Summary
Tropism and efficiency of skeletal muscle depend on the complex balance between anabolic and catabolic factors. This balance gradually deteriorates with aging, leading to an age-related decline in muscle quantity and quality, called sarcopenia: this condition plays a central role in physical and functional impairment in late life. The knowledge of the mechanisms that induce sarcopenia and the ability to prevent or counteract them, therefore, can greatly contribute to the prevention of disability and probably also mortality in the elderly. It is well known that skeletal muscle is the target of numerous hormones, but only in recent years studies have shown a role of skeletal muscle as a secretory organ of cytokines and other peptides, denominated myokines (IL6, IL8, IL15, Brain-derived neurotrophic factor, and leukaemia inhibitory factor), which have autocrine, paracrine, or endocrine actions and are deeply involved in inflammatory processes. Physical inactivity promotes an unbalance between these substances towards a pro-inflammatory status, thus favoring the vicious circle of sarcopenia, accumulation of fat – especially visceral – and development of cardiovascular diseases, type 2 diabetes mellitus, cancer, dementia and depression, according to what has been called “the diseasome of physical inactivity”.
Keywords: skeletal muscle, sarcopenia, aging, myokine
Skeletal muscle and aging
The dynamic balance between anabolic and catabolic status of human skeletal muscle is related to many factors, such as mechanical and nervous stimuli, age, hormonal changes, and nutrient intake, which tightly interact to determine muscle vitality and trophism. However, a decrease in skeletal muscle mass, by 3–8 % per decade after the age of 30 years (1), is a universal consequence of aging. Since the mid-’90s, the age-related “lack of flesh” has been termed sarcopenia (2), which indicates a deterioration in muscle quantity and quality leading to a gradual slowing of movement, a decline in strength and power, and an increased risk of falls and fall-related injuries. These features are considered distinctive components of sarcopenia and they have been incorporated also in the recent definition of the European Working Group on Sarcopenia in Older People (EWGSOP) (3).
Based upon epidemiological studies, where sarcopenia was defined as appendicular muscle mass two standard deviations below gender-specific reference data for young adults, the prevalence of this condition is estimated to be approximately 30% in individuals over 60 years of age and as high as 50% in those over 80 years (1). Sarcopenia is considered a risk factor for physical disability, independent of age, ethnicity, obesity, socioeconomic status, morbidity, and health behaviors (1). Thus, efforts are made to improve our understanding of the mechanisms that induce sarcopenia, finalized to its prevention or postponement, in the assumption that this would reduce morbidity, disability and mortality in the elderly population.
The myokines
Among other mechanisms involved in sarcopenia, the effects of hormones on skeletal muscle have received a great deal of attention. Thus, it has been known for decades that excessive glucocorticoids and thyroid hormones, as well as diminished testosterone, estrogen, and growth hormone, lead to muscle atrophy. Subsequent investigations pointed out the anabolic actions of IGF-1 and ghrelin (4,5). In addition, evidence has been provided, in recent years, that skeletal muscles produce a variety of molecules, denominated “myokines”, which act in an autocrine, paracrine, or endocrine hormone-like fashion (6). The most important of these substances are interleukin (IL)-6, IL-8, IL-15, Brain-Derived Neurotrophic Factor (BDNF), and Leukemia Inhibitory Factor (LIF).
