor are measured and used to approximate the size of the ablation zone relative to the tumor. The margin of the freeze zone is variable and cytotoxic temperatures may not have been achieved, so a more accurate estimate of tissue necrosis can be evaluated by subtracting a 3- to 7-mm margin from the diameter of the low-density ablated region.32
Currently, there are two commercially available percutaneous, argon-based cryoablation devices available in the United States: Presice (Galil Medical, Arden Mills, MN) and Cryocare (Endocare, Irvine, CA). These cryoablation systems allow the placement of 1 to 15 1.5- to 2.4-mm-diameter cryoprobes and can achieve local tumor necrosis with a single freeze-thaw-freeze cycle.
Irreversible Electroporation
IRE is a new nonthermal ablation modality being investigated for the treatment of solid malignancies. Through the use of high-voltage electrical currents, IRE disrupts the lipid bilayer of the cellular membrane by forming permanent nanopores, which in turn disrupts cellular homeostasis and leads to apoptotic cell death.33,34,35 This technology can also be applied in a reversible fashion; very short electrical pulses can destabilize the electrical potential across the cell membrane, creating reversible membrane holes. Nonpermeable chemical substances, such as drugs, can then be transported through this membrane defect.36
IRE is similar to thermal ablation techniques because it is also a minimally invasive technique utilized with the intended goal of eliminating small volumes of malignant tissue. However, IRE does not encounter some of the limitations associated with thermal ablation, including anatomical restrictions due to possible thermal injury to collateral tissues, such as hollow viscera, airways, nerves, and skin.24 Histologic studies have shown that IRE produces a well-demarcated ablation zone, preserving structures and maintaining blood flow in adjacent vascular structures.36 If IRE can ablate tumors close to the chest wall, hilum, and mediastinum, without concern for blood flow interference or thermal injury to adjacent healthy parenchyma, then many patients with thoracic malignancies may benefit.37
At our institution, IRE has only been evaluated in a swine model (Fig. 4). Nine domestic swine were treated under general anesthesia, and a neuromuscular blockade was used to counteract the muscle contractions caused by the high direct-current voltages. Before administering pancuronium (paralytic), the thoracic wall was sterilely prepped and a small thoracic incision was made at the insertion point of the IRE electrode. The electrocardiogram (ECG), heart rate, respiratory rate, temperature, pulse oximetry, and end tidal volume were continuously monitored. If necessary, muscle relaxation was reversed using a mixture of edrophonium and atropine. Ablation zones were created in close proximity to the pulmonary hilum, with care taken to avoid the main stem bronchi and major cardiopulmonary vasculature.38 There is currently one IRE system approved by the Food and Drug Administration (NanoKnife, AngioDynamics, Latham, NY). The available IRE electrodes are monopolar electrodes with a retractable sheath, which allows for an adjustable exposure length ranging from 1 to 4 cm. The generator (AngioDynamics, Latham, NY) allows for the simultaneous use of up to six electrodes with a maximum delivery of 50 A and 3000 V.
Figure 4.
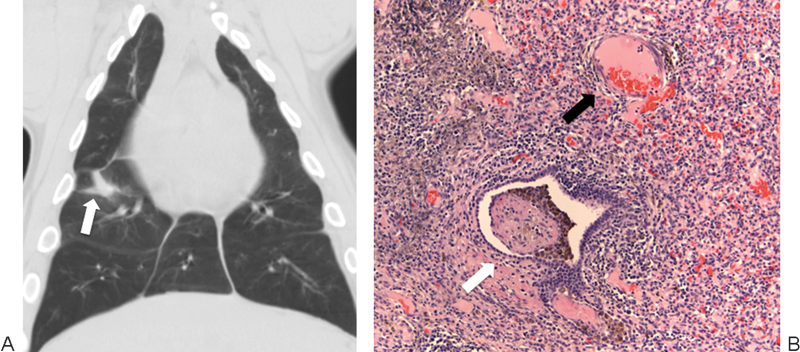
Irreversible electroporation (IRE) in a swine lung model. (A) Coronal reconstruction of a chest computed tomography image 2 weeks after lung IRE shows a fibrotic scar in the right lung (arrow). (B) High-power magnification with hematoxylin and eosin staining of an IRE ablation zone 2 weeks after treatment shows inflammatory infiltrate with intact veins (black arrow) and bronchioles (white arrow).
