Summary
Introduction.
Osteoporosis is a chronic condition leading to an increased risk of developing fractures, with high morbidity and mortality in aging population. Efficacy of anti-osteoporotic treatment is based on drug potency but also on compliance and persistence to treatment regimen, which is very low, as already described for other diseases. Teriparatide (TPTD) is the first anabolic agent developed for the treatment of osteoporosis.
Since it appears that persistence to Teriparatide declines over time, aim of this pilot multicenter observational study was to evaluate persistence and adherence to TPTD (20 μg daily injection regimen for 18 months) treatment (PATT) in patients affected by severe osteoporosis in an every day clinical practice.
Methods.
Patients affected by severe osteoporosis were selected among those who referred to 5 different specialized centers for osteoporosis in North, Center and South of Italy. A sample of 475 women with severe postmenopausal osteoporosis treated with TPTD in accordance to the Italian osteoporosis guidelines was included. At the beginning of TPTD treatment patients were instructed on the use of the device by the referring specialist of the center, a resident fellow or a nurse. Bone biochemical markers were evaluated the same morning and after 1, 3, 6, 12 and 18 months. Patients were visited at time 0 and after 6, 12 and 18 months for clinical follow up.
Results.
The results included observations of 441/475 patients (98% women) who completed the 18 months treatment; mean age for women was 73±8 and for men 65±9. After 6 months of TPTD treatment persistence was of 89,79%, 87,75% after 12 months and 86,85% after 18 months. Adherence was of 100% at 6,12 and 18 months. Total dropouts were 13,15% (71/441), which was usually higher within the first 6 months of TPTD treatment. Most common adverse events (arthralgies 2,7%, dizziness 1,8%, migraine 1,8%, depression 1,6%, hypertension 1,1%) were reported in 62/441 patients (14%) of patients, but were not reason for stopping treatment.
Conclusions.
The persistence and adherence to TPTD treatment obtained in this multicenter observational real life study was very high as compared to studies performed by others. These encouraging results suggest that different key factors such quality of information, frequency of visits, motivations given to patients, opportunity to call the doctor might play a pivotal role in the high persistence and adherence to TPTD treatment obtained in our study and need to be carefully considered before prescribing chronic anti-osteoporotic therapy.
Keywords: osteoporosis, pharmacological treatment, teriparatide, adherence, persistence
Introduction
It is well known that improvement of life conditions has significantly increased life expectancy leading to an increased number of aged subjects suffering chronic disorders, such as obesity, diabetes, cardiovascular diseases, and osteoporosis (1,2). Although osteoporosis is not considered a directly life threatening condition, it has to be considered a key risk factor for fragility fractures in an aging population (3–5). It has been calculated that the 10 year risk of a fragility fracture in a 50 year old post-menopausal woman with osteoporosis is 5%, but it increases to 20% by the age of 65 (3). One of six patients aged 50–55 after hip fracture is discharged from the hospital to a nursing home; this rate increases exponentially with increasing age (6, 7) while the mortality rates are on average 20% higher within 1 year of hip fracture (1). Despite these alarming figures, which link osteoporosis to major disabilities and high mortality rate in aging population, the perceived risks from osteoporosis among affected patients are low (5, 8), since they do not feel any direct benefit from taking an anti-osteoporotic drug leading to a high therapy discontinuation (9).
Indeed, several pharmacological treatments have been developed in the recent years, which are all effective in decreasing the risk of fragility fractures when evaluated in controlled clinical trials. However, therapeutic benefits are highly dependent upon medication adherence (synonym: compliance) (10), which is usually defined as the extent to which patients take medication as prescribed by their physicians and it is expressed as a percent of prescribed doses taken over a specified period, and also persistence with a medication, defined as continuing to take the prescribed therapy (10) for the period indicated.
A new therapeutic agents recently introduced for the cure of osteoporosis, Teriparatide (TPTD), the human (1–34) PTH peptide, is the first effective anabolic agent (11) which requires daily 20 μg subcutaneously injections (self-administered or performed by another individual). This anabolic agent has been demonstrated to increase osteoblast activity with consequent new bone formation on trabecular and cortical surfaces, increase in bone mass and improvement of bone strength (11). In a large randomized clinical trial of 1637 postmenopausal women TPTD significantly reduced the risk of vertebral fracture by 65% and the risk of non-vertebral fracture by 53%, as compared to placebo (11, 12).
