Abstract
Although radiofrequency ablation for lung cancer is generally safe (with a mortality rate <1%), it may cause various complications. Common complications include pneumothorax, pleural effusion, and parenchymal hemorrhage. Although most complications can be treated conservatively or with minimal therapy, physicians should be aware of rare but serious complications. Potentially fatal complications include massive hemorrhage, intractable pneumothorax due to bronchopleural fistula, pulmonary artery pseudoaneurysm, systemic air embolism, and pneumonitis. Other serious complications include injury to the nearby tissues (e.g., brachial nerve plexus, phrenic nerve, diaphragm, and chest wall), needle tract seeding, lung abscess, empyema, and skin burn. Although cavitation of the ablation zone is usually insignificant clinically, such a cavity occasionally ruptures, leading to pneumothorax and bleeding. Cavities may also serve as a scaffold for fungal colonization. Precautions to minimize risk should be taken whenever possible. Nevertheless, serious complications may occur, and thus physicians should be aware of the appropriate treatments for these complications. This article reviews complications associated with lung cancer ablation.
Keywords: radiofrequency ablation, lung cancer, complication, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify the complications of lung radiofrequency ablation and the treatment methods used for such complications.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Primary lung cancer is the most common malignancy and the leading cause of cancer-related death worldwide. In addition, the lungs are the second most frequent site of metastasis from extrathoracic cancers and the only site of metastasis in 20% of such cases. Surgical resection is the first-line treatment for non-small cell lung cancers and generally considered the best treatment option. Surgery is also accepted as a treatment option for selected patients with metastatic lung cancer. However, surgical resection is not suitable for many patients mainly because of the advanced stage of cancer, compromised lung function, and/or comorbidities. Although chemotherapy, radiation therapy, and a combination of these modalities are alternative treatments for such patients, complete remission of the disease is rarely achieved. Therefore, in recent decades research has extensively focused on alternative therapies for lung cancer.
Radiofrequency ablation (RFA) was initially developed as a therapy for liver tumors. The favorable outcomes for RFA in the liver have encouraged the application of this technique to cancer in other organs. In 2000, Dupuy et al1 first reported the clinical application of this technique in the lungs. Since this work, RFA has rapidly gained popularity as a treatment for lung cancer. RFA of lung cancer is usually performed under computed tomography (CT) guidance, and the techniques are simple and similar to those used for CT-guided lung biopsy. Although early results (e.g., local control and patient survival) of RFA for both primary and metastatic lung cancer appear promising, the procedure may be accompanied by various complications. An international survey2 revealed that the rate of lung RFA-related death was 0.4% (2 of 493). Although most of these complications are minor, complications can be severe and even fatal. In 2007, the Food and Drug Administration in the United States made a public announcement regarding deaths following RFA for lung tumors.
Pneumothorax
Pneumothorax is the most common complication following RFA; pneumothorax may be accompanied by subcutaneous emphysema. The incidence of pneumothorax after RFA of lung cancer is 11 to 52%.3,4,5,6 Risk factors for pneumothorax included male gender, no history of pulmonary surgery, high number of tumors ablated, treated tumors in the lower lungs, increased length of the aerated lung traversed by the electrode, pulmonary emphysema, advanced age, small tumors, and traversal of the major fissure by the electrode.3,4,5,6 In 6 to 29% of pneumothorax cases, chest tube placement for drainage is required.3,4,5,6 The risk factors for chest tube placement for pneumothorax include no history of pulmonary surgery, the use of a cluster electrode, and involvement of the upper lobe.3 Pneumothorax may develop after RFA at a later stage; delayed pneumothorax occurs after ~10% of the procedures.7,8
Bronchopleural Fistula
Although most pneumothorax cases can usually be treated conservatively or via the placement of a chest tube, pneumothorax occasionally becomes intractable because of the development of a bronchopleural fistula. Sakurai et al9 reported the incidence of intractable pneumothorax due to bronchopleural fistula as 0.6% (2 of 334). In both cases, RFA-induced necrosis of the lung tissue between the pleural space and the bronchus was noted, and the bronchopleural fistula formed after sloughing of the necrotic tissue. Management of the bronchopleural fistula was challenging requiring various techniques such as pleurodesis, endobronchial management, and/or surgical repair. In one case, air leakage persisted despite these efforts, and the patient died of acute pneumonia.
Kodama et al10 also reported a case of bronchopleural fistula. Twelve days after RFA of a 3.4-cm primary lung cancer lesion, pneumothorax developed, and it was treated with chest tube placement. However, air leakage persisted for 1 month secondary to bronchopleural fistula. The several bronchi that developed fistulae were endoscopically embolized successfully using a silicone embolic material. Cannella et al11 also reported two cases of bronchopleural fistula. Both of these cases were successfully treated with only chest tube placement. A case of bronchopleural fistula at the author's institution is illustrated in Fig. 1. The patient was first treated with chest tube placement, but air leakage persisted. Pleurodesis was performed twice, and the tube was finally removed 11 days after RFA.
