Abstract
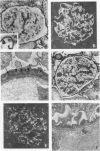
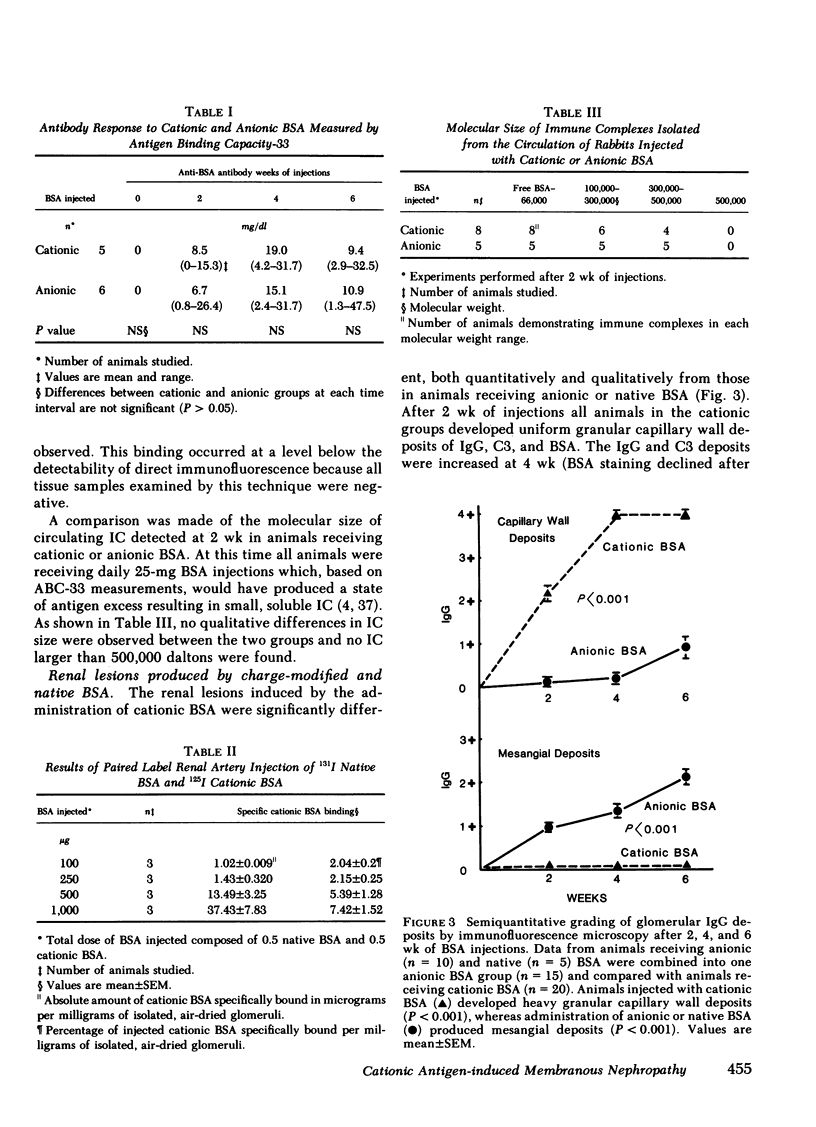
We examined the role of antigenic electrical charge as a determinant of glomerular immune complex localization in the rabbit. Serum sickness nephritis was induced in groups of New Zealand white rabbits by daily 25-mg intravenous injections of bovine serum albumin (BSA) chemically modified to be cationic (pI > 9.5) or more anionic (pI, 3.5-4.6); an additional group received unmodified native BSA (pI, 4.5-5.1). Factors known to influence immune complex localization, e.g., molecular size of the administered antigen and resulting circulating immune complexes, immunogenicity, and disappearance time from the circulation were examined and found to be similar for both anionic and cationic BSA. Charge modification did increase the nonimmune clearance of cationic and anionic BSA compared with native BSA. Injected cationic BSA was shown in paired label experiments to bind directly to glomeruli compared with native BSA. The renal lesion produced by cationic BSA was markedly different from that found in rabbits given anionic or native BSA. Animals receiving cationic BSA uniformly developed generalized diffuse granular capillary wall deposits of IgG, C3, and BSA detected after 2 wk of injections and increasing until death at 6 wk. Qualitatively similar deposits were produced by the administration of low doses of cationic BSA of only 1 or 10 mg/d. In contrast, the injection of both anionic and native BSA resulted in mesangial deposits at 2 and 4 wk with capillary wall deposits appearing by 6 wk. Ultrastructural examination of animals receiving cationic BSA revealed pure, extensive formation of dense deposits along the lamina rara externa of the glomerular basement membrane whereas such deposits were absent or rare in animals injected with the anionic or native BSA. Albuminuria was significantly greater at 6 wk in the groups receiving cationic BSA with a mean of 280 mg/24 h compared with 53 mg/24 h in the combined groups injected with anionic or native BSA. Blood urea nitrogen values were similar in all groups at 2 and 4 wk but higher in the animals receiving cationic BSA at 6 wk.
