Abstract
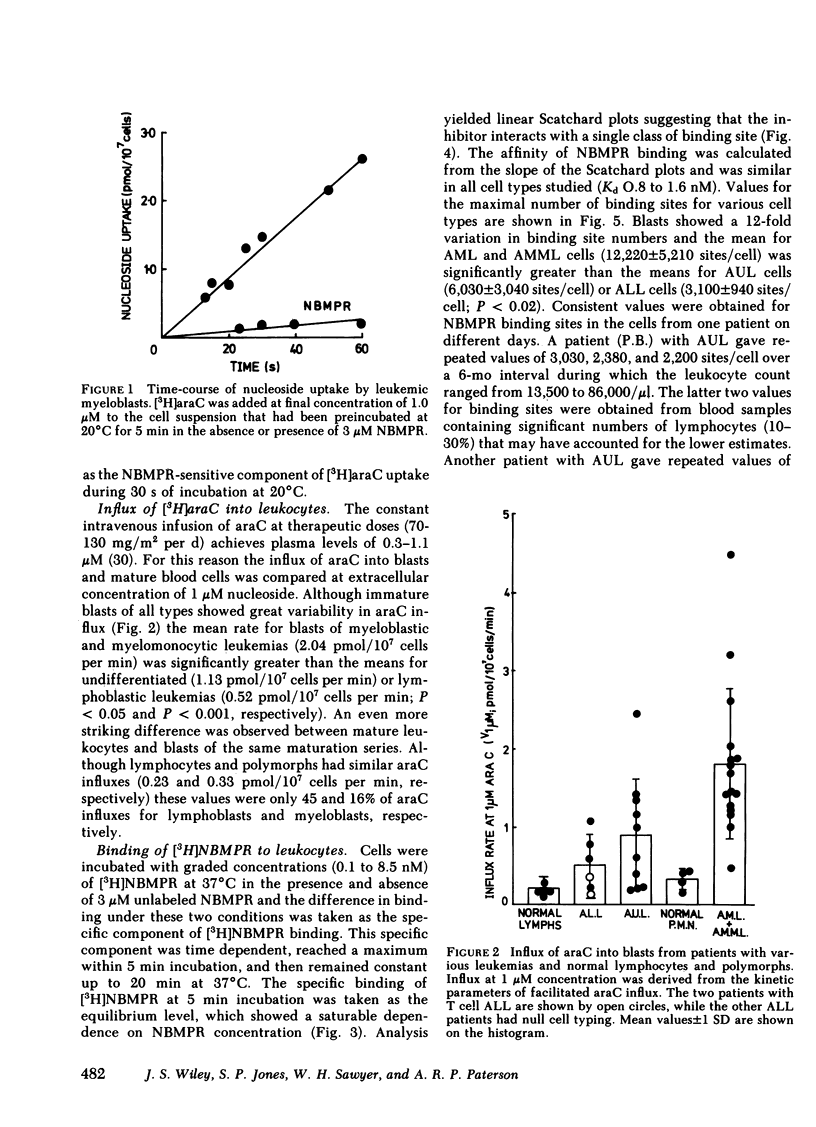
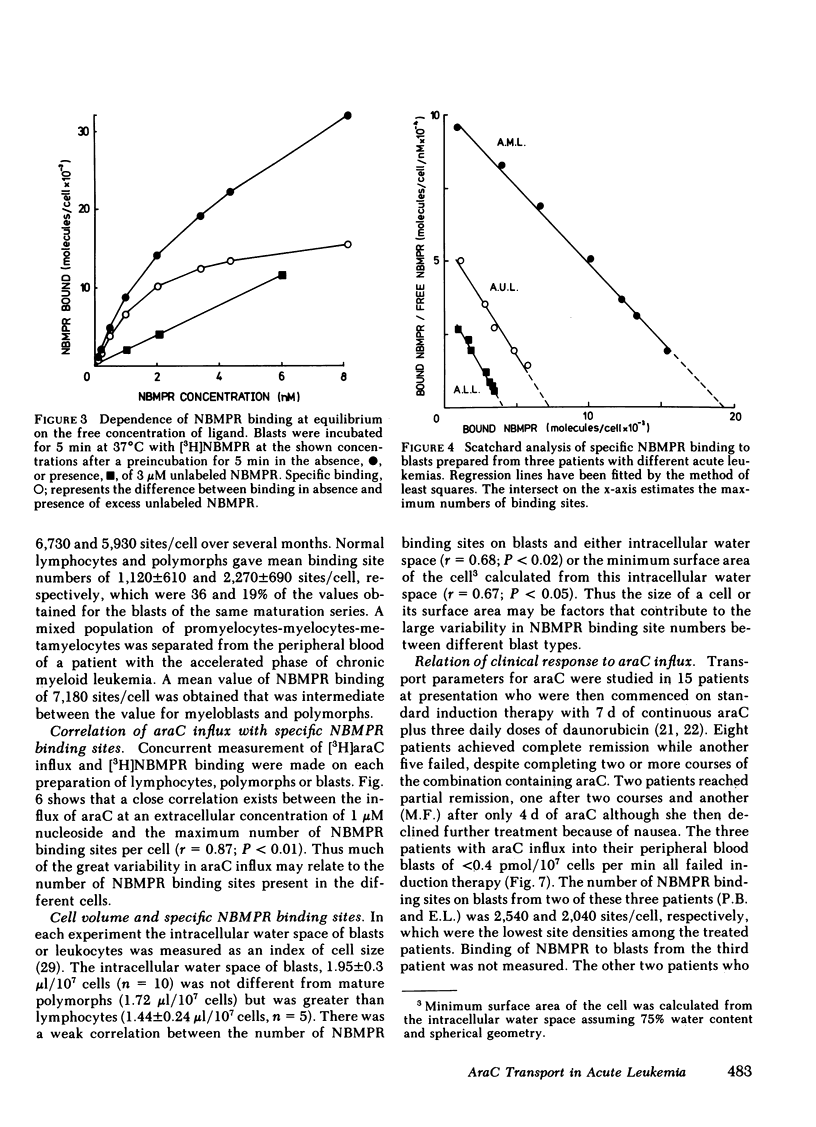
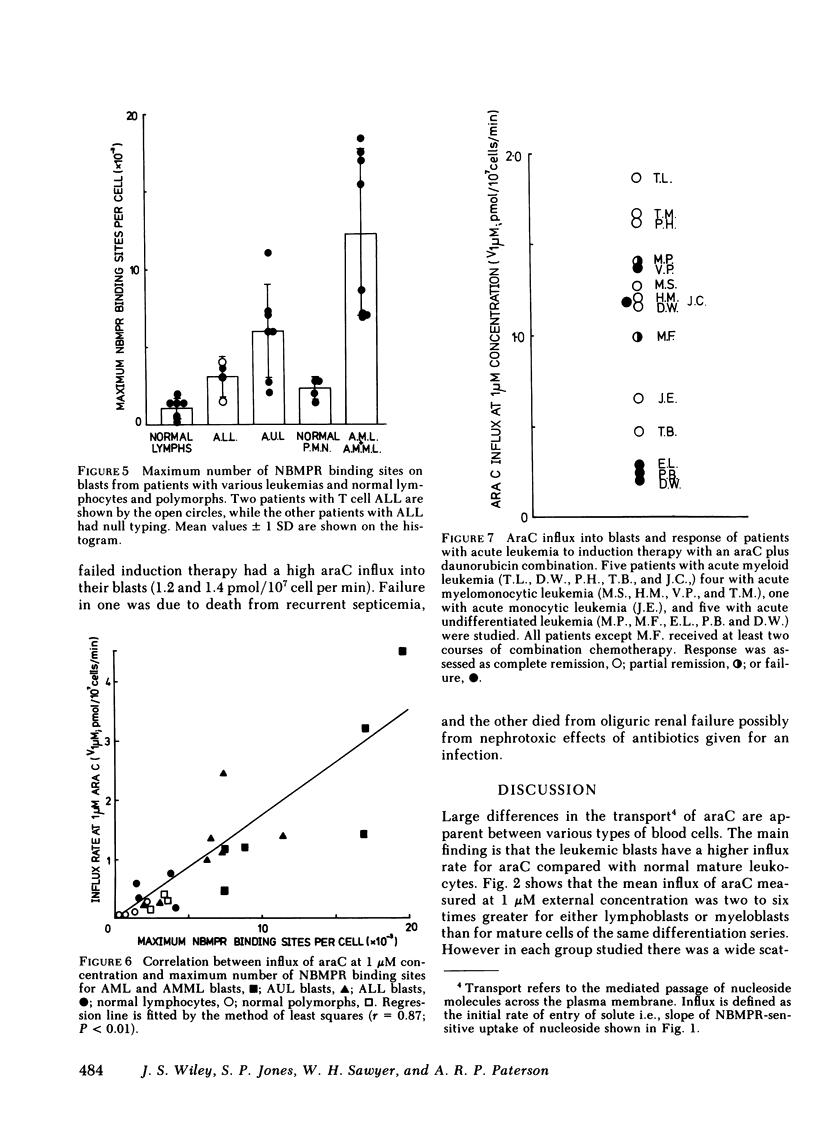
Although cytosine arabinoside (araC) can induce a remission in a majority of patients presenting with acute myeloblastic leukemia (AML), a minority fail to respond and moreover the drug has less effect in acute lymphoblastic leukemia (ALL). The carrier-mediated influx of araC into purified blasts from patients with AML, ALL, and acute undifferentiated leukemia (AUL) has been compared to that of normal lymphocytes and polymorphs. Blasts showed a larger mediated influx of araC than mature cells, since mean influxes for myeloblasts and lymphoblasts were 6- and 2.3-fold greater than polymorphs and lymphocytes, respectively. Also, the mean influx for myeloblasts was fourfold greater than the mean for lymphoblasts. The number of nucleoside transport sites was estimated for each cell type by measuring the equilibrium binding of [3H]nitrobenzylthioinosine (NBMPR), which inhibits nucleoside fluxes by binding with high affinity to specific sites on the transport mechanism. The mean binding site numbers for myeloblasts and lymphoblasts were 5- and 2.8-fold greater, respectively, than for the mature cells of the same maturation series. The mean number of NBMPR binding sites for myeloblasts was fourfold greater than for lymphoblasts. Patients with AUL were heterogeneous since blasts from some gave values within the myeloblastic range and others within the lymphoblastic range. The araC influx correlated closely with the number of NBMPR binding sites measured in the same cells on the same day. Transport parameters were measured on blasts from 15 patients with AML or AUL who were then treated with standard induction therapy containing araC. Eight patients entered complete remission, while seven failed therapy, among whom were the three patients with the lowest araC influx (<0.4 pmol/107 cells per min) and NBMPR binding (<3,000 sites/cell) for the treated group. In summary, myeloblasts have both higher araC transport rates and more nucleoside transport sites than lymphoblasts and this factor may contribute to the greater