Of note, IRE requires general anesthesia with complete neuromuscular blockade, to avoid generalized muscle contractions. Also, an ECG-gated delivery of high-voltage pulses is necessary to avoid serious cardiac arrhythmias. These requirements make the preoperative planning of IRE treatment more complex than the usual minimally invasive interventional radiology procedure.36,39
Follow-Up
A comprehensive knowledge of the evolution of ablated tumors on imaging studies is important for accurately assessing the efficacy of ablative therapy and for the early identification of recurrent disease. The goal of surveillance is to detect recurrent malignant disease early, to allow for retreatment with ablation or with another therapy. There is currently no consensus on which imaging modality or which time interval postablation best detects treatment failure, recurrent disease, or new tumor. Extracellular contrast agents are used to help differentiate between nonenhancing coagulated tissue and enhancing viable and/or residual tumor.40,41 A more comprehensive follow-up regimen is described elsewhere in this compilation, but an overview is provided here.
During RFA and up to 72 hours afterward, postablation changes can be seen on CT. These findings include ground-glass opacification, wrinkling of the edges of the tumor, vaporization, and the “cockade phenomenon” (concentric rings with varying densitometric characteristics).42 Based on tumor type, the tumor diameter may decrease or it may not change during the treatment. Cavitation can occur in as many as 25% of lesions and is associated with ablation areas that vastly exceed the pretreatment tumor size.43 Imaging 1 month post-RFA demonstrates a lesion that appears consolidated and/or nodular with a mean diameter much larger than the preablation size; there are sometimes associated cavitations or bubble lucencies.8,42,43 CT scans 2 to 6 months after the ablation should show no change or increased size from the post-RFA baseline study, assuming that the patient had a complete treatment response. Jin and colleagues demonstrated that partially ablated lesions showed similar changes to completely treated tumors up until 6 months, at which point interval increase in tumor size was noted in the partially ablated lesions.44 Pleural changes following RFA have been noted within the first 3 months of treatment. These changes include pleural thickening, usually along the trajectory traversed by the electrode, due to pleural heating from the electrode. Reactive pleural effusions are rare.
Nodule CT densitometry has been applied to RFA-treated nodules and helps to distinguish between more highly vascularized malignant nodules that enhance after the administration of contrast.45 Successfully treated tumors should show a decrease in contrast enhancement. Positron emission tomography (PET) can also be useful in the follow-up evaluation of treated RF-ablated tumors. A decrease or complete absence of PET activity suggests complete treatment with tumor necrosis. Residual or recurrent tumors tend to exhibit PET uptake, most often along the peripheral margins of the tumor. It is not uncommon, however, to see false-positive PET uptake within the first 6 months of RFA, likely resulting from an inflammatory reaction. Yoo et al recently reported on the use of fluorodeoxyglucose-PET scans for early and 6-month follow-up, in 26 patients with early stage NSCLC. The results of this study indicated that PET/CT 6-months post-RFA versus ≤4 days after the procedure more accurately correlated with the clinical outcomes at 1 year.46 At our institution, an overall increase in the size of the soft tissue ablation region that is 1.25 times greater than the baseline CT is believed to represent local progression, as is any soft tissue focus >9 mm in greatest diameter that shows significant enhancement. A uniform enhancing rim of soft tissue <5 mm in thickness around the ablation zone is considered reactive.
Imaging performed immediately after MWA demonstrates tumors with thermally induced coagulative necrosis. A hazy ground-glass opacification is most commonly seen within and extending from the zone of ablation, penetrated by well-defined antennae tracts. At 1-, 3-, and 6-month intervals, ablation zones increase in size, as a result of the thermal changes to the surrounding lung tissue. This is followed by a persistent reduction in lesion diameter, consistent with consolidation. Cavitary changes have also been noted and at our institution are associated with a significant inverse relationship to cancer-specific mortality. Pleural thickening is a common finding (34%) in ablated tumors directly abutting the visceral pleura.27
Kawamura et al reported imaging findings for 20 patients who underwent cryoablation. The response of the tumors was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) protocol. The response rate according to RECIST was 50%. Even with marked scar formation around the tumor postprocedure, CT images still provided an accurate evaluation of tumor size: 79% of tumors diagnosed as stable disease showed no signs of recurrence at 14-month follow-up.21
The limited data available on the use of IRE in human lungs makes it difficult to evaluate imaging findings.
