Abstract
Computed tomography-guided percutaneous needle biopsy of the lung is an indispensable tool in the evaluation of pulmonary abnormalities due to its high diagnostic accuracy in the detection of malignancy. Percutaneous biopsy in the lung plays a critical role in obtaining pathologic proof of malignancy, guiding staging and planning treatment. This article reviews biopsy techniques and their related efficacy and complications.
Keywords: interventional radiology, lung cancer, biopsy, percutaneous
Objectives: Upon completion of this article, the reader will be able to discuss the indications for percutaneous lung biopsies, identify the technical factors important in performing such a procedure, and explain the complications associated with the procedure.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Percutaneous needle biopsy via computed tomography (CT) is an indispensable tool in the evaluation of pulmonary abnormalities because of its high diagnostic accuracy, sensitivity, and specificity for the detection of malignancy. Needle biopsy in the lung plays a critical role in obtaining pathologic proof of malignancy, guiding staging and planning treatment.
Biopsy Technique
Biopsy Needles
Many options are available for performing percutaneous needle biopsies including aspiration needles and cutting needles. Needle choice is based on the size of the lesion, intended needle trajectory, information sought from the pathologic sample, and operator preference. Some of the more commonly used fine-needle aspiration (FNA) devices include Chiba (Cook Medical, Inc., Bloomington, IN), Franseen (Cook), Westcott (Angiotech, Vancouver, BC, Canada), MaxiCELL (Angiotech, Vancouver, BC, Canada), Greene (Cook), and Turner (Cook) needles. The Chiba, Franseen, Greene, and Turner needles have circumferentially sharpened tips allowing for sampling; the Westcott needle has a beveled side cutting notch (Fig. 1). Core biopsy needles are designed to collect a small piece of tissue intended for surgical pathology analysis rather than cytologic evaluation. Core biopsy needles are designed as end-cutting or side-cutting devices. The most commonly used core biopsy needle is the Tru-Cut (Baxter Healthcare, Deerfield, IL), which consists of an outer cutting cannula and an inner slotted stylet. The Temno (CareFusion, Waukegan, IL) core biopsy device is similar to the Tru-Cut device and is also a commonly used automatic core biopsy needle. The Biopince full core (Inter-V, Gainesville, FL) is an automated end-cutting needle that produces a full cylindrical core specimen.
Figure 1.
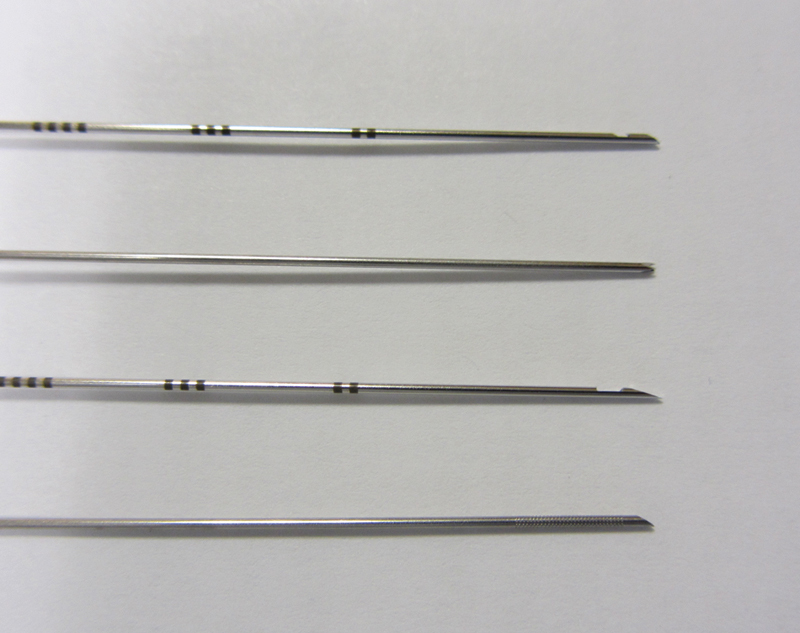
Small sampling of various biopsy needles. Top to bottom: MaxiCELL (Angiotech, Vancouver, BC, Canada), Franseen (Cook Medical, Inc., Bloomington, IN), Westcott (Angiotech), and Chiba (Cook).
The decision to perform core biopsy or both core biopsy and FNA is multifactorial and highly institution/operator dependent. FNA biopsy has a relatively high sensitivity of 82 to 99%, specificity of 86 to 100%, and accuracy of 64 to 97% for the diagnosis of malignancy.1,2 However, a definitive benign diagnosis can only be made in 20 to 50% of cases.1,2 Obtaining a core biopsy specimen increases the rate of a definite benign diagnosis from 52% to 91%.1,3,4,5,6,7 Core histologic material also allows for characterization of different cell types and is especially useful in lymphoproliferative disorders. Although performing core biopsy improves the diagnostic yield of benign diagnosis, there is a slightly higher rate of complications such as pneumothorax and pulmonary hemorrhage.1,6,8 Small lesions <10 mm are more likely to result in a false negative for malignancy, which may suggest an increased utility of core biopsy in those cases (even in the presence of a higher rate of pneumothorax).9 The risk of complications and the diagnostic yield should be considered on a case-by-case basis when choosing a biopsy technique for the safest and most accurate diagnosis. Fig. 2 demonstrates an example of a typical CT-guided lung biopsy.