sensitivity of AML to this drug. AraC transport varied >10-fold between leukemic blasts and normal leukocytes, but transport capacity related directly to the number of nucleoside transport sites on the cell. Finally, low araC transport rates or few NBMPR binding sites on blasts were observed in a subset of patients with acute leukemia who failed to achieve remission with drug combinations containing araC.
Full text
PDF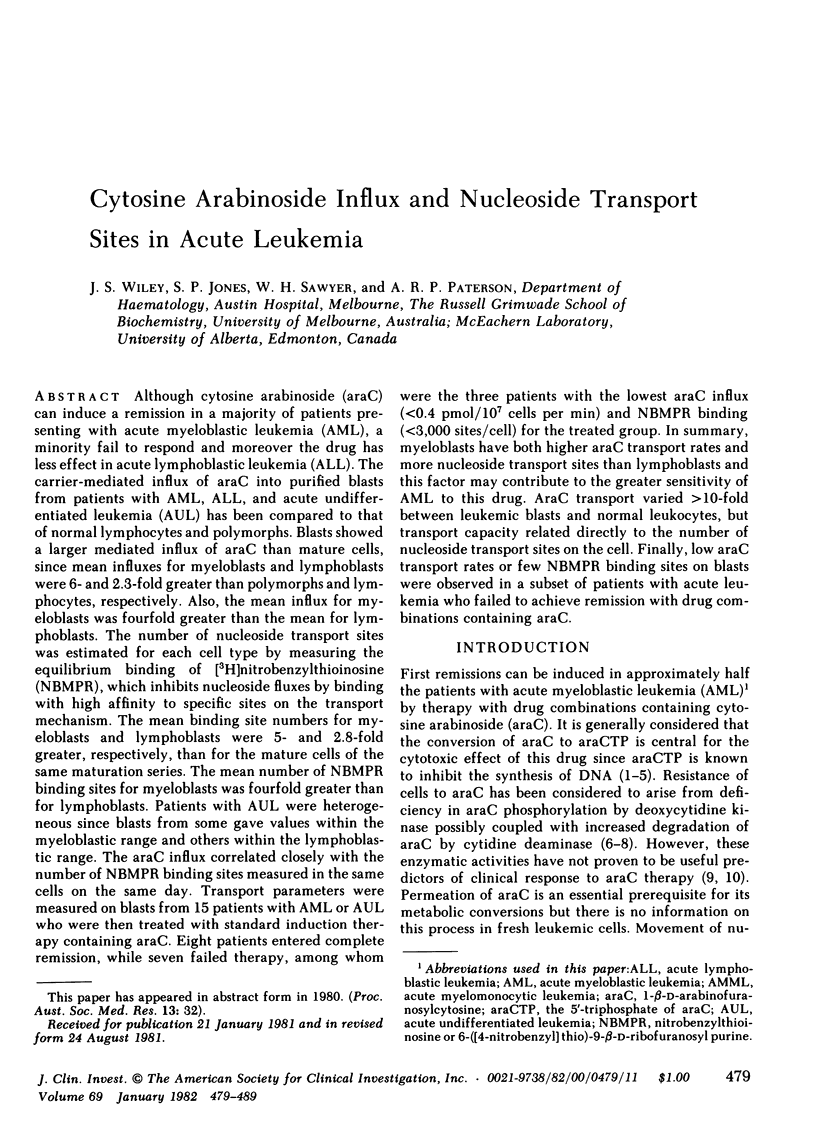






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. A., Schaumburg B. P., Osterline K., Vinten J., Gammeltoft S., Gliemann J. A rapid technique for separation of thymocytes from suspensions by centrifugation through silicone oil. Anal Biochem. 1974 Jun;59(2):610–616. doi: 10.1016/0003-2697(74)90314-5. [DOI] [PubMed] [Google Scholar]
- Arlin Z. A., Fried J., Clarkson B. D. Therapeutic role of cell kinetics in acute leukaemia. Clin Haematol. 1978 Jun;7(2):339–362. doi: 10.1016/s0308-2261(78)80009-5. [DOI] [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Bury R. W., Keary P. J. Determination of cytosine arabinoside in human plasma by high-pressure liquid chromatography. J Chromatogr. 1978 Sep 1;146(2):350–353. doi: 10.1016/s0378-4347(00)81902-7. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Dahlig E., Lau E. Y., Lynch T. P., Paterson A. R. Fluctuations in nucleoside uptake and binding of the inhibitor of nucleoside transport, nitrobenzylthioinosine, during the replication cycle of HeLa cells. Cancer Res. 1979 Apr;39(4):1245–1252. [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Cassileth P. A., Katz M. E. Chemotherapy for adult acute nonlymphocytic leukemia with daunorubicin and cytosine arabinoside. Cancer Treat Rep. 1977 Nov;61(8):1441–1445. [PubMed] [Google Scholar]
- Chou T. C., Arlin Z., Clarkson B. D., Phillips F. S. Metabolism of 1-beta-D-arabinofuranosylcytosine in human leukemic cells. Cancer Res. 1977 Oct;37(10):3561–3570. [PubMed] [Google Scholar]
- Cohen A., Ullman B., Martin D. W., Jr Characterization of a mutant mouse lymphoma cell with deficient transport of purine and pyrimidine nucleosides. J Biol Chem. 1979 Jan 10;254(1):112–116. [PubMed] [Google Scholar]
