Abstract
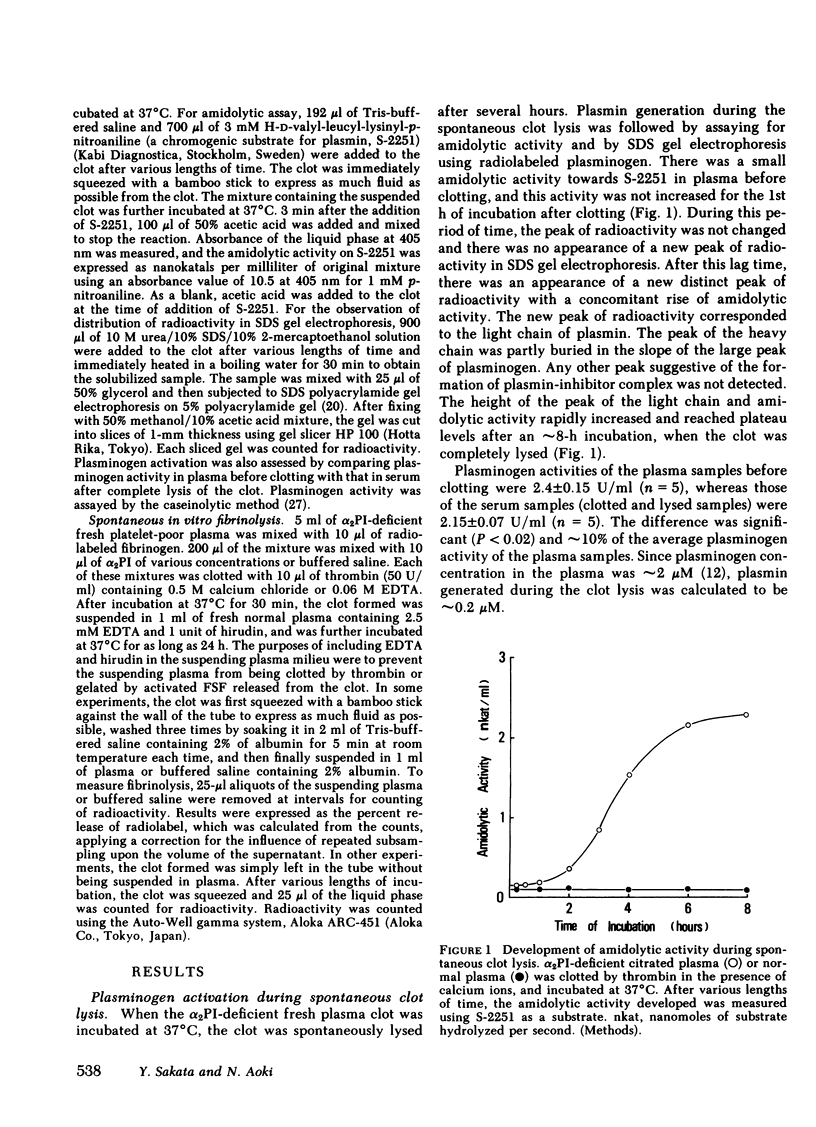
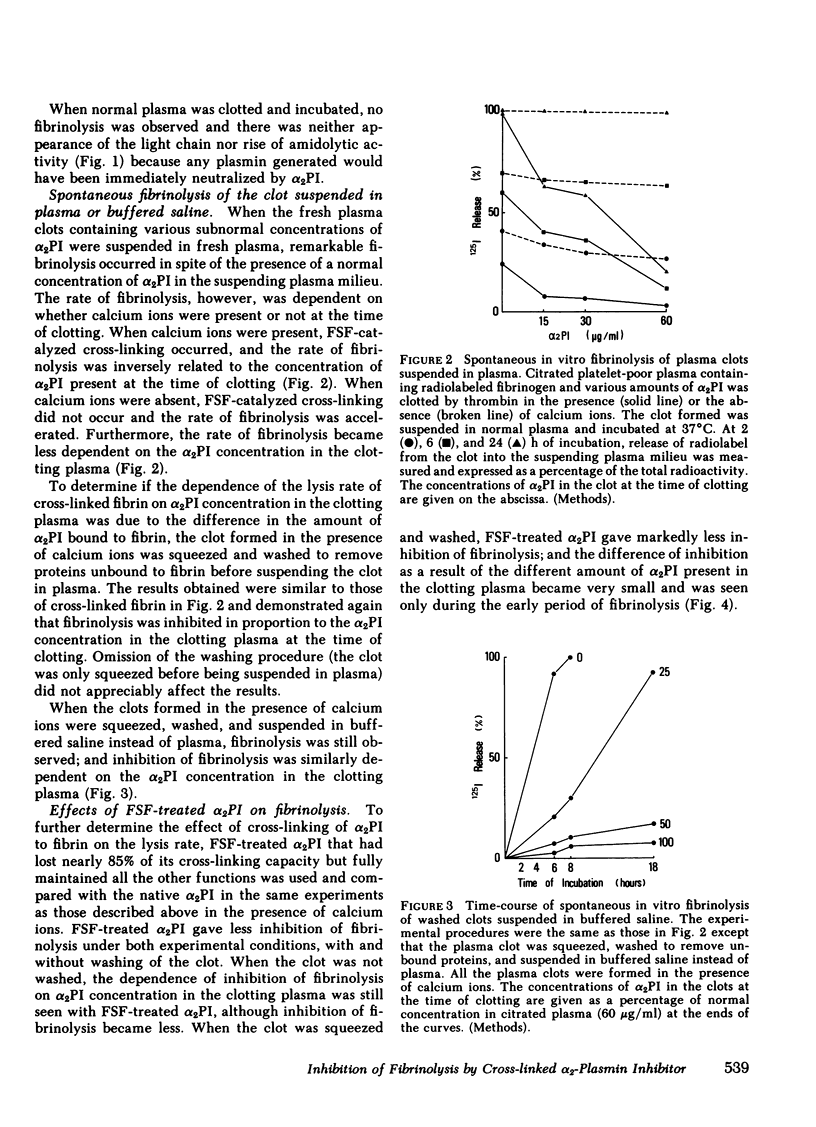
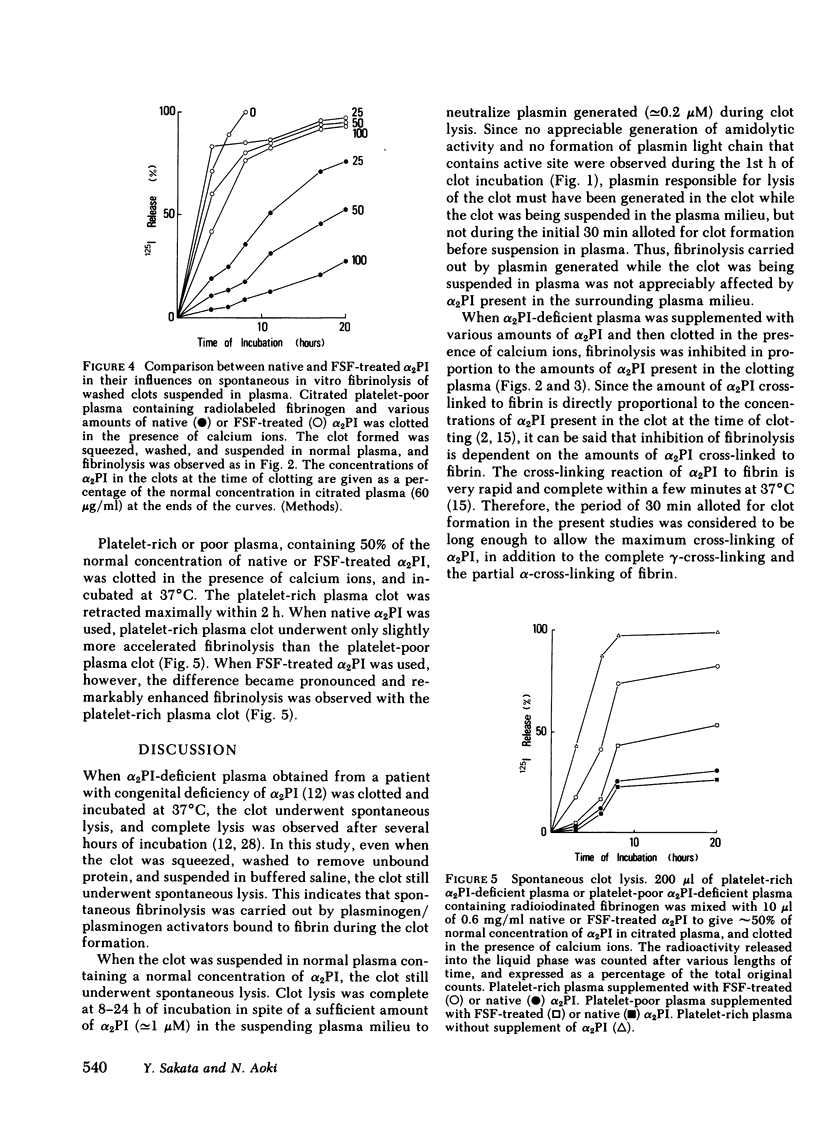
When blood is clotted, α2-plasmin inhibitor (α2PI) is cross-linked to fibrin by activated fibrin-stabilizing factor (activated coagulation Factor XIII, plasma transglutaminase). The amount of cross-linked α2-PI is proportional to the amount of α2PI present at the time of clotting. Plasma from a patient with congenital deficiency of α2PI was supplemented with various amounts of purified α2PI. Clots were prepared from these plasmas and were suspended in plasma containing a normal concentration of α2PI, and spontaneous clot lysis was observed. When the clot was formed in the presence of calcium ions and thereby allowing cross-linking to occur, the rate and extent of fibrinolysis were found to be inversely proportional to the concentrations of α2PI present in the clot at the time of clotting. When the clot was formed in the absence of calcium ions so that no cross-linking occurred, the clot underwent fibrinolysis at similar rates, regardless of the concentrations of α2PI in the clot. When the clot formed in the presence of calcium ions was squeezed and washed to remove unbound proteins before being suspended in plasma, the extent of fibrinolysis was also inversely proportional to the amount of α2PI cross-linked to fibrin. Similar results were obtained when the clot was suspended in buffered saline instead of plasma. These observations suggest that spontaneous fibrinolysis is mainly carried out by plasminogen/plasminogen activator bound to fibrin, and this fibrinolysis caused by fibrin-associated activation of plasminogen was mainly inhibited by α2PI cross-linked to fibrin. To further support this concept, α2PI treated with activated