However, efficacy of a specific drug, including TPTD, is due not only to drug potency but also to compliance and persistence to the specific treatment regimen. Indeed new drug efficacy is evaluated in randomized clinical trials (RCT) which imply selection of patients, usually high motivated, agreement in RCT protocol, preorganized follow up visit.
Thus, low therapeutic adherence is a major issue faced by physicians when they need to treat patients for chronic diseases such as osteoporosis in a daily clinical practice care (13). Indeed, long-term compliance and persistence with any therapy are very poor, therefore they are most common causes of low effectiveness of pharmacological treatments. Several recent studies have evaluated fragility fractures burden by different models in terms of both high economic costs and health related quality of life showing a pivotal role in the lack of efficacy due to poor adherence and persistence of patients to therapy (14–16).
For instance, recent studies have shown that less than half of patients affected by this skeletal disorder are adherent to bisphosphonate therapy regimens (17), showing that only one-third of postmenopausal women who were prescribed daily bisphosphonates, and just less than half on weekly bisphosphonate therapy, had adequate adherence to this regimen (17). The most frequent reasons for discontinuation of bisphosphonate treatment were insufficient motivation, fear of adverse events or drug related side effects, regimen treatment (18–20). Thus, aim of this pilot multicenter study was to evaluate adherence and persistence to TPTD treatment (daily injection regimen) in individuals affected by severe osteoporosis in an everyday clinical practice care.
Material and methods
Patients
Postmenopausal women or men affected by severe osteoporosis were enrolled in 5 different osteoporosis centers (one rheumatologic, two orthopaedics, one endocrinology, one physiatric) located in the north, center and south of Italy. Patients were prescribed TPTD therapy according to the Italian reimbursement criteria for osteoporosis (National Health Service 79), which requires diagnosis of severe osteoporosis (3 or more prevalent severe vertebral fractures; or 2 prevalent severe vertebral fractures and a historical proximal hip fracture; or an incident vertebral fracture or hip fracture during treatment), including patients affected by glucocorticoid-induced osteoporosis (GIO).
Demographic variables were collected for all patients at the baseline observation as shown in Table 1. Informations were also obtained regarding risk factors (history of fragility fracture after age of 40; history of fragility fracture in mother; smoking status; alcohol use; regular exercise; current chronic diseases and/or chronic therapies that could affect bone metabolism/fracture risk) and previous anti-osteoporotic treatments. Historical vertebral fractures were confirmed by radiographs, non vertebral fractures were confirmed by radiographs anytime this was possible and by medical records in all other cases.
Table 1.
Baseline characteristics of patients initiating Teriparatide treatment.
| General features | |
|---|---|
| Gender, (n) | |
| Women | 434 |
| Men | 7 |
| Women Age (years) | |
| Mean ± SD | 73,28 |
| Range | 8,14 |
| Men Age (years) | |
| Mean ± SD | 64,43 |
| Range | 9,38 |
| Time from menopause (years) | |
| Mean ± SD | 34,5 |
| Range | 10,7 |
| Women BMI | |
| Mean ± SD | 23,22 |
| Range | 3,85 |
| Men BMI | |
| Mean ± SD | 24,57 |
| Range | 2,93 |
|
| |
| Family history, n (%) | |
| Mother with osteoporosis | |
| Mother with fragility fractures | 25,29 |
|
| |
| Number of previous fractures per patients | |
|
| |
| Femoral (n=83) | 18,8 % |
| Vertebral (n=) | |
| mean ± SD | 3,08 ± 2,41 |
| range | 1–12 |
All patients were supplemented with 500–1000mg/day calcium and 400–800 UI/day vitamin D. All patients signed an informed consent and this observational study was approved by ethical committee of each site.
Methods
In our study, we considered as indicator of persistence to Teriparatide treatment, the percentage of patients still on treatment at 6, 12 and 18-months after first drug prescription. We assumed that each patient took out the drug prescribed every 28-days from the Local Hospital Pharmacy. Rates of adherence were reported as the percentage of the prescribed doses of the medication taken by the patient over a specified period with properly administration conforming to the recommendations made by the provider (21).
