Abstract
Recent studies have indicated that omega-3 (n3) polyunsaturated fatty acids (PUFAs) decrease adipose tissue inflammation in rodents and in morbidly obese humans. We investigated whether a diet rich in n3 PUFAs from both marine and plant sources reduces adipose tissue and systemic inflammation in overweight to moderately obese adults. We conducted a randomized, single-blind, parallel-design, placebo-controlled feeding trial. Healthy men and women with a body mass index between 28 and 33 kg/m2 consumed a diet rich in n3 PUFAs (3.5% of energy intake; n = 11) from plant and marine sources or a control diet (0.5% of energy intake from n3 PUFAs; n = 13). These diets were consumed for 14 wk (ad libitum for 12 wk). All foods were provided for the entire study period. Subcutaneous abdominal adipose tissue and fasting plasma were collected after the first 2 wk with the control diet and again at the end of the 14-wk dietary period. The primary outcome of this ex post analysis was the adipose tissue gene expression of 13 key mediators of inflammation. Adipose tissue gene expression of inflammatory mediators did not differ between the 2 groups, after adjustment for weight change. Furthermore, none of the 5 plasma markers of systemic inflammation differed significantly as an effect of diet treatment. We conclude that a relatively high dose of n3 PUFAs from plant and marine sources did not significantly lower adipose tissue or systemic inflammation in overweight to moderately obese healthy men and women over 14 wk.
Introduction
Low-grade, chronic adipose tissue inflammation is associated with obesity and plays a key role in the development of insulin resistance leading to type 2 diabetes. Proinflammatory cytokines such as tumor necrosis factor (TNF)9 α and interleukin (IL)-6 secreted largely by classically activated macrophages disrupt insulin signaling in adipocytes (1). Adipose tissue inflammation may also affect insulin sensitivity in the liver and muscle—for example, by reducing the adipocyte secretion of the major insulin-sensitizing adipokine adiponectin (2). A focus in the field is to identify modifiable factors affecting adipose tissue inflammation.
A dietary factor with proposed antiinflammatory actions are the long-chain n3 PUFAs (3). Several antiinflammatory mechanisms have been proposed for the n3 PUFAs EPA (20:5n3) and DHA (22:6n3), in particular. First, eicosanoids synthesized from EPA are less potent in their proinflammatory actions than those synthesized from the n6 PUFA arachidonic acid (4). Second, EPA and DHA are substrates for the synthesis of resolvins and protectins, endogenous compounds involved in the active resolution of inflammation (5). Third, EPA and DHA are ligands for GPCR120, a G-protein coupled receptor (GPCR) expressed in adipose tissue that potently inhibits inflammatory signaling pathways (6).
In rodent models, high-dose fish-oil administration strongly attenuated several aspects of adipose tissue inflammation triggered by a high-fat diet (7–9). In contrast, the effect of n3 PUFAs on adipose tissue inflammation in humans is less clear. For example, morbidly obese, nondiabetic adults receiving 3.36 g EPA/DHA capsules daily for 8 wk before undergoing bariatric surgery exhibited significantly lower inflammatory gene expression compared with controls consuming an equivalent amount of butter fat (10). Although this study had some notable strengths, such as the assessment of inflammatory gene expression in both subcutaneous and intraabdominal adipose tissue depots, it suffered from the fact that gene expression analysis was conducted at the conclusion of the intervention only, i.e., not compared with a baseline value. Moreover, whereas the study provided convincing evidence that marine n3 PUFAs may lower adipose tissue inflammation in morbidly obese participants, effects among individuals with a lower level of adiposity are less clear. In another study, 27 diabetic women were randomly assigned to receive either 3 g fish oil or placebo (paraffin oil) daily for 2 mo (11). The expression of some genes involved in inflammation was reduced after fish oil but not placebo (11). However, data on major mediators of inflammation such as cytokines, chemokines, or adhesion molecules were not reported, indicating that these were either not studied or were not differentially regulated by fish-oil treatment. Questions therefore remain as to whether n3 PUFAs, in practically relevant doses, have the potential to lower adipose tissue inflammation in overweight to moderately obese individuals.
We previously conducted a well-controlled dietary intervention during which overweight to mildly obese men and women consumed either a diet rich in n3 PUFAs (3.5% of energy from n3 PUFAs) or a control diet (0.5% from n3 PUFAs) for 14 wk (12, 13). Subcutaneous adipose tissue samples were collected at baseline and again after 14 wk, which enabled us to investigate the effect of the study diets on the expression of genes encoding major mediators of inflammation. We also collected fasting plasma to assess the effect of the study diets on circulating markers of inflammation. The primary aim of this study was to test the hypothesis that a diet rich in n3 PUFAs lowers body weight in overweight and moderately obese men and women.