Figure 1.
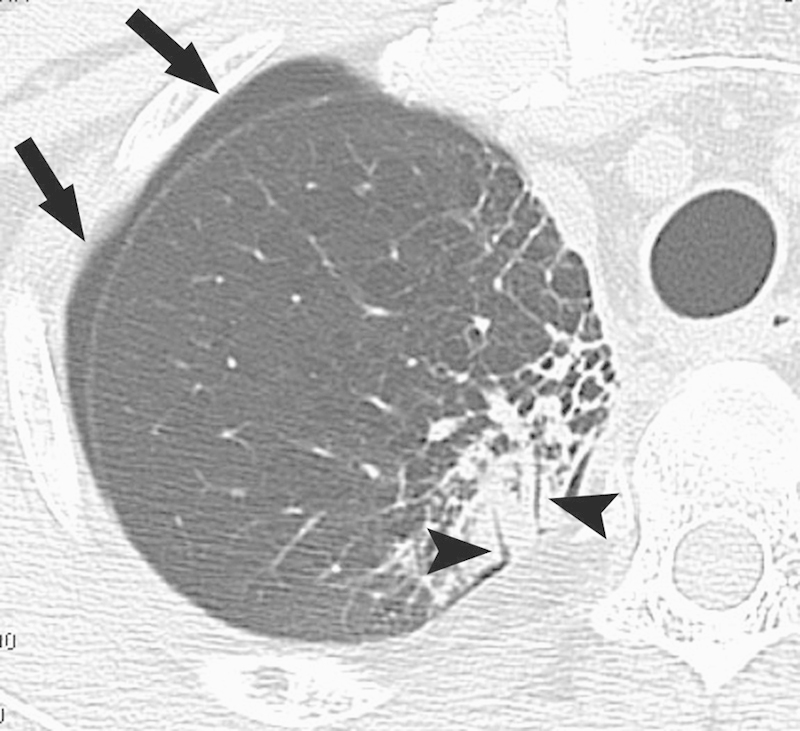
Bronchopleural fistula. Computed tomography image 6 days after radiofrequency ablation shows that two bronchi (arrowheads) in the ablation zone are contiguous with the pleural space, causing pneumothorax (arrows).
Pleural Effusion
The incidence of pleural effusion is reported to be 6 to 19%.3,5,12 Significant risk factors for the development of pleural effusion are the use of a cluster electrode, decreased distance to the nearest pleura, and a decrease in the length of the aerated lung that is traversed by the electrode.3 Tajiri et al13 investigated the relationship between pleural temperature and pleural effusion after RFA of lung tumors. The occurrence of pleural effusion was associated with increased pleural temperatures during the procedure, which may indicate that pleural effusion is related to pleuritis induced by thermal injury. Nevertheless, aseptic pleural effusion after RFA can usually be treated conservatively.
Hemorrhage
The incidence of hemoptysis after RFA is 3 to 9%, but the incidence of all the forms of hemorrhage are approximately double that rate.5,14,15 Steinke et al16 reported that the incidence of pulmonary hemorrhage during RFA was 6%; Nour-Eldin et al17 demonstrated an incidence of 18% (44 of 248 procedures). Risk factors for intraparenchymal hemorrhage include lesions <1.5 cm in diameter, basal and middle lung zone lesions, the needle track traversing the lung parenchyma by >2.5 cm, traversing pulmonary vessels in the track of ablation, and the use of multi-tined electrodes.
Although the vast majority of parenchymal hemorrhages are self-limiting, the hemorrhages are occasionally massive and even fatal.17,18,19,20 The study by Nour-Eldin et al17 included a case of uncontrollable hemorrhage resulting in the patient's death during RFA. Vaughn et al18 also reported massive intraparenchymal and extrapleural hemorrhage during RFA. The patient died of pulmonary aspiration on postoperative day 23. In addition, Sano et al15 reported a case of massive fatal hemoptysis from thermal injury of bronchial mucosa 28 days after RFA of hilar lymph nodes. In addition to parenchymal hemorrhage, physicians should be aware of hemothorax primarily caused by injury to an intercostal artery. A case of just such an injury at the author's institution is illustrated in Fig. 2, in which the patient was successfully treated with coil embolization of the intercostal artery.
Figure 2.