All subjects underwent clinical examination (time 0, and after 6, 12 and 18 months), routine and bone-specific biochemical exams (time 0, after 1, 3, 6 12 and 18 months), dorso-lumbar XRay (time 0, and if required by clinical examination, at 18 months), lumbar and femoral BMD measurements by dual X-ray absorptiometry (DXA) were performed (time 0 and after 18 months). Biochemical exams results were sent by patients (by fax or email) to assigned physician, who recalled patient only if abnormal results were found.
Patients, and/or close relatives if they were accompanied by, received a 30 minutes one-to-one explanation (or in small group of patients) by a physician regarding the importance of taking treatment. During the same visit patient received a training regarding delivery and use of Teriparatide pen. All physicians involved in this study gave similar kind of information to patients (and eventually to accompanying relatives). Blood test and DXA exam was performed on the same morning. DXA instruments were both, either GE Lunar or HOLOGIC Bone Densitometer.
All patients who began the treatment had the possibility to call a dedicated telephone number to have answers of any questions about the new medication prescribed.
Statistical analysis
Data management and analyses were centralized, and evaluated by a Chi-square test to evaluate statistical significance. Data are expressed as mean ±SD.
Results
A total of 475 patients were evaluated in this pilot multicenter study performed in 5 Italian centres. In particular at the time of statistical evaluation 441 patients were prescribed TPTD and completed the 18 months course treatment, 388 subjects performed 6 months visit, 376 were visited after 12 months and 370 were evaluated at 18 months end point.
Baseline features of the individuals analyzed and prescribed TPTD treatment are summarized in Table 1. As previously mentioned, most of the patients were women (98% women), with a mean body mass index (BMI) 25,27 kg/m2, elderly (mean age was 73±8 years for women and 65±9 years for men). Mean age at menopause was 48±5.
Most of the patients reported no physical activity (however none of them were bed-ridden) and relatively few patients had other known risk factors including regular alcohol consumption and current or former smoking. A subset of patients (25,9%) reported a maternal history positive for fragility fractures. Patients had received a diagnosis of severe osteoporosis based on bone mineral density evaluation (mean T Score) and fractures (vertebral and / or femoral) and all of them had previously performed anti-osteoporotic treatments.
Biochemical markers on bone remodeling and densitometric evaluation (data not shown) reflected the modifications previously published by Neer et al. (11).
In the treatment of osteoporosis good adherence has generally been defined as the use of 80% or more of prescribed medication (22). In our study, persistence has been defined as the percentage of patients still on treatment at the end of the 18-months course.
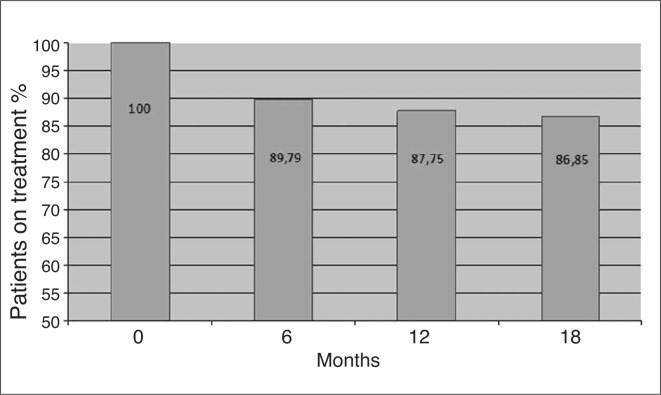
In particular, after 6 months of TPTD treatment persistence was of 89,79%, 87,75% after 12 months and 86,85% after 18 months (Figure 1). Adherence, as previously described (22) was of 100% at 6, 12 and 18 months.
Figure 1.
Rate of persistence, expressed as % of total patients initiating TPTD therapy.
Total dropouts was 13,15% (71/441), which was usually higher within the first 6 months of TPTD treatment with a almost total persistence the year-after.
Most common adverse events (arthralgies 2,7%, dizziness 1,8%, emicrania 1,8%, depression 1,6%, hyperthension 1,1%) were reported in 62/441 patients (14%) of patients.
Drop-out most common causes were: nausea and vomiting (8,6%), dizziness (4,1%), hypertension (5,2%), emicrania (8,6%). For more detailed description see Table 2. One patient over 441 discontinued therapy disclaiming stress of daily therapy. Three patients died: one for car accident, the others for causes judged not related to TPTD treatment.
Table 2.
