Summary
Severe hyperparathyroidism can affect bone metabolism and be in the origine of multiple brown tumours (generalized osteitis fibrosa cystica). When associated with fibro-ossifying tumours of the jaw, it realizes a rare genetic syndrome referred as Hyperparathyroidism-jaw tumour HPT-JT.
We report the case of a patient we treated for HPT-JT, and literature review.
Keywords: hyperparathyroidism, osteitis fibrosa cystica, ossifying fibroma
Introduction
Brown tumours, also called osteitis fibrosa cystica, are relatively rare non-neoplastic osteolytic lesions of bones that appear in an advanced stage of hyperparathyroidism and involve mostly axial skeleton and femora. These lesions may produce awful pain and pathologic fractures (1–3).
Association of primary hyperparathyroidism and ossifying fibroma of the jaw is seen in a rare hereditary syndrome referred as hyperparathyroidism-jaw tumour HPT-JT.
We report a case of a female patient who was treated for generalized osteitis fibrosa cystica related to a primary hyperparathyroidism associated to an ossifying fibroma of the jaw.
Case report
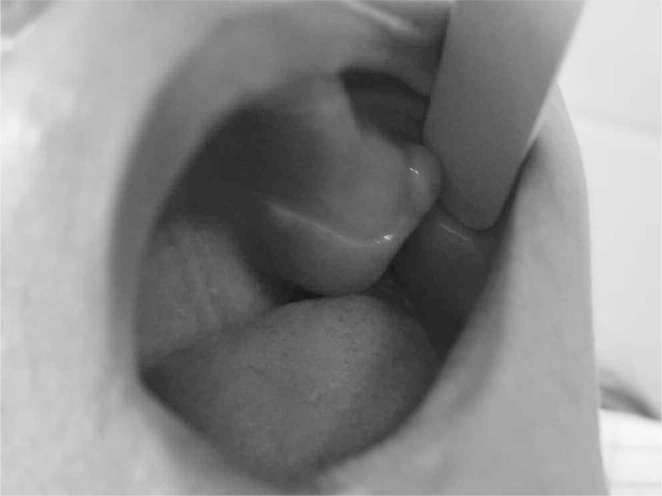
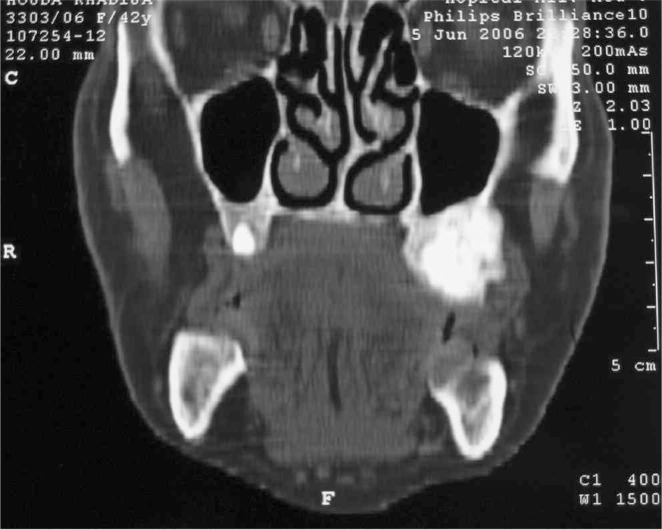
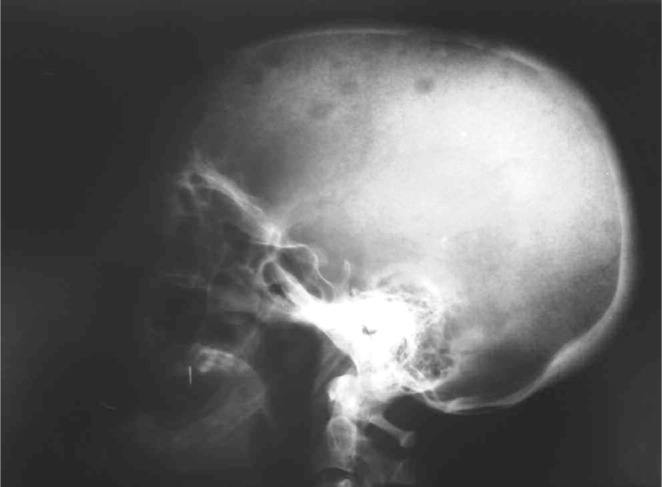
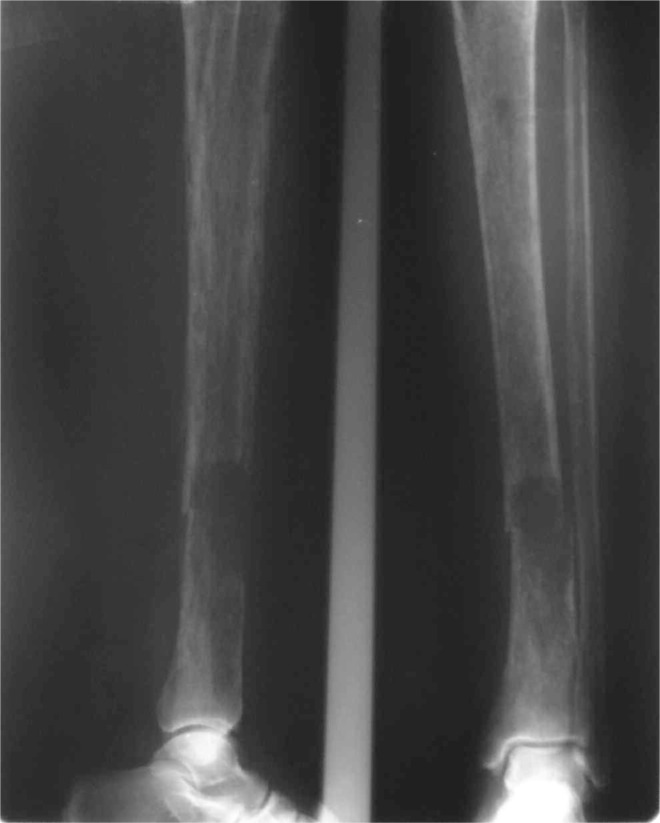
A 41-year-old woman suffering from diabetes for 8 years under glibenclamide, treated for pulmonary tuberculosis 3 years ago, who underwent surgery for radial bone osteitis 12 years ago, toothless, and without history of radiotherapy or family disease, was referred to our department for a palpable but indolent tumour of the left maxilla (Figure 1). Orthopantomography and Computed tomography (Figure 2) found a well-defined osteo-condensing lesion in the left maxillary tuberosity and a silent premaxillar cyst. We removed the maxillary tuberosity tumour which revealed an ossifying fibroma and we decided to follow up the premaxillar cyst. Six months later, the patient returned to the hospital suffering from an extreme fatigue, a severe weight loss (24 kg in 6 months), and also intolerable spontaneous pain