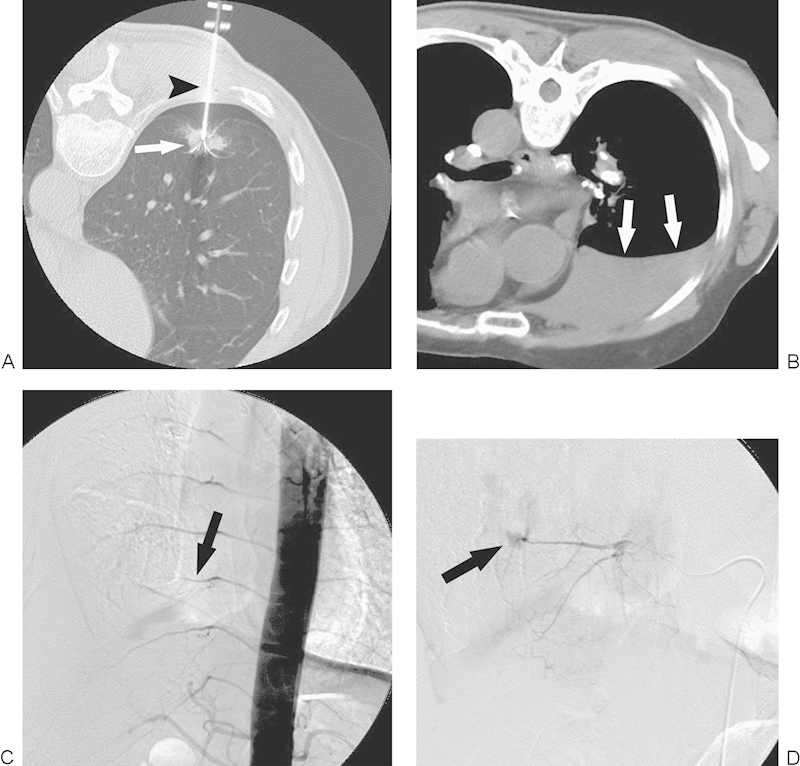
Hemothorax. (A) Computed tomography (CT) image during radiofrequency ablation (RFA) with a patient in a prone position reveals that a multi-tined expandable electrode (arrowhead) is introduced into the tumor (arrow). (B) CT image immediately after RFA with a patient in a prone position reveals massive hemothorax (arrows). (C) Thoracic aortography shows truncation of an intercostal artery (arrow). (D) Intercostal arteriography demonstrates extravasation of contrast medium (arrow), compatible with active hemorrhage.
Pulmonary Artery Pseudoaneurysm
Pulmonary artery pseudoaneurysm is a serious and potentially fatal complication. Sakurai et al21 reported a case of pulmonary artery pseudoaneurysm related to RFA of a lung tumor directly adjacent to a branch of the pulmonary artery. Seventeen days later, the patient complained of hemoptysis, and a contrast-enhanced CT scan revealed a pseudoaneurysm in the ablation zone. The pseudoaneurysm was successfully treated using transcatheter coil embolization. The incidence of pseudoaneurysm in this series was 0.2% (1 of 538 sessions). Yamakado et al22 reported a similar case in which the patient presented with massive hemoptysis 1 week after RFA. Contrast-enhanced CT revealed a pseudoaneurysm that was successfully treated using coil embolization. Soh et al23 also reported a case of pulmonary artery pseudoaneurysm leading to massive hemothorax and intrapulmonary hematoma that was treated using transcatheter coil embolization followed by lobectomy.
The experience with these cases suggests that if a patient presents with a significant hemorrhagic event after RFA, contrast-enhanced CT should be performed to investigate the development of a pseudoaneurysm, and as soon as a pseudoaneurysm is detected, it should be treated. We experienced a case of pseudoaneurysm that was revealed but missed on contrast-enhanced CT images, resulting in subsequent fatal hemoptysis (Fig. 3).
Figure 3.
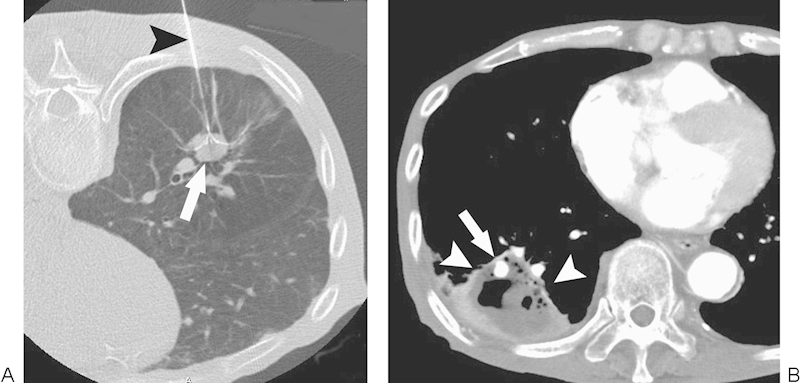
Pulmonary artery pseudoaneurysm. (A) Computed tomography (CT) image during radiofrequency ablation (RFA) with a patient in a prone position demonstrates a multi-tined expandable electrode (arrowhead) introduced into the tumor (arrow). (B) CT image 10 days after RFA shows a pulmonary artery pseudoaneurysm (arrow) in the ablation zone (arrowheads).
