Abstract
Objective
To summarize and determine the appropriate use for the new and old management tools for genital warts.
Sources of information
The following databases were searched: MEDLINE, PubMed, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, ACP Journal Club, and Trip. The bibliographies of retrieved papers were also reviewed. Clinical trials, qualitative review articles, consensus reports, and clinical practice guidelines were retrieved.
Main message
Symptomatic warts are prevalent in at least 1% of the population between the ages of 15 and 49, with estimates of up to 50% of the population being infected with human papillomavirus at some point in their lifetime. Imiquimod and podophyllotoxin are 2 new treatments for external genital warts that are less painful and can be applied by patients at home. In addition, the quadrivalent human papillomavirus vaccine has been shown to be efficacious in preventing genital warts and cervical cancer. There is still a role for the older treatment methods in certain situations, such as intravaginal, urethral, anal, or recalcitrant warts; or for pregnant patients.
Conclusion
The new treatments of external genital warts can reduce the pain of treatment and the number of office visits. Other treatment methods are still useful in certain situations.
Case introduction
A 24-year-old woman presents to the office with a 3-month history of tender, itchy “bumps” on her vulva. She is a competitive cyclist and finds that biking irritates the bumps and causes them to bleed at times. She is not currently sexually active, but has had 4 male sexual partners in the past, with the most recent relationship ending 6 months ago. On examination you find multiple papillomatous lesions on her outer labia that are consistent with the appearance of warts. As you reach for the podophyllin, you remember hearing about some new topical treatments for genital warts that are more convenient and less toxic. You also wonder if you should be discussing the human papillomavirus (HPV) vaccine with your patient.
Sources of information
The databases MEDLINE, PubMed, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, ACP Journal Club, and Trip were searched up to March 2011. Searches were conducted for each treatment individually and were limited to English-language articles. The search terms used were warts or condyloma acuminata or papilloma virus and venereal or genital or vaginal and the specific treatment. The bibliographies of retrieved papers were also scanned for relevant articles. Excluded were articles examining cervical neoplasia and studies done in subjects who were immunocompromised, HIV-positive, or homosexual men.
Seventy-seven relevant articles were retrieved and their abstracts were assessed for inclusion in this evidence-based review, with preference given to high-quality systematic reviews from the Cochrane Collaboration. Of the retrieved papers, 49 were read and 30 were included in this review.
The recommendations and the level of evidence were graded using the Canadian Task Force on Preventive Health Care system (Table 1).1
Table 1.
Grades of recommendations and levels of evidence from the Canadian Task Force on Preventive Health Care
| GRADE OR LEVEL | RECOMMENDATION OR EVIDENCE |
|---|---|
| A | There is good evidence to recommend the clinical preventive action |
| B | There is fair evidence to recommend the clinical preventive action |
| C | The existing evidence is conflicting and does not allow a recommendation for or against the use of the clinical preventive action; however, other factors might influence decision making |
| D | There is fair evidence to recommend against the clinical preventive action |
| E | There is good evidence to recommend against the clinical preventive action |
| F | There is insufficient evidence (in quantity or quality) to make a recommendation; however, other factors might influence decision making |
| I | At least 1 properly conducted randomized controlled trial, systematic review, or meta-analysis |
| II | Other comparison trials, non-randomized studies, cohort studies, case-control studies, or epidemiologic studies, and preferably more than 1 study |
| III | Expert opinion or consensus statements |
Adapted from the Canadian Task Force on Preventive Health Care.1
Main message
Genital warts are a common cause of morbidity. Symptomatic warts are prevalent in at least 1% of the population between the ages of 15 and 49,2,3 with estimates of up to 50% of the population being infected with HPV at some point in their lifetime.4 New treatments have become available in the past decade that have pushed some of the old treatments, such as podophyllin, into lesser favour. This review presents the new approaches to the treatment and management of genital warts, while including possible roles for some of the old treatments (Table 2 and Figures 1 and 2).5–21
Table 2.