These experiments describe the reproducible induction of epimembranous immune deposits by administration of an exogenous cationic antigen. They suggest that antigenic charge can play an important role in the pathogenesis of membranous nephropathy by permitting direct glomerular binding of an antigen and predisposing to in situ immune complex formation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batsford S. R., Takamiya M., Vogt A. A model of in situ immune complex glomerulonephritis in the rat employing cationized ferritin. Clin Nephrol. 1980 Nov;14(5):211–216. [PubMed] [Google Scholar]
- Bennett C. M., Glassock R. J., Chang R. L., Deen W. M., Robertson C. R., Brenner B. M., Troy J. L., ueki I. R., Rasmussen B. Permselectivity of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using dextran sulfate. J Clin Invest. 1976 May;57(5):1287–1294. doi: 10.1172/JCI108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Humes H. D., Glassock R. J., Robertson C. R., Brenner B. M. Permselectivity of the glomerular capillary wall. Facilitated filtration of circulating polycations. J Clin Invest. 1978 Jan;61(1):72–78. doi: 10.1172/JCI108927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Kamil E. S., Ward H. J., Cohen A. H. Antigenic changes as a determinant of immune complex localization in the rat glomerulus. Lab Invest. 1981 Nov;45(5):442–449. [PubMed] [Google Scholar]
- Border W. A., Wilson C. B., Dixon F. J. Failure of heparin to affect two types of experimental glomerulonephritis in rabbits. Kidney Int. 1975 Sep;8(3):140–148. doi: 10.1038/ki.1975.93. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Cameron J. S. Pathogenesis and treatment of membranous nephropathy. Kidney Int. 1979 Jan;15(1):88–103. doi: 10.1038/ki.1979.12. [DOI] [PubMed] [Google Scholar]
- Chang R. L., Deen W. M., Robertson C. R., Bennett C. M., Glassock R. J., Brenner B. M., Troy J. L., Ueki I. F., Rasmussen B. Permselectivity of of the glomerular capillary wall. Studies of experimental glomerulonephritis in the rat using neutral dextran. J Clin Invest. 1976 May;57(5):1272–1286. doi: 10.1172/JCI108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. H., Border W. A., Glassock R. J. Nehprotic syndrome with glomerular mesangial IgM deposits. Lab Invest. 1978 May;38(5):610–619. [PubMed] [Google Scholar]
- Collins A. B., Andres G. A., McCluskey R. T. Lack of evidence for a role of renal tubular antigen in human membranous glomerulonephritis. Nephron. 1981;27(6):297–301. doi: 10.1159/000182074. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Salant D. J. In situ immune complex formation and glomerular injury. Kidney Int. 1980 Jan;17(1):1–13. doi: 10.1038/ki.1980.1. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON F. J., FELDMAN J. D., VAZQUEZ J. J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J Exp Med. 1961 May 1;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Satvat B., Jamieson J. M. Theoretical model for glomerular filtration of charged solutes. Am J Physiol. 1980 Feb;238(2):F126–F139. doi: 10.1152/ajprenal.1980.238.2.F126. [DOI] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuren G., Grond J., Hoedemaeker P. J. In situ formation of subepithelial glomerular immune complexes in passive serum sickness. Kidney Int. 1980 May;17(5):631–637. doi: 10.1038/ki.1980.74. [DOI] [PubMed] [Google Scholar]
- Gallo G. R., Caulin-Glaser T., Lamm M. E. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. J Clin Invest. 1981 May;67(5):1305–1313. doi: 10.1172/JCI110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germuth F. G., Jr, Senterfit L. B., Dreesman G. R. Immune complex disease. V. The nature of the circulating complexes associated with glomerular alterations in the chronic BSA-rabbit system. Johns Hopkins Med J. 1972 Jun;130(6):344–357. [PubMed] [Google Scholar]
- Germuth F. G., Jr, Taylor J. J., Siddiqui S. Y., Rodriguez E. Immune complex disease. VI. Some determinants of the varieties of glomerular lesions in the chronic bovine serum albumin-rabbit system. Lab Invest. 1977 Aug;37(2):162–169. [PubMed] [Google Scholar]