Figure 2.
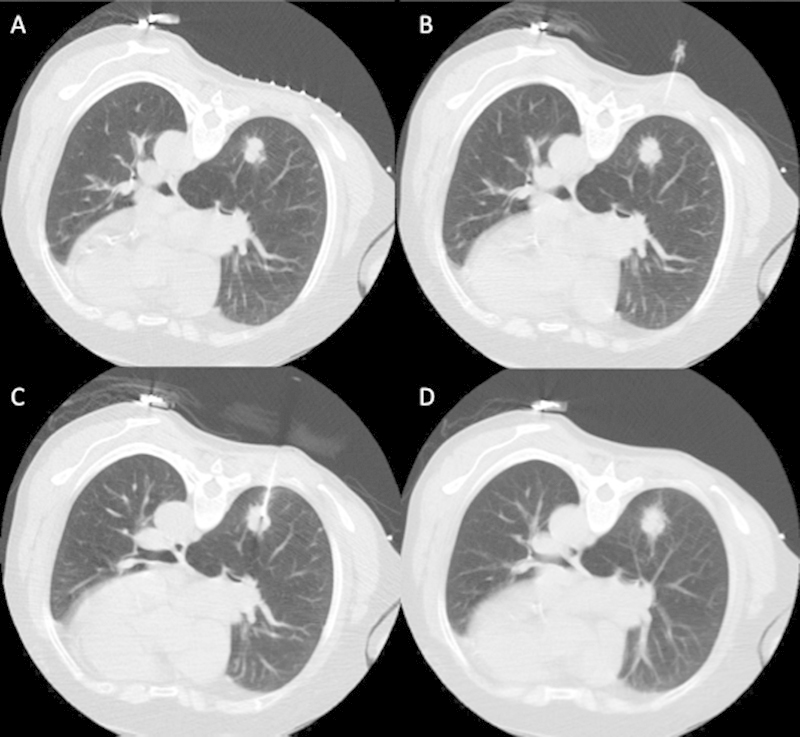
(A) Example of a normal lung biopsy in a patient with a right lower lobe lung nodule showing the preprocedure scan with a grid on the skin. (B) A 25-gauge needle in the subcutaneous tissues following lidocaine injection. (C) The outer cannula of a coaxial biopsy device within the lesion. (D) A postbiopsy image through the region of biopsy.
Patient Selection and Preprocedural Evaluation
Prior to performing a lung biopsy, all patients should have a CT or positron emission tomography/CT scan. If preprocedural imaging demonstrates extrapulmonary disease that is accessible, biopsy of those lesions may be performed to determine both the diagnosis and staging of disease. Imaging also aids in determining the safest approach for biopsy. Bronchoscopy is preferred for central endobronchial or peribronchial lesions, whereas surgical biopsy is usually performed for suspected interstitial lung disease.
Percutaneous biopsy should be performed to establish a malignant diagnosis, establish a benign diagnosis, or obtain material for culture. Imaging findings in the lung can have a broad differential diagnosis; therefore, biopsy of the lung is indicated for indeterminate pulmonary nodules or masses, and in patients with a history of extrapulmonary malignancy. As targeted therapies become a larger part of the armamentarium in the treatment of lung cancer, percutaneous needle biopsy of the lung may be performed to obtain tissue for molecular testing.
Relative contraindications to percutaneous lung biopsy include uncooperative patients, positive pressure ventilation, severe respiratory compromise, pulmonary artery hypertension, severe interstitial lung disease, small lesions close to the diaphragm, and central lesions adjacent to large vessels or bronchi. The platelet count, prothrombin time, international normalized ratio (INR), and activated partial thromboplastin time should be routinely checked prior to the procedure. Although local standards apply, according to recent consensus guidelines, INR should be corrected if >1.5, and transfusion should be performed for platelet counts <50,000/µL. Plavix should be held for 5 days prior to the procedure. Aspirin does not need to be held, but the last dose of low molecular weight heparin should be held prior to the procedure.10
Procedural Considerations
Moderate sedation is preferred during percutaneous lung biopsy because it allows the patient to lie motionless during the procedure and have regular respirations. Sedation can also be useful for patient anxiety and for elderly patients who have difficulty lying still due to pain. A balance must be reached when using sedatives because oversedation can result in irregular respiration increasing the difficulty of the biopsy. Intravenous midazolam and fentanyl are most commonly used for providing sedation.