Preferred and alternative treatment options: Ranges in rates of clearance and recurrence reflect the variation of results across studies.
| TREATMENT, LEVEL OF EVIDENCE, AND GRADE OF RECOMMENDATION | CLEARANCE, % | RECURRENCE, % | APPLICATION REGIMEN | ADVERSE EFFECTS | SAFE FOR INTRAVAGINAL OR INTRA-ANAL USE | SAFE FOR USE IN PREGNANCY OR LACTATION |
|---|---|---|---|---|---|---|
| Patient-applied treatments | ||||||
| Imiquimod 5% cream5–7 (grade B, level I)* Imiquimod 3.75% has not been evaluated for the treatment of genital warts |
51 | 22–63 | Apply with finger 3 nights/wk for 16 wk Wash off in the morning | Localized erythema, burning, inflammation; rarely, hypopigmentation Might weaken latex condoms and diaphragms | No | No; safety unknown |
| Podophyllotoxin 0.5% solution or gel7 (grade B, level I)* More efficacious and less toxic than podophyllin |
56 | 2–90 | Apply with swab or finger 2 times/d for 3 d, then 4 d off, for up to 4 cycles. Limit of 10 cm2/d of skin surface or 0.5 mL/d | Localized burning, pain, itching, erosion, inflammation | No | No; it is an extract of podophyllin, which is teratogenic |
| Provider-applied treatments | ||||||
| Cryotherapy8–12 (grade B, level I)* | 27–88 | 25–55 | Once per wk with cotton swab, spray, or cryoprobe (not in the vagina) | Localized pain, inflammation, scarring Risk of vaginal perforation if using cryoprobe | Yes | Yes |
| TCA10,11,13 (grade B, level I)* | 63–70 | 35 | Once per wk with cotton swab or toothpick | Localized pain and ulceration Can neutralize with sodium bicarbonate solution |
Yes | Yes |
| Excision14 (grade B, level I)† | 35–72 | 19–79 | Local anesthesia then excision of lesions | Pain, bleeding, infection | Yes | Yes |
| Electrocautery14 (grade B, level II)† | 61–94 | 22 | Local anesthesia then destruction of lesions with cautery tools | Pain, bleeding, infection Operator should wear a virus-filtering mask | Yes | Yes |
| Alternative treatments | ||||||
| Podophyllin 25% in tincture of benzoin14–16 (grade C, level I)‡ | 23–72 | 23–65 | Once per wk with cotton swab or toothpick Limit 10 cm2 of skin surface or 0.5 mL per treatment |
Localized burning, pain, itching, erosion, inflammation | Limit < 2 cm2 of skin surface | No; is teratogenic |
| Sinecatechins (green tea extract) 15% ointment17,18 (grade B, level II; 2 trials)‡ | 57 | 6.5 | Apply 3 times/d for up to 16 wk | Localized erythema, burning, pain, rash, ulceration | No; not studied | No; not studied |
| Fluorouracil 1% gel or 5% cream19,20 (grade C, level II)‡ Useful for large numbers of vaginal or urethral-meatal warts in which cryotherapy or TCA are not tolerated |
80–90 | No data | Insert applicatorful into vagina 3 nights/wk | Erythema, erosion, edema | Yes | No; is teratogenic |
| Interferon21 (grade C, level II)‡ | 44.4 | 21.1 | One applicatorful in vagina twice daily for 5 d/wk for 4 wk | Headache, tenderness, transient fever | Yes | No; not studied |
| Observation*14 | 40–60 | No data | No data | No data | No data | No data |
TCA—trichloroacetic acid.
First-choice therapy.
Second-choice therapy.
Not-generally-recommended therapy.
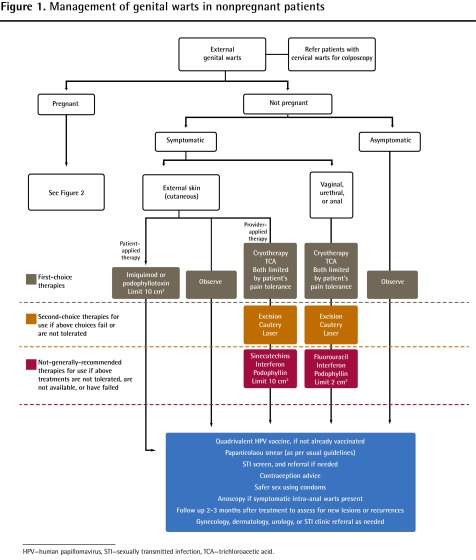
Figure 1.