Participants and Methods
Study design and participants
Details of the study design, dietary interventions, and participant recruitment, enrollment, and randomization have been previously described (12, 13). In brief, 24 overweight to moderately obese participants (Table 1) completed a 16-wk controlled dietary period in which all foods were provided. During the first 2-wk lead-in period all participants consumed a control diet, which consisted of conventional foods typically found in a mixed American diet rich in MUFAs (Table 2). The caloric content of the diet was calculated on the basis of individual 3-d diet records completed by the participants and the Mifflin formula (14) multiplied by an activity factor based on a physical activity questionnaire to maintain each participant’s weight to within 1 kg of baseline weight. The principal sources of fat for the control diet were high-oleic safflower and high-oleic sunflower oil and margarines based on these oils, as well as capsules containing high-oleic safflower oil. Subsequently, all participants were randomized into 2 groups, one of which continued to consume the control diet, whereas the other group received the diet enriched in n3 PUFAs (Table 2). Participants remained on these 2 diets for the next 14 wk, the first 2 of which were again isocaloric, followed by 12 wk of ad libitum consumption. The n3 PUFA diet was identical to the control diet in all respects apart from the principal sources of dietary fat, in that canola and flaxseed oils were substituted for high-oleic safflower and sunflower oils. Both oils are rich in the n3 PUFA α-linolenic acid. In addition, subjects in the n3 PUFA group were asked to take capsules containing fish oil rather than high-oleic safflower oil, which was calculated to provide 1.4% of energy in the form of long-chain n3 PUFAs. Adherence of the study participants to the dietary regimen was assessed on the basis of twice-weekly reviews of the dietary records between study staff and participants, along with weights of returned foods and counts of returned capsules (12). Mean (±SD) capsule intake was 99.2 ± 1.4% of those administered, and median (range) intake was 99.9% (94.4, 100%). During the course of the study, each subject was admitted to the University of Washington Clinical Research Center (CRC) on d 14 (CRC1), d 28 (CRC2), and d 112 (CRC3). At each CRC visit, study participants were weighed in a hospital gown and a fasting blood specimen was obtained by intravenous collection. Blood specimens were centrifuged immediately at 2500 × g for 10 minutes, and plasma was stored at −70°C until analyses. In addition, at CRC1 and CRC3, a subcutaneous adipose tissue biopsy was performed under sterile conditions as described previously (15). Harvested adipose tissue was rinsed and flash frozen on dry ice before storage at −70°C until analysis. Periumbilical subcutaneous adipose tissue biopsies were selected for this study because they are well tolerated and easy to perform (15). All study procedures were approved by the University of Washington Human Subjects Committee and were in compliance with the Helsinki Declaration. All participants provided informed written consent.
TABLE 1.
Baseline characteristics of the overweight to moderately obese men and women who completed all study procedures1
| Control | n3 PUFA | |
| Participants, n (F/M) | 13 (8/5) | 11 (8/3) |
| Age, y | 37.8 ± 13.6 | 40.5 ± 13.1 |
| Body weight, kg | 85.4 ± 9.3 | 85.2 ± 11.4 |
| BMI, kg/m2 | 30.1 ± 1.1 | 30.4 ± 1.3 |
| Fasting plasma glucose, mg/dL | 96.6 ± 7.4 | 99.6 ± 5.7 |
| 96 (85, 113) | 99 (91, 109) | |
| Plasma hsCRP, mg/L | 1.4 ± 2.3 | 2.1 ± 2.4 |
| 0.6 (0.2, 8.9) | 0.8 (0.6, 7.2) | |
| Plasma IL-6, pg/mL | 2.5 ± 1.0 | 2.8 ± 1.0 |
| Plasma total adiponectin, μg/mL | 4.3 ± 1.9 | 4.3 ± 2.2 |
| Adipose tissue TNFα, mRNA copy number, normalized | 8.2 ± 1.7 | 10.3 ± 6.3 |
| 8.0 (4.7, 10.9) | 7.8 (5.0, 26.3) | |
| Adipose tissue ADIPOQ, mRNA copy number, normalized | 7310 ± 1800 | 7630 ± 1340 |
Values are means ± SDs or median (range) unless otherwise indicated. The 2 groups did not differ significantly with regard to any of these variables (in independent-samples t tests or Mann-Whitney U tests, P = 0.05). ADIPOQ, adiponectin; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; TNFα, tumor necrosis factor α.
TABLE 2.
Composition of the diets1
| Lead-in period (2 wk) |
Isocaloric period (2 wk) |
Ad libitum period (12 wk) |
|||
| Control diet | Control diet | n3 PUFA diet | Control diet | n3 PUFA diet | |
| Energy intake, MJ/d | 10.7 ± 1.7 | 10.9 ± 1.3 | 10.6 ± 1.7 | 10.2 ± 1.9 | 10.4 ± 2.4 |
| Protein, % of energy | 15.9 ± 0.1 | 15.9 ± 0.0 | 15.8 ± 0.1 | 15.8 ± 0.5 | 16.1 ± 0.4 |
| Carbohydrates, % of energy | 49.8 ± 0.4 | 49.8 ± 0.2 | 49.5 ± 0.2 | 49.5 ± 1.0 | 49.1 ± 1.2 |
| Alcohol, % of energy | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.6 ± 1.2 |
| Fat, % of energy | 34.3 ± 0.4 | 34.3 ± 0.2 | 34.7 ± 0.1 | 34.0 ± 0.8 | 34.2 ± 0.6 |
| SFAs, % of energy | 8.6 ± 0.1 | 8.5 ± 0.1 | 8.4 ± 0.0 | 8.5 ± 0.3 | 8.3 ± 0.3 |
| MUFAs, % of energy | 18.5 ± 0.5 | 18.7 ± 0.1 | 15.5 ± 0.0 | 18.3 ± 0.4 | 15.2 ± 0.3 |
| n6 PUFAs, % of energy | 4.8 ± 0.0 | 4.8 ± 0.0 | 4.9 ± 0.0 | 4.8 ± 0.2 | 4.8 ± 0.1 |
| n3 PUFAs, % of energy | 0.5 ± 0.0 | 0.5 ± 0.0 | 3.6 ± 0.1 | 0.5 ± 0.0 | 3.5 ± 0.1 |
| α-Linolenic acid, % of energy | 0.5 ± 0.0 | 0.5 ± 0.0 | 2.2 ± 0.0 | 0.5 ± 0.0 | 2.0 ± 0.1 |
| Long-chain n3 PUFAs, % of energy | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 0.1 | 0.0 ± 0.0 | 1.4 ± 0.1 |
| EPA, % of energy | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.66 ± 0.03 | 0.0 ± 0.0 | 0.68 ± 0.06 |
| DHA, % of energy | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.46 ± 0.02 | 0.0 ± 0.0 | 0.48 ± 0.04 |
| Vitamin E, mg α-TE/1000 kcal | 25.8 ± 1.1 | 26.3 ± 0.5 | 26.5 ± 0.8 | 26.1 ± 0.8 | 26.6 ± 1.7 |
| Cholesterol, mg/1000 kcal | 67.7 ± 2.7 | 67.1 ± 2.6 | 60.1 ± 0.3 | 69.6 ± 3.4 | 64.9 ± 3.1 |
| Fiber, g/1000 kcal | 11.1 ± 0.1 | 11.0 ± 0.1 | 11.1 ± 0.0 | 10.7 ± 0.8 | 10.7 ± 0.3 |
Values are means ± SDs; n = 24 (lead-in period), n = 13 (control diet), or n = 11 (n3 PUFA diet). α-TE, α tocopherol equivalents.