Outcomes and Comparison of Thermal Ablation Techniques
Tumor ablation of thoracic malignancies should be considered a viable treatment option for patients with early stage primary or secondary lung cancers who are not surgical candidates or for patients in whom palliation of tumor-related symptoms is the intent. The goal of ablation of thoracic malignancies is threefold: Clinicians should aim to ablate the entire tumor and a margin of normal parenchyma surrounding it, they should avoid injury to critical structures, and they should create a large ablation area quickly. Data regarding outcomes of thermal ablation are diverse because study groups are heterogeneous and follow-up periods and reporting standards vary. Additionally, there is a relative paucity of prospective blinded studies that compare various therapies either to other ablation modalities or to surgical resection.
The major advantage of RFA over other ablative techniques is experience because the technology has been used for pulmonary malignancies for over a decade and numerous studies have been conducted evaluating the safety and efficacy of the therapy. The major disadvantage of RFA is that it is generally to be avoided when treating tumors in the mediastinum or high in the lung apex because it can cause mechanical or thermal injury to vascular structures, airways, and nerves.15 Additionally, the thermal conductance of RF energy is impeded by tissue charring. There is a theoretical disadvantage of systemic embolic events, caused by gas microbubbles generated during charring; however, we have no documented embolic events in our patient cohort, and in the literature there is only one reported case of acute stroke that was likely unrelated to ablation treatment.44 In a large single center experience of 153 patients treated with RFA for pulmonary malignancies, a subset of 75 patients with stage I NSCLC showed a median survival time of 29 months. Additionally, Kaplan-Meier survival estimates at 1, 2, 3, 4, and 5 years for stage I NSCLC were 78%, 57%, 36%, 27%, and 27%, respectively, thus indicating a survival benefit with such treatment. A key finding in this study was that tumor size was a statistically significant predictor of local tumor progression, with tumors >3 cm associated with a 12-month median time to progression versus a 45-month median time to progression in those tumors <3 cm.7
MWA has emerged as a potentially superior treatment option to RFA due to a much broader energy deposition, capable of producing a larger zone of active heating.47,48 As a result, MWA can produce higher intratumoral temperatures, larger tumor ablation volumes, and faster ablation times. Additionally, currently available MW systems allow for the use of multiple applicators and can provide more effective heating of cystic masses, less char effect, less procedural pain, and elimination of grounding pad injuries associated with RFA.49,50,51 RFA is limited by tissue boiling and charring because both water vapor and char act as electrical insulators, which can prevent the thermal necrosis of the periphery of a lesion.49 Notably, MWA is associated with less procedural pain relative to RFA, perhaps due to the lack of electrical nerve stimulation associated with RF. Multiple large single-institution studies have been published suggesting that MWA can provide promising control of malignancies. Cancer-specific mortalities in three studies, each with >50 patients, yielded 1-year survival rates ranging from 47.6% to 83%, 2-year survival rates ranging from 23.8% to 73%, and 3-year survival rates ranging from 14.3% to 61%.27,28,29
Cryoablation is associated with many of the same advantages as MWA over RFA. It can achieve larger tumor ablation volumes, allows for the use of multiple applicators, creates less procedural pain due to the analgesic effects of freezing, and it does not require grounding pads, thus eliminating grounding pad injuries. Compared with heat-based thermal ablation therapies, cryoablation allows for the preservation of collagenous and other cellular architecture in virtually any frozen tissue.52,53,54 Theoretical disadvantages of cryoablation include bleeding requiring intervention, such as tract coagulation with fibrin glue. Another disadvantage of cryoablation is the long procedural times required in the freeze-thaw-freeze cycle and the many probes needed to generate adequate coverage. Wang and colleagues reported on their experience and the safety profile of cryoablation in the treatment of 187 patients with peripheral lung masses. The mean coverage of peripheral masses <4 cm was 99%, with tumor size and location the most significant predictors of tumor ice coverage. Survival estimates could not be accurately calculated due to the limited follow-up data; however, the authors noted that patients experienced palliative benefits including increased dietary intake and weight gain.55
There are few reports in humans regarding the safety and/or efficacy of IRE for the treatment of thoracic malignancies. Theoretical benefits include a narrow transition between the zone of tissue necrosis and healthy tissue, the ability to overcome thermal sinks, and preservation of underlying structure.24,35 In our evaluation of IRE of the lung in a swine model, histologic analysis revealed that bronchioles and blood vessels within the area of IRE were intact and did not show signs of tissue injury. In smaller structures in the IRE zone, including arterioles between 0.2 and 1.0 mm and bronchioles between 0.3 and 1.5 mm, there were mild inflammatory changes within the walls as well as hemosiderin deposition; however, structures remained intact without evidence of thrombosis or collapse.38 Thomson and colleagues treated 38 cancer patients with IRE, 3 of whom had advanced lung malignancies. Although IRE was not associated with postprocedure hemoptysis, pleuritic pain, or pleural effusion, none of the patients had a satisfactory treatment response.56
Conclusion
Given the high rates of mortality attributable to lung cancer, the emergence of new and efficacious treatment options remains a priority. Research evaluating the ideal tumor histology, size, and location continues to be performed and we hope will enable interventional radiologists and device manufacturers to further optimize tumor ablation treatments. Additionally, further studies should aim to establish guidelines for the appropriate type and timing of postprocedure imaging, to determine treatment success or the need for retreatment of recurrent or new disease. As the field of tumor ablation progresses, investigators should delineate which ablation modalities are best suitable for various clinical scenarios. Newer devices should focus on generating greater ablation volumes of diseased tissue in shorter time periods and with fewer treatment restrictions.