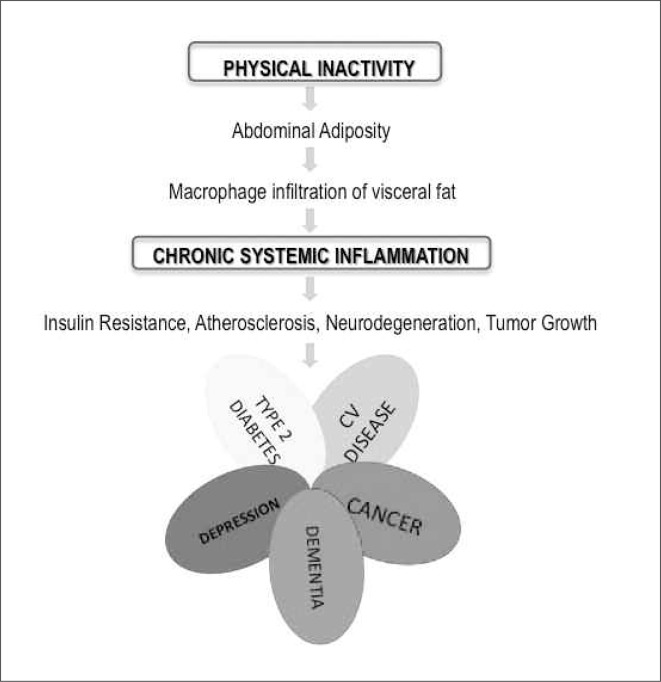
Physical activity has a favorable role in the delicate balance between myokines, which is definitively pushed towards a proinflammatory status by a sedentary lifestyle: inflammation, in turn, enhances sarcopenia and accumulation of fat within the context of skeletal muscle, in a vicious circle that decreases muscle strength and further favors physical inactivity. Moreover, visceral fat accumulation is a risk factor for cardiovascular disease, type 2 diabetes mellitus, cancer, dementia, and depression, according to what has been called “the diseasome of physical inactivity” (Figure 1) (7): fat tissue, indeed, sustains chronic inflammation, which is involved in the pathogenesis of insulin resistance, atherosclerosis, neurodegeneration, and tumor growth. Evidence suggests that the protective effect of exercise towards cardiovascular disease and other chronic degenerative disorders may, to some extent, be ascribed to the anti-inflammatory effect of regular exercise. The finding that muscles produce and release myokines provides a plausible biological explanation to the observation that exercise influences metabolism and exerts anti-inflammatory effects (8–10). According to Pedersen, contracting skeletal muscles release myokines, which work in a hormone-like fashion, exerting specific endocrine effects on visceral fat. Other myokines act locally, i.e. within the muscle, via paracrine mechanisms, working on signaling pathways involved in fat oxidation.
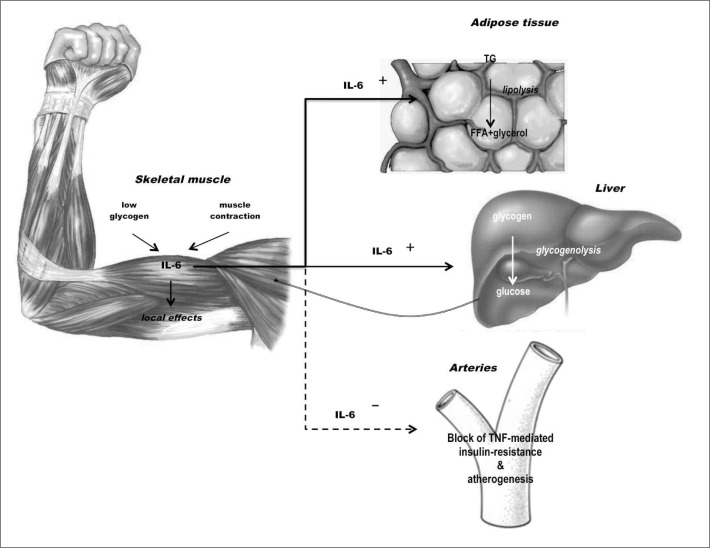
Figure 1.
Biological role of contraction-induced IL-6. Modified from Febbraio MA, et al. (18).
IL-6 was the first cytokine to be proposed as a myokine by Pedersen et al. in 2003 (11): in the year 2000 it was observed that IL-6 plasma levels increase with exercise (12), but only subsequent research highlighted that muscle-derived IL-6 is an important player in metabolism (13). It was later shown that the production of IL-6 during exercise is particularly high when muscle levels of glycogen are low, as a possible response of the muscle itself to a specific metabolic demand (14). IL-6 is produced by both type I and II fibres in response to muscle contraction (14–17) and it subsequently exerts its effects locally and remotely. Within skeletal muscle, IL-6 activates AMPK and/or PI3-kinase to increase glucose uptake and fat oxidation, but it is also released into the bloodstream to reach the liver, where it increases glucose production during exercise, and the adipose tissue, where it enhances lipolysis (Figure 2) (18). Taken together, local and distant actions of muscle-derived IL-6 synergistically increase the availability of energetic substrates to contracting muscles.