fibrin-stabilizing factor and that had lost most of its cross-linking capacity was used in similar experiments. This modified α2PI had the same inhibitory activity on plasmin as the native inhibitor, but gave significantly less inhibition of fibrinolysis in every experiment, particularly when the clot was compacted by platelet-mediated clot retraction or by squeezing. Thus, it was concluded that α2PI cross-linked to fibrin plays a significant role in inhibition of physiologically occurring fibrinolysis. It is further suggested that the absence of cross-linked α2PI contributes to accelerated fibrinolysis and hemorrhagic tendency in patients with congenital deficiency of fibrin-stabilizing factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Moroi M., Matsuda M., Tachiya K. The behavior of alpha2-plasmin inhibitor in fibrinolytic states. J Clin Invest. 1977 Aug;60(2):361–369. doi: 10.1172/JCI108784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Moroi M., Sakata Y., Yoshida N., Matsuda M. Abnormal plasminogen. A hereditary molecular abnormality found in a patient with recurrent thrombosis. J Clin Invest. 1978 May;61(5):1186–1195. doi: 10.1172/JCI109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Moroi M., Tachiya K. Effects of alpha2-plasmin inhibitor on fibrin clot lysis. Its comparison with alpha2-macroglobulin. Thromb Haemost. 1978 Feb 28;39(1):22–31. [PubMed] [Google Scholar]
- Aoki N. Natural inhibitors of fibrinolysis. Prog Cardiovasc Dis. 1979 Jan-Feb;21(4):267–286. doi: 10.1016/0033-0620(79)90014-8. [DOI] [PubMed] [Google Scholar]