Management of genital warts in nonpregnant patients
HPV—human papillomavirus, STI—sexually transmitted infection, TCA—trichloroacetic acid.
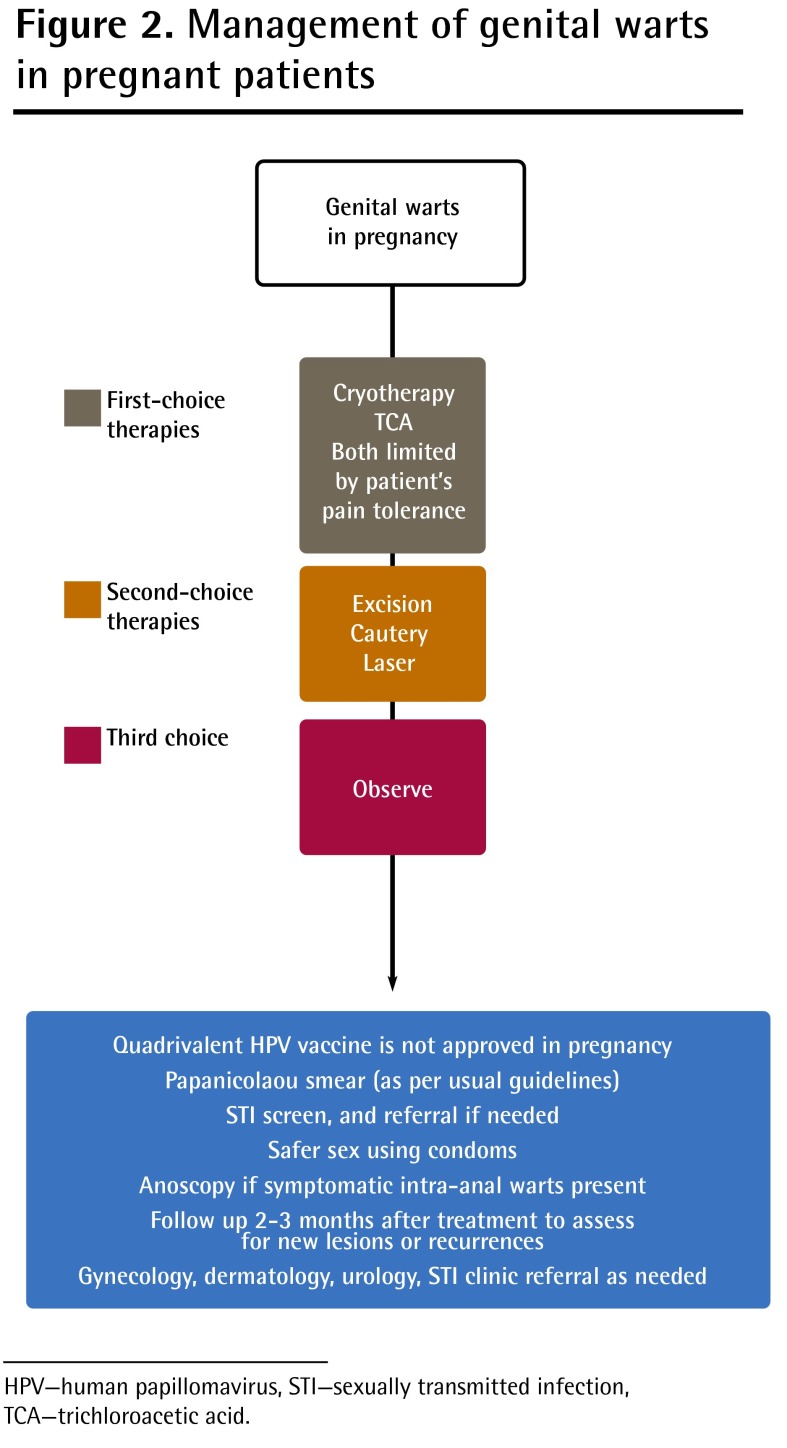
Figure 2.