Image guidance for biopsy is performed using CT or CT fluoroscopy for its clear visualization of pulmonary structures and abnormalities. Compared with conventional CT, CT fluoroscopy is faster and requires fewer needle passes, resulting in 27.1% shorter procedure times.11 CT fluoroscopy has also been associated with fewer complications compared with conventional CT, predominantly due to shorter procedure times and fewer needles passes.11,12 Heck et al12 showed a trend toward a lower pneumothorax rate in the CT-fluoroscopy group. The study by Kim et al11 showed a statistically significant decrease in pneumothorax rate from 27.1% to 11.1% when using CT fluoroscopy. However, CT fluoroscopy results in significantly increased radiation doses to both the patient and the radiologist, which may limit its widespread adoption.11,12,13 Radiation doses can be minimized by using the “quick check” method of CT fluoroscopy, which is what is most commonly used.14 Fig. 3 demonstrates images from a biopsy that used CT fluoroscopy with the “quick check” method of obtaining three images intermittently after advancement or repositioning of the needle.
Figure 3.
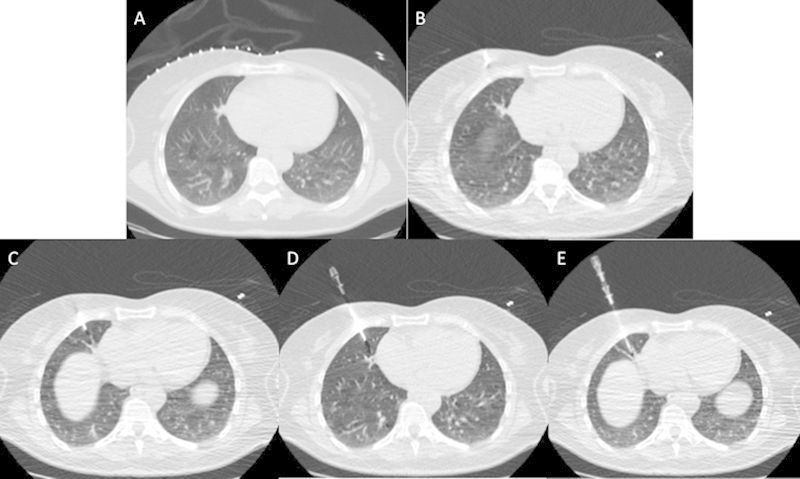
Example of a computed tomography (CT)-guided lung biopsy that utilized CT fluoroscopy with the “quick check” method. (A) The preprocedure planning CT with a grid on the patient's skin. (B) The needle within the subcutaneous tissues. (C-E) Sequential images showing advancement of the biopsy needle into the lesion of interest.
Patient positioning should be chosen based on the location of the lesion, size of the lesion, and the patient's ability to tolerate positioning. The prone position is preferred because the posterior ribs move less than the anterior ribs, the posterior intercostal spaces are wider than the anterior intercostal spaces, and prone positioning prevents the patient from visualizing the needle during the procedure, which may decrease anxiety. The oblique and decubitus positions are less desirable because they are not as stable, but they can be utilized if necessary for lateral subpleural lesions.
Once the patient is positioned on the procedure table, an access route is planned using CT images. The important factors in choosing an access route include avoiding chest wall vessels such as subclavian, internal mammary, intercostal, and intrapulmonary vessels. It is also important to minimize pleural transgression by performing a single pleural puncture and avoiding fissures if possible. Large bullae should also be avoided. If biopsy is being performed for a lesion of mixed CT attenuation, biopsy should be targeted toward the solid component of the nodule or mass. Similarly, if there is central necrosis, biopsy should be directed at the wall of the lesion.
It is our belief that subpleural lesions should not be targeted directly, but a path transgressing some normal lung parenchyma should be chosen. Subpleural nodules are often pushed away by the biopsy needle or trocar, and thus a direct path may result in multiple pleural punctures. Fig. 4 demonstrates percutaneous biopsy of subpleural nodules in two different patients demonstrating both a direct approach and an approach transgressing normal lung parenchyma.
Figure 4.
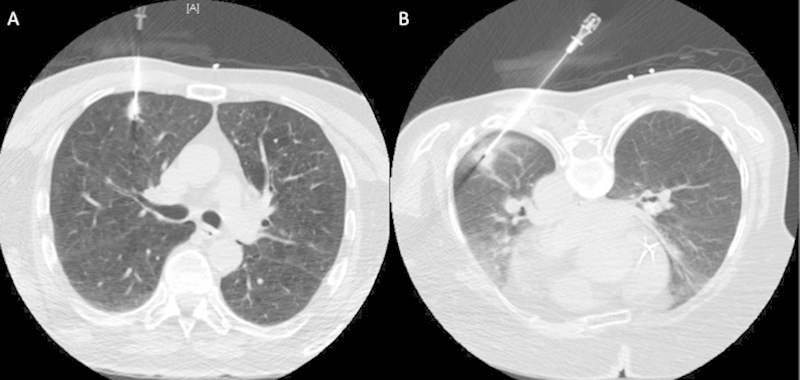
Example of biopsies of subpleural nodules in two different patients. (A) A direct path approach. (B) A path transgressing more normal lung parenchyma.
