Abstract
NASA’s extra-vehicular activities (EVAs) involve exposure to high energy photons while breathing 100% oxygen. Using previously verified mouse models, our laboratory is studying whether low dose irradiation under these hyperoxic conditions could lead to an increase in carcinogenic potential. To simulate the environment astronauts encounter during an EVA, enclosed chambers were constructed that allowed for mouse movement, controlled gas conditions, and uniform radiation dose delivery. Custom-built gas chambers with input/output gas valves and dividers that allowed for uniform gas flow were used to keep 6 unanesthetized mice separated while they were irradiated. The chambers were supplied with 100% oxygen or air using ball valves linked together with T-splitters. A calibrated ion chamber was used to verify the radiation dose distribution across an entire chamber. Mice were placed in the gas environments for 0.5 h, irradiated with a 10 or 18 MV photon beam from a medical linear accelerator, and left in their gas environment for 2 h post-irradiation. We irradiated 200 mice (5 different doses between 0–1000 mGy) under normoxic or 100% oxygen conditions. For the next step of this research, these mice will be euthanized 9 months post-irradiation, and lung tumors will be counted and sized to determine if hyperoxia increases the carcinogenic effect for this model.
Keywords: lung, carcinogenesis, radiation, hyperoxia, NASA, spacewalk, oxygen chamber, mouse model
INTRODUCTION
Our research group is currently studying the effects of low dose radiation using sensitive lung carcinogenesis mouse models. It has been estimated that approximately 29,000 excess tumors result from exposure to x-ray computed tomography (CT) radiation in the U.S. each year [1, 2]. However, these approximations are based on many assumptions to predict the carcinogenic risk of low-energy, low-dose levels of CT radiation. A primary objective of our group is to directly measure the carcinogenic potential of low-energy, low-dose exposures by using a clinical CT scanner and a previously validated in vivo mouse model developed at our institution for carcinogenesis studies [3]. Our studies may also challenge the accuracy of the widely accepted Linear No-Threshold radiation response model; one of our initial studies showed that there was no dose response when multiple CT exposures were given to our mouse model to a total of 80–160 mGy. The same study also showed that there appears to be an increase in the carcinogenic risk for individuals that express cancer susceptibility genes [4].
In previous studies, we used a transgenic mouse model developed in our laboratory that contains a mutant form of the human Ki-ras gene [3, 4]. Ki-ras mutations have been implicated as one of the key genetic alterations that drive tumorigenesis in approximately 30% of human lung adenocarcinomas [5–8]. Adenocarcinoma is a common form of non-small cell lung cancer and its incidence has been increasing [9,10]. The bitransgenic CCSP/Ki-ras mice used in these experiments were generated on an FVB/N background and expressed the Ki-rasG12C gene only in the lungs when given doxycycline (DOX) in their drinking water. FVB/N is a common mouse strain utilized in transgenic experiments, both because of its excellent reproductive ability and because the fertilized eggs’ large pronuclei facilitate DNA modification [11]. Our DOX treated bitransgenic mice exhibit, i] small, hyperplastic foci in their lungs after 12 days, ii] extensive epithelial hyperplasia of the alveolar region of the lungs after 5 weeks, iii] a few lung adenomas < 1 mm in size by 3 months, and iv] these adenomas increase to a maximum number of 15–20 adenomas/lung by 6–9 months [4]. These adenomas rarely progress to adenocarcinoma, making it possible to evaluate the effects of radiation exposures on this transition. We have shown that exposure of DOX-treated mice to lung tumor promoters will result in increased tumor multiplicity and the development of adenocarcinomas [12].
We are currently examining how the added variable of hyperoxia in combination with radiation affects carcinogenesis. NASA routinely conducts extra-vehicular activities (EVAs) on their space missions. During these EVAs, astronauts are supplied with 100% oxygen for respiration. Furthermore, without the added shielding that the spaceship provides, the astronauts are subject to greater high energy radiation exposure during these spacewalks. Oxygen is a known radiosensitizer; oxygenated cells are more susceptible to radiation effects than hypoxic cells [13]. Additionally, above normal oxygen concentrations may lead to oxidative stress, which in combination with radiation exposure, may contribute to tumorigenesis through both DNA damage and alterations in gene expression [14]. This provides the motivation to investigate the effects of breathing 100% oxygen under radiation conditions that attempt to mimic those encountered during an EVA. The main objective of this project is to describe the technique used to create a controlled oxygen environment and uniform dose radiation exposure for unanesthetized mice.