of bones and joints involving especially right arm and leg. Clinical examination found a frontal bone tumour, dorsal and lumbar spinous processes pains and a cervical node attached to the right lobe of the thyroid. Bones radiography revealed generalized demineralization, multiple osteolytic lesions of the skeleton affecting long bones and frontal bone (Figure 3) and hands. Multiple fractures were involving costal bones, pelvic girdle, right humerus and tibia (Figure 4) and left fibula. Ultrasonography and CT of the neck showed a 30 mm node contiguous to the right lobe of the thyroid. The biology exams revealed an inflammatory anaemia, severe hypercalcemia (corrected calcemia: 199 mg/l), and functional renal failure. Phosphataemia was at a normal level.
Figure 1.
The tumour of the left maxilla for which the patient was first seen.
Figure 2.
The CT is showing an osteocondensing lesion of the left maxillary tuberosity.
Figure 3.
Brown tumours affecting the skull.
Figure 4.
Brown tumour and pathological fracture of the tibia.
Taken as a metabolic emergency, the patient was treated by immediate rehydration and diphosphonates. PTH 1–84 was increased to a very high level 2366 pg/ml (15–65 pg/ml) suggesting hyperparathyroidism. Blood protein electrophoresis and bone marrow examination eliminated the differential diagnosis of multiple myeloma. The levels of tumour markers were all within normal limits (AFP, ACE, CA 15-3, CA 19-9, CA 125, NSE and CYFRA), and the full body computed tomography found no visceral tumour. We therefore eliminated the hypothesis of metastatic lesions of bones and paraneoplastic syndromes.
This hyperparathyroidism was therefore considered as primary and we supposed that osteolytic lesions were brown tumours. The patient was treated by cast immobilization for her fractures, associated to heparin therapy and analgesics. A surgery was performed to explore the thyroid gland. A necrotic parathyroid gland was found which suggested cancer. Large right isthmolobectomy was realised. The histology examination revealed a parathyroid adenoma.