A coaxial technique is preferred at our institution to decrease the number of pleural punctures. However, one study showed no difference in complication rates of pneumothorax and pulmonary hemorrhage in cases using an outer cannula compared with those using a single-pass needle.15 Following adequate sedation and standard sterile precautions, the coaxial needle is advanced into the subcutaneous tissues of the chest wall incrementally to assure proper needle angulation and direction prior to pleural puncture. Pleural puncture should be performed in a single deliberate motion directed at the target lesion. If repositioning is necessary, the needle can usually be adjusted without exiting the lung. The outer cannula or introducer needle should be advanced into the peripheral aspect of the target lesion.
Once the position is confirmed with CT images, FNA can be performed using one of the aspiration needles described earlier. These samples should be immediately delivered to a cytotechnologist or cytopathologist on site for rapid interpretation of cellularity and presence of malignant cells. If a specific diagnosis cannot be obtained, repeat FNA or core biopsy can be performed through the introducer sheath based on patient risk as described previously. Core biopsy specimens should be placed directly in formalin for fixation. However, if there is a concern for a lymphoproliferative disorder, a sample should be placed in normal saline or Roswell Park Memorial Institute (RPMI) solution for flow cytometry.
Following removal of the biopsy needle, numerous techniques have been described in regard to sealing the biopsy tract, one of which is creating a blood patch with autologous venous blood. Several studies have demonstrated that injection of autologous blood clot into the biopsy tract results in a decreased rate of postbiopsy pneumothorax, reducing the need for treatment and hospitalization.16 One study showed that instillation of autologous blood clot in addition to aspiration in patients with postbiopsy pneumothorax decreased the need for chest tube placement.17 However, several older studies failed to demonstrate the added utility of a blood patch technique.18,19 We do not routinely use the blood patch technique at our institution.
Following removal of the needles, it is advantageous to place the patient in a position with the biopsy side down. Talking, moving, and coughing should be discouraged to minimize increases in intrathoracic pressure that could result in a pneumothorax. The patient should be monitored in a recovery area for a minimum of 2 hours following the procedure. Our routine is to obtain upright chest radiographs immediately following the biopsy and 2 hours after biopsy to evaluate for pneumothorax.
If a pneumothorax occurs during or following a lung biopsy procedure, the air should be aspirated from the pleural space prior to removal of the needle. Aspiration of excess pleural air results in improved apposition of the visceral and parietal pleura, minimizing the need for chest tube insertion. One study showed that when a large amount of air (>670 mL) is aspirated postprocedure, it may be prudent to insert a chest tube.20
Chest tube insertion should be performed for symptomatic or enlarging pneumothoraces. If the pneumothorax develops intraprocedurally, it is wise to use the coaxial biopsy needle for access to the pleural space and placement of the chest tube. Otherwise, CT or fluoroscopic guidance can be used to place a small caliber (8 to 10F) pigtail tube as a chest tube in the anterior second to third intercostal space. Although the management of chest tubes varies considerable among different institutions, one algorithm involves starting with continuous low wall suction, converting to water seal after a 2-hour interval without recurrent pneumothorax, clamping the chest tube for 2 hours, and removing the tube if the patient remains asymptomatic after clamping and the pneumothorax does not recur.21,22 This requires inpatient admission to the hospital for observation and intermittent chest radiographs, but the patient can typically be discharged from the hospital within 24 hours.