- Aoki N., Saito H., Kamiya T., Koie K., Sakata Y., Kobakura M. Congenital deficiency of alpha 2-plasmin inhibitor associated with severe hemorrhagic tendency. J Clin Invest. 1979 May;63(5):877–884. doi: 10.1172/JCI109387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Sakata Y. Influence of alpha 2-plasmin inhibitor on adsorption of plasminogen to fibrin. Thromb Res. 1980 Jul 1;19(1-2):149–155. doi: 10.1016/0049-3848(80)90414-4. [DOI] [PubMed] [Google Scholar]
- Aoki N., Sakata Y., Matsuda M., Tateno K. Fibrinolytic states in a patient with congenital deficiency of alpha 2-plasmin inhibitor. Blood. 1980 Mar;55(3):483–488. [PubMed] [Google Scholar]
- Aoki N., Yamanaka T. The alpha2-plasmin inhibitor levels in liver diseases. Clin Chim Acta. 1978 Mar 1;84(1-2):99–105. doi: 10.1016/0009-8981(78)90481-3. [DOI] [PubMed] [Google Scholar]
- Bohn H., Haupt H. Eine quantitative Bestimmung von Faktor 13 mit Anti-Faktor-13-Serum. Thromb Diath Haemorrh. 1968 Jul 31;19(3):309–315. [PubMed] [Google Scholar]
- Chung S. I., Folk J. E. Kinetic studies with transglutaminases. The human blood enzymes (activated coagulation factor 13 and the guinea pig hair follicle enzyme. J Biol Chem. 1972 May 10;247(9):2798–2807. [PubMed] [Google Scholar]
- Collen D. Identification and some properties of a new fast-reacting plasmin inhibitor in human plasma. Eur J Biochem. 1976 Oct 1;69(1):209–216. doi: 10.1111/j.1432-1033.1976.tb10875.x. [DOI] [PubMed] [Google Scholar]
- Collen D., Wiman B. Fast-acting plasmin inhibitor in human plasma. Blood. 1978 Apr;51(4):563–569. [PubMed] [Google Scholar]
- Curtis C. G., Lorand L. Fibrin-stabilizing factor (factor XIII). Methods Enzymol. 1976;45:177–191. doi: 10.1016/s0076-6879(76)45018-8. [DOI] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L. A rapid method for the purification of bovine thrombin and the inhibition of the purified enzyme wtih phenylmethylsulfonyl fluoride. Biochemistry. 1971 Jun 22;10(13):2501–2506. doi: 10.1021/bi00789a012. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Inhibition of proteases in coagulation, kinin-forming and complement systems by alpha2-plasmin inhibitor. J Biochem. 1977 Oct;82(4):969–972. doi: 10.1093/oxfordjournals.jbchem.a131801. [DOI] [PubMed] [Google Scholar]
