Abstract
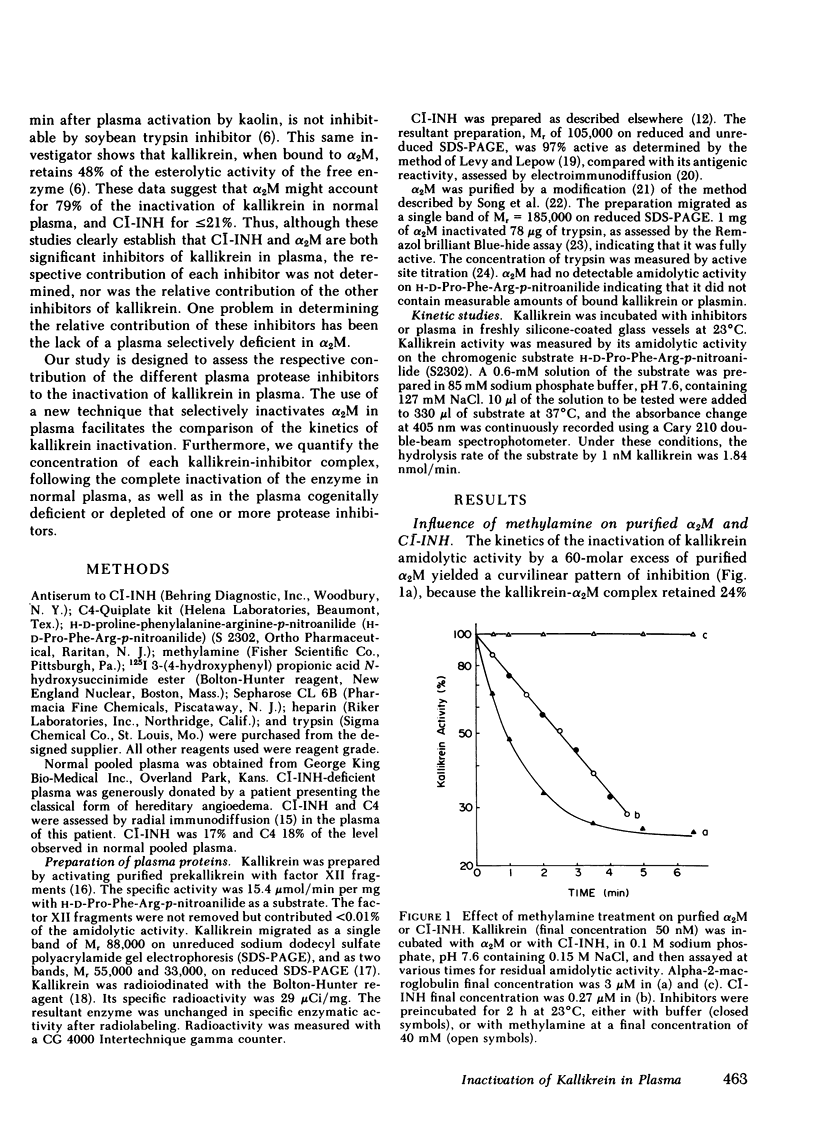
Although C̄l-inhibitor (C̄l-INH) and α2-macroglobulin (α2M) have been reported as the major inhibitors of plasma kallikrein in normal plasma, there is little quantitative support for this conclusion. Thus, we studied the inactivation of purified kallikrein in normal plasma, as well as in plasma congenitally deficient in C̄l-INH, or artificially depleted of α2M by chemical modification of the inhibitor with methylamine. Under pseudo-first-order conditions, the inactivation rate constant of kallikrein in normal plasma was 0.60 min−1. This rate constant was reduced to 0.35, 0.30, and 0.06 min−1, in plasma deficient respectively in C̄l-INH, α2M, or both inhibitors. Thus C̄l-INH (42%) and α2M (50%) were found to be the major inhibitors of kallikrein in normal plasma. Moreover all the other protease inhibitors present in normal plasma contributed only for 8% to the inactivation of the enzyme. To confirm these kinetic results, 125I-kallikrein (Mr 85,000) was completely inactivated by various plasma samples, and the resulting mixtures were analyzed by gel filtration on Sepharose 6B CL for the appearance of 125I-kallikrein-inhibitor complexes. After inactivation by normal plasma, 52% of the active enzyme were found to form a complex (Mr 370,000) with C̄l-INH, while 48% formed a complex (Mr 850,000) with α2M. After inactivation by C̄l-INH-deficient plasma, >90% of the active 125I-kallikrein was associated with α2M. A similar proportion of the label was associated with C̄l-INH in plasma deficient in α2M. After inactivation by plasma deficient in both C̄l-INH and α2M, 125I-kallikrein was found to form a complex of Mr 185,000. This latter complex, which may involve antithrombin III, α1-protease inhibitor, and/or α1-plasmin inhibitor, was not detectable in appreciable concentrations in the presence of either C̄l-INH or α2M, even after the addition of heparin (2 U/ml). These observations demonstrate that C̄l-INH and α2M are the only significant inhibitors of kallikrein in normal plasma confirming previous predictions based on experiments in purified systems. Moreover, in the absence of either C̄l-INH or α2M, the inactivation of kallikrein becomes almost entirely dependent on the other major inhibitor.