MATERIALS & METHODS
i. Chamber Construction
Our typical rodent irradiation protocols involve anesthetizing rodents for the irradiation procedure. However, because our goal was to model astronauts performing EVAs, a temporary restraining device had to be constructed that allowed for rodent movement while still ensuring a uniform dose delivery in an efficient manner. Plexiglas was chosen because of its relatively low cost and easy handling. Chloroform can be used as an adhesive to bond Plexiglas. By melting a small amount of Plexiglas into a container of chloroform, an adhesive paste is created that, once applied and left to dry for ~1 h, will sufficiently bind sheets of Plexiglas together. In this manner, ¼ inch thick Plexiglas was used to construct a 30 × 30 × 9 cm3 box with a removable lid. The inside height of the box was 1½ inches. This allowed the mice the freedom for planar movement while still preventing them from standing fully upright and potentially complicating the dosimetry. The platform that the mice stood on was elevated from the floor, i.e., there was an air gap of 1⅜ inches, in order to minimize backscatter exposure to the mice. The dimensions of the box were designed entirely in cooperation with our institution’s animal care and use committee to ensure compliance with acceptable mice housing guidelines. Dividers with holes were used to keep six mice separate while still allowing for steady and uniform air flow through the chamber. Gas valves were fitted into holes at opposite ends of the box to allow for gas entry and exit. To feed the chambers with oxygen or breathing air, ⅜ inch ball valves were linked together with T-splitters. This configuration improved efficiency because we could incubate multiple chambers simultaneously from the same gas canister. Once the multi-valve system was constructed, air flow was qualitatively verified across all four boxes. The lid of the box was sealed with a layer of petroleum jelly to help ensure that the direction of air flow was purely through the entry and exit valves. Once a steady stream of oxygen was verified exiting the box, it was assumed that, after incubation for ~10 min to reach a steady state, the initial gas concentration in the box was entirely displaced by the oxygen (or regular air for the control group) that was being pumped in. Figure 1 shows an example of a chamber and the gas flow design.
FIG. 1.
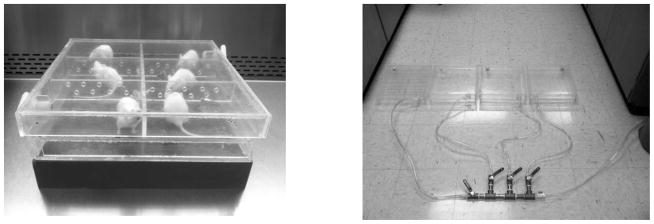
Left: A photograph of six mice in the completed chamber, placed on top of a water-equivalent solid phantom. The lid is removable, and the dividers with holes for air flow can be configured to meet different geometric needs. Right: A photograph of four chambers all connected in parallel to the ball valve setup. Each chamber has an independent handle to individually regulate the flow into each chamber.
ii. Mouse Model
All procedures in this study were approved by the institutional animal care and use committee (IACUC). We used a sensitive bitransgenic mouse model to measure the potential of radiation and/or oxygen to exacerbate lung carcinogenesis. This model was developed by our laboratory to conditionally express a mutant human Ki-rasG12C gene in a doxycycline (DOX) inducible and lung specific manner [3]. At 8 weeks of age, DOX is administered to the mice in their drinking water in order to induce the mutant Ki-ras expression. One week later, mice were irradiated as described below. DOX administration continued until euthanized 9 months post-irradiation. This process results in the formation of small, benign, early-stage lesions. All mice were maintained on an AIN-76A diet and drinking water, either with or without 500 mg/mL of DOX [15]. The lung morphology of CCSP/Ki-ras mice that do not receive DOX in their drinking water is normal [3].
iii. Radiation Delivery
A calibrated medical linear accelerator was used to deliver high energy photon radiation. An 18 MV photon beam was initially used until facility changes required the use of a 10 MV beam as the highest possible energy choice. The high energy beam was chosen to best simulate space radiation, which includes a moderate component of particle production [16]. A calibrated ion chamber, wrapped in appropriately thick bolus material to simulate the volume of a mouse, was utilized to verify a uniform dose distribution across the entire box (Figure 2). This is essential since the mice are divided into different compartments within the box. Ten cm of water-equivalent solid material was placed on top of the box to allow for the desired radiation dose buildup (secondary electron equilibrium) and to achieve a flat dose profile. Additionally, 5 cm of water-equivalent solid material was placed under the box to minimize any backscatter dose from a metal plate on the floor (Figure 3). Water-equivalent material is a solid manufactured to match the radiation characteristics of water while also being volumetrically similar.