- Moroi M., Aoki N. Isolation and characterization of alpha2-plasmin inhibitor from human plasma. A novel proteinase inhibitor which inhibits activator-induced clot lysis. J Biol Chem. 1976 Oct 10;251(19):5956–5965. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Müllertz S., Clemmensen I. The primary inhibitor of plasmin in human plasma. Biochem J. 1976 Dec 1;159(3):545–553. doi: 10.1042/bj1590545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K., Aoki N. Assay of alpha2-plasmin inhibitor activity by means of a plasmin specific tripeptide substrate. Thromb Res. 1978 Jun;12(6):1147–1156. doi: 10.1016/0049-3848(78)90069-5. [DOI] [PubMed] [Google Scholar]
- Saito H., Goldsmith G. H., Moroi M., Aoki N. Inhibitory spectrum of alpha 2-plasmin inhibitor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2013–2017. doi: 10.1073/pnas.76.4.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J Clin Invest. 1980 Feb;65(2):290–297. doi: 10.1172/JCI109671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Tamaki T., Aoki N. Cross-linking of alpha 2-plasmin inhibitor and fibronectin to fibrin by fibrin-stabilizing factor. Biochim Biophys Acta. 1981 Oct 13;661(2):280–286. doi: 10.1016/0005-2744(81)90016-4. [DOI] [PubMed] [Google Scholar]
- Wallén P., Wiman B. Characterization of human plasminogen. II. Separation and partial characterization of different molecular forms of human plasminogen. Biochim Biophys Acta. 1972 Jan 26;257(1):122–134. doi: 10.1016/0005-2795(72)90261-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



