Abstract
Background
Acute exposure to outdoor air pollutants has been associated with increased pediatric asthma morbidity. However, the impact of sub-chronic exposures is largely unknown.
Objective
To examine the association between sub-chronic exposure to six outdoor air pollutants (PM2.5, PM10, O3, NO2, SO2, CO) and pediatric asthma hospitalization length of stay, charges, and costs.
Methods
We linked pediatric asthma hospitalization discharge data from a nationally representative dataset, the 1999-2007 Nationwide Inpatient Sample, with outdoor air pollution data from the Environmental Protection Agency. Hospitals with no air quality data within 10 miles were excluded. Our predictor was the average concentration of six pollutants near the hospital during the month of admission. We conducted bivariate analyses using Spearman correlations and multivariable analyses using Poisson regression for length of stay and linear regression for log-transformed charges and costs, controlling for patient demographics, hospital characteristics, and month of admission.
Results
In unadjusted analyses, all six pollutants had minimal correlation with the three outcomes (rho<0.1, p<0.001). In multivariable analyses, a 1-unit (μg/m3) increase in monthly PM2.5 led to a $123 increase in charges (95% CI $40-249) and a $47 increase in costs (95% CI $15-93). No other pollutants were significant predictors of charges or costs, or length of stay.
Conclusion
Sub-chronic PM2.5 exposure is associated with increased costs for pediatric asthma hospitalizations. Policy changes to reduce outdoor sub-chronic pollutant exposure may lead to improved asthma outcomes as well as substantial savings in healthcare spending.
Background
Asthma is one of the most common chronic illnesses of children in the United States (US), causing a significant health and economic burden. Morbidity from this disease results in many preventable hospital admissions and considerable use of healthcare dollars.1-4 Asthma prevalence is highest among children living in inner cities.5-7 The development and severity of asthma is multi-factorial, but ambient air pollutants are known risk factors.8,9 The economic burden from air pollutants and asthma is estimated to be substantial,3 but the only previous analysis relied on expert judgment about attributable burden of disease. No epidemiological studies have been conducted to quantify costs attributable to individual pollutants.
In the US, air pollutant levels are regulated by the Environmental Protection Agency (EPA), which has set standards for six criteria pollutants, including particulate matter, ozone (O3), nitrogen oxides (NO2), sulfur oxides (SO2), carbon monoxide (CO), and lead. Particulate matter can be further classified into aerodynamic diameter ≤10μm (PM10) or ≤2.5μm (PM2.5).
Increasing evidence supports the association between air pollutants and asthma morbidity, including worsening asthma symptoms,10-13 increased emergency department visits,14-18 and decreased lung function.19-21 This evidence is primarily from studies of exposures in limited geographic areas,10, 17, 20, 22-23 although some national multi-city studies exist as well.19, 24-26 Of the six EPA criteria pollutants, PM2.5 and O3 have most often been associated with asthma morbidity.10, 12, 13, 27-29
The existing literature on air pollutants and asthma has primarily focused on acute exposures; data regarding more chronic exposures are limited.22,24 In general, exposure duration of less than 14 days is acute, more than 14 days up to one year is sub-chronic, and greater than one year is chronic.30 Higher chronic ozone exposure is associated with increased risk of asthma hospitalization,22 and higher chronic exposure to both ozone and particulate matter is associated with increased risk of current asthma and symptom exacerbation.24 Both sub-chronic and chronic PM2.5 exposure have been associated with increased risk of hospitalization for infant bronchiolitis, another pediatric respiratory illness.31
The objective of this exploratory study was to examine the association between sub-chronic exposure to air pollutants and length of stay, total charges, and total costs for pediatric asthma hospitalizations. To accomplish this, we linked two nationally representative datasets: the Nationwide Inpatient Sample (NIS), an annual survey of hospital discharge data, and the Environmental Protection Agency (EPA) Aerometric Information Retrieval System (AIRS), containing US air pollutant data.
The NIS allows quantification of hospitalization costs, an important step towards evaluating the economic impact made by air pollutants on pediatric asthma. Patient data in the NIS is de-identified, and the dataset contains no information on individual residence. Therefore, merging with the EPA air pollutant data occurs at the level of the hospital, not the patient. Although this is an important deficiency to consider, the NIS represents the largest dataset available to measure increases in asthma hospitalization costs associated with air pollutant levels. We therefore proceeded to perform descriptive, bivariate, and multivariable analysis of the 1999-2007 NIS to quantify the economic impact of air pollutants on pediatric asthma hospitalizations.