Full text
PDF
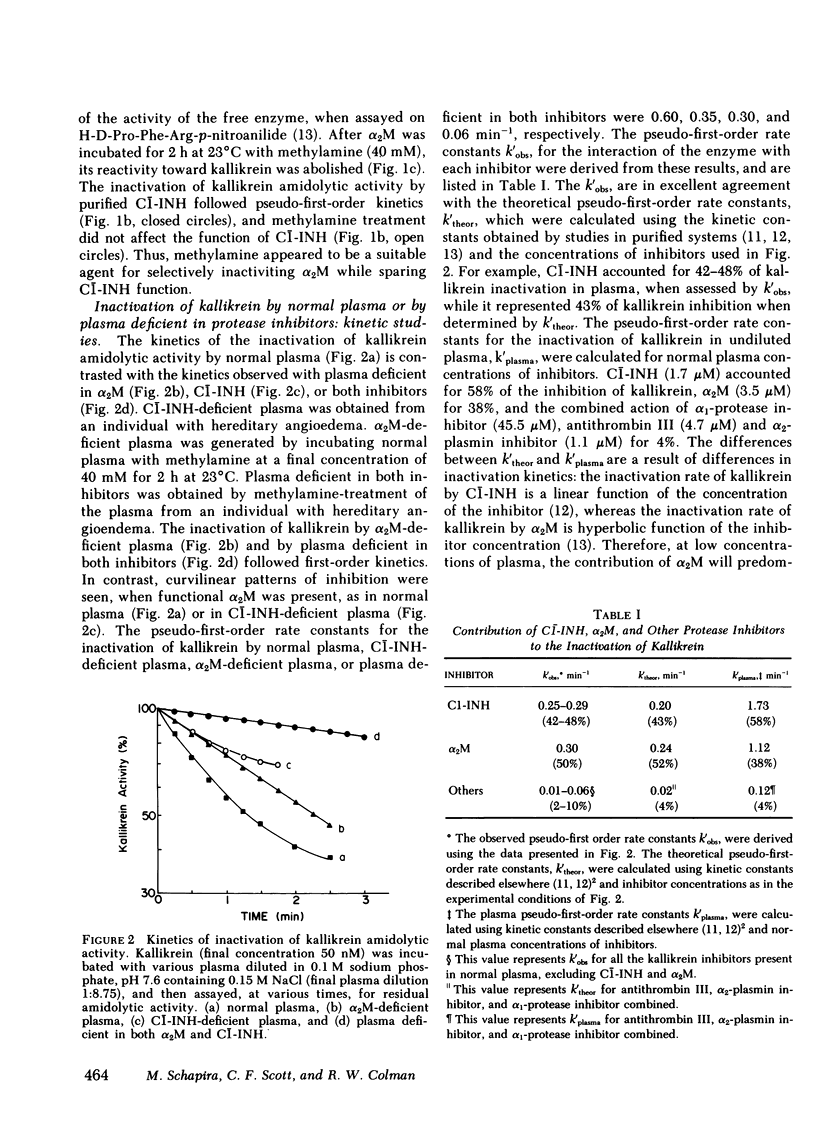

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. L., Begué-Cantón M. L., Blakeley R. L., Brubacher L. J., Feder J., Gunter C. R., Kézdy F. J., Killheffer J. V., Jr, Marshall T. H., Miller C. G. The determination of the concentration of hydrolytic enzyme solutions: alpha-chymotrypsin, trypsin, papain, elastase, subtilisin, and acetylcholinesterase. J Am Chem Soc. 1966 Dec 20;88(24):5890–5913. doi: 10.1021/ja00976a034. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
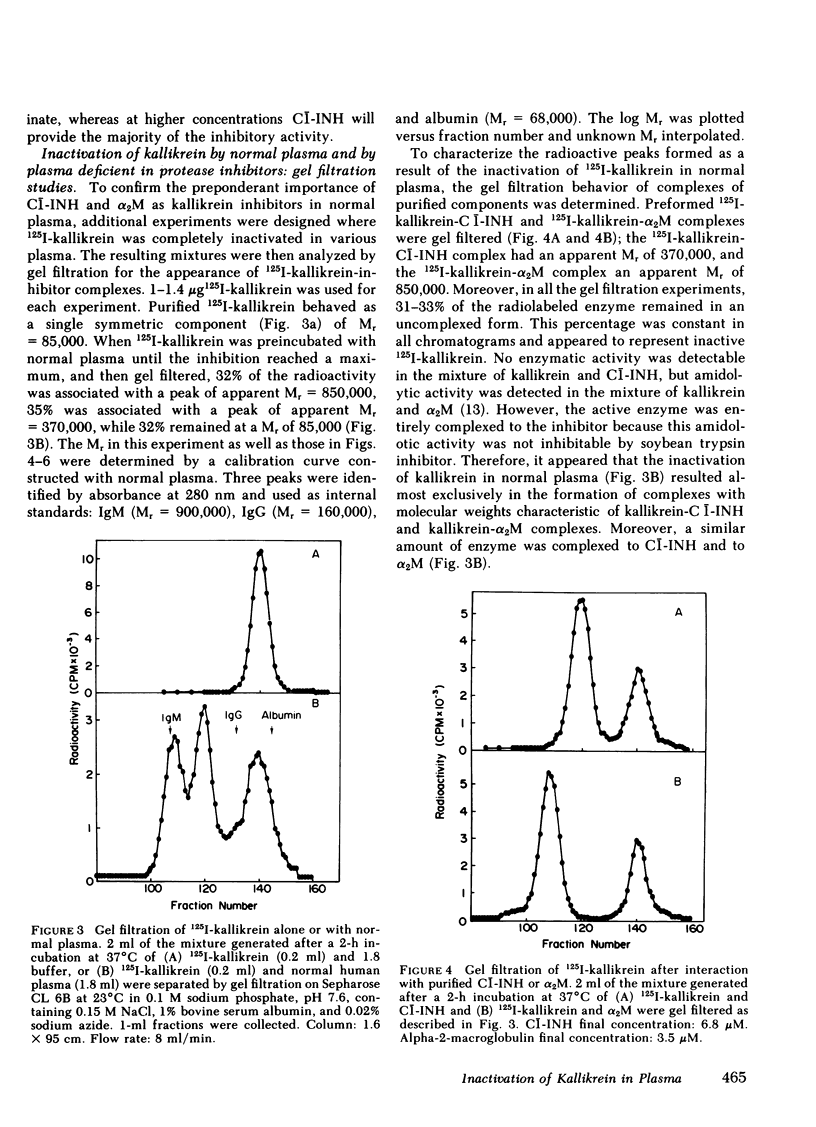
- Colman R. W., Mason J. W., Sherry S. The kallikreinogen-kallikrein enzyme system of human plasma. Assay of components and observations in disease states. Ann Intern Med. 1969 Oct;71(4):763–773. doi: 10.7326/0003-4819-71-4-763. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Schapira M., Scott C. F. Regulation of the formation and inhibition of human plasma kallikrein. Ann N Y Acad Sci. 1981;370:261–270. doi: 10.1111/j.1749-6632.1981.tb29739.x. [DOI] [PubMed] [Google Scholar]
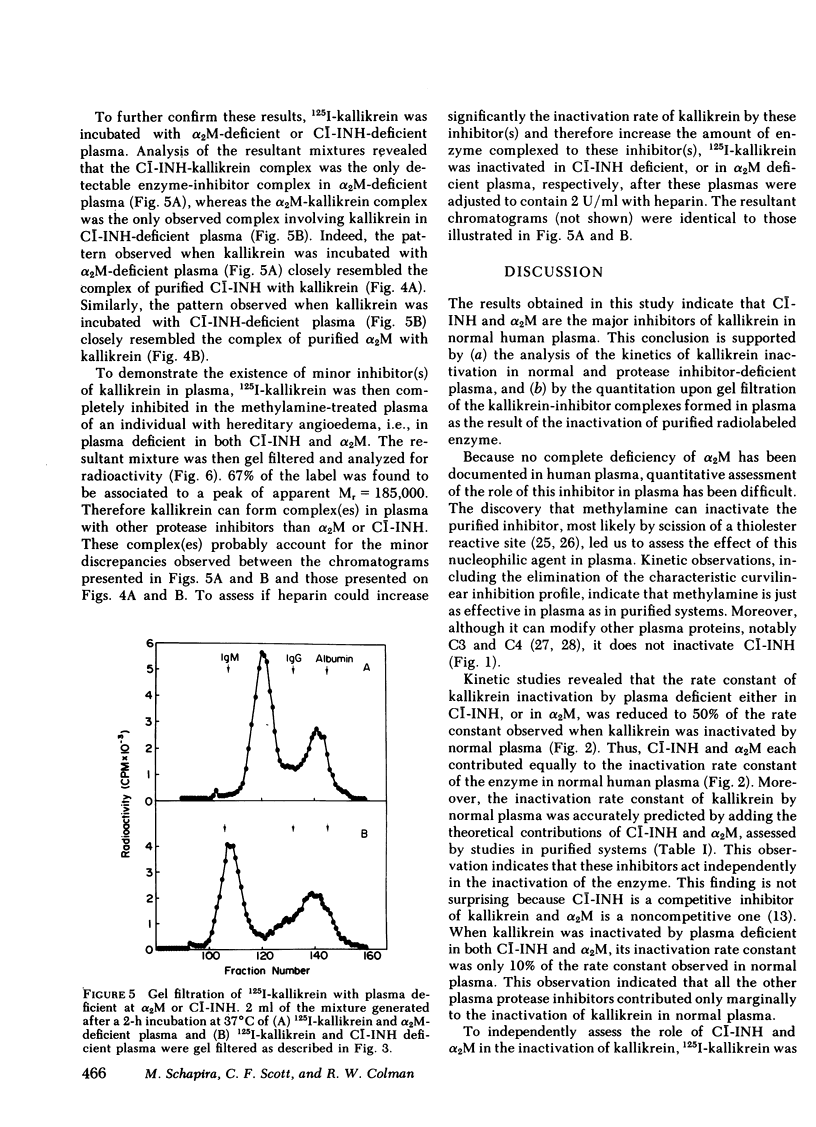
- Davie E. W., Fujikawa K., Kurachi K., Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv Enzymol Relat Areas Mol Biol. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- Fritz H., Wunderer G., Kummer K., Heimburger N., Werle E. -Antitrypsin und c 1-inaktivator: progressiv-inhibitoren für serumkallikreine von mensch und schwein. Hoppe Seylers Z Physiol Chem. 1972 Jun;353(6):906–910. [PubMed] [Google Scholar]