Most Common Causes of Drop Outs in patients taking Teriparatide.
| Nausea, Vomiting | 8,6 % (5/58) |
| Migraine | 8,6 % (5/58) |
| Dizziness | 5,2 % (3/58) |
| Death (*) | 5,2 % (3/58) |
| Hypertension | 5,2 % (3/58) |
| Renal Failure | 3,4 % (2/58) |
| Chest pain | 3,4 % (2/58) |
| Allergy | 3,4 % (2/58) |
| Unknown reason | 39,6% (23/58) |
One patient died in a car accident
Discussion
This observational study was the first multicenter Italian study (PATT study) assessing persistence and adherence of patients affected by severe osteoporosis treated with TPTD for 18 months in a everyday clinical practice care.
It is well known that persistence and adherence are key factors to achieve full therapeutic benefits of all anti-osteoporotic drugs, including TPTD treatment.
Interventions to improve osteoporosis medication persistence and adherence have evaluated effects of different regimens, patient monitoring, and education. Several studies (8, 19, 23, 24) show that oral weekly bisphosphonates may improve adherence versus daily regimen; however other studies pointed out that, even for the patients taking bisphosphonates dosed weekly, persistence and adherence were suboptimal. Although it is possible that a less frequently dosed drugs might further improve adherence (8), the literature suggests that enhancing medication adherence is much more complicated than changes in frequency of dosing (25–28). Indeed, optimal adherence is associated with fewer osteoporotic fractures, and the impact appear more evident among patients with prior fractures.
Data by Clowes et al. suggested that patient education might be considered an important strategy to improve therapy adherence (29–31) and additional studies on patients with chronic diseases such as rheumatoid arthritis and asthma show that face-to-face counseling from healthcare providers can significantly improve adherence (32).
For instance, clinicians might have had some concern prescribing TPTD treatment believing that patients would have not been as adherent or persistent as with other treatments for osteoporosis due to the medication’s daily injectable route of administration, older age of patients and worse health status due to a more severe osteoporosis than patients on other anti-osteoporotic drugs (33, 34).
Our data obtained in this multicenter observational study showed that persistence to TPTD therapy has been maintained high through the 18 months of treatment regimen (86,85%), whereas adherence was optimal (100%). Most common adverse events (arthralgies, dizziness, emicrania, depression, hyperthension) were reported in 62/441 patients (14%) of patients. None of patient discontinued therapy because of the above mentioned adverse events which, if present, disappeared within first few weeks of treatment, except arthralgias that persisted over the all treatment period. Patients well tolerated these minimal adverse events compared to the improvement of vertebral fracture back pain.
After first visit when therapy was prescribed, all our patients were met or contacted after 1 and 3 months (to control biochemical serum parameters, including potential calcium fluctuation) and visited again after six and 12 months to accomplish to the National health care prescription protocol.
Training on self-administered subcutaneous injection (patients and/or their relatives), motivation instilled in the patients, subsequent visits to the first meeting set, possibility of contact by telephone (for potential presence of any problems or side effects), monitoring of blood test during the first months, when is higher the risk of treatment dropout, were all likely reasons which justify the high persistence and adherence to Teriparatide in our study as compared to others evaluating the same molecule (35–36).
Adverse events were few and modest; the self-administered subcutaneous injection of TPTD resulted easy for patients. In particular the adherence to TPTD during the first semester treatment has allowed patients to obtain improvement of quality of life, and a strong stimulus for TPTD treatment persistence and adherence.
Other studies have been performed in different countries (34–38) to examine persistence and adherence to the same treatment have found high persistence to the therapy but with lower rate (81%) after 18 months (35) while others have published data only at 12 months (37).
A limitation of our study could be found in the fact that our patients suffered from severe osteoporosis and have previously sustained several fragility fractures, which had significantly impaired the quality of life and undoubtedly accentuated their awareness of the seriousness of the disease and willingness to continue the pharmacological treatment.
Strengths of our study include the use of a typical community population versus a clinical trial population, where volunteers may be different from common everyday patients; additionally multiple specialty centers (rheumatology, orthopedic, endocrinology and physiatric); different Italian regions (north, center south) where medical approach might not be the same.
In particular, a pivotal role seems to be played by the prescribing physician in terms of the quality of information and motivations given to patients (risk of the disease and efficacy of the prescribed treatment), frequency of visit, opportunity to call the doctor were all points which need careful valuation before prescribing TPTD treatment in order to have high adherence and persistence.