Methods
Study Design
This was a multi-year cross-sectional study, including hospitalizations between 1999 and 2007 for children ages 2-17 with a primary diagnosis of asthma (ICD-9 code 493.XX). Children under 2 were excluded because wheezing at this age is often transient and diagnosis of asthma is difficult.32 Hospitalizations with no corresponding air pollutant data were excluded. Weights accounting for sampling design were included in the NIS; these weights were incorporated into all analyses except descriptive pollutant data. Analyses were conducted using SAS-callable SUDAAN software version 10.0.1 (Research Triangle Institute, NC) to take into account the complex sampling design used by NIS. This research involved previously collected, de-identified data and was exempt from review by the Institutional Review Board of Mount Sinai School of Medicine.
Databases
The NIS is the largest all-payer inpatient care database in the US, containing data from approximately 8 million hospitalizations annually.33 Details regarding sample design, data collection and weighting are described elsewhere.33 The survey includes diagnostic codes and basic patient demographics, as well as length of stay and total hospital charges.
The EPA AIRS contains pollutant data recorded at defined intervals, ranging from hourly to every few days,34 available to the public for download.35 We obtained text files for six air pollutants (PM2.5, PM10, O3, NO2, SO2, CO) from 1999 through 2007 to merge with the NIS hospitalization data. To assess for any systematic missing data, we compared the number of measurements available per month, day and hour for each pollutant for each year to the total number of measurements possible; we observed no systematic patterns of missing data.
Main outcomes: hospitalization data
Outcomes in this study included length of stay, total charges, and total costs for pediatric asthma hospitalizations. The length of stay was provided in days. Total charges represent the amount billed for each hospitalization. Charges were controlled for inflation by adjusting to 2005 dollars using the Healthcare Consumer Price Index from the Bureau of Labor.36 Costs represent the amount of money actually paid to the hospital, generally significantly less than the charges. To estimate costs, charges were converted using hospital-specific cost-to-charge ratio files in a dataset accompanying the NIS. These cost-to-charge ratio files are constructed using all-payer inpatient cost and charge information from the Centers for Medicare and Medicaid Services.37
Cost-to-charge ratios were not available for data from 1999–2000; these years were excluded when analyzing cost. Overall, many hospitals did not have cost-to-charge ratios available, and therefore cost analyses represent only a fraction of all hospitalizations; also, as explained above, costs are only an estimate based on a ratio assigned to the hospital overall and not specific to the visit. The majority of our analyses were conducted to explore the nature of the relationship between pollutants and healthcare spending. For these analyses, we used charges as the outcome, allowing a greater number of hospitalizations to be included and more accuracy in our results. However, for analyses used to estimate potential healthcare savings in real healthcare dollars, we used costs as the outcome; charges as the outcome for this purpose would over-represent any potential savings.
Main predictors: air pollutants
The predictors of interest were average monthly air pollutant levels. Because the NIS does not contain identifying information about patient residence, air pollutant levels were determined for a defined area surrounding the hospital.
For all NIS hospitals with available addresses, we determined the latitude and longitude, which were verified using a geocoding website38 and Google maps. The latitude and longitude for the AIRS monitors are available on a publicly available website.39 We determined the distance between NIS hospitals and AIRS monitors using the following equation, calculating the hypotenuse of a right triangle formed by the two locations: D= cos−1(sin(lat1)*sin(lat2)+cos(lat1)*cos(lat2)*cos(lon2- lon1))*6371*0.62137 where D is distance in miles between a hospital and a monitor, lat1 and lat2 are latitudes of first and second locations respectively and lon1 and lon2 are longitudes of those locations.
To calculate the monthly average air pollutant levels, we averaged data from all monitors located within 10 miles of the hospital. We then linked the NIS hospital data to average monthly levels for all six pollutants. Two-month averages (which included the month of admission as well as the month prior) as well as three-, four-, and five-month averages were also calculated and linked to the hospital data.
Statistical Analyses
We used Spearman correlations to examine the unadjusted association between all six pollutants and the three outcomes: length of stay, charges, and costs. Recognizing that pollutants arising from similar sources (e.g. motor vehicles) can be highly correlated,40,41 we performed correlations among the pollutants to determine which ones to include in multivariable analyses. Any two pollutants with rho ≥ absolute value of 0.3 were not included together in multivariable models, and we prioritized the inclusion of PM2.5 and O3, the pollutants with the strongest evidence linked to adverse asthma outcomes.
Early in our analysis, we found an extremely narrow distribution of LOS among pediatric asthma hospitalizations, which are coded as ordinal numbers of days rather than hours. With the caveat that the limited distribution of LOS might make detection of pollutant effects unlikely, we used Poisson regression to account for the skewed distribution of the variable. For total charges and costs, we log10-transformed the variables. We included air pollutants in linear regression models based on results