In one study, 93% of patients requiring a chest tube could be managed safely as an outpatient without hospital admission by using a Heimlich valve.23 Patients with underlying emphysema of the lungs are more likely to undergo additional interventions or prolonged chest tube drainage, which may require inpatient admission for underwater suction and pain control, but many of these patients can be managed on an outpatient basis.23
Efficacy
Because screening for lung cancer with low-dose CT has been shown to reduce mortality, a minimally invasive technique of tissue sampling such as percutaneous CT-guided needle biopsy is an essential part of the workup algorithm. Thoracostomy performed for pulmonary nodules results in 18 to 50% of those nodules having a benign diagnosis, with a high relative morbidity and mortality.24,25,26 Percutaneous FNA is considered to have a very low false-positive rate of 0 to 0.2%, which imparts a high level of confidence in a positive biopsy result.27,28 However, the false-negative rate of FNA varies, occurring in 6 to 54% of biopsies.28,29,30,31 The sensitivity, specificity, and accuracy for FNA of pulmonary lesions is 82 to 99%, 86 to 100%, and 64 to 97%, respectively.1
Core biopsy has been shown to have a slightly higher overall sensitivity, specificity, and accuracy of 89%, 97%, and 93%, respectively.32 Performing a core biopsy compared with FNA also improves the definitive diagnosis of benign lesions from 52 to 91%.1,3,4,5,6,7 Other factors that decrease the accuracy of percutaneous lung biopsies include lesion size <10 to 15 mm, and lesions >5 cm that likely demonstrate necrosis.8,9,12 Decreased diagnostic accuracy for small lesions may be related to sampling error from increased difficulty localizing the lesion.8 The degree to which diagnostic accuracy is affected is variable among studies but associated with the number of biopsy samples. Tsukada et al33 found a 67% accuracy rate for lesions <10 mm and an average of 1.4 needle passes per lesion. Others found diagnostic accuracy rates of 84 to 88% for lesions <15 to 20 mm and 2.5 to 3 samples per lesion.6,8
Due to the increased complication rates and decreased diagnostic accuracy, core biopsy is not recommended for lesions <10 mm.34 A smaller decrease in diagnostic accuracy is seen with larger necrotic lesions (>5 cm), with an accuracy rate of 93%.8 When performing a biopsy on such large lesions, it is important to have a preprocedural contrast-enhanced CT to be able to determine the necrotic portion of the lesion in advance and to target the biopsy at the nonnecrotic enhancing margin. Ultimately, the decision for obtaining fine-needle aspirates or core biopsy specimens is determined in close discussion with the pathologists/cytopathologists.
Immediate cytologic assessment on site in the Radiology Department is common practice at most institutions when performing a lung biopsy. Evaluation of both touch preparations of core biopsy samples and/or separate FNA samples result in fewer passes required for adequate diagnosis and improved diagnostic yield.9,20,35
Complications
Pneumothorax
Pneumothorax is the most common complication of CT-guided percutaneous lung biopsy, with a reported rate of 17 to 26.6% based in part on technique.22,36,37,38,39 The incidence of a pneumothorax requiring chest tube placement is much lower ranging from 1 to 14.2%.22,36,37,38,39 Although some are controversial, various factors have been associated with increased risk of pneumothorax such as lesion size, lesion depth, and the performing radiologist.39 Although some studies demonstrated a pneumothorax rate of 33 to 60% for lesions <2 cm, another study showed no difference in pneumothorax rate for lesions less than or greater than 2 cm.22,34,36,39,40 As described earlier, biopsy of smaller lesions is technically more difficult, requiring longer procedure times and potentially more needle adjustments that can result in higher complication risks. Lesion depth also affects pneumothorax rate, although this is debated in the literature.
Yeow et al39 demonstrated a significant increase in pneumothorax rates from 13% for lesions abutting the pleural surface to 29% for lesions where the needle traverses aerated lung, which is likely due to decreased stability of the needle in a short intrapulmonary course leading to pleural tears. One study showed a lower pneumothorax rate and a higher success rate for biopsy of subpleural lesions when a longer transpulmonary course was utilized.41 Others support that a needle path >4 cm is associated with a higher incidence of pneumothorax.22,36,37 Radiologist experience is the third major risk factor for pneumothorax, with a 17% pneumothorax rate for biopsies performed by experienced radiologists compared with 30% for the remainder of radiologists.39 Imaging findings and pulmonary function tests suggestive of chronic obstructive pulmonary disease increase the risk of pneumothorax to 47% from 7% in patients without suchfindings.42,43
The rate of pneumothorax is also increased by procedural factors such as increased number of pleural punctures and needle insertion at an angle other than perpendicular to the pleural surface.43 Factors such as prior surgery to the ipsilateral lung and prevention of traversing aerated lung for chest wall, mediastinal, pleural, or subpleural lesions are considered protective and associated with lower rates of pneumothorax.22,36,43 Table 1 illustrates the increased pneumothorax rates associated with particular findings.
Table 1. Known risk factors for pneumothorax following percutaneous lung biopsy and their effect on the pneumothorax rate.