Laboratory methods
Inflammation is a complex process that cannot be quantified by a single measure alone. Therefore, we measured several markers in both plasma and adipose tissue that are known key inflammatory molecules. Target genes in adipose tissue included the antiinflammatory and insulin-sensitizing adipokine adiponectin; the key inflammatory cytokines TNFα and IL6; the adhesion molecule intercellular adhesion molecule 1 (ICAM1); the macrophage cell surface markers CD14, CD206 (mannose receptor), and CD284 (Toll-like receptor 4); and the chemokines chemokine (C-C motif) ligand 2 (CCL2 or monocyte chemotactic protein-1) and serum amyloid A1 (SAA1). We also included inducible nitric oxide synthase (NOS2), an enzyme produced by classically activated macrophages (16) and plasminogen activator inhibitor 1 (PAI-1), a risk factor for cardiovascular disease that is elevated in humans in instances of acute inflammation (17) and the expression of which is increased by proinflammatory cytokines (18). Last, we included endothelial nitric oxide synthase (NOS3) and hyaluronan synthase 2 (HAS2). NOS3 mediates the interaction between endothelial cells and leukocytes and plays a key role in cell rolling, adhesion, and extravasation (19). HAS2 is responsible for the production of hyaluronan, a glycan that binds and retains macrophages in adipose tissue (20). Taken together, these 13 genes provide a diverse and comprehensive picture of the inflammatory status in adipose tissue. In plasma, we measured the concentrations of the acute-phase protein C-reactive protein (CRP), the major proinflammatory cytokine IL-6, the chemokine CCL2, and the soluble TNF receptors 1 and 2, together providing a comprehensive picture of systemic inflammation.
Plasma analysis.
In plasma, concentrations of the cytokine IL-6, CCL2, and the soluble TNF receptors 1 and 2 were measured by using high-sensitivity ELISA assays (R&D Systems). These assays were performed in duplicate in the Biomarker Laboratory at Fred Hutchinson Cancer Research Center. Intra- and interassay CVs were 4.1 and 0.3% for IL-6, 2.5 and 16.4% for CCL2, 0.8 and 8.1% for soluble TNF receptor 1, and 1.0 and 20.4% for soluble TNF receptor 2, respectively. High-sensitivity CRP (hsCRP) was measured by immunonephelometry at the Northwest Lipid Research Laboratories in Seattle, WA. Two additional participants in the n3 PUFA group (n = 13) were included in the plasma analysis only because they did not undergo biopsy at CRC3.
RNA isolation and gene expression analysis.
Total RNA was extracted from adipose tissue by using RNeasy mini kits (Qiagen) and quantified by using the RiboGreen RNA Quantitation Kit (Invitrogen). cDNA synthesis was carried out on 0.5–1.5 μg of total RNA by using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and PCR was performed by using predesigned ABI TaqMan Gene Expression Assays on an Mx3005P Multiplex QPCR System (Stratagene). By including a standard curve on each plate, Ct values were converted to copy numbers of all target genes, and all results are expressed as target gene copy number per nanogram of RNA. These data were then normalized by applying a normalization factor based on 3 housekeeping genes: β-glucuronidase, phosphoglycerate kinase 1, and 18s rRNA. These 3 genes are stably expressed in adipose tissue (21). Using the geNorm application (22), we assessed the actual variability of these 3 housekeeping genes in our data set. All 3 showed low levels of variability, and were therefore all included in the calculation of the normalization factor.
Statistical analysis
All statistical analyses were performed by using the Statistical Package for the Social Sciences, version 11.5 (SPSS). The distribution of variables was analyzed by checking histograms and normal plots of the data, and normality was tested by means of Kolmogorov-Smirnov and Shapiro-Wilk tests. Baseline characteristics of the groups were compared by means of independent samples t tests or Mann-Whitney U tests. Changes in body weight, adipose tissue gene expression, and plasma markers were compared by repeated-measures ANOVA (RM-ANOVA), with the 2 time points for which adipose tissue was available (CRC1 and CRC3) as the 2 levels of the within-subjects factor (time) and treatment (control vs. n3 PUFAs) as a between-subjects factor, adjusted for the change in weight between CRC1 and CRC3. We also conducted these analyses including gender as a covariate to explore whether the response to treatment or weight change may have differed between men and women. RM-ANOVAs were conducted with (natural) log-transformed variables if the residuals were not normally distributed, which was the case for adipose tissue TNFα, ICAM1, CCL2, IL6, PAI-1, and NOS2 mRNA and for plasma hsCRP. Of note, our 13 target genes and 5 plasma markers address different aspects of the same biological process, inflammation. These need to be considered together, i.e., a single significant finding would not be strongly indicative of an effect on “inflammation” in the absence of changes in other genes; we therefore decided not to adjust for multiple testing. To assess the strength and direction of the observed interactions, we conducted follow-up multiple linear regression analyses. These included the adipose tissue or plasma marker of inflammation as the dependent variable and weight change, gender, and diet group as independent variables. The level of significance was set at P < 0.05 for all analyses.