Figure 2.
The pathogenic hypothesis of “diseasoma of physical inactivity”. Modified from Pedersen BK, et al. (7).
Further evidence supports the role of IL-6 on glucose metabolism in the liver. Indeed, it has been shown that this myokine inhibits glycogen synthase and accelerates glycogen phosphorylase activity (19). Moreover, it may increase basal and insulin-stimulated glucose uptake via increased GLUT4 translocation from the intracellular compartment to the plasma membrane of muscle cells (20), suggesting an important role of this myokine and of skeletal muscle in maintaining glucose homeostasis. These data have been confirmed by other studies, which showed delayed onset of diabetes mellitus and increased survival in transgenic non-obese diabetic (NOD) mice that overexpress IL-6, compared to NOD mice with normal IL-6 expression (21), whereas IL-6-deficient mice have higher basal blood glucose and markedly impaired glucose disposal during intravenous glucose tolerance test (22). It, therefore, appears the IL-6 would be produced by skeletal muscle in order to maintain glucose homeostasis during periods of altered metabolic demand or under insulin stimulus (20).
IL-6 has been always considered a pro-inflammatory cytokine: this is, indeed, its role when it is produced by monocytemacrophages in response to infectious stimuli. However, contraction-induced production of IL-6 by skeletal muscles occurs in the absence of other inflammatory mediators, mainly IL-10 and TNF-a indicating that the cytokine cascade induced by physical activity is not resembling inflammation (13); quite the opposite, exercise increases circulating levels of anti-inflammatory cytokines, such as IL-1ra, IL-10, and soluble TNF-R, a naturally occurring inhibitor of TNF-a (13). During exercise, IL-6 itself might display anti-inflammatory effects, as data suggest that this myokine is able to suppress IL-1 and TNF-a synthesis (23) and stimulate production of IL-1ra and IL-10 (24). In conclusion, it can be hypothesized that physical activity, by stimulating IL-6 production, counteracts systemic inflammation and modulates glucose and lipid metabolism through the mechanisms described above: this would explain the well-known favorable effects of physical activity towards the diseases associated with a sedentary lifestyle (7) (Figure 2).
IL-15 was identified as an anabolic factor, because it can stimulate muscle growth. In addition, it is seemingly implicated in lipid metabolism (25), as an inverse correlation has been shown between plasma levels of IL-15 and trunk fat mass, and overexpression of skeletal muscle IL-15 determines a reduction of visceral fat in murine models (26).
Another myokine whose production is increased after exercise is the BDNF (27), a protein that may play a crucial role in regulating survival, growth, and maintenance of neurons, therefore interfering with information processing, learning and memory. Individuals with Alzheimer’s disease have low levels of plasma BDNF (28), whereas post-mortem studies have found decreased BDNF expression in hippocampal specimens from their brains (29). Decreased blood levels of BDNF have been shown also in subjects suffering from major depression (30), acute coronary syndromes (31), and type 2 diabetes mellitus (32). Although BDNF mRNA and protein increase in human skeletal muscle with physical exercise (27), this myokine, unlike others, is not released into the circulation. Its biological effect is to enhance fat oxidation in an AMPK-dependent fashion, within skeletal muscles, with a consequent reduction of adipose tissue bulk (27).
Taken together, the available evidence indicates that muscle activity would improve lipid metabolism and reduce visceral fat, thus ultimately reducing the risk of cardiovascular diseases, diabetes mellitus, dementia (33), and some types of cancer (34,35), at least in part by stimulating the production of BDNF and IL-15.
IL-8 is a well-known chemokine for neutrophils, but it also acts as an angiogenic factor. Plasma levels of IL-8 increase in response to exhaustive exercise which involves eccentric muscle contractions (36) but not during regular physical activity (37), suggesting that it is a myokine with paracrine activity. What is its role within the muscle is to be clarified further.
Finally, LIF is a pleiotropic cytokine that has been suggested to have positive effects on myogenesis (38) by increasing survival of myoblasts (39).