- Gallimore M. J., Amundsen E., Larsbraaten M., Lyngaas K., Fareid E. Studies on plasma inhibitors of plasma kallikrein using chromogenic peptide substrate assays. Thromb Res. 1979;16(5-6):695–703. doi: 10.1016/0049-3848(79)90213-5. [DOI] [PubMed] [Google Scholar]
- Gigli I., Mason J. W., Colman R. W., Austen K. F. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970 Mar;104(3):574–581. [PubMed] [Google Scholar]
- Gorski J. P., Howard J. B. Effect of methylamine on the structure and function of the fourth component of human complement, C4. J Biol Chem. 1980 Nov 10;255(21):10025–10028. [PubMed] [Google Scholar]
- Harpel P. C. Alpha2-plasmin inhibitor and alpha2-macroglobulin-plasmin complexes in plasma. Quantitation by an enzyme-linked differential antibody immunosorbent assay. J Clin Invest. 1981 Jul;68(1):46–55. doi: 10.1172/JCI110253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel P. C. Human plasma alpha 2-macroglobulin. An inhibitor of plasma kallikrein. J Exp Med. 1970 Aug 1;132(2):329–352. doi: 10.1084/jem.132.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B. Methylamine reaction and denaturation-dependent fragmentation of complement component 3. Comparison with alpha2-macroglobulin. J Biol Chem. 1980 Aug 10;255(15):7082–7084. [PubMed] [Google Scholar]