Results
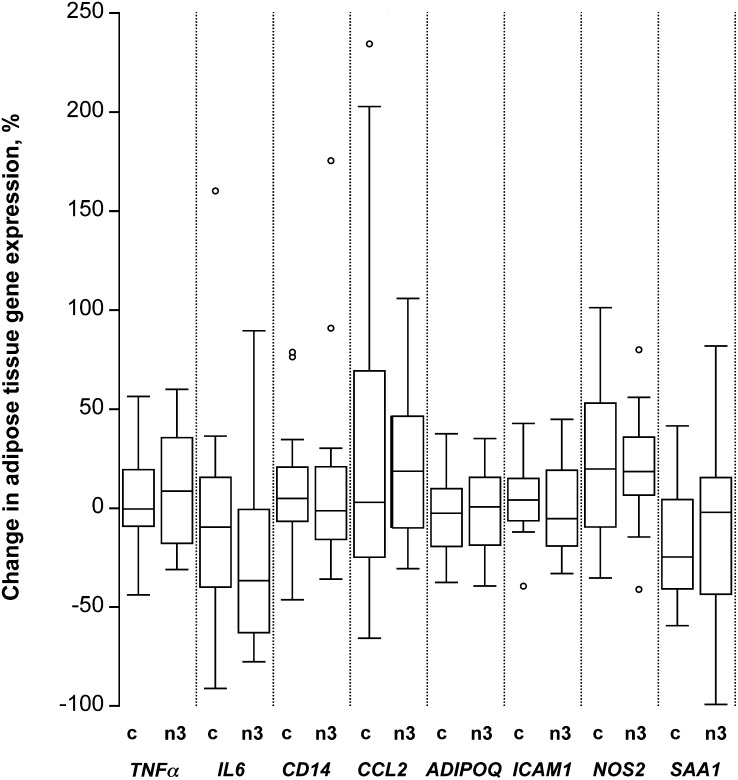
As reported previously, changes in body weight were similar in the n3 PUFA and control groups, whereas the level of physical activity among participants in either group remained unchanged throughout the study (12). Because there was substantial interindividual variation in weight change, however, we adjusted the RM-ANOVA for the change in weight between CRC1 and CRC3. Adjusting for weight change, there were no significant time by treatment interactions for any of the 13 genes encoding major mediators of inflammation (Table 3, Fig. 1). As is apparent from Supplemental Figure 1, which shows the gene expression data for each participant, unadjusted for weight change, the gene expression levels changed in some individuals, sometimes substantially. However, there was no consistent trend for a change in gene expression levels in the n3 PUFA group compared with the control group for any of the genes.
TABLE 3.
Adipose tissue expression of genes encoding mediators of inflammation in overweight to moderately obese men and women before and after 14 wk of consuming a diet rich in n3 PUFAs vs. a control diet low in n3 PUFAs1
| mRNA copies |
P value (RM-ANOVA) |
||||
| Post lead-in period (CRC1) | Post ad libitum period (CRC3) | Time | Time × treatment | Time × weight change | |
| n/ng total mRNA | |||||
| TNFα | |||||
| Control | 7.99 (7.14, 9.39) | 8.70 (7.33, 9.32) | 0.91 | 0.70 | 0.42 |
| n3 PUFA | 7.75 (6.99, 9.87) | 8.87 (7.58, 12.2) | |||
| IL6 | |||||
| Control | 23.3 (22.1, 34.1) | 26.4 (16.9, 37.3) | 0.11 | 0.10 | 0.79 |
| n3 PUFA | 24.1 (20.6, 56.1) | 20.6 (14.5, 24.8) | |||
| CD14 | |||||
| Control | 256 ± 52.5 | 277 ± 85.1 | 0.21 | 0.96 | 0.42 |
| n3 PUFA | 296 ± 76.5 | 317 ± 92.3 | |||
| CCL2 | |||||
| Control | 2520 (2180, 3030) | 2370 (1930, 5230) | 0.19 | 0.95 | 0.72 |
| n3 PUFA | 2230 (1410, 2600) | 2220 (1990, 2870) | |||
| ADIPOQ | |||||
| Control | 7310 ± 1800 | 6950 ± 1960 | 0.38 | 0.89 | 0.04 |
| n3 PUFA | 7630 ± 1340 | 7470 ± 1870 | |||
| ICAM1 | |||||
| Control | 55.7 (49.4, 62.8) | 58.9 (49.7, 67.1) | 0.90 | 0.61 | 0.87 |
| n3 PUFA | 61.6 (52.5, 66.5) | 58.2 (44.5, 59.5) | |||
| SAA1 | |||||
| Control | 17,400 ± 8660 | 14,400 ± 9060 | 0.81 | 0.59 | 0.05 |
| n3 PUFA | 15,500 ± 7590 | 14,400 ± 9470 | |||
| CD206 | |||||
| Control | 214 ± 53.4 | 226 ± 53.4 | 0.82 | 0.64 | 0.59 |
| n3 PUFA | 248 ± 30.1 | 251 ± 51.7 | |||
| CD284 | |||||
| Control | 161 ± 27.6 | 167 ± 21.1 | 0.27 | 0.30 | 0.21 |
| n3 PUFA | 165 ± 40.4 | 155 ± 39.6 | |||
| PAI-1 | |||||
| Control | 14.2 (6.88, 20.4) | 13.4 (10.9, 16.5) | 0.97 | 0.30 | 0.14 |
| n3 PUFA | 15.6 (7.35, 19.5) | 12.1 (6.41, 32.5) | |||
| NOS2 | |||||
| Control | 1.38 (1.03, 3.77) | 1.71 (1.35, 3.59) | 0.49 | 0.99 | 0.12 |
| n3 PUFA | 1.68 (1.19, 4.62) | 2.62 (1.41, 4.28) | |||
| NOS3 | |||||
| Control | 23.2 ± 8.26 | 21.4 ± 4.94 | 0.56 | 0.71 | 0.83 |
| n3 PUFA | 21.7 ± 6.41 | 21.2 ± 6.58 | |||
| HAS2 | |||||
| Control | 480 ± 154 | 538 ± 136 | 0.35 | 0.30 | 0.66 |
| n3 PUFA | 430 ± 147 | 426 ± 131 | |||
Values are means ± SDs or medians (IQRs); n = 13 (control) or n = 11 (n3 PUFAs). ADIPOQ, adiponectin; CCL2, chemokine (C-C motif) ligand 2; CD, cluster of differentiation; CRC, Clinical Research Center; HAS2, hyaluronan synthase 2; ICAM1, intercellular adhesion molecule 1; IL6, interleukin 6; NOS2, inducible nitric oxide synthase; NOS3, endothelial nitric oxide synthase; PAI-1, plasminogen activator inhibitor 1; RM-ANOVA, repeated-measures ANOVA; SAA1, serum amyloid A1; TNFα, tumor necrosis factor α.