- Ellison R. R., Holland J. F., Weil M., Jacquillat C., Boiron M., Bernard J., Sawitsky A., Rosner F., Gussoff B., Silver R. T. Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults. Blood. 1968 Oct;32(4):507–523. [PubMed] [Google Scholar]
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- Fridland A. Inhibition of deoxyribonucleic acid chain initiation: a new mode of action for 1-beta-D-arabinofuranosylcytosine in human lymphoblasts. Biochemistry. 1977 Nov 29;16(24):5308–5312. doi: 10.1021/bi00643a023. [DOI] [PubMed] [Google Scholar]
- Gingrich R. D., Armitage J. O., Burns C. P. Treatment of adult acute lymphoblastic leukemia with cytosine arabinoside, vincristine, and prednisone. Cancer Treat Rep. 1978 Sep;62(9):1389–1391. [PubMed] [Google Scholar]
- Heichal O., Ish-Shalom D., Koren R., Stein W. D. The kinetic dissection of transport from metabolic trapping during substrate uptake by intact cells. Uridine uptake by quiescent and serum-activated Nil 8 hamster cells and their murine sarcoma virus-transformed counterparts. Biochim Biophys Acta. 1979 Feb 20;551(1):169–186. doi: 10.1016/0005-2736(79)90363-8. [DOI] [PubMed] [Google Scholar]
- Ho D. H., Carter C. J., Brown N. S., Hester J., McCredie K., Benjamin R. S., Freireich E. J., Bodey G. P. Effects of tetrahydrouridine on the uptake and metabolism of 1-beta-D-arabinofuranosylcytosine in human normal and leukemic cells. Cancer Res. 1980 Jul;40(7):2444–2446. [PubMed] [Google Scholar]
- Howard J. P., Albo V., Newton W. A., Jr Cytosine arabinoside. Results of a cooperative study in acute childhood leukemia. Cancer. 1968 Mar;21(3):341–345. doi: 10.1002/1097-0142(196803)21:3<341::aid-cncr2820210302>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Genetic control of nucleoside transport in sheep erythrocytes. Biochem Genet. 1978 Oct;16(9-10):1035–1043. doi: 10.1007/BF00483754. [DOI] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in human and sheep erythrocytes. Evidence that nitrobenzylthioinosine binds specifically to functional nucleoside-transport sites. Biochem J. 1980 Aug 15;190(2):377–383. doi: 10.1042/bj1900377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez de Asua L., Rozengurt E. Multiple control mechanisms underlie initiation of growth in animal cells. Nature. 1974 Oct 18;251(5476):624–626. doi: 10.1038/251624a0. [DOI] [PubMed] [Google Scholar]