Footnotes
Funding Information Erica Alexander has no conflicts of interest to disclose. Damian E. Dupuy is a consultant for Perfint and Biocompatibles, receives grant support from NeuWave and MedWaves, and is a board member of BSD Medical.
References
- 1.American Cancer Society . Atlanta, GA: American Cancer Society; 2012. Cancer facts and figures 2012. [Google Scholar]
- 2.Rusch V W. Philadelphia, PA: Churchill Livingston; 2008. Lung metastasis; pp. 873–884. [Google Scholar]
- 3.Robinson L A Ruckdeschel J C Wagner H Jr Stevens C W American College of Chest Physicians Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition) Chest 2007132(3, Suppl):243S–265S. [DOI] [PubMed] [Google Scholar]
- 4.Ruckdeschel J C, Schwarz A G, Bepler G, Philadelphia, PA: Elsevier; 2008. Cancer of the lung—NSCLC and SCLC; pp. 1649–1743. [Google Scholar]
- 5.Zemlyak A, Moore W H, Bilfinger T V. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211(1):68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Pennathur A Abbas G Gooding W E et al. Image-guided radiofrequency ablation of lung neoplasm in 100 consecutive patients by a thoracic surgical service Ann Thorac Surg 20098851601–1606.; discussion 1607-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon C J, Dupuy D E, DiPetrillo T A. et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 8.de Baère T, Palussière J, Aupérin A. et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240(2):587–596. doi: 10.1148/radiol.2402050807. [DOI] [PubMed] [Google Scholar]
- 9.Lencioni R, Crocetti L, Cioni R. et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9(7):621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 10.Kong F M, Ten Haken R K, Schipper M J. et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63(2):324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Sura S, Yorke E, Jackson A, Rosenzweig K E. High-dose radiotherapy for the treatment of inoperable non-small cell lung cancer. Cancer J. 2007;13(4):238–242. doi: 10.1097/PPO.0b013e31813ffd7b. [DOI] [PubMed] [Google Scholar]
- 12.Bogart J A, Hodgson L, Seagren S L. et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol. 2010;28(2):202–206. doi: 10.1200/JCO.2009.25.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onishi H, Shirato H, Nagata Y. et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7) 03:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 14.Timmerman R, Paulus R, Galvin J. et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain S K, Dupuy D E, Cardarelli G A, Zheng Z, DiPetrillo T A. Percutaneous radiofrequency ablation of pulmonary malignancies: combined treatment with brachytherapy. AJR Am J Roentgenol. 2003;181(3):711–715. doi: 10.2214/ajr.181.3.1810711. [DOI] [PubMed] [Google Scholar]
- 16.Dupuy D E, Mayo-Smith W W, Abbott G F, DiPetrillo T A. Clinical applications of radio-frequency tumor ablation in the thorax. Radiographics. 2002;22(Spec No):S259–S269. doi: 10.1148/radiographics.22.suppl_1.g02oc03s259. [DOI] [PubMed] [Google Scholar]
- 17.McTaggart R A, Dupuy D E, Dipetrillo T. New York, NY: Cambridge University Press; 2008. Image guided ablation in the thorax; pp. 440–474. [Google Scholar]
- 18.Suh R, Reckamp K, Zeidler M, Cameron R. Radiofrequency ablation in lung cancer: promising results in safety and efficacy. Oncology (Williston Park) 2005;19(11) 04:12–21. [PubMed] [Google Scholar]