| Pneumothorax risk | Pneumothorax rate (%) |
|---|---|
| Size <2 cm | 33-60 |
| Lesion depth >4 cm | 14-32 |
| Experienced radiologist | 17 |
| Inexperienced radiologist | 30 |
| Imaging findings of chronic obstructive pulmonary disease | 47 |
Pulmonary Hemorrhage
Pulmonary hemorrhage is the second most common complication of percutaneous lung biopsy at a rate of 4 to 27%.36,39 CT imaging findings of perilesional ground-glass opacity representing hemorrhage occurs in 27 to 30% of patients. Hemoptysis occurs in closer to 4% of patients.36,39 Higher bleeding rates are associated with smaller lesions (<2 cm), longer needle paths (>4 cm), absence of pleural effusion, and multiple pleural punctures.36,39 Higher rates of bleeding are thought to occur with smaller lesions because of the increased technical difficulty requiring more needle corrections and longer dwell times.36 Other factors, such as needle size, number of biopsy specimens, pleural puncture site, positioning after needle biopsy, location of the lung lesions, patient age, and CT evidence of emphysema, do not appear to be associated with an increased risk of bleeding.36,39 Intrapulmonary hemorrhage may be protective against the development of pneumothorax, effectively sealing the biopsy tract.36,44 Although bleeding can be worrisome to patients, especially if it results in hemoptysis, 86% of pulmonary bleeding is minor alveolar hemorrhage along the needle tract only visible as ground-glass opacity on CT, and it is rarely life threatening.39
Preprocedural patient selection and planning is extremely important in minimizing the complications of hemorrhage. Anticoagulants should be held as described in the preprocedural evaluation section. Central and large pulmonary vessels should be avoided when planning a trajectory for the biopsy needle so as to avoid bleeding complications. If the patient develops significant hemoptysis, the procedure should be terminated immediately. Once the needles are removed, the patient should be placed in a position with the biopsy side down.
Air Embolism
Air embolism is an extremely rare but life-threatening complication of lung biopsy, occurring in ~0.061% of cases.45 Air embolism occurs when the needle traverses or enters into a pulmonary vein and a negative pressure gradient from inspiration sucks air into the biopsy needle. Symptoms of air embolism include arrhythmias, hypoxia, cardiovascular collapse, and rarely stroke. In an attempt to minimize air embolism, the biopsy needle should always be occluded by the inner stylet, drops of saline, or a finger. If air embolism is suspected, hemodynamic support should be initiated, the patient should immediately be placed in the left lateral decubitus position to relieve the air lock in the right side of the heart, oxygen should be administered, and further air entry should be prevented.46
Tumor Seeding
Tumor seeding of the lung, pleura, or chest wall is exceeding rare, occurring in 0.01 to 0.06% of patients undergoing percutaneous lung biopsy.45,47
Conclusion
Percutaneous CT-guided lung biopsy is an effective, highly accurate, and safe method of obtaining tissue for the diagnosis of indeterminate pulmonary lesions. Appropriate preprocedural planning, patient preparation, and adherence to strict procedural routine can minimize the risks associated with lung biopsy. However, it is important to counsel patients appropriately in advance of the procedure regarding their individual procedural risk of complications such as pneumothorax and pulmonary hemorrhage with regard to their lesion, presence or absence of underlying lung disease, and local expertise.
References
- 1.Beslic S, Zukic F, Milisic S. Percutaneous transthoracic CT guided biopsies of lung lesions; fine needle aspiration biopsy versus core biopsy. Radiol Oncol. 2012;46(1):19–22. doi: 10.2478/v10019-012-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connor S, Dyer J, Guest P. Image-guided automated needle biopsy of 106 thoracic lesions: a retrospective review of diagnostic accuracy and complication rates. Eur Radiol. 2000;10(3):490–494. doi: 10.1007/s003300050082. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa H, Nakajima Y, Kurihara Y, Niimi H, Ishikawa T. CT-guided transthoracic needle biopsy: a comparison between automated biopsy gun and fine needle aspiration. Clin Radiol. 1996;51(7):503–506. doi: 10.1016/s0009-9260(96)80191-7. [DOI] [PubMed] [Google Scholar]
- 4.Boiselle P M, Shepard J A, Mark E J. et al. Routine addition of an automated biopsy device to fine-needle aspiration of the lung: a prospective assessment. AJR Am J Roentgenol. 1997;169(3):661–666. doi: 10.2214/ajr.169.3.9275873. [DOI] [PubMed] [Google Scholar]
- 5.Haramati L B. CT-guided automated needle biopsy of the chest. AJR Am J Roentgenol. 1995;165(1):53–55. doi: 10.2214/ajr.165.1.7785631. [DOI] [PubMed] [Google Scholar]