FIG. 2.
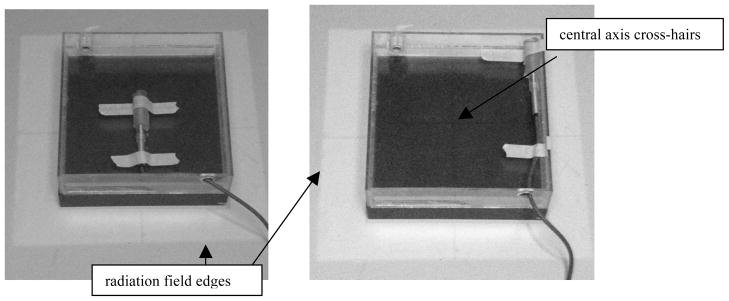
A calibrated ion chamber was used to verify the dosimetry on (left) and off (right) the central axis. The illuminated light field surrounding the chamber (representative of the radiation field) and the central axis cross-hairs (used for positioning) can be seen.
FIG. 3.
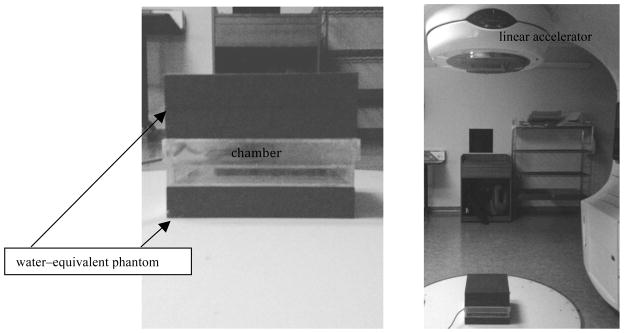
Left: The water-equivalent block configuration. Right: Our irradiation setup for the clinical linear accelerator.
The dose distribution was verified using a calibrated ion chamber wrapped in tissue-equivalent bolus material to approximate a mouse phantom in order to determine our central axis dose calibration factor and to verify off-axis dose uniformity. A variation in dose across the radiation field in an attenuating medium is to be expected from the “horn effect.” This effect is due to a linear accelerator’s flattening filter geometry and results in a variation of the dose rate in a given plane perpendicular to the incident radiation beam. For shallow depths, this variation leads to an increase in radiation beam intensity, or “hot spot,” at locations away from the central axis until the radiation field edge is approached. Since the beam profile flattens out at depth, 10 cm of solid water-equivalent material was placed on top of the chamber. The dose was measured at several locations (Figure 4) within the gas chamber to verify that the dose uniformity was within an acceptable tolerance of < 5% variation.
FIG. 4.
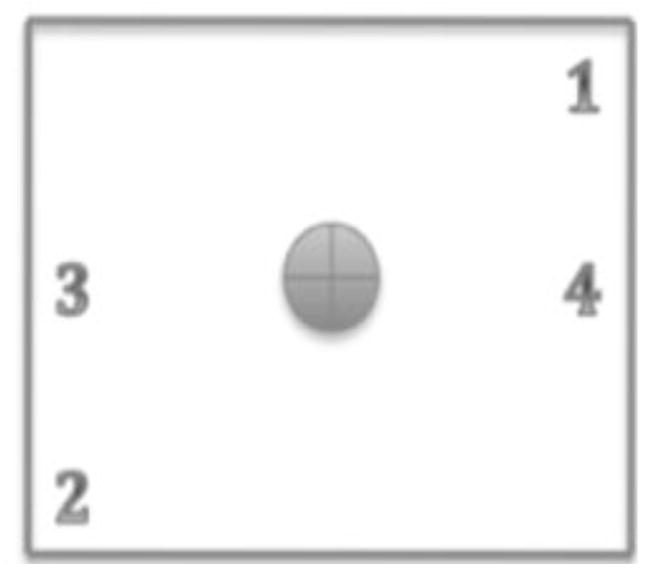
Illustration showing the locations where ion chamber measurement were made in the gas chambers to verify dose uniformity.