- Glassock R. J., Edgington T. S., Watson J. I., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. II. The pathogenetic mechanism. J Exp Med. 1968 Mar 1;127(3):573–588. doi: 10.1084/jem.127.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbus S. M., Wilson C. B. Experimental glomerulonephritis induced by in situ formation of immune complexes in glomerular capillary wall. Kidney Int. 1979 Aug;16(2):148–157. doi: 10.1038/ki.1979.116. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Hourani M. R., Mayor G. H., Greenbaum D. S., Hugget D. O., Patterson M. J. Hepatitis B surface antigen in urine of hemodialysis patients. Kidney Int. 1978 Apr;13(4):324–328. doi: 10.1038/ki.1978.46. [DOI] [PubMed] [Google Scholar]
- Janatova J., Crandall R. E., Andrade J. D. An analysis of the heterogeneity of albumin. Prep Biochem. 1980;10(4):405–430. doi: 10.1080/00327488008061740. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Sutherland D. E., Howard R. J., Fish A. J., Najarian J. S., Michael A. F. The glomerular mesangium. 3. Acute immune mesangial injury: a new model of glomerulonephritis. J Exp Med. 1973 Mar 1;137(3):553–570. doi: 10.1084/jem.137.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. Radioiodination of proteins by the use of the chloramine-T method. Methods Enzymol. 1980;70(A):210–213. doi: 10.1016/s0076-6879(80)70050-2. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Purtell J. N., Pesce A. J., Clyne D. H., Miller W. C., Pollak V. E. Isoelectric point of albumin: effect on renal handling of albumin. Kidney Int. 1979 Sep;16(3):366–376. doi: 10.1038/ki.1979.139. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennke H. G., Venkatachalam M. A. Chemical modification of horseradish peroxidase. Preparation and characterization of tracer enzymes with different isoelectric points. J Histochem Cytochem. 1979 Oct;27(10):1352–1353. doi: 10.1177/27.10.41873. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Venkatachalam M. A. Glomerular permeability: in vivo tracer studies with polyanionic and polycationic ferritins. Kidney Int. 1977 Jan;11(1):44–53. doi: 10.1038/ki.1977.6. [DOI] [PubMed] [Google Scholar]
- Salant D. J., Belok S., Stilmant M. M., Darby C., Couser W. G. Determinants of glomerular localization of subepithelial immune deposits: effects of altered antigen to antibody ratio, steroids, vasoactive amine antagonists, and aminonucleoside of puromycin on passive Heymann nephritis in rats. Lab Invest. 1979 Jul;41(1):89–99. [PubMed] [Google Scholar]
- Stilmant M. M., Couser W. G., Cotran R. S. Experimental glomerulonephritis in the mouse associated with mesangial deposition ofautologous ferritin immune complexes. Lab Invest. 1975 Jun;32(6):746–756. [PubMed] [Google Scholar]
- Szabó T., Szabó J., Balázs C., Lustyik G. Experimental glomerular lesions induced by chronic immune complex formation. I. Formation and elimination of the immune complex (relationship between the immune status and the glomerular changes). Int Urol Nephrol. 1979;11(2):119–125. doi: 10.1007/BF02082232. [DOI] [PubMed] [Google Scholar]
- Thorpe L. W., Cavallo T. Renal tubule brush border antigens: failure to confirm a pathogenetic role in human membranous glomerulonephritis. J Clin Lab Immunol. 1980 Mar;3(2):125–127. [PubMed] [Google Scholar]
- UNANUE E. R., DIXON F. J. EXPERIMENTAL GLOMERULONEPHRITIS. V. STUDIES ON THE INTERACTION OF NEPHROTOXIC ANTIBODIES WITH TISSUE OF THE RAT. J Exp Med. 1965 May 1;121:697–714. doi: 10.1084/jem.121.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Dixon F. J. Experimental glomerulonephritis: immunological events and pathogenetic mechanisms. Adv Immunol. 1967;6:1–90. doi: 10.1016/s0065-2776(08)60521-0. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Rennke H. G. The structural and molecular basis of glomerular filtration. Circ Res. 1978 Sep;43(3):337–347. doi: 10.1161/01.res.43.3.337. [DOI] [PubMed] [Google Scholar]