Management of genital warts in pregnant patients
HPV—human papillomavirus, STI—sexually transmitted infection, TCA—trichloroacetic acid.
Visible genital warts can be psychologically and physically distressing for patients. While warts are often asymptomatic, at other times they can cause pain, itching, burning, irritation against clothing, and occasionally bleeding.4 They can also cause pain and bleeding during sexual activity.
Treatment of benign, symptomatic genital warts is aimed at alleviation of physical symptoms and cosmetic improvement. From 40% to 60% of untreated warts will spontaneously resolve in 9 to 12 months,14 but many patients are psychologically distressed by the presence of warts and require intervention to eradicate them (Box 1).
Box 1. Getting started.
Discuss the treatment options with the patient, including observation, and consider the location of the warts, extent of involvement for painful treatments (cryotherapy or trichloroacetic acid), and the possibility of pregnancy
Order a pregnancy test and discuss contraception with fertile women if treating with imiquimod, podophyllotoxin, or podophyllin
Start treatment with a first-choice therapy and continue for the recommended duration
Reassess at the end of the treatment and repeat the treatment if lesions are not cleared, or if new ones have appeared
If lesions do not seem to be responding to treatment after 2 or 3 cycles, try an alternate first-choice therapy. If there is a response to treatment, you can keep going with the same therapy, repeating cycles of treatment as needed
Continue in this manner until you find the treatment that works for this patient. Choose a second-choice therapy if you fail to find a suitable first-choice therapy
In situations in which the first-choice therapies have failed, are contraindicated, or are not tolerated by the patient, and the second-choice therapies are not available, or not feasible (eg, periurethral warts), try a not-generally-recommended therapy. They are not usually recommended because of lack of evidence, more severe side effects, or teratogenicity, but are still useful in difficult cases
If all available options for treatment have failed, then refer the patient to a local sexually transmitted infection clinic, gynecologist, dermatologist, or urologist for treatment
Genital warts are caused by several strains of HPV and are spread by skin-to-skin contact during sexual activity. They are, therefore, considered a sexually transmitted infection (STI).22 A number of different treatments are available. Some of these treatments can be self-applied by the patients, while others require treatment by a nurse or physician. In this article, I have included older treatments (eg, podophyllin) that might now be relegated to use only in difficult cases, as they are familiar to many general practitioners who have been in practice for decades. It might be useful for them to know where these treatments now stand. I have also included some new, less-used treatments (interferon, sinecatechins) because readers might have heard about them and wondered about their use.