- Kessel D., Hall T. C., Rosenthal D. Uptake and phosphorylation of cytosine arabinoside by normal and leukemic human blood cells in vitro. Cancer Res. 1969 Feb;29(2):459–463. [PubMed] [Google Scholar]
- Killmann S. A. Proliferative activity of blast cells in leukemia and myelofibrosis. Morphological differences between proliferating and non-proliferating blast cells. Acta Med Scand. 1965 Sep;178(3):263–280. doi: 10.1111/j.0954-6820.1965.tb04271.x. [DOI] [PubMed] [Google Scholar]
- Koren R., Shohami E., Yeroushalmi S. A kinetic analysis of the uptake of cytosine-beta-D-arabinoside by rat-B77 cells. Differentiation between transport and phosphorylation. Eur J Biochem. 1979 Apr 2;95(2):333–339. doi: 10.1111/j.1432-1033.1979.tb12970.x. [DOI] [PubMed] [Google Scholar]
- Lauzon G. J., Paran J. H., Paterson A. R. Formation of 1-beta-D-arabinofuranosylcytosine diphosphate choline in cultured human leukemic RPMI 6410 cells. Cancer Res. 1978 Jun;38(6):1723–1729. [PubMed] [Google Scholar]
- Lauzon G. J., Paterson A. R. Binding of the nucleoside transport inhibitor nitrobenzylthioinosine to HeLa cells. Mol Pharmacol. 1977 Sep;13(5):883–891. [PubMed] [Google Scholar]
- Lieu T. S., Hudson R. A., Brown R. K., White B. C. Transport of pyrimidine nucleosides across human erythrocyte membranes. Biochim Biophys Acta. 1971 Sep 14;241(3):885–893. [PubMed] [Google Scholar]
- MAUER A. M., FISHER V. Comparison of the proliferative capacity of acute leukaemia cells in bone marrow and blood. Nature. 1962 Mar 17;193:1085–1086. doi: 10.1038/1931085a0. [DOI] [PubMed] [Google Scholar]
- Madsen N. P. Use of toluene/triton X-100 scintillation mixture for counting C14-protein radioactivity. Anal Biochem. 1969 Jun;29(3):542–544. doi: 10.1016/0003-2697(69)90341-8. [DOI] [PubMed] [Google Scholar]