- 6.Klein J S, Salomon G, Stewart E A. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology. 1996;198(3):715–720. doi: 10.1148/radiology.198.3.8628859. [DOI] [PubMed] [Google Scholar]
- 7.Goralnik C H, O'Connell D M, el Yousef S J, Haaga J R. CT-guided cutting-needle biopsies of selected chest lesions. AJR Am J Roentgenol. 1988;151(5):903–907. doi: 10.2214/ajr.151.5.903. [DOI] [PubMed] [Google Scholar]
- 8.Yeow K-M, Tsay P-K, Cheung Y-C, Lui K-W, Pan K-T, Chou A S-B. Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol. 2003;14(5):581–588. doi: 10.1097/01.rvi.0000071087.76348.c7. [DOI] [PubMed] [Google Scholar]
- 9.Montaudon M, Latrabe V, Pariente A, Corneloup O, Begueret H, Laurent F. Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol. 2004;14(7):1234–1240. doi: 10.1007/s00330-004-2250-3. [DOI] [PubMed] [Google Scholar]
- 10.Patel I J, Davidson J C, Nikolic B. et al. Standards of Practice Committee, with Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Endorsement . Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim G R, Hur J, Lee S M. et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol. 2011;21(2):232–239. doi: 10.1007/s00330-010-1936-y. [DOI] [PubMed] [Google Scholar]
- 12.Heck S L, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol. 2006;16(6):1387–1392. doi: 10.1007/s00330-006-0152-2. [DOI] [PubMed] [Google Scholar]
- 13.Prosch H, Stadler A, Schilling M. et al. CT fluoroscopy-guided vs. multislice CT biopsy mode-guided lung biopsies: accuracy, complications and radiation dose. Eur J Radiol. 2012;81(5):1029–1033. doi: 10.1016/j.ejrad.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 14.Paulson E K, Sheafor D H, Enterline D S, McAdams H P, Yoshizumi T T. CT fluoroscopy—guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001;220(1):161–167. doi: 10.1148/radiology.220.1.r01jl29161. [DOI] [PubMed] [Google Scholar]
- 15.Küçük C U, Yilmaz A, Yilmaz A, Akkaya E. Computed tomography-guided transthoracic fine-needle aspiration in diagnosis of lung cancer: a comparison of single-pass needle and multiple-pass coaxial needle systems and the value of immediate cytological assessment. Respirology. 2004;9(3):392–396. doi: 10.1111/j.1440-1843.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 16.Lang E K, Ghavami R, Schreiner V C, Archibald S, Ramirez J. Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology. 2000;216(1):93–96. doi: 10.1148/radiology.216.1.r00jl3293. [DOI] [PubMed] [Google Scholar]
- 17.Wagner J M, Hinshaw J L, Lubner M G. et al. CT-guided lung biopsies: pleural blood patching reduces the rate of chest tube placement for postbiopsy pneumothorax. AJR Am J Roentgenol. 2011;197(4):783–788. doi: 10.2214/AJR.10.6324. [DOI] [PubMed] [Google Scholar]
- 18.Bourgouin P M, Shepard J A, McLoud T C, Spizarny D L, Dedrick C G. Transthoracic needle aspiration biopsy: evaluation of the blood patch technique. Radiology. 1988;166(1 Pt 1):93–95. doi: 10.1148/radiology.166.1.3336708. [DOI] [PubMed] [Google Scholar]
- 19.Herman S J, Weisbrod G L. Usefulness of the blood patch technique after transthoracic needle aspiration biopsy. Radiology. 1990;176(2):395–397. doi: 10.1148/radiology.176.2.2367653. [DOI] [PubMed] [Google Scholar]
- 20.Yamagami T, Kato T, Hirota T, Yoshimatsu R, Matsumoto T, Nishimura T. Usefulness and limitation of manual aspiration immediately after pneumothorax complicating interventional radiological procedures with the transthoracic approach. Cardiovasc Intervent Radiol. 2006;29(6):1027–1033. doi: 10.1007/s00270-005-0368-6. [DOI] [PubMed] [Google Scholar]
- 21.Brown K T, Brody L A, Getrajdman G I, Napp T E. Outpatient treatment of iatrogenic pneumothorax after needle biopsy. Radiology. 1997;205(1):249–252. doi: 10.1148/radiology.205.1.9314993. [DOI] [PubMed] [Google Scholar]
- 22.Covey A M, Gandhi R, Brody L A, Getrajdman G, Thaler H T, Brown K T. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(5):479–483. doi: 10.1097/01.rvi.0000124951.24134.50. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Hicks M E, Wallace M J, Ahrar K, Madoff D C, Murthy R. Outpatient management of postbiopsy pneumothorax with small-caliber chest tubes: factors affecting the need for prolonged drainage and additional interventions. Cardiovasc Intervent Radiol. 2008;31(2):342–348. doi: 10.1007/s00270-007-9250-z. [DOI] [PubMed] [Google Scholar]
- 24.Crestanello J A, Allen M S, Jett J R. et al. Thoracic surgical operations in patients enrolled in a computed tomographic screening trial. J Thorac Cardiovasc Surg. 2004;128(2):254–259. doi: 10.1016/j.jtcvs.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Midthun D E. Solitary pulmonary nodule: time to think small. Curr Opin Pulm Med. 2000;6(4):364–370. doi: 10.1097/00063198-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Allen M S Darling G E Pechet T T et al. ACOSOG Z0030 Study Group Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial Ann Thorac Surg 20068131013–1019.; discussion 1019-1020 [DOI] [PubMed] [Google Scholar]