Once the dose distribution was verified, we began the process of irradiating 200 total mice at doses of 0, 80, 300, 600, and 1000 mGy, with either 100% oxygen or normal breathing air (20 mice in each group). Mice were placed in their individual compartments within the box and the lid sealed with a layer of petroleum jelly. The boxes were then incubated with the appropriate gas environment for 30 min prior to irradiation to ensure a uniform gas distribution. They were then transferred to the clinical linear accelerator room, where they were connected to the in-room oxygen/air supply and arranged in the correct geometry to match the dosimetry calculations. After irradiation with the prescribed dose, the box was re-connected to the appropriate gas canister for 2 h so that the gaseous environment was maintained during cellular repair. Control mice (0 mGy) were placed in the chamber with the appropriate gas environment for the same ~2.5 h, but remained unirradiated. After this irradiation process, all mice were housed in our Animal Research Program facilities in ALAC approved housing. After 9 months, the mice will be sacrificed, their lungs excised, and tumors counted and sized with digital calipers.
RESULTS & DISCUSSION
Table 1 shows the resulting dosimetry characteristics for our controlled-environment gas chambers. Note that the variation is < 3% which is within our acceptable tolerance of 5%. Monitor Units (MU) are the settings on a medical linear accelerator used to deliver a desired dose. Our linear accelerators are calibrated to deliver 1 cGy/MU to the isocenter at the depth of maximum dose for a 10 × 10 cm2 field in water with a radiation source to surface distance equal to 100 cm. From this and knowledge of a given geometry, dose at any depth and location can be calculated. This ensures that all the mice receive approximately the same dose of radiation. These experiments were all conducted with the chamber on the floor below the radiation source, i.e., the furthest possible distance from the source. The source to floor distance was 231 cm. This irradiation geometry allowed for a more uniform dose delivery to all six mice, while also providing for a low dose rate to mimic the radiation dose rate in space. The final linear accelerator irradiation parameters are given in Table 2.
Table 1.
Dose Uniformity Verification within the Chamber
| Calculated Dosimetry Data | ||
|---|---|---|
| Position | Dose Rate (mGy/MU) | Deviation from CAX |
| Central Axis (CAX) | 2.05 | ----- |
| Position 1 | 2.01 | 1.95% |
| Position 2 | 1.99 | 2.93% |
| Position 3 | 2.07 | 0.98% |
| Position 4 | 2.08 | 1.46% |
Table 2.
Irradiation Parameters
| Linac Parameters | |
|---|---|
| Beam Energy | 18 MV or 10 MV |
| Field Size | 20 × 20 cm2 (at isocenter) |
| Buildup Region | 10 cm water-equivalent material |
| Backscatter Region | 5 cm water-equivalent material |
| Dose | 0, 80, 300, 600, or 1000 mGy |
| Dose Rate | 410 mGy/min |
The linked ball valve system greatly improved the efficiency of running the experiment, as it permitted us to incubate up to four chambers simultaneously on the same gas tank. The flow rate of the oxygen or breathing air was set to ~10 L/min, and the levers for each valve were adjusted so that there was a noticeable and equal flow of gas leaving each exit port. Since the gas chambers are sealed besides this exit port, the flowing gas follows the path of least resistance and quickly saturates the chamber to nearly 100% concentration. All the chambers were flushed for 10 min with the appropriate gas to ensure that all of the initial gas was equilibrated for each exposure. Then the mice were quickly loaded (< 1 min) into their individual compartments, and the lids were sealed with a layer of petroleum jelly. Following a 30 min incubation period, one of the chambers was disconnected from the main tank (while the other three remained attached) and quickly moved (~3 min) to the linear accelerator room, where it was attached to the in-room gas supply. After irradiation, the chamber was transported back to the lab and reattached to the main tank, where it remained in the gas environment for 2 h to ensure that the desired gas conditions were present throughout the time needed for sufficient cellular repair after irradiation. The mice did not exhibit any outward physical signs of stress in response to breathing 100% oxygen. In assembly line fashion, the remaining chambers underwent the same process. The ability to separately disconnect individual boxes while maintaining the gas environment for the remaining chambers, all while utilizing the same gas tank, maximized our efficiency.