The diagnosis of genital warts is made by visual inspection for the appearance of lesions consistent with warts. They can appear as papillomatous plaques or flat lesions, and can be singular or multiple, or can coalesce into condylomata acuminata. They can vary from flesh-coloured to white, pink, or brown. The locations involved in women can be the cervix, vagina, vulva, urethral meatus, and perianal region. In men, the scrotum, penis shaft, corona and under the foreskin, and perianal region can be involved.23
The differential diagnosis includes sebaceous glands, seborrheic keratoses, molluscum contagiosum, psoriasis, lichen planus, melanocytic nevi, fibroepitheliomas, neoplasia, and condylomata lata (syphilis).23 Occasionally, a biopsy is indicated to confirm the diagnosis and rule out malignancy. Testing for acetowhitening with a 3% to 5% acetic acid solution (household vinegar) is not recommended because it is considered too nonspecific to be useful.4 Human papillomavirus types 16 and 18 cause more than 70% of cases of invasive cervical cancer.24 More than 90% of cases of benign disease (genital warts) are caused by types 6 and 11.15
Transmission of HPV to a neonate can result in laryngeal papillomatosis in the newborn. As this is a rare, non-malignant condition, and it is unclear whether the newborn becomes infected during birth or post partum, cesarean section is not recommended for prevention (grade C, level II).4
There is a consensus of expert opinion that attempts should be made to reduce the HPV load in pregnant women by treating genital warts before vaginal delivery, although there is no evidence that such treatment reduces viral load (levels I to III).4 There is a lack of evidence that treatment of visible warts eradicates the HPV infection or prevents transmission of the virus.4,25
Other management and prevention
Quadrivalent HPV vaccine protects against HPV types 6, 11, 16, and 18, the strains that most commonly cause benign warts and cervical cancer. Initial studies in women showed that it is 90% to 100% efficacious in preventing genital warts.26 After the introduction of a vaccination program in 2007, 4-year follow-up of young, sexually active women in Australia showed a marked decline in the prevalence of genital warts from 11.7% to 4.8% by 2009, and an ongoing, slower decline since then. There has been an associated, but less dramatic, decline in the incidence of warts in heterosexual men as well, presumably through reduced exposure to the virus as more of their partners were vaccinated.27,28
At this time, Canada has approved the quadrivalent HPV vaccine for women aged 9 to 26 years. It is recommended that women who present with genital warts and who have not yet been vaccinated should be offered the vaccine (3 doses at 0, 2, and 6 months). While it does not clear current HPV infection, it might help prevent reinfection with other strains (in particular the higher risk types 16 or 18). There is grade A, level I evidence that the quadrivalent HPV vaccine prevents cervical cancer3,4 (not addressed in this paper) and grade B, level I evidence that it prevents genital warts.23,27
The quadrivalent HPV vaccine has an increased cost utility; it reduces the cost burden, as it prevents both genital warts and cervical cancer, compared with a vaccination for cervical cancer alone.3
Other than administering the quadrivalent HPV vaccine, the following recommendations are made to prevent and manage genital warts:
All women should have Papanicolaou smears to screen for co-infection with oncogenous strains.
Women should be screened for other STIs according to STI guidelines. Screening partners for warts is not indicated.4 Genital warts do not need to be treated unless they are symptomatic.
Anoscopy should be performed to confirm the diagnosis of symptomatic (itching, painful, bleeding) intra-anal warts. Asymptomatic warts can be observed, so a diagnosis is unnecessary. They can occur without a history of anal-receptive intercourse.
Contraception might need to be discussed and prescribed, particularly if using a wart treatment that is contraindicated in pregnancy. Keep in mind that imiquimod might weaken latex barrier devices such as condoms and diaphragms.
Condoms do offer protection, although incomplete protection, against transmission of HPV (grade B, level II).29,30
Follow-up at 2 to 3 months after achieving wart clearance is recommended, to check for recurrence or new lesions.
Patients with recalcitrant (difficult to treat), symptomatic warts should be referred to local STI clinics, gynecologists, dermatologists, or urologists.
Case resolution
Your diagnosis is genital warts. You do a Pap smear and screen for gonorrhea and chlamydia. You advise the patient that condoms might help to prevent transmission. After discussion of treatment versus observation, she decides that she would like to treat the warts because they are bothering her. You prescribe her an oral contraceptive before initiating treatment, and you also arrange for her to receive the quadrivalent HPV vaccine. You prescribe imiquimod 5% cream, to be applied at home 3 nights a week for 16 weeks. When you see her again, 2 months after she finishes the treatment, there are no longer any visible warts.
Limitations
This review was limited by the evidence available, in English, at the time of writing. Systematic reviews were available for 5 of the therapies, but even so, some of the studies used in the reviews were not of high quality (eg, studies of fluorouracil).20 Other therapies have a lot more research behind them (eg, imiquimod). Information on safety in pregnancy is lacking for imiquimod, interferon, and sinecatechins. And some research results are still pending (eg, for the HPV vaccine). This topic would be worth reviewing again in 4 years.