The follow-up showed clinical improvement, bone pain lack and normalization of calcemia and PTH rate (27 pg/ml after 3 weeks). A long term follow-up couldn’t be carried because of the lost sight of the patient after clinical improvement.
Discussion
Parathyroid hormone (PTH) is secreted by parathyroid glands under the control of the ionized calcium level. PTH regulates serum calcium levels and bone metabolism. Hyperparathyroidism means the presence of high concentrations of PTH or PTH-related peptide PTHrP. Hyperparathyroidism causes bone metabolic disorders represented in an increase bone resorption and also an increased frequency of bone remodelling leading to increased bone porosity. Microhemorrhages and microfractures lead to blood by-products deposition such as hemosiderin which confers reddish-brown hue, hence the name of brown tumours (4). Brown tumours of bones are a late manifestation of severe hyperparathyroidism and affect mostly cortical bones. When brown tumours occur in head and neck, they usually involve the mandible, a cortical bone whereas the affection of the maxilla as seen in this case is rare (2).
Primary hyperparathyroidism is defined by an increased PTH production related to a parathyroid adenoma in most cases (85%), followed by parathyroid hyperplasia and parathyroid carcinoma (1%). Biology examination often reveals hypercalcaemia and low or normal serum phosphate level. Kidney stones, or intestinal disorders may occur, and less than 5% of cases are recognized by the presence of brown tumours. Secondary hyperparathyroidism happens when parathyroid glands are stimulated to produce amount of hormones to correct abnormally low serum calcium levels as seen in renal failure context. Biology tests show hypocalcaemia and hyperphosphataemia. Hypercalcaemia related symptoms are missing, but osteitis fibrosa cystica may occur. It is referred as renal osteodystrophy also named Von Recklinghausen’s disease of the bone. Tertiary hyperparathyroidism represents transformation of a hypocalcaemic to a hypercalcaemic state when parathyroid glands become autonomous. At last, paraneoplastic syndrome or hypercalcaemia of malignancy occurs in a malignant neoplasm context, such as the presence of multiple myeloma, bronchogenic, gynaecologic, or renal carcinoma which can produce PTHrP. PTHrP is not identified as PTH radioimmunoassay but can mimic all its physiological functions on bones, kidneys, intestines and calcium metabolism. Both PTH and PTHrP have the same 13 first amino acids and share a similar tertiary structural configuration (2, 3).
Association of ossifying fibroma of the jaw and primary hyperparathyroidism as seen in this case seems exceptional. This syndrome is defined as hyperparathyroidism-jaw tumour HPTJT. It is a rare autosomal dominant disorder with incomplete penetrance and variable expression (5).
Fibro-ossifying jaw tumours are seen in 30%, and uterine tumours in 40% of female patients. Renal disease can also occur as: hamartomas, polycystic disease, Wilms tumours or adenocarcinoma. HPT-JT pathogenesis involves an inactivation of HRPT2 gene (located in 1q25) who codes for a 531 amino acid protein called parafibromin. Allelic loss of 1q24–q32 has been identified in some but not all parathyroid tumours associated with HPT-JT, which suggested a tumour suppressor role of HRPT2. Compared to sporadic hyperparathyroidism, HPT-JT is classically considered as more aggressive with frequent multiglandular involvement and risk of developing parathyroid carcinomas (24%). These data may be overestimated since most papers focused prevalently on parathyroid carcinoma. Recent literature reports a single-gland involvement in 89% (5, 6).
Even if a consensual treatment isn’t yet established, these data may suggest that the key treatment in HRPT2-related hyperparathyroidism, when imaging techniques show a unique parathyroid lesion, is a limited and focused excision of grossly enlarged parathyroid glands unless parathyroid cancer is suspected. This procedure offers the advantage of causing lower risk of hypoparathyroidism and less tissues injury facilitating re-operations in recurrent cases (5–7).
PTH intraoperative assay may be interesting to confirm the single-gland disease, when its rate decreases significantly after a minimal parathyroidectomy (a decrease of more than 50% of the PTH level, ten minutes after excision). Some authors prefer extensive parathyroidectomy to avoid recurrences and theoretical parathyroid carcinomas (5, 6). The potential association between HRPT2 mutations and parathyroid carcinomas suggests the need of a long term monitoring of patients with parathyroid adenomas in HRPT2-related hyperparathyroidism context.