FIGURE 1.
Percentage change in a selection of markers of inflammation in overweight to moderately obese men and women after 14 wk of either a control diet or an n3 PUFA–enriched diet. Percentage change calculated at wk 14 (CRC3) minus baseline (CRC1) in adipose tissue gene expression. Box plots depict the IQR, median (horizontal line), and outliers (open circles) for the control diet group (“c”, n=13) and the n3 PUFA diet group (“n3”, n=11). ADIPOQ, adiponectin; CCL2, chemokine (C-C motif) ligand 2; CRC, Clinical Research Center; ICAM1, intercellular adhesion molecule 1; IL6, interleukin 6; NOS2, inducible nitric oxide synthase; SAA1, serum amyloid A1; TNFα, tumor necrosis factor α.
Adjusting the RM-ANOVA for changes in body weight allowed us to also assess whether there were any changes in the measures of adipose tissue inflammation over time that were dependent on weight change (i.e., “time × weight-change interaction”). We found a significant time by weight-change interaction for the adipose tissue expression of ADIPOQ and SAA1 between CRC1 and CRC3 (P-interaction = 0.04 and P-interaction = 0.05, respectively; Table 3). In multiple regression analyses adjusted for diet group, weight change was significantly and positively associated with change in adipose tissue expression of the SAA1 gene (β = 0.411, P = 0.05) and the ADIPOQ gene (β = 0.431, P = 0.04). There was no other significant time by weight-change interaction for any of the other markers of adipose tissue inflammation. In subsequent analyses, we adjusted for gender to determine whether gender-specific differences may influence the response of inflammatory mediators to treatment or weight change (“time × treatment × gender interaction” and “time × weight change × gender interaction”). Adipose tissue expression of PAI-1 exhibited a significant time by weight change by gender interaction (P-interaction = 0.04). In follow-up multiple regression analysis adjusted for diet group, weight change was inversely associated with the change in adipose tissue PAI-1 expression in women [β = (−0.624), P = 0.011], but not in men [β = (−0.210), P = 0.606] (data not shown). There were no other significant time by weight change by gender interactions.
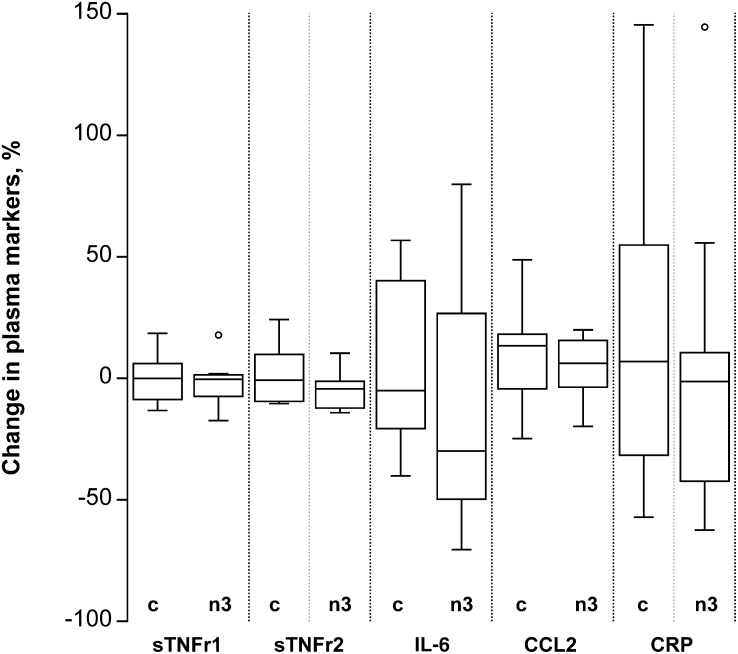
There was also no time by treatment interaction for any of the 5 plasma markers (Table 4, Fig. 2). Individual concentrations either changed minimally over the 14-wk intervention (hsCRP, CCL2, soluble TNF receptors 1 and 2) or there was no trend in the individual changes in either group (IL-6) (Supplemental Fig. 2). In fasting plasma, adjusted for the dietary treatment group, there was a significant time by weight change interaction for hsCRP concentrations that was independent of gender (P-interaction = 0.004; Table 4). In multiple regression analysis adjusted for diet group, the change in body weight between CRC1 and CRC3 was significantly and positively associated with the change in hsCRP (β = 0.558, P = 0.004). There was no other significant time by weight change interaction for any of the other markers of systemic inflammation. For fasting hsCRP, we also had data on CRC2, the time point after the initial 2-wk isocaloric phase on either the control or the n3 PUFA diet. Again, the time by diet group interaction did not indicate a significant effect of diet group (P-interaction = 0.14; data not shown). Similarly, when gender was included in the model as a covariate, there were no significant time by treatment by gender interactions among the plasma markers of inflammation (data not shown).
TABLE 4.