- 19.Alexander E S, Healey T T, Martin D W, Dupuy D E. Use of endobronchial valves for the treatment of bronchopleural fistulas after thermal ablation of lung neoplasms. J Vasc Interv Radiol. 2012;23(9):1236–1240. doi: 10.1016/j.jvir.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 20.VanSonnenberg E, Shankar S, Morrison P R. et al. Radiofrequency ablation of thoracic lesions: part 2, initial clinical experience—technical and multidisciplinary considerations in 30 patients. AJR Am J Roentgenol. 2005;184(2):381–390. doi: 10.2214/ajr.184.2.01840381. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura M, Izumi Y, Tsukada N. et al. Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates. J Thorac Cardiovasc Surg. 2006;131(5):1007–1013. doi: 10.1016/j.jtcvs.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 22.Solomon S B, Thornton R H, Dupuy D E, Downey R J. Protection of the mediastinum and chest wall with an artificial pneumothorax during lung ablations. J Vasc Interv Radiol. 2008;19(4):610–615. doi: 10.1016/j.jvir.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Dupuy D E, Mayo-Smith W W, Abbott G F, DiPetrillo T. Clinical applications of radio-frequency tumor ablation in the thorax. Radiographics. 2002;22(Spec No):S259–S269. doi: 10.1148/radiographics.22.suppl_1.g02oc03s259. [DOI] [PubMed] [Google Scholar]
- 24.Nahum Goldberg S, Dupuy D E. Image-guided radiofrequency tumor ablation: challenges and opportunities—part I. J Vasc Interv Radiol. 2001;12(9):1021–1032. doi: 10.1016/s1051-0443(07)61587-5. [DOI] [PubMed] [Google Scholar]
- 25.Beland M D, Dupuy D E. Current and future applications of percutaneous radiofrequency ablation in the treatment of lung neoplasms. Appl Radiol. 2006;35(12):21–28. [Google Scholar]
- 26.Feng W, Liu W, Li C. et al. Percutaneous microwave coagulation therapy for lung cancer. Zhonghua Zhong Liu Za Zhi. 2002;24(4):388–390. [PubMed] [Google Scholar]
- 27.Wolf F J, Grand D J, Machan J T, Dipetrillo T A, Mayo-Smith W W, Dupuy D E. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247(3):871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q, Cao W, Huang L. et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol. 2012;10:80. doi: 10.1186/1477-7819-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belfiore G, Ronza F, Belfiore M P. et al. Patients' survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82(1):177–181. doi: 10.1016/j.ejrad.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Gage A A, Baust J G. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 31.Izumi Y Oyama T Ikeda E Kawamura M Kobayashi K The acute effects of transthoracic cryoablation on normal lung evaluated in a porcine model Ann Thorac Surg 2005791318–322.; discussion 322 [DOI] [PubMed] [Google Scholar]
- 32.Hinshaw J L, Lee F T Jr, Laeseke P F, Sampson L A, Brace C. Temperature isotherms during pulmonary cryoablation and their correlation with the zone of ablation. J Vasc Interv Radiol. 2010;21(9):1424–1428. doi: 10.1016/j.jvir.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davalos R V, Mir I L, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 34.Lee E W, Chen C, Prieto V E, Dry S M, Loh C T, Kee S T. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255(2):426–433. doi: 10.1148/radiol.10090337. [DOI] [PubMed] [Google Scholar]
- 35.Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 36.Rempp H, Boss A, Helmberger T, Pereira P. The current role of minimally invasive therapies in the management of liver tumors. Abdom Imaging. 2011;36(6):635–647. doi: 10.1007/s00261-011-9749-2. [DOI] [PubMed] [Google Scholar]
- 37.McTaggart R A, Dupuy D E. Thermal ablation of lung tumors. Tech Vasc Interv Radiol. 2007;10(2):102–113. doi: 10.1053/j.tvir.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Dupuy D E, Aswad B, Ng T. Irreversible electroporation in a swine lung model. Cardiovasc Intervent Radiol. 2011;34(2):391–395. doi: 10.1007/s00270-010-0091-9. [DOI] [PubMed] [Google Scholar]