- Ohnuma T., Arkin H., Minowada J., Holland J. F. Differential chemotherapeutic susceptibility of human T-lymphocytes and B-lymphocytes in culture. J Natl Cancer Inst. 1978 Apr;60(4):749–752. doi: 10.1093/jnci/60.4.749. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Babb L. R., Paran J. H., Cass C. E. Inhibition by nitrobenzylthioinosine of adenosine uptake by asynchronous HeLa cells. Mol Pharmacol. 1977 Nov;13(6):1147–1158. [PubMed] [Google Scholar]
- Paterson A. R., Lau E. Y., Dahlig E., Cass C. E. A common basis for inhibition of nucleoside transport by dipyridamole and nitrobenzylthioinosine? Mol Pharmacol. 1980 Jul;18(1):40–44. [PubMed] [Google Scholar]
- Paterson A. R., Naik S. R., Cass C. E. Inhibition of uridine uptake in HeLa cells by nitrobenzylthioinosine and related compounds. Mol Pharmacol. 1977 Nov;13(6):1014–1023. [PubMed] [Google Scholar]
- Paterson A. R., Paran J. H., Yang S., Lynch T. P. Protection of mice against lethal dosages of nebularine by nitrobenzylthioinosine, an inhibitor of nucleoside transport. Cancer Res. 1979 Sep;39(9):3607–3611. [PubMed] [Google Scholar]
- Paterson A. R., Yang S. E., Lau E. Y., Cass C. E. Low specificity of the nucleoside transport mechanism of RPMI 6410 cells. Mol Pharmacol. 1979 Nov;16(3):900–908. [PubMed] [Google Scholar]
- Paul B., Chen M. F., Paterson A. R. Inhibitors of nucleoside transport. A structure-activity study using human erythrocytes. J Med Chem. 1975 Oct;18(10):968–973. doi: 10.1021/jm00244a003. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Marz R., Wohlhueter R. M. Transport and metabolism of deoxycytidine and 1-beta-D-arabinofuranosylcytosine into cultured Novikoff rat hepatoma cells, relationship to phosphorylation, and regulation of triphosphate synthesis. Cancer Res. 1978 Apr;38(4):978–989. [PubMed] [Google Scholar]
- Rivera G., Aur R. J., Dahl G. V., Pratt C. B., Wood A., Avery T. L. Combined VM-26 and cytosine arabinoside in treatment of refractory childhood lymphocytic leukemia. Cancer. 1980 Mar 15;45(6):1284–1288. doi: 10.1002/1097-0142(19800315)45:6<1284::aid-cncr2820450604>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustum Y. M., Preisler H. D. Correlation between leukemic cell retention of 1-beta-D-arabinofuranosylcytosine 5'-triphosphate and response to therapy. Cancer Res. 1979 Jan;39(1):42–49. [PubMed] [Google Scholar]