Needle Tract Seeding
Hiraki et al24 reported two cases of needle tract seeding after RFA for lung cancer (2 of 661 procedures; 0.3% incidence). Needle biopsy was performed immediately before RFA in one case. In both of these cases, RFA was performed with a single internally cooled electrode; the electrode tip temperature was <60°C immediately after radiofrequency application, and the electrode was removed without cauterizing the electrode tract. The seeded tumor in both cases was completely treated with RFA. The conclusions of the study were that tumor seeding was attributed to the biopsy performed immediately before RFA or the detachment of viable cancer cells at the electrode tip while removing the electrode without cauterizing the tract.
Yamakado et al25 also reported a case of needle tract seeding after RFA in a patient with poorly differentiated adenocarcinoma. The incidence at their institution was 0.7% (1 of 144 procedures). In this case, percutaneous biopsy was performed before RFA. RFA was performed with a single internally cooled electrode, the electrode tip temperature was 52°C immediately after radiofrequency application, and the electrode was removed without cauterizing the electrode tract. All of these findings were similar to the case reported by Hiraki et al. The seeded tumor was treated using RFA.
These cases indicated that the risk factors for needle tract seeding after lung RFA are assumed to be the use of a internally cooled electrode, an electrode tip temperature of <60°C immediately after RFA, absence of tract ablation, biopsy prior to RFA, and poor differentiation of cancers. To reduce the risk of this complication, we recommend that the electrode be removed with cauterization of the electrode tract.
Thermal Injury to Nearby Structures
Peripheral nerves are sensitive to heat, and thus RFA may injure peripheral nerves located near the ablation site. For example, the caudal elements of the brachial plexus course immediately above the lung apex. Hiraki et al26 reported four cases of brachial nerve injury, the incidence of which was 0.5% (4 of 733 procedures). As expected, the treated tumor was in the lung apex in all cases. When the analysis was confined to procedures for apical lung cancer, the incidence of brachial nerve injury was 15% (4 of 26 procedures). The patients developed symptoms at the medial side of the forearm and upper arm and in the fourth and fifth fingers, indicating an injury of the caudal brachial plexus comprising the C8 and T1 nerves. Despite partially receding over time, symptoms included a grade 2 sensory dysfunction in three patients, and a grade 3 motor dysfunction in one patient (graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v.4.0).
Matsui et al27 studied phrenic nerve injury after lung RFA. Phrenic nerve injury developed after 10 of 786 procedures (1.3%). According to multivariate analysis, the only independent risk factor for phrenic nerve injury was the proximity of the phrenic nerve to the tumor (<10 mm). The patients who developed phrenic nerve injury lost a mean of ~20% of their vital capacity and forced expiratory volume at 1 second. They suggested that referred pain in the shoulder, teeth, or mandible during the procedure may be predictive of phrenic nerve injury after RFA. A case of phrenic nerve injury in the our institution is illustrated in Fig. 4. The patient lost a significant proportion (1 L) of his vital capacity because of phrenic nerve paralysis.
Figure 4.
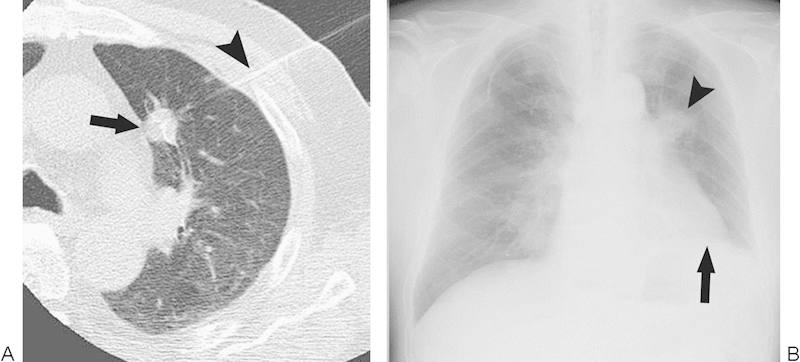
Phrenic nerve injury. (A) Computed tomography image during radiofrequency ablation (RFA) show a multi-tined expandable electrode (arrowhead) introduced into a tumor (arrow) close to the pulmonary trunk. (B) Chest radiograph after RFA demonstrates elevation of the diaphragm on the treated side (arrow). The arrowhead denotes the ablation zone.