Finally the results of this multicenter study indicate that persistence with TPTD in our patients affected by severe osteoporosis seems to be higher than expected with other anti-osteoporotic drugs likely for therapy effectiveness but also for a special relationship patient-physician which needs to be increased to add value for health-care systems.
Conclusions
The persistence and adherence to TPTD treatment obtained in this multicenter observational real life study was very high as compared to results obtained by others. These encouraging results suggest that different key factors such quality of information, frequency of visits, motivations given to patients, opportunity to call the doctor might play a pivotal role in the high persistence and adherence to TPTD treatment obtained in our study and need to be carefully considered before prescribing chronic anti-osteoporotic therapy.
Acknowledgements and disclosure
No competing interests exist for any authors.
Abbreviations
- (PATT)
Persistence Adherence Teriparatide Treatment
- TPTD
Teriparatide
- RCT
randomized clinical trials
- DXA
dual X-ray absorptiometry
- BMI
body mass index
Footnotes
Author’s contribution
SM, GR, AL,UT, GI, NM conceived the study, participated in its design and coordination and helped to draft the manuscript
FG carried out follow up organization of patients, performed the statistical analysis and helped to draft the manuscript
AD, NM performed statistical analysis
AB, RF, GDP, IC, RM, MC, MF carried out visits, organization and follow up of patients
All authors read and approved the final manuscript.
References
- 1.Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103(Suppl):12–17. doi: 10.1016/s0002-9343(97)90022-x. [DOI] [PubMed] [Google Scholar]
- 2.Oleksik A, Lips P, Dawson A, et al. Health-related quality of life in post-menopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15:1384–92. doi: 10.1359/jbmr.2000.15.7.1384. [DOI] [PubMed] [Google Scholar]
- 3.Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013–22. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 4.Raisz LG. Screening for osteoporosis. N Engl J Med. 2005;353:164–71. doi: 10.1056/NEJMcp042092. [DOI] [PubMed] [Google Scholar]
- 5.Gold DT, Silverman SL. Compliance with osteoporosis medications: challenges for healthcare providers. Medscape Ob/Gyn & Women’s Health. 2005;10:1–5. [Google Scholar]
- 6.Ettinger MP. Aging bone and osteoporosis. Arch Intern Med. 2003;163:2237–46. doi: 10.1001/archinte.163.18.2237. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int. 2004;152:108–12. doi: 10.1007/s00198-003-1516-y. [DOI] [PubMed] [Google Scholar]
- 8.Reginster JY. Adherence and persistence: impact on outcomes and health care resources. Bone. 2006;38(Suppl2):18–21. doi: 10.1016/j.bone.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Kothwala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 10.Silverman SL, Gold DT, Cramer JA. Reduced fracture rates observed only in patients with proper persistence and compliance with bisphosphonate therapies. South Med. 2007;100:1214–1218. doi: 10.1097/SMJ.0b013e31815a9685. [DOI] [PubMed] [Google Scholar]
- 11.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher JC, Genant HK, Crans GG, Vargas SJ, Krege JH. Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. J Clin Endocrinol Metab. 2005;903:1583–7. doi: 10.1210/jc.2004-0826. [DOI] [PubMed] [Google Scholar]
- 13.Rabenda V, Hiligsmann M, Reginster JY. Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother. 2009;10:2303–15. doi: 10.1517/14656560903140533. [DOI] [PubMed] [Google Scholar]
- 14.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–928. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Seeman E, Compston J, Adachi J, Brandi ML, Cooper C, Dawson-Hughes B, Jönsson B, Pols H, Cramer JA. Non-compliance: the Achilles’ heel of anti-fracture efficacy. Osteoporosis Int. 2007;18:711–719. doi: 10.1007/s00198-006-0294-8. [DOI] [PubMed] [Google Scholar]