- Howard J. B. Reactive site in human alpha 2-macroglobulin: circumstantial evidence for a thiolester. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2235–2239. doi: 10.1073/pnas.78.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P. Initiation of the intrinsic coagulation and fibrinolytic pathways of man: the role of surfaces, hageman factor, prekallikrein, high molecular weight kininogen, and factor XI. Prog Hemost Thromb. 1978;4:127–175. [PubMed] [Google Scholar]
- LANDERMAN N. S., WEBSTER M. E., BECKER E. L., RATCLIFFE H. E. Hereditary angioneurotic edema. II. Deficiency of inhibitor for serum globulin permeability factor and/or plasma kallikrein. J Allergy. 1962 Jul-Aug;33:330–341. doi: 10.1016/0021-8707(62)90032-1. [DOI] [PubMed] [Google Scholar]
- LEVY L. R., LEPOW I. H. Assay and properties of serum inhibitor of C'l-esterase. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:608–611. doi: 10.3181/00379727-101-25034. [DOI] [PubMed] [Google Scholar]
- Lahiri B., Bagdasarian A., Mitchell B., Talamo R. C., Colman R. W. Antithrombin-heparin cofactor: an inhibitor of plasma kallikrein. Arch Biochem Biophys. 1976 Aug;175(2):737–747. doi: 10.1016/0003-9861(76)90567-1. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McConnell D. J. Inhibitors of kallikrein in human plasma. J Clin Invest. 1972 Jul;51(7):1611–1623. doi: 10.1172/JCI106962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnoff O. D., Pensky J., Ogston D., Naff G. B. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C'1r subcomponent of the first component of complement by serum C'1 esterase inhibitor. J Exp Med. 1969 Feb 1;129(2):315–331. doi: 10.1084/jem.129.