The chest wall may be injured during RFA of a tumor close to the lung surface. When this occurs, patients complain of pain along the rib after RFA, a result from injury to the intercostal nerve. Le et al28 reported a case of osteonecrosis of the rib after RFA of a pleural-based tumor. Some possible techniques can be used to separate the tumor from the chest wall including the creation of an artificial pneumothorax (which has proven useful for pain relief during RFA),29 the administration of artificial pleural effusion, and the use of thoracoscopic guidance instead of the percutaneous approach.
Hiraki et al30 reported a case of diaphragmatic hernia occurring after RFA for pulmonary metastasis in the lung base. The incidence of diaphragmatic hernia after lung RFA at their institution was 0.1% (1 of 859 procedures). Herniation of the liver occurred, but the patient was asymptomatic and thus was treated conservatively. Herniation of the intestine would have been more dangerous because of the risk of strangulation. Although diaphragmatic hernia is a well-known complication of liver RFA, it may be also caused by RFA for lung cancer close to the diaphragm.
Pneumonitis
Nomura et al12 reported two cases of interstitial pneumonia that resulted in patient death after RFA of lung cancer. Both patients underwent radiation therapy before RFA. The authors suggested that the pneumonia was radiation pneumonitis induced by RFA. Thus the authors concluded that the indication for RFA in patients with a history of prior radiation should be carefully determined. Hiraki et al31 reported three cases of bronchiolitis obliterans organizing pneumonia-like reactive pneumonitis following RFA for lung cancer. The incidence of bronchiolitis obliterans organizing pneumonia at their institution was ~0.4% (3 of 840 sessions). The patients presented with nonspecific symptoms (e.g., fever, cough, sputum, malaise, and/or dyspnea). CT images revealed consolidation or ground-glass opacity in a peripheral-dominant distribution and/or patchy air-space opacities. Antibiotics were not effective, but the disease responded favorably to pulse steroid therapy. A case of a bronchiolitis obliterans organizing pneumonitis after RFA at the our institution is illustrated in Fig. 5.
Figure 5.
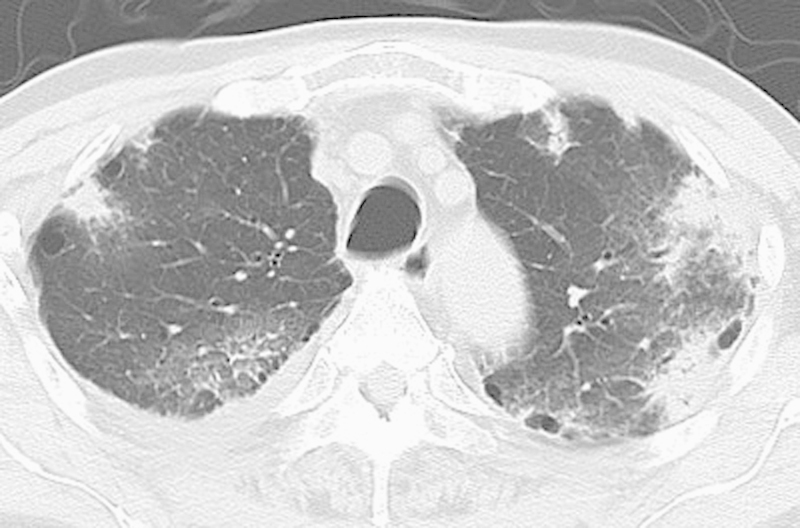
Bronchiolitis obliterans organizing pneumonia. Computed tomography image after radiofrequency ablation reveals patchy consolidations or ground-glass opacities predominantly in the subpleural area of the bilateral upper lobes, compatible with bronchiolitis obliterans organizing pneumonia.
Lee et al14 described a case of acute respiratory distress syndrome, although the details were not described in the report. Okuma et al32 reported a case of acute deterioration of interstitial pneumonia. A patient with interstitial pneumonia underwent RFA for a squamous cell carcinoma lesion measuring 3.4 cm. After RFA, a pneumothorax developed, and the patient was treated with chest tube placement. However, because of the lack of improvement of the pneumothorax, pleurodesis was performed with OK-342 on day 8. The following day, acute exacerbation of interstitial pneumonia occurred. Despite intubation and steroid pulse therapy, the patient died in the evening of the same day.
Systemic Air Embolism
Systemic air embolism is well known as a complication of percutaneous lung biopsy; this potentially fatal complication may also be caused by lung RFA. Okuma et al33 reported a case of systemic air embolism during lung RFA. Immediately after a coughing episode, the patient suddenly became unresponsive and developed respiratory arrest. The patient was successfully resuscitated and discharged 8 days after the event without any neurologic deficit. Ghaye et al34 also reported a case of systemic air embolism during lung RFA. RFA was performed for a pulmonary metastasis 11 mm in size under general anesthesia requiring positive-pressure ventilation. Although the patient was asymptomatic, massive systemic air was observed on CT images. The patient was treated with 100% oxygen, resulting in the disappearance of systemic air. The authors concluded that positive-pressure ventilation was one of the factors that increased the risk of this complication.