- 16.Warriner AH, Curtis JR. Adherence to Osteoporosis Treatments: Room for Improvement. Curr Opin Rheumatol. 2009;21:356–362. doi: 10.1097/BOR.0b013e32832c6aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papaioannou A, Kennedy CC, Dolovich L, Lau E, Adachi JD. Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging. 2007;24:37–55. doi: 10.2165/00002512-200724010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Carr AJ, Thompson PW, Cooper C. Factors associated with adherence and persistence to bisphosphonate therapy in osteoporosis: a cross-sectional survey. Osteoporos Int. 2006;17:1638–44. doi: 10.1007/s00198-006-0166-2. [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Amonkar MM, Hebborn A, Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with post-menopausal osteoporosis. Curr Med Res Opin. 2005;21:1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 20.Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, Adami S. Treatment of Osteoporosis in clinical Practice (TOP) Study Group. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int. 2006;17:914–21. doi: 10.1007/s00198-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 21.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication Compliance and Persistence: Terminology and Definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 22.Gold DT, Alexander IM, Ettinger MP. How can osteoporosis patients benefit more from their therapy? Adherence issues with bisphosphonate therapy. Ann Pharmacot. 2006;40:1143–1150. doi: 10.1345/aph.1G534. [DOI] [PubMed] [Google Scholar]
- 23.Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–861. doi: 10.4065/80.7.856. [DOI] [PubMed] [Google Scholar]
- 24.Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM. Determinants of persistence with bisphosphonates: a study in women with post-menopausal osteoporosis. Clin Ther. 2006;28:236–242. doi: 10.1016/j.clinthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Emkey RD, Ettinger M. Improving compliance and persistence with bisphosphonate therapy for osteoporosis. Am J Med. 2006;119(Suppl4):18–24. doi: 10.1016/j.amjmed.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 26.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. doi: 10.1001/jama.288.22.2868. [published correction appears in JAMA 2003;289:3242.] [DOI] [PubMed] [Google Scholar]
- 27.Cramer JA, Silverman S. Persistence with bisphosphonate treatment for osteoporosis: finding the root of the problem. Am J Med. 2006;119(Suppl):12–17. doi: 10.1016/j.amjmed.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Osterberg R, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 29.Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:1117–1123. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 30.Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Brookhart MA. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414–2419. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 31.Varenna M, Sinigaglia L. Adherence to treatment of osteoporosis: an open question. Reumatismo. 2009;61:4–9. doi: 10.4081/reumatismo.2009.4. [DOI] [PubMed] [Google Scholar]
- 32.Miller NH. Compliance with treatment regimens in chronic asymptomatic diseases. Am J Med. 1997;102:43–49. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- 33.Foster SA, Foley KA, Meadows ES, Johnston JA, Wang S, Pohl GM, Long SR. Characteristics of patients initiating teriparatide for the treatment of osteoporosis. Osteoporos Int. 2008;19:373–377. doi: 10.1007/s00198-007-0455-4. [DOI] [PubMed] [Google Scholar]
- 34.Foster SA, Foley KA, Meadows ES, Johnston JA, Wang SS, Pohl GM, Long SR. Adherence and persistence with teriparatide among patients with commercial, Medicare, and Medicaid insurance. Osteop Int. 2011;22:551–7. doi: 10.1007/s00198-010-1297-z. [DOI] [PubMed] [Google Scholar]
- 35.Briot K, Ravaud P, Dargent-Molina P, Zylberman M, Liu-Leage S, Roux C. Persistence with teriparatide in postmenopausal osteoporosis; impact of a patient education and follow-up program: the French experience. Osteoporos Int. 2009;20:625–30. doi: 10.1007/s00198-008-0698-8. [DOI] [PubMed] [Google Scholar]
- 36.Mulgund M, Beattie KA, Wong AKO, Papaioannou A, Adachi JD. Assessing adherence to teriparatide therapy, causes of non adherence and effect of adherence and effect of adherence on bone mineral density measurements in osteoporotic patients at high risk for fracture. Ther Adv Muskol Disease. 2009;1:5–11. doi: 10.1177/1759720X09339551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziller V, Zimmermann SP, Kalder M, Ziller M, Seker-Pektas B, Hellmeyer L, Hadji P. Adherence and persistence in patients with severe osteoporosis treated with teriparatide. Current Medical Research & Opinion. 2010;26:675–681. doi: 10.1185/03007990903538409. [DOI] [PubMed] [Google Scholar]
- 38.Arden NK, Earl S, Fisher DJ, Cooper C, Carruthers S, Goater M. Persistence with teriparatide in patients with osteoporosis: the UK experience. Osteoporos Int. 2006;17:1626–1629. doi: 10.1007/s00198-006-0171-5. [DOI] [PubMed] [Google Scholar]