Plasma markers of inflammation in overweight to moderately obese men and women before and after 14 wk of consuming a diet rich in n3 PUFAs vs. a control diet low in n3 PUFAs1
| Post lead-in period (CRC1) | Post ad libitum period (CRC3) |
P value (RM-ANOVA) |
|||
| Time | Time × treatment | Time × weight change | |||
| sTNFr1, pg/mL | |||||
| Control | 1030 ± 113 | 1030 ± 120 | 0.90 | 0.58 | 0.41 |
| n3 PUFA | 936 ± 203 | 911 ± 200 | |||
| sTNFr2, pg/mL | |||||
| Control | 1740 (1550, 1960) | 1800 (1620, 1990) | 0.15 | 0.12 | 0.35 |
| n3 PUFA | 1730 (1350, 2090) | 1670 (1320, 1950) | |||
| IL-6, pg/mL | |||||
| Control | 2.49 ± 1.04 | 2.56 ± 0.82 | 0.93 | 1.00 | 0.74 |
| n3 PUFA | 2.70 ± 0.96 | 2.75 ± 1.91 | |||
| CCL2, pg/mL | |||||
| Control | 126 (97.8, 170) | 142 (108, 171) | 0.20 | 0.66 | 0.85 |
| n3 PUFA | 117 (104, 136) | 126 (99, 139) | |||
| CRP, mg/L | |||||
| Control | 0.58 (0.38, 1.68) | 0.79 (0.52, 1.20) | 0.03 | 0.32 | 0.004 |
| n3 PUFA | 0.80 (0.65, 2.77) | 0.97 (0.40, 2.75) | |||
Values are means ± SDs or medians (IQRs); n = 13 (control) or n = 13 (n3 PUFAs) (analysis included an additional 2 participants who did not undergo biopsy). CCL2, chemokine (C-C motif) ligand 2; CRC, Clinical Research Center; CRP, C-reactive protein; IL-6, interleukin 6; RM-ANOVA, repeated-measures ANOVA; sTNFr1, soluble tumor necrosis factor α receptor.
FIGURE 2.
Percentage change in systemic markers of inflammation in overweight to moderately obese men and women after 14 wk of either a control diet or an n3 PUFA–enriched diet. Percentage change calculated at wk 14 (CRC3) minus baseline (CRC1) in plasma. Box plots depict the IQR, median (horizontal line), and outliers (open circles) for the control diet group (“c”, n=13) and the n3 PUFA diet group (“n3”, n=13). CCL2, chemokine (C-C motif) ligand 2; CRC, Clinical Research Center; CRP, C-reactive protein; IL-6, interleukin 6; sTNFr1, soluble tumor necrosis factor α receptor.
To ascertain that the participants in this study exhibited low-grade inflammation, as their elevated BMI would suggest, we compared inflammatory mediators in plasma and adipose tissue with those in five normal-weight participants who were studied separately (Supplemental Table 1). For this purpose, we normalized the gene expression levels across both studies by using a normalization factor based on 3 housekeeping genes, as described above. Adipose tissue gene expression and plasma markers of inflammation differed substantially between these normal-weight participants and the overweight and moderately obese participants studied here (Supplemental Table 1). Thus, when compared with healthy, normal-weight individuals, low-grade inflammation was present in both plasma and adipose tissue in the population studied here.
Discussion
The major finding of this randomized, controlled, dietary intervention trial in overweight and moderately obese participants was that neither adipose tissue nor plasma markers of inflammation differed significantly between participants consuming a diet enriched in n3 PUFAs and participants consuming a control diet low in n3 PUFAs. These findings are consistent with our previous observation that n3 PUFAs did not affect plasma total or high-molecular-weight adiponectin concentrations in this population (13).
Our findings are in contrast to animal models that suggest that n3 PUFAs, specifically the long-chain fatty acids EPA and DHA in fish oil, are antiinflammatory. In the present study, participants were provided with 3.5% of total energy in the form of n3 PUFAs, of which 1.4% of total energy intake were long-chain n3 PUFAs of marine origin. This dose is approximately equal to the amount of n3 PUFAs in a daily portion of 125–250 g fatty fish, such as Chinook salmon. This is on the high end of what humans can be expected to consume on a regular basis. In animal models of n3 PUFAs and adipose tissue inflammation, mice were typically fed 12 to 18% of total energy in the form of long-chain n3 PUFAs, which is equivalent to a human dose of at least 27 g/d of long-chain n3 PUFAs based on a 2000-kcal diet. In these experiments, high-fat-diet–induced infiltration of macrophages into adipose tissue was prevented if the diet was supplemented with n3 PUFAs (7). In a similar rodent study, when EPA was supplemented along with a high-fat diet for 11 wk, mice did not exhibit the increase in tissue Pai-1 or Ccl2 expression seen in mice fed a low-EPA, high-fat diet (8). A third group found that mice fed a high-fat diet supplemented with fish oil for 12 wk exhibited lower accumulation of macrophages in white adipose tissue as measured by the macrophage markers MAC-1 and CD68, as well as a downregulation of Tnfα, matrix metalloproteinase 3, and Saa3 compared with mice fed a high-fat diet supplemented with olive oil (9). The discrepancy between these mouse studies and our human study may be explained by species differences, differences in the n3 PUFA dose administered, or the fact that the effects of n3 PUFA were assessed in the context of a proinflammatory condition (high-fat diet) in the mouse studies.