The normalization of PTH levels will often cause the brown tumours to regress. In fact, the radiographic appearance of a brown tumour after parathyroidectomy varies with the pathological type of lesion. The cystic brown tumour will not show radiographic ossification after parathyroidectomy, in the opposite of not cystic brown tumours. Removal of brown tumours of bones is not necessary when hyperparathyroidism resolved. However, some authors have reported that they resected any large, symptomatic or remaining brown tumour after hyperparathyroidism resolved (8, 9).
Ossifying fibromas are benign tumours touching the jaw composed by fibrocellular tissue and mineralized material. Complete surgical removal is the recommended treatment, but recurrences may appear after surgery in patients with HRPT2-related hyperparathyroidism (10). In the case we reported, clinical symptoms and osteolytic bone lesions and pathologic fractures suggested first multiple myeloma, especially in lack of renal disease history. Serum protein electrophoresis and bone marrow examination were performed first to eliminate this diagnosis. Biological findings allowed us to consider the bone lesions as brown tumours.
We proceeded first to the management of the severe hypercalcaemia, an orthopaedic treatment was established for bones fractures, and the resection of parathyroid adenoma was performed as the key treatment. The clinical, biological and radiological improvement seen during the follow-up proved the appropriate strategy performed, and reassured us of our patient health status.
For this patient and her family, screening of HRPT2 gene mutation was not carried because of the lost of sight of the patient after clinical improvement. We also have no information about clinical and radiographic outcome 3 years later.
References
- 1.El Abdi B, Berrkia I, Mohssine A, El Hassani MR, El Quessar A, Chakir N, Boukhrissi N, Jiddane M. Tumeur maxillaire révélant une hyperparathyroïdie primitive : à propos d’un cas. J Radiol. 2006;87:1705–7. doi: 10.1016/s0221-0363(06)74151-8. [DOI] [PubMed] [Google Scholar]
- 2.Selvi F, Cakarer S, Tanakol R, Guler SD, Keskin C. Brown tumour of the maxilla and mandible: a rare complication of tertiary hyperparathyroidism. Dentomaxillofac Radiol. 2009;38:53–8. doi: 10.1259/dmfr/81694583. [DOI] [PubMed] [Google Scholar]
- 3.Triantafillidou K, Zouloumis L, Karakinaris G, Kalimeras E, Iordanidis F. Brown tumors of the jaws associated with primary or secondary hyperparathyroidism. A clinical study and review of the literature. Am J Otolaryngol. 2006;27:281–6. doi: 10.1016/j.amjoto.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Mafee MF, Yang G, Tseng A, Keiler L, Andrus K. Fibro-osseous and giant cell lesions, including brown tumor of the mandible, maxilla, and other craniofacial bones. Neuroimaging Clin N Am. 2003;13:525–40. doi: 10.1016/s1052-5149(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 5.Iacobone M, Masi G, Barzon L, Porzionato A, Macchi V, Ciarleglio FA, Palù G, De Caro R, Viel G, Favia G. Hyperparathyroidism-jaw tumor syndrome: a report of three large kindred. Langenbecks Arch Surg. 2009;394:817–25. doi: 10.1007/s00423-009-0511-y. [DOI] [PubMed] [Google Scholar]
- 6.Rekik N, Ben Naceur B, Mnif M, Mnif F, Mnif H, Boudawara T, Abid M. Hyperparathyroidism-jaw tumor syndrome: a case report. Ann Endocrinol. 2010;71:121–6. doi: 10.1016/j.ando.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Carling T, Udelsman R. Parathyroid surgery in familial hyperparathyroid disorders. Journal of Internal Medicine. 2005;257:27–37. doi: 10.1111/j.1365-2796.2004.01428.x. [DOI] [PubMed] [Google Scholar]
- 8.Jouan A, Zabraniecki L, Vincent V, Poix E, Fournié B. An unusual presentation of primary hyperparathyroidism: severe hypercalcemia and multiple brown tumors. Joint Bone Spine. 2008;75:209–11. doi: 10.1016/j.jbspin.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Keyser J, Postma G. Brown Tumor of the Mandible. American Journal of Otolaryngology. 1996;17:407–10. doi: 10.1016/s0196-0709(96)90075-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita Y, Akiyama T, Mizusawa N, Yoshimoto K, Goto M. A case of hyperparathyroidism jaw tumour syndrome found in the treatment of an ossifying fibroma in the maxillary bone. Int J Oral Maxillofac Surg. 2007;36:365–9. doi: 10.1016/j.ijom.2006.08.007. [DOI] [PubMed] [Google Scholar]