- 27.Gelbman B D, Cham M D, Kim W. et al. Radiographic and clinical characterization of false negative results from CT-guided needle biopsies of lung nodules. J Thorac Oncol. 2012;7(5):815–820. doi: 10.1097/JTO.0b013e31824abd9c. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber G McCrory D C Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence Chest 2003123(1, Suppl):115S–128S. [DOI] [PubMed] [Google Scholar]
- 29.Savage C, Walser E M, Schnadig V, Woodside K J, Ustuner E, Zwischenberger J B. Transthoracic image-guided biopsy of lung nodules: when is benign really benign? J Vasc Interv Radiol. 2004;15(2 Pt 1):161–164. doi: 10.1097/01.rvi.0000109397.74740.8d. [DOI] [PubMed] [Google Scholar]
- 30.Calhoun P, Feldman P S, Armstrong P. et al. The clinical outcome of needle aspirations of the lung when cancer is not diagnosed. Ann Thorac Surg. 1986;41(6):592–596. doi: 10.1016/s0003-4975(10)63066-4. [DOI] [PubMed] [Google Scholar]
- 31.Anderson J M, Murchison J, Patel D. CT-guided lung biopsy: factors influencing diagnostic yield and complication rate. Clin Radiol. 2003;58(10):791–797. doi: 10.1016/s0009-9260(03)00221-6. [DOI] [PubMed] [Google Scholar]
- 32.Oikonomou A, Matzinger F R, Seely J M, Dennie C J, Macleod P J. Ultrathin needle (25 G) aspiration lung biopsy: diagnostic accuracy and complication rates. Eur Radiol. 2004;14(3):375–382. doi: 10.1007/s00330-003-2076-4. [DOI] [PubMed] [Google Scholar]
- 33.Tsukada H, Satou T, Iwashima A, Souma T. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol. 2000;175(1):239–243. doi: 10.2214/ajr.175.1.1750239. [DOI] [PubMed] [Google Scholar]
- 34.Laurent F, Latrabe V, Vergier B, Montaudon M, Vernejoux J M, Dubrez J. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm: results with an automated 20-gauge coaxial cutting needle. Clin Radiol. 2000;55(4):281–287. doi: 10.1053/crad.1999.0368. [DOI] [PubMed] [Google Scholar]
- 35.Mullan C P, Kelly B E, Ellis P K, Hughes S, Anderson N, McCluggage W G. CT-guided fine-needle aspiration of lung nodules: effect on outcome of using coaxial technique and immediate cytological evaluation. Ulster Med J. 2004;73(1):32–36. [PMC free article] [PubMed] [Google Scholar]
- 36.Khan M F, Straub R, Moghaddam S R. et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008;18(7):1356–1363. doi: 10.1007/s00330-008-0893-1. [DOI] [PubMed] [Google Scholar]
- 37.Saji H, Nakamura H, Tsuchida T. et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest. 2002;121(5):1521–1526. doi: 10.1378/chest.121.5.1521. [DOI] [PubMed] [Google Scholar]
- 38.Wu C C, Maher M M, Shepard J-AO. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011;196(6):W678-82. doi: 10.2214/AJR.10.4659. [DOI] [PubMed] [Google Scholar]
- 39.Yeow K-M, Su I-H, Pan K-T. et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126(3):748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 40.Cox J E, Chiles C, McManus C M, Aquino S L, Choplin R H. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212(1):165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka J, Sonomura T, Shioyama Y. et al. “Oblique path”—the optimal needle path for computed tomography-guided biopsy of small subpleural lesions. Cardiovasc Intervent Radiol. 1996;19(5):332–334. [PubMed] [Google Scholar]
- 42.Fish G D, Stanley J H, Miller K S, Schabel S I, Sutherland S E. Postbiopsy pneumothorax: estimating the risk by chest radiography and pulmonary function tests. AJR Am J Roentgenol. 1988;150(1):71–74. doi: 10.2214/ajr.150.1.71. [DOI] [PubMed] [Google Scholar]
- 43.Ko J P, Shepard J O, Drucker E A. et al. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology. 2001;218(2):491–496. doi: 10.1148/radiology.218.2.r01fe33491. [DOI] [PubMed] [Google Scholar]
- 44.Topal U, Berkman Y M. Effect of needle tract bleeding on occurrence of pneumothorax after transthoracic needle biopsy. Eur J Radiol. 2005;53(3):495–499. doi: 10.1016/j.ejrad.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Tomiyama N, Yasuhara Y, Nakajima Y. et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59(1):60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Mirski M A, Lele A V, Fitzsimmons L, Toung T J. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106(1):164–177. doi: 10.1097/00000542-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 47.Ayar D, Golla B, Lee J Y, Nath H. Needle-track metastasis after transthoracic needle biopsy. J Thorac Imaging. 1998;13(1):2–6. doi: 10.1097/00005382-199801000-00002. [DOI] [PubMed] [Google Scholar]