Jin et al35 reported a case of nonfatal cerebral infarction occurring immediately after RFA of atypical pulmonary carcinoid. The authors speculated that the cause of the infarction was systemic air embolism, although the exact cause was not determined. Yamamoto et al36 performed an interesting study in which they evaluated microbubbles in the carotid artery on sonography during lung RFA. Microbubbles were demonstrated in the carotid artery on sonography during 3 of 17 RFA sessions. However, no new infarction was detected using postoperative brain magnetic resonance imaging, and no clinical sequelae were noted.
If systemic air embolism occurs, 100% oxygen should be immediately administered to promote the replacement of nitrogen within the embolized air by oxygen, thereby facilitating the resorption of the air. Although appropriate patient positioning is controversial, many researchers recommend the Trendelenburg position or flat supine position. The mainstay of therapy is hyperbaric oxygen therapy, which reduces gas volume substantially and promotes the replacement of nitrogen with oxygen.
Cavitation
Okuma et al37 studied cavitation after lung RFA, the incidence of which was 14% (14 of 100 procedures). Most patients who developed cavitation were asymptomatic. Risk factors for cavitation were the proximity of the tumor to the chest wall, primary lung cancer, and pulmonary emphysema. Additionally, in the our experience, most cases of cavitation do not result in clinically important events. Rarely, however, such a cavity may enlarge over time and rupture, leading to pneumothorax and hemorrhage (Fig. 6). Furthermore, such a cavity may be a scaffold for Aspergillus infection, as reported by Hiraki et al.38
Figure 6.
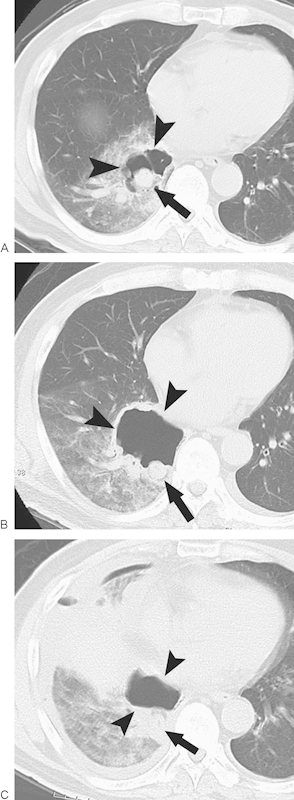
Cavitation. (A) Computed tomography (CT) image 1 day after radiofrequency ablation (RFA) reveals a cavity (arrowheads) around an ablated tumor (arrow). (B) CT image 4 days after RFA illustrates an enlarged cavity (arrowheads) with a thickened wall. The arrow denotes the ablated tumor. (C) CT image 6 days after RFA reveals shrinkage of the cavity (arrowheads) along with the development of pneumothorax and pleural effusion, which suggest rupture of the cavity. The arrow denotes the ablated tumor.
Other Complications
Other serious reported complications include lung abscess,5,12,15,39 empyema,12,15,39 and skin burns.15 These complications are all serious but exceedingly uncommon.
Conclusions
Although RFA for lung cancer is generally safe, it may cause significant complications. Most complications can be treated conservatively or with minimal therapy; however, operators should be aware of the rare but serious complications including massive hemorrhage, intractable pneumothorax, pneumonitis, pulmonary artery pseudoaneurysm, injury of nearby important tissues, systemic air embolism, needle tract seeding lung abscess, empyema, and skin burn. Precautions to minimize the risk of serious complications should be taken, if possible. If serious complications occur despite these precautions, appropriate treatment should be provided immediately.