The methods described above successfully allowed for a controlled and uniform dose delivery to unanesthetized mice under normoxic and hyperoxic conditions. The next step of this research will examine the resulting number of lung tumors and their sizes in each mouse to determine if there is i] a radiation dose-dependent response and ii] if an increase in the carcinogenic potential exists in our model due to hyperoxic breathing conditions.
CONCLUSION
A practical device was developed to control murine breathing conditions during irradiation. This chamber could be easily constructed at any facility and adapted to individual needs and specifications. At our own facility, the dimensions of the chambers were easily modified to accommodate a similar project conducted with rats. A key advantage is that the mice are not anesthetized during irradiation. This produces a more natural, translatable environment for radiation studies that strive to mimic NASA extra-vehicular activities, while also removing the complications of an additional variable from the anesthesia. The next phase of this research will directly evaluate the comparative levels of carcinogenesis for this methodology and mouse model.
Acknowledgments
This study was supported by a National Cancer Institute supplement (NASA sponsored) R01-CA136910-S1 to NIH/NCI grant R01-CA136910, and a Wake Forest University Cancer Center Support Grant, P30-CA12197.
References
- 1.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231:440–5. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 2.Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floyd HS, Farnsworth CL, Kock ND, Mizesko MC, Little JL, Dance ST, et al. Conditional expression of the mutant Ki rasG12C allele results in formation of benign lung adenomas: Development of a novel mouse lung tumor model. Carcinogenesis. 2005;26:2196–206. doi: 10.1093/carcin/bgi190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munley MT, Moore JE, Walb MC, Isom SP, Olson JD, Zora JG, Kock ND, Wheeler KT, Miller MS. Cancer Prone Mice Expressing the Ki-rasG12C Gene Show Increased Lung Carcinogenesis Following CT Screening Procedures. Radiat Res. 2011;176:842–8. doi: 10.1667/rr2649.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li ZH, Zheng J, Weiss LM, Shibata D. c-K-ras and p53 mutations occur very early in adenocarcinoma of the lung. Am J Pathol. 1994;144:303–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Westra WH, Slebos RJ, Offerhaus GJ, Goodman SN, Evers SG, Kensler TW, et al. “K-ras oncogene activation in lung adenocarcinomas from former smokers”. Evidence that K-ras mutations are an early and irreversible event in the development of adenocarcinoma of the lung. Cancer. 1993;72:432–8. doi: 10.1002/1097-0142(19930715)72:2<432::aid-cncr2820720219>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Westra WH, Baas IO, Hruban RH, Askin FB, Wilson K, Offerhaus GJ, et al. K-ras oncogene activation in atypical alveolar hyperplasias of the human lung. Cancer Res. 1996;56:2224–8. [PubMed] [Google Scholar]
- 8.Reynolds SH, Wiest JS, Devereux TR, Anderson MW, You M. Protooncogene activation in spontaneously occurring and chemically induced rodent and human lung tumors. In: Klein-Szanto AJP, Anderson MW, Barrett JC, Slaga TJ, editors. Comparative molecular carcinogenesis. New York: Wiley-Liss; 1992. pp. 303–20. [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 10.Alberg AJ, Samet JM. Epidemiology of lung cancer. In: Kane MA, Bunn PA Jr, editors. Biology of lung cancer. New York: Marcel Dekker; 1998. pp. 11–51. [Google Scholar]
- 11.Taketo M, Schroeder AC, Hansen CT, et al. FVB/N: An inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci. 1991;88(6):2065–9. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dance-Barnes ST, Kock ND, Moore JE, Lin EY, Mosley LJ, D’Agostino RB, Jr, et al. Lung tumor promotion by curcumin. Carcinogenesis. 2009;30:1016–1023. doi: 10.1093/carcin/bgp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall EJ. Radiobiology for the Radiologist. 4. J.B. Lippincott Company; Philadelphia, PA: 1994. [Google Scholar]
- 14.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2011 Dec 16; doi: 10.1016/j.canlet.2011.12.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bieri JG, Stoewsand GS, Knapka JJ, et al. Report of the American Institution of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 16.LaBel KA. The Natural Space Radiation Hazard. 2000 [Online]. Available: http://radhome.gsfc.nasa.gov/radhome/Nat_Space_Rad_Haz.htm.