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Silverman P., Geokas M. C., Haverback B. J. Determination of trypsin and chymotrypsin with remazolbrilliant blue-hide. Clin Chim Acta. 1970 May;28(2):239–246. doi: 10.1016/0009-8981(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Goldsmith G. H., Moroi M., Aoki N. Inhibitory spectrum of alpha 2-plasmin inhibitor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2013–2017. doi: 10.1073/pnas.76.4.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Scott C. F., Colman R. W. Protection of human plasma kallikrein from inactivation by C1 inhibitor and other protease inhibitors. The role of high molecular weight kininogen. Biochemistry. 1981 May 12;20(10):2738–2743. doi: 10.1021/bi00513a006. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Liu C. Y., Colman R. W. Human plasma prekallikrein: a rapid high-yield method for purification. Eur J Biochem. 1979 Oct;100(1):77–83. doi: 10.1111/j.1432-1033.1979.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Song M. K., Adham N. F., Rinderknecht H. Large scale purification of alpha2-macroglobulin from human plasma. Biochem Med. 1975 Oct;14(2):162–169. doi: 10.1016/0006-2944(75)90033-2. [DOI] [PubMed] [Google Scholar]
- Vischer T. L., Berger D. Activation of macrophages to produce neutral proteinases by endocytosis of alpha 2-macroglobulin-trypsin complexes. J Reticuloendothel Soc. 1980 Nov;28(5):427–435. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