- 39.Mali B, Jarm T, Corovic S. et al. The effect of electroporation pulses on functioning of the heart. Med Biol Eng Comput. 2008;46(8):745–757. doi: 10.1007/s11517-008-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieco C A, Simon C J, Mayo-Smith W W, Dipetrillo T A, Ready N E, Dupuy D E. Image-guided percutaneous thermal ablation for the palliative treatment of chest wall masses. Am J Clin Oncol. 2007;30(4):361–367. doi: 10.1097/COC.0b013e318033e76a. [DOI] [PubMed] [Google Scholar]
- 41.Suh R D, Wallace A B, Sheehan R E, Heinze S B, Goldin J G. Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation—preliminary results. Radiology. 2003;229(3):821–829. doi: 10.1148/radiol.2293021756. [DOI] [PubMed] [Google Scholar]
- 42.Gadaleta C, Mattioli V, Colucci G. et al. Radiofrequency ablation of 40 lung neoplasms: preliminary results. AJR Am J Roentgenol. 2004;183(2):361–368. doi: 10.2214/ajr.183.2.1830361. [DOI] [PubMed] [Google Scholar]
- 43.Steinke K, King J, Glenn D, Morris D L. Radiologic appearance and complications of percutaneous computed tomography-guided radiofrequency-ablated pulmonary metastases from colorectal carcinoma. J Comput Assist Tomogr. 2003;27(5):750–757. doi: 10.1097/00004728-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Jin G Y, Lee J M, Lee Y C, Han Y M, Lim Y S. Primary and secondary lung malignancies treated with percutaneous radiofrequency ablation: evaluation with follow-up helical CT. AJR Am J Roentgenol. 2004;183(4):1013–1020. doi: 10.2214/ajr.183.4.1831013. [DOI] [PubMed] [Google Scholar]
- 45.Swensen S J, Viggiano R W, Midthun D E. et al. Lung nodule enhancement at CT: multicenter study. Radiology. 2000;214(1):73–80. doi: 10.1148/radiology.214.1.r00ja1473. [DOI] [PubMed] [Google Scholar]
- 46.Yoo D C, Dupuy D E, Hillman S L. et al. Radiofrequency ablation of medically inoperable stage IA non-small cell lung cancer: are early posttreatment PET findings predictive of treatment outcome? AJR Am J Roentgenol. 2011;197(2):334–340. doi: 10.2214/AJR.10.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brace C L, Hinshaw J L, Laeseke P F, Sampson L A, Lee F T Jr. Pulmonary thermal ablation: comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251(3):705–711. doi: 10.1148/radiol.2513081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dupuy D E. Microwave ablation compared with radiofrequency ablation in lung tissue—is microwave not just for popcorn anymore? Radiology. 2009;251(3):617–618. doi: 10.1148/radiol.2513090129. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg S N, Gazelle G S, Solbiati L, Rittman W J, Mueller P R. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3(8):636–644. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 50.Simon C J, Dupuy D E, Mayo-Smith W W. Microwave ablation: principles and applications. Radiographics. 2005;25 01:S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 51.Yu N C, Lu D S, Raman S S. et al. Hepatocellular carcinoma: microwave ablation with multiple straight and loop antenna clusters—pilot comparison with pathologic findings. Radiology. 2006;239(1):269–275. doi: 10.1148/radiol.2383041592. [DOI] [PubMed] [Google Scholar]
- 52.Maiwand M O. The role of cryosurgery in palliation of tracheo-bronchial carcinoma. Eur J Cardiothorac Surg. 1999;15(6):764–768. doi: 10.1016/s1010-7940(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 53.Maiwand M O, Homasson J P. Cryotherapy for tracheobronchial disorders. Clin Chest Med. 1995;16(3):427–443. [PubMed] [Google Scholar]
- 54.Sanderson D R, Neel H B III, Fontana R S. Bronchoscopic cryotherapy. Ann Otol Rhinol Laryngol. 1981;90(4 Pt 1):354–358. doi: 10.1177/000348948109000414. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Littrup P J, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235(1):289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 56.Thomson K R, Cheung W, Ellis S J. et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011;22(5):611–621. doi: 10.1016/j.jvir.2010.12.014. [DOI] [PubMed] [Google Scholar]