- Shumak K. H., Baker M. A., Taub R. N., Coleman M. S. Myeloblastic and lymphoblastic markers in acute undifferentiated leukemia and chronic myelogenous leukemia in blast crisis. Cancer Res. 1980 Nov;40(11):4048–4052. [PubMed] [Google Scholar]
- Smyth J. F., Robins A. B., Leese C. L. The metabolism of cytosine arabinoside as a predictive test for clinical response to the drug in acute myeloid leukaemia. Eur J Cancer. 1976 Jul;12(7):567–573. doi: 10.1016/0014-2964(76)90164-x. [DOI] [PubMed] [Google Scholar]
- Steuart C. D., Burke P. J. Cytidine deaminase and the development of resistance to arabinosyl cytosine. Nat New Biol. 1971 Sep 22;233(38):109–110. doi: 10.1038/newbio233109a0. [DOI] [PubMed] [Google Scholar]
- Tattersall M. H., Ganeshaguru K., Hoffbrand A. V. Mechanisms of resistance of human acute leukaemia cells to cytosine arabinoside. Br J Haematol. 1974 May;27(1):39–46. doi: 10.1111/j.1365-2141.1974.tb06772.x. [DOI] [PubMed] [Google Scholar]
- Traggis D. G., Dohlwitz A., Das L., Jaffe N., Moloney W. C., Hall T. C. Cytosine arabinoside in acute leukemia of childhood. Cancer. 1971 Oct;28(4):815–818. doi: 10.1002/1097-0142(1971)28:4<815::aid-cncr2820280402>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Wantzin G. L., Karle H., Killmann S. A. Nuclear DNA-polymerase estimation in human leukaemic myeloblasts. Br J Haematol. 1976 Jul;33(3):329–334. doi: 10.1111/j.1365-2141.1976.tb03548.x. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Kraft N., Cooper I. A. The binding of ouabain to normal and chronic lymphocytic leukemic lymphocytes. Blood. 1979 Nov;54(5):994–1000. [PubMed] [Google Scholar]
- Woodcock D. M., Fox R. M., Cooper I. A. Evidence for a new mechanism of cytotoxicity of 1-beta-D arabinofuranosylcytosine. Cancer Res. 1979 Apr;39(4):1418–1424. [PubMed] [Google Scholar]
- Woodruff R. The management of adult acute lymphoblastic leukaemia. Cancer Treat Rev. 1978 Jun;5(2):95–113. doi: 10.1016/s0305-7372(78)80009-7. [DOI] [PubMed] [Google Scholar]
- Yates J. W., Wallace H. J., Jr, Ellison R. R., Holland J. F. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973 Nov-Dec;57(4):485–488. [PubMed] [Google Scholar]