Two recent interventions studied the effect of n3 PUFAs on adipose tissue inflammation in humans. The first was conducted in 27 postmenopausal women with type 2 diabetes who were randomly assigned to receive either 3 g/d fish oil or placebo (paraffin oil) for 2 mo. Subcutaneous adipose tissue was collected for a subset of 7 participants in each diet group, and gene expression was measured for several inflammation-related genes by using microarray analysis. The investigators found that among 800 genes included in the array, “some adipose tissue inflammation-related genes were also reduced” in the fish-oil group (11). However, data on major mediators of inflammation such as cytokines, chemokines, or adhesion molecules were not reported (11). It remains unclear from their article whether these were not included in the microarray or not differentially regulated by fish-oil treatment. It therefore is unknown whether their data are consistent with ours. The second study was conducted in 55 healthy, morbidly obese adults undergoing elective bariatric surgery (10). Participants were randomly assigned to receive either 3.36 g EPA/DHA or the equivalent amount of butterfat each day for 8 wk. At the end of the intervention, the expression of 5 key inflammatory genes was lower in subcutaneous adipose tissue collected from individuals in the n3 PUFA group compared with controls. These included CCL2, the macrophage inflammatory protein 1α (CCL3), hypoxia-inducible factor 1 (HIF1A), transforming growth factor β (TGFβ1), and the costimulatory molecule CD40. Additionally, the adipose tissue concentrations of resolvins and protectins were greater in the n3 PUFA group compared with controls. The data from this study are clearly in contrast to ours. It may be that n3 PUFAs exerted antiinflammatory effects in this morbidly obese population but not in our overweight to moderately obese individuals because baseline levels of adipose tissue inflammation were likely higher in the more obese participants included in the Itariu et al. (10) study. It is also possible that their results differed from ours due to differences in the specific genes chosen to assess adipose tissue inflammation. Last, Itariu et al. (10) compared adipose tissue expression of genes involved in inflammation at 1 time point only in participants after fish oil vs. placebo, which did not allow comparison with baseline as in our study.
We also found that n3 PUFA supplementation did not influence plasma markers of systemic inflammation. Our finding is consistent with a recent review of randomized trials investigating the influence of long-chain n3 PUFAs on inflammatory biomarkers (23). Summarizing the evidence from 26 trials, Rangel-Huerta et al. (23) concluded that long-chain n3 PUFAs have no effect on systemic inflammation in healthy individuals, but a modest antiinflammatory effect in patients with cardiovascular or other chronic disease.
Although we saw no changes in inflammatory markers as a result of dietary treatment, we did see a minor effect of weight loss. Individuals in both diet groups lost a small, yet similar amount of weight (12). However, there was substantial interindividual variation in weight change in both groups, and that change in weight was associated with changes in the tissue expression of ADIPOQ, PAI-1, and SAA1 and the plasma concentration of hsCRP over the 14-wk intervention. Although the observed changes in ADIPOQ and SAA1 mRNA expression and plasma CRP concentrations were independent of gender, changes in adipose tissue PAI-1 differed between men and women. Considering that adiponectin plasma concentrations are inversely associated with adiposity in most studies (24–26), our finding of a positive association between weight change and adipose tissue ADIPOQ expression was surprising. In contrast, the positive association between weight change on the one hand and adipose tissue SAA1 expression and plasma hsCRP concentrations on the other was consistent with previously reported findings by other groups (27, 28). When considering these individual findings, it is important to emphasize again that we did not adjust for multiple testing; some or all of these may be false-positive findings due to an inflated overall α-error.
Our study has a number of important strengths and limitations. Among its strengths are the strict control of dietary intakes over an extended period of time, the length of the study (14 wk, n3 PUFA vs. control), and our use of quantitative real-time RT-PCR focused on 13 genes encoding proteins involved in a variety of different aspects of inflammation. The latter, in particular, is notable, because it reduces the risk of false-positive results as seen in gene expression microarray analyses. The primary limitation is that our study was originally designed to investigate the effects of n3 PUFAs on energy balance and body weight and may not have been ideal to study the effect of these diets on measures of inflammation. Specifically, we did not explicitly recruit individuals with high levels of adipose tissue or systemic inflammation. It is possible that n3 PUFAs may have an antiinflammatory effect in participants with more substantial levels of inflammation at baseline, as suggested by the Itariu et al. (10) study discussed above. Our comparison of baseline levels of inflammation in both adipose tissue and plasma in healthy, normal-weight individuals, however, suggests that low-grade inflammation was present in this group of overweight to moderately obese participants, and that the lack of an effect of n3 PUFAs was not due to the absence of inflammation at baseline. Furthermore, it is possible that the study was not sufficiently powered to detect a clinically significant difference in plasma or tissue markers of inflammation between groups. However, it seems unlikely that we missed an effect of n3 PUFAs due to lack of power because there was not even a trend toward significance in the expression of any of the 13 genes or 5 plasma markers of inflammation. Finally, there remains the distinct possibility that differences in visceral and subcutaneous adipose tissue depots may obscure or mislead findings. Subcutaneous and visceral fat depots in humans are known to exhibit differences in metabolic function and secretory capacity (29–31). However, genes involved in metabolic pathways, such as those pertaining to energy/electron transport and lipid, fatty acid, amino acid, and pyruvate metabolism, were negatively associated with body fat mass and insulin resistance in both subcutaneous and visceral adipose tissue depots (32). Genes involved in inflammation were positively associated with body fat mass and insulin resistance, again similarly in both depots. Furthermore, macrophage gene expression within adipose tissue was essentially identical between these 2 depots, increasing in both with adiposity (33). This suggests that despite functional differences among fat depots, inflammatory processes associated with increasing adiposity are likely similar. Because these markers reflect different aspects of the same biological process, inflammation, one would expect that at least a trend would have become apparent for several of these markers if n3 PUFAs had a clinically relevant antiinflammatory effect. We therefore conclude that a high, but practically relevant dose of n3 PUFAs does not affect adipose tissue or systemic inflammation in healthy overweight to moderately obese men and women.