References
- 1.Dupuy D E, Zagoria R J, Akerley W, Mayo-Smith W W, Kavanagh P V, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174(1):57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 2.Steinke K, Sewell P E, Dupuy D. et al. Pulmonary radiofrequency ablation—an international study survey. Anticancer Res. 2004;24(1):339–343. [PubMed] [Google Scholar]
- 3.Hiraki T, Tajiri N, Mimura H. et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology. 2006;241(1):275–283. doi: 10.1148/radiol.2411051087. [DOI] [PubMed] [Google Scholar]
- 4.Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T. Pneumothorax as a complication of percutaneous radiofrequency ablation for lung neoplasms. J Vasc Interv Radiol. 2006;17(10):1625–1629. doi: 10.1097/01.RVI.0000236607.05698.4A. [DOI] [PubMed] [Google Scholar]
- 5.Okuma T, Matsuoka T, Yamamoto A. et al. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Intervent Radiol. 2008;31(1):122–130. doi: 10.1007/s00270-007-9225-0. [DOI] [PubMed] [Google Scholar]
- 6.Nour-Eldin N E, Naguib N N, Saeed A S. et al. Risk factors involved in the development of pneumothorax during radiofrequency ablation of lung neoplasms. AJR Am J Roentgenol. 2009;193(1):W43-8. doi: 10.2214/AJR.08.1457. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimatsu R, Yamagami T, Terayama K, Matsumoto T, Miura H, Nishimura T. Delayed and recurrent pneumothorax after radiofrequency ablation of lung tumors. Chest. 2009;135(4):1002–1009. doi: 10.1378/chest.08-1499. [DOI] [PubMed] [Google Scholar]
- 8.Clasen S, Kettenbach J, Kosan B. et al. Delayed development of pneumothorax after pulmonary radiofrequency ablation. Cardiovasc Intervent Radiol. 2009;32(3):484–490. doi: 10.1007/s00270-008-9489-z. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai J, Hiraki T, Mukai T. et al. Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol. 2007;18(1 Pt 1):141–145. doi: 10.1016/j.jvir.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kodama H, Yamakado K, Murashima S. et al. Intractable bronchopleural fistula caused by radiofrequency ablation: endoscopic bronchial occlusion with silicone embolic material. Br J Radiol. 2009;82(983):e225–e227. doi: 10.1259/bjr/23975691. [DOI] [PubMed] [Google Scholar]
- 11.Cannella M, Cornelis F, Descat E. et al. Bronchopleural fistula after radiofrequency ablation of lung tumours. Cardiovasc Intervent Radiol. 2011;34 02:S171–S174. doi: 10.1007/s00270-010-9826-x. [DOI] [PubMed] [Google Scholar]
- 12.Nomura M, Yamakado K, Nomoto Y. et al. Complications after lung radiofrequency ablation: risk factors for lung inflammation. Br J Radiol. 2008;81(963):244–249. doi: 10.1259/bjr/84269673. [DOI] [PubMed] [Google Scholar]
- 13.Tajiri N, Hiraki T, Mimura H. et al. Measurement of pleural temperature during radiofrequency ablation of lung tumors to investigate its relationship to occurrence of pneumothorax or pleural effusion. Cardiovasc Intervent Radiol. 2008;31(3):581–586. doi: 10.1007/s00270-007-9283-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee J M, Jin G Y, Goldberg S N. et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology. 2004;230(1):125–134. doi: 10.1148/radiol.2301020934. [DOI] [PubMed] [Google Scholar]
- 15.Sano Y, Kanazawa S, Gobara H. et al. Feasibility of percutaneous radiofrequency ablation for intrathoracic malignancies: a large single-center experience. Cancer. 2007;109(7):1397–1405. doi: 10.1002/cncr.22541. [DOI] [PubMed] [Google Scholar]
- 16.Steinke K, King J, Glenn D, Morris D L. Pulmonary hemorrhage during percutaneous radiofrequency ablation: a more frequent complication than assumed? Interact Cardiovasc Thorac Surg. 2003;2(4):462–465. doi: 10.1016/S1569-9293(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 17.Nour-Eldin N E, Naguib N N, Mack M, Abskharon J E, Vogl T J. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur Radiol. 2011;21(1):197–204. doi: 10.1007/s00330-010-1889-1. [DOI] [PubMed] [Google Scholar]
- 18.Vaughn C, Mychaskiw G II, Sewell P. Massive hemorrhage during radiofrequency ablation of a pulmonary neoplasm. Anesth Analg. 2002;94(5):1149–1151. doi: 10.1097/00000539-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Dupuy D E, Mayo-Smith W W, Abbott G F, DiPetrillo T. Clinical applications of radio-frequency tumor ablation in the thorax. Radiographics. 2002;22(Spec No):S259–S269. doi: 10.1148/radiographics.22.suppl_1.g02oc03s259. [DOI] [PubMed] [Google Scholar]
- 20.Herrera L J, Fernando H C, Perry Y. et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125(4):929–937. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai J, Mimura H, Gobara H, Hiraki T, Kanazawa S. Pulmonary artery pseudoaneurysm related to radiofrequency ablation of lung tumor. Cardiovasc Intervent Radiol. 2010;33(2):413–416. doi: 10.1007/s00270-009-9565-z. [DOI] [PubMed] [Google Scholar]