Conclusion
When, and how, to treat genital warts is a decision that the patient and clinician should make together. The choice of treatment should consider the severity of the symptoms—both psychological and physical—and weigh that against the adverse effects of the treatment. The final decision might depend on the extent of the warts and the tolerance the patient has for painful treatments, or the time that the patient is willing to invest in repeated treatments. The advent of patient-applied therapies reduces the burden of repeated visits to a clinic.
EDITOR’S KEY POINTS
Nearly all external genital warts are benign and not associated with cervical or penile cancer.
Treatment of genital warts is aimed at alleviation of symptoms and emotional distress. Most cases of genital warts will resolve spontaneously, usually within 12 months, if left untreated.
There is no evidence that treating visible genital warts will prevent transmission of the virus.
The quadrivalent human papillomavirus vaccine is efficacious in preventing genital warts, but will not clear those that are already present.
Footnotes
This article has been peer reviewed.
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de juillet 2013 à la page e304.
Competing interests
None declared
References
- 1.Canadian Task Force on Preventive Health Care New grades for recommendations from the Canadian Task Force on Preventive Health Care. CMAJ. 2003;169(3):207–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Gall SA. Female genital warts: global trends and treatments. Infect Dis Obstet Gynecol. 2001;9(3):149–54. doi: 10.1155/S1064744901000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Money DM, Roy M. Canadian consensus guidelines on human papillomavirus. J Obstet Gynaecol Can. 2007;29(8 Suppl 3):S1–56. doi: 10.1016/S1701-2163(16)32573-7. [DOI] [PubMed] [Google Scholar]
- 4.Workowski KA, Berman S, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 5.Gotovtseva EP, Kapadia AS, Smolensky MH, Lairson DR. Optimal frequency of imiquimod (Aldara) 5% cream for the treatment of external genital warts in immunocompetent adults: a meta-analysis. Sex Transm Dis. 2008;35(4):346–51. doi: 10.1097/OLQ.0b013e31815ea8d1. [DOI] [PubMed] [Google Scholar]
- 6.Moore RA, Edwards JE, Hopwood J, Hicks D. Imiquimod for the treatment of genital warts: a quantitative systematic review. BMC Infect Dis. 2001;1:3. doi: 10.1186/1471-2334-1-3. Epub 2001 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan J, Chen SL, Wang HN, Wu TX. Meta-analysis of 5% imiquimod and 0.5% podophyllotoxin in the treatment of condylomata acuminata. Dermatology. 2006;213(3):218–23. doi: 10.1159/000095039. [DOI] [PubMed] [Google Scholar]
- 8.Stefanaki C, Katzouranis I, Lagogianni E, Hadjivassiliou M, Nicolaidou E, Panagiotopoulos A, et al. Comparison of cryotherapy to imiquimod 5% in the treatment of anogenital warts. Int J STD AIDS. 2008;19(7):441–4. doi: 10.1258/ijsa.2007.007196. Erratum in: Int J STD AIDS 2008;19(10):722. [DOI] [PubMed] [Google Scholar]
- 9.Stone KM, Becker TM, Hadgu A, Kraus SJ. Treatment of external genital warts: a randomised clinical trial comparing podophyllin, cryotherapy, and electrodesiccation. Genitourin Med. 1990;66(1):16–9. doi: 10.1136/sti.66.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdullah AN, Walzman M, Wade A. Treatment of external genital warts comparing cryotherapy (liquid nitrogen) and trichloroacetic acid. Sex Transm Dis. 1993;20(6):344–5. [PubMed] [Google Scholar]
- 11.Godley MJ, Bradbeer CS, Gellan M, Thin RN. Cryotherapy compared with trichloroacetic acid in treating genital warts. Genitourin Med. 1987;63(6):390–2. doi: 10.1136/sti.63.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yliskoski M, Saarikoski S, Syrjänen K, Syrjänen S, Castrén O. Cryotherapy and CO2-laser vaporization in the treatment of cervical and vaginal human papillomavirus (HPV) infections. Acta Obstet Gynecol Scand. 1989;68(7):619–25. doi: 10.3109/00016348909013281. [DOI] [PubMed] [Google Scholar]