Supplementary Material
Acknowledgments
The authors thank Pamela Y. Yang, Allison M. Shircliff, Holly Edelbrock, Barbara H. Burke, and Malinda M. Gehrke for excellent technical assistance. The fish-oil and control capsules were purchased from Capsugel (Peapack, NJ). Study margarines were provided by Unilever Foods NA (Englewood Cliffs, NJ). M.K. and D.S.W. initiated the project and were responsible for the design and implementation of the study and the collection of the data; M.K. and J.N.K. completed statistical analysis of data and the first draft of the manuscript; D.K.H. provided technical assistance and review of the manuscript; B.v.Y. measured expression of genes in adipose tissue; C.C.M. and H.S.C. designed and calculated the study diets and were responsible for the preparation of all study meals; and all authors contributed to the preparation of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ADIPOQ, adiponectin; CCL2, chemokine (C-C motif) ligand 2; CCL3, macrophage inflammatory protein 1α CD, cluster of differentiation; CRC, Clinical Research Center; CRP, C-reactive protein; GPCR, G protein–coupled receptor; HAS2, hyaluronan synthase 2; HIF1A, hypoxia-inducible factor 1; hsCRP, high-sensitivity C-reactive protein; ICAM1, intercellular adhesion molecule 1; IL-6, interleukin 6; MAC-1, macrophage-1 antigen; NOS2, inducible nitric oxide synthase; NOS3, endothelial nitric oxide synthase; PAI-1, plasminogen activator inhibitor-1; RM-ANOVA, repeated-measures ANOVA; SAA1, serum amyloid A1; sTNFr, soluble tumor necrosis factor α receptor; TGFβ1, transforming growth factor β TLR, Toll-like receptor; TNF, tumor necrosis factor.
Literature Cited
- 1.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 2.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. [DOI] [PubMed] [Google Scholar]
- 3.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–55. [DOI] [PubMed] [Google Scholar]
- 6.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhausl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–19. [DOI] [PubMed] [Google Scholar]
- 8.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J Nutr. 2010;140:1915–22. [DOI] [PubMed] [Google Scholar]
- 9.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J Nutr. 2007;137:1776–82. [DOI] [PubMed] [Google Scholar]
- 10.Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K, Bohdjalian A, Mascher D, Vangala S, Schranz M, et al. Long-chain n–3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am J Clin Nutr. 2012;96:1137–49. [DOI] [PubMed] [Google Scholar]
- 11.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, et al. Treatment for 2 mo with n–3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr. 2007;86:1670–9. [DOI] [PubMed] [Google Scholar]
- 12.Kratz M, Callahan HS, Yang PY, Matthys CC, Weigle DS. Dietary n-3-polyunsaturated fatty acids and energy balance in overweight or moderately obese men and women: a randomized controlled trial. Nutr Metab (Lond). 2009;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kratz M, Swarbrick MM, Callahan HS, Matthys CC, Havel PJ, Weigle DS. Effect of dietary n–3 polyunsaturated fatty acids on plasma total and high-molecular-weight adiponectin concentrations in overweight to moderately obese men and women. Am J Clin Nutr. 2008;87:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 15.Campbell KL, Makar KW, Kratz M, Foster-Schubert KE, McTiernan A, Ulrich CM. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prev Res (Phila). 2009;2:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. [DOI] [PubMed] [Google Scholar]
- 17.Ekström M, Liska J, Eriksson P, Sverremark-Ekstrom E, Tornvall P. Stimulated in vivo synthesis of plasminogen activator inhibitor-1 in human adipose tissue. Thromb Haemost. 2012;108:485–92. [DOI] [PubMed] [Google Scholar]
- 18.Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost. 2003;1:1575–9. [DOI] [PubMed] [Google Scholar]
- 19.Cirino G, Fiorucci S, Sessa WC. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends Pharmacol Sci. 2003;24:91–5. [DOI] [PubMed] [Google Scholar]
- 20.Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and Resadhesion. Diabetes. 2007;56:2260–73. [DOI] [PubMed] [Google Scholar]
- 21.Catalán V, Gomez-Ambrosi J, Rotellar F, Silva C, Rodriguez A, Salvador J, Gil MJ, Cienfuegos JA, Fruhbeck G. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 2007;39:495–500. [DOI] [PubMed] [Google Scholar]
- 22.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1–.11. [DOI] [PMC free article] [PubMed]
- 23.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107 suppl 2:S159–70. [DOI] [PubMed] [Google Scholar]
- 24.Illán-Gómez F, Gonzalvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragon-Alonso A, Pascual-Diaz M, Perez-Paredes M, Lozano-Almela ML. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–5. [DOI] [PubMed] [Google Scholar]
- 25.Appachi S, Kelly KR, Schauer PR, Kirwan JP, Hazen S, Gupta M, Kashyap SR. Reduced cardiovascular risk following bariatric surgeries is related to a partial recovery from “adiposopathy”. Obes Surg. 2011;21:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86:3815–9. [DOI] [PubMed] [Google Scholar]
- 27.Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, Campbell KL, Duggan C, Foster-Schubert KE, Kong A, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72:2314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, He X, Shi X, Huang C, Liu J, Zhou S, Heng CK. Association between serum amyloid A and obesity: a meta-analysis and systematic review. Inflamm Res. 2010;59:323–34. [DOI] [PubMed] [Google Scholar]
- 29.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. [DOI] [PubMed] [Google Scholar]
- 30.Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, Wang F, Hull RL, Boyko EJ, Retzlaff BM, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–15. [DOI] [PubMed] [Google Scholar]
- 31.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes. 2003;52:172–9. [DOI] [PubMed] [Google Scholar]
- 32.Klimcáková E, Roussel B, Marquez-Quinones A, Kovacova Z, Kovacikova M, Combes M, Siklova-Vitkova M, Hejnova J, Sramkova P, Bouloumie A, et al. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. J Clin Endocrinol Metab. 2011;96:E73–82. [DOI] [PubMed] [Google Scholar]
- 33.Klimcakova E, Roussel B, Kovacova Z, Kovacikova M, Siklova-Vitkova M, Combes M, Hejnova J, Decaunes P, Maoret JJ, Vedral T, et al. Macrophage gene expression is related to obesity and the metabolic syndrome in human subcutaneous fat as well as in visceral fat. Diabetologia. 2011;54:876–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.