- 22.Yamakado K, Takaki H, Takao M. et al. Massive hemoptysis from pulmonary artery pseudoaneurysm caused by lung radiofrequency ablation: successful treatment by coil embolization. Cardiovasc Intervent Radiol. 2010;33(2):410–412. doi: 10.1007/s00270-009-9564-0. [DOI] [PubMed] [Google Scholar]
- 23.Soh J, Toyooka S, Gobara H. et al. A case of delayed massive hemothorax caused by the rupture of a pulmonary artery pseudoaneurysm after radiofrequency ablation of lung tumors. Jpn J Clin Oncol. 2012;42(7):646–649. doi: 10.1093/jjco/hys068. [DOI] [PubMed] [Google Scholar]
- 24.Hiraki T, Mimura H, Gobara H. et al. Two cases of needle-tract seeding after percutaneous radiofrequency ablation for lung cancer. J Vasc Interv Radiol. 2009;20(3):415–418. doi: 10.1016/j.jvir.2008.12.411. [DOI] [PubMed] [Google Scholar]
- 25.Yamakado K, Akeboshi M, Nakatsuka A. et al. Tumor seeding following lung radiofrequency ablation: a case report. Cardiovasc Intervent Radiol. 2005;28(4):530–532. doi: 10.1007/s00270-004-0246-7. [DOI] [PubMed] [Google Scholar]
- 26.Hiraki T, Gobara H, Mimura H. et al. Brachial nerve injury caused by percutaneous radiofrequency ablation of apical lung cancer: a report of four cases. J Vasc Interv Radiol. 2010;21(7):1129–1133. doi: 10.1016/j.jvir.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Matsui Y, Hiraki T, Gobara H. et al. Phrenic nerve injury after radiofrequency ablation of lung tumors: retrospective evaluation of the incidence and risk factors. J Vasc Interv Radiol. 2012;23(6):780–785. doi: 10.1016/j.jvir.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Le T X, Andrews R T. Thermal osteonecrosis of the rib after radiofrequency ablation in the thorax. J Vasc Interv Radiol. 2008;19(6):940–944. doi: 10.1016/j.jvir.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Hiraki T, Gobara H, Shibamoto K. et al. Technique for creation of artificial pneumothorax for pain relief during radiofrequency ablation of peripheral lung tumors: report of seven cases. J Vasc Interv Radiol. 2011;22(4):503–506. doi: 10.1016/j.jvir.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Hiraki T, Gobara H, Masaoka Y, Toyooka S, Kanazawa S. Diaphragmatic hernia after percutaneous radiofrequency ablation of lung tumor. J Vasc Interv Radiol. 2011;22(12):1777–1778. doi: 10.1016/j.jvir.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Hiraki T, Gobara H, Kato K, Toyooka S, Mimura H, Kanazawa S. Bronchiolitis obliterans organizing pneumonia after radiofrequency ablation of lung cancer: report of three cases. J Vasc Interv Radiol. 2012;23(1):126–130. doi: 10.1016/j.jvir.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Okuma T, Matsuoka T, Hamamoto S, Nakamura K, Inoue Y. Percutaneous computed tomography-guided radiofrequency ablation of lung tumors complicated with idiopathic interstitial pneumonia. Ann Thorac Surg. 2009;87(3):948–950. doi: 10.1016/j.athoracsur.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 33.Okuma T, Matsuoka T, Tutumi S, Nakmura K, Inoue Y. Air embolism during needle placement for CT-guided radiofrequency ablation of an unresectable metastatic lung lesion. J Vasc Interv Radiol. 2007;18(12):1592–1594. doi: 10.1016/j.jvir.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Ghaye B, Bruyère P J, Dondelinger R F. Nonfatal systemic air embolism during percutaneous radiofrequency ablation of a pulmonary metastasis. AJR Am J Roentgenol. 2006;187(3):327–328. doi: 10.2214/AJR.06.0179. [DOI] [PubMed] [Google Scholar]
- 35.Jin G Y, Lee J M, Lee Y C, Han Y M. Acute cerebral infarction after radiofrequency ablation of an atypical carcinoid pulmonary tumor. AJR Am J Roentgenol. 2004;182(4):990–992. doi: 10.2214/ajr.182.4.1820990. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto A, Matsuoka T, Toyoshima M. et al. Assessment of cerebral microembolism during percutaneous radiofrequency ablation of lung tumors using diffusion-weighted imaging. AJR Am J Roentgenol. 2004;183(6):1785–1789. doi: 10.2214/ajr.183.6.01831785. [DOI] [PubMed] [Google Scholar]
- 37.Okuma T, Matsuoka T, Yamamoto A. et al. Factors contributing to cavitation after CT-guided percutaneous radiofrequency ablation for lung tumors. J Vasc Interv Radiol. 2007;18(3):399–404. doi: 10.1016/j.jvir.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Hiraki T, Gobara H, Mimura H. et al. Aspergilloma in a cavity formed after percutaneous radiofrequency ablation for lung cancer. J Vasc Interv Radiol. 2009;20(11):1499–1500. doi: 10.1016/j.jvir.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 39.Chua T C, Sarkar A, Saxena A, Glenn D, Zhao J, Morris D L. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol. 2010;21(10):2017–2022. doi: 10.1093/annonc/mdq098. [DOI] [PubMed] [Google Scholar]