Conclusions
According to some authors (40,41), a major component of the aging phenotype may be explained by an imbalance between inflammatory and anti-inflammatory networks, resulting in the low-grade, chronic pro-inflammatory status that Franceschi and Bonafè called inflammaging(42). Systemic low-grade inflammation, defined as two-to four-fold elevation in circulating levels of pro-inflammatory and anti-inflammatory cytokines, appears to contribute to the development of atherosclerosis, insulin resistance, tumor growth, and neurodegeneration (43). High levels of IL-6 are associated with lower muscle strength and mass (44) and with a two-to three-fold increased risk of muscle strength decline in older persons (45). Thus, it has been hypothesized that inflammation plays a causal role in the functional decline associated with aging, likely through sarcopenia (45). Low-grade inflammation has been associated also with frailty, defined as a multi-system impairment with increased vulnerability to stress in old age, distinct from, although inter-related with, comorbidity and disability (46,47). Within this perspective, data collected by Baggio et al., showing high levels of IL-6 in healthy centenarians, represent an apparent paradox (48), which might be explained by a shift in the biological significance of this cytokine in centenarians; indeed, it can be speculated that in the very old plasma IL-6 is mainly produced by skeletal muscle as a myokine, thus with a completely different biological impact.
Over the past decades, several studies have shown a protective effect of regular physical activity on morbidity and all-cause mortality (49), and myokines released by contracting skeletal muscles, by creating a systemic anti-inflammatory environment and exerting endocrine effects on visceral fat and glucose and lipid metabolism, may be, at least partially, responsible for the beneficial effects of exercise. In this light, healthy aging can be viewed as the ability not only to power the age-associated proinflammatory state, but also to stimulate and potentiate anti-inflammatory mechanisms. Many studies are still needed, but the evidence collected so far clearly indicates that skeletal muscle, under the simple, physiological stimulus of exercise, may behave as an endocrine organ, producing a variety of agents with favorable metabolic actions. This shed some light to the mechanisms through which physical exercise protects against the development of disease and promotes longevity, thus opening fascinating venues to research on prevention and treatment.
References
- 1.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon AM, Bouloux PMG. Modifying muscle mass – the endocrine perspective. J Endocrinol. 2006;191:349–360. doi: 10.1677/joe.1.06837. [DOI] [PubMed] [Google Scholar]
- 5.Sakuma K, Yamaguchi A. Sarcopenia and age-related endocrine function. Int J Endocrinol. 2012;2012:127362. doi: 10.1155/2012/127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen BK. Muscle and their myokines. J Exp Biol. 2011;214:337–346. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen BK. The diseasome of physical inactivity- and role of myokines in muscle – fat cross talk. J Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt C, Pedersen BK. The Role of Exercise-Induced Myokines in Muscle Homeostasis and the Defense against Chronic Diseases. J Biomed Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78:819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 10.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen BK, Steensberg A, Fischer C, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle res Cell Motil. 2003;24:113–9. doi: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- 12.Steensberg A, van Hall G, Osada T, et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen BK, Febbraio MA. Muscle as endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 14.Keller C, Steensberg A, Pilegaard H, et al. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- 15.Hiscock N, Chan MH, Bisucci T, et al. Skeletal myocytes are a source a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–4. doi: 10.1096/fj.03-1259fje. [DOI] [PubMed] [Google Scholar]
- 16.Malm C, Nyberg P, Engstrom M, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penkowa M, Keller C, Keller P, et al. Immunohistochemical detection of interleukin-& in human skeletal muscle fibers following exercise. FASEB J. 2003;17:2166–2168. doi: 10.1096/fj.03-0311fje. [DOI] [PubMed] [Google Scholar]
- 18.Febbraio MA, Pedersen BK. Muscle-derived inteleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;11:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 19.Kanemaki T, Kitade H, Kaibori M, et al. Interleukin 1 beta and interleukin 6, but not tumor necrosis factor alpha, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology. 1998;27:1296–1303. doi: 10.1002/hep.510270515. [DOI] [PubMed] [Google Scholar]