- 13.Sherrard J, Riddell L. Comparison of the effectiveness of commonly used clinic-based treatments for external genital warts. Int J STD AIDS. 2007;18(6):365–8. doi: 10.1258/095646207781024711. [DOI] [PubMed] [Google Scholar]
- 14.Wiley DJ, Douglas J, Beutner K, Cox T, Fife K, Moscicki AB, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35(Suppl 2):S210–24. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]
- 15.Mayeaux EJ, Jr, Dunton C. Modern management of external genital warts. J Low Genit Tract Dis. 2008;12(3):185–92. doi: 10.1097/LGT.0b013e31815dd4b4. [DOI] [PubMed] [Google Scholar]
- 16.BASHH HPV Special Interest Group . United Kingdom national guideline on the management of ano-genital warts, 2007. Macclesfield, UK: British Association for Sexual Health and HIV; 2007. Available from: www.bashh.org/documents/86/86.pdf. Accessed 2013 May 28. [Google Scholar]
- 17.Tatti S, Stockfleth E, Beutner KR, Tawfik H, Elsasser U, Weyrauch P, et al. Polyphenon E: a new treatment for external anogenital warts. Br J Dermatol. 2010;162(1):176–84. doi: 10.1111/j.1365-2133.2009.09375.x. Epub 2009 Jul 27. [DOI] [PubMed] [Google Scholar]
- 18.Tatti S, Swinehart JM, Thielert C, Tawfik H, Mescheder A, Beutner KR. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1371–9. doi: 10.1097/AOG.0b013e3181719b60. [DOI] [PubMed] [Google Scholar]
- 19.Batista CS, Atallah AN, Saconato H, da Silva EM. 5-FU for genital warts in non-immunocompromised individuals. Cochrane Database Syst Rev. 2010;(4):CD006562. doi: 10.1002/14651858.CD006562.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syed TA, Qureshi ZA, Ahmad SA, Ali SM. Management of intravaginal warts in women with 5-fluorouracil (1%) in vaginal hydrophilic gel: a placebo-controlled double-blind study. Int J STD AIDS. 2000;11(6):371–4. doi: 10.1258/0956462001916074. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Pu YG, Zeng ZM, Yu ZJ, Huang N, Deng QW. Interferon for the treatment of genital warts: a systematic review. BMC Infect Dis. 2009;9:156. doi: 10.1186/1471-2334-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ault KA. Epidemiology and natural history of human papillomavirus infections in the female genital tract. Infect Dis Obstet Gynecol. 2006;2006(Suppl):40470. doi: 10.1155/IDOG/2006/40470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Australia and New Zealand HPV Project . Guidelines for the management of genital HPV in Australia and New Zealand. 5th ed. Viral Sexually Transmitted Infection Education Foundation; 2007. [Google Scholar]
- 24.Ogunmodede F, Yale SH, Krawisz B, Tyler GC, Evans AC. Human papillomavirus infections in primary care. Clin Med Res. 2007;5(4):210–7. doi: 10.3121/cmr.2007.751. Epub 2007 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacey CJ. Therapy for genital human papillomavirus-related disease. J Clin Virol. 2005;32(Suppl 1):S82–90. doi: 10.1016/j.jcv.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 26.FUTURE I/II Study Group. Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis. 2011;11(1):39–44. doi: 10.1016/S1473-3099(10)70225-5. Epub 2010 Nov 8. [DOI] [PubMed] [Google Scholar]
- 28.Fairley CK, Hocking JS, Gurrin LC, Chen MY, Donovan B, Bradshaw CS. Rapid decline in presentations of genital warts after the implementation of a national quadrivalent human papillomavirus vaccination programme for young women. Sex Transm Infect. 2009;85(7):499–502. doi: 10.1136/sti.2009.037788. Epub 2009 Oct 16. [DOI] [PubMed] [Google Scholar]
- 29.Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis. Sex Transm Dis. 2002;29(11):725–35. doi: 10.1097/00007435-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Winer RL, Hughes JP, Feng Q, O’Reilly S, Kiviat NB, Holmes KK, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354(25):2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]