- 20.Carey AL, Stenberg GR, Macaulay SL, et al. IL-6 increases insulin stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMPK. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 21.DiCosmo BF, Picarella D, Flavell RA. Local production of human IL6 promotes insulitis but retards the onset of-insulin-dependent diabetes mellitus in non-obese diabetic mice. Int Immunol. 1994;6:1829–1837. doi: 10.1093/intimm/6.12.1829. [DOI] [PubMed] [Google Scholar]
- 22.Wallenius W, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 23.Schindler R, Mancilla J, Endres S, et al. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- 24.Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:433–437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen AR, Pedersen BK. The biological roles of exercise-induced cytokines: IL-6, IL-8, and IL-15. Appl Physiol Nutr Metab. 2007;32:833–9. doi: 10.1139/H07-054. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen AR, Hojman P, Erikstrup C, et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab. 2008;11:4486–93. doi: 10.1210/jc.2007-2561. [DOI] [PubMed] [Google Scholar]
- 27.Matthews VB, Aström MB, Chan MH, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–18. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 28.Laske C, Stransky E, Leyhe T, et al. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. 2006;113:1217–24. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- 29.Conner JM, Lauterborn JC, Yan Q, et al. Distribution of Brain-Derived Neurotrophic Factor (BDNF) Protein and mRNA in the Normal Adult Rat CNS: Evidence for Anterograde Axonal Transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karege F, Perret G, Bondolfi G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 31.Manni L, Nikolova V, Vyagova D. Reduced plasma levels of NGF and BDNF in patients with acute coronary syndromes. Int J Cardiol. 2005;102:169–171. doi: 10.1016/j.ijcard.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 32.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–8. doi: 10.1007/s00125-006-0537-4. [DOI] [PubMed] [Google Scholar]
- 33.Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 35.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86:823–35. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 36.Nieman DC, Henson DA, Smith LL, et al. Cytokine changes after marathon race. J Appl Physiol. 2001;91:109–114. doi: 10.1152/jappl.2001.91.1.109. [DOI] [PubMed] [Google Scholar]
- 37.Akerstrom TC, Steensberg A, Keller P, et al. Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol. 2005;563:507–516. doi: 10.1113/jphysiol.2004.077610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Austin L, Bower J, Kurek J, Vakakis N. Effects of leukaemia inhibitory factor and other cytokines on murine and human myoblast proliferation. Journal of the neurological sciences. 1992;11:185–191. doi: 10.1016/0022-510x(92)90149-f. [DOI] [PubMed] [Google Scholar]
- 39.Hunt L, Tudor E, White J. Leukaemia inhibitory facto-dependent increase in myoblast cell number is associated with phosphotidylinositol 3-kinase-mediated inhibition of apoptosis and not mitosis. Experimental Cell Research. 2010;316:1002–1009. doi: 10.1016/j.yexcr.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Ostan R, Bucci L, Capri M, et al. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15:224–40. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- 41.Candor G, Caruso C, Colonna-Romano G. Inflammation, genetic background and longevity. Biogerontology. 2010;11:565–573. doi: 10.1007/s10522-010-9286-3. [DOI] [PubMed] [Google Scholar]
- 42.Franceschi C, Bonafè M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31:457–61. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- 43.Handschin C, Spiegelman BM. The role of exercise and PGC1alfa in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visser M, Pahor M, Taaffe DR, et al. Relationship of IL6 and TNF-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC study. J Geront A Bio Sci Med Sci. 2002;57:326–332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 45.Shaap L, Pluijm S, Deeg D, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J med. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 46.Fried LP, Tangen CM, Waltson J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Bio Sci Med Sci. 2001;56:146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 47.Wong CH, Weiss D, Sourial N, et al. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in montreal: a cross-sectional study. Aging Clin Exp Res. 2010;22:54–62. doi: 10.1007/BF03324816. [DOI] [PubMed] [Google Scholar]
- 48.Baggio G, Donazzan S, Monti D, et al. Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J. 1998;12:433–437. doi: 10.1096/fasebj.12.6.433. [DOI] [PubMed] [Google Scholar]
- 49.Nocon M, Hiemann T, Muller-Riemenschneider F, et al. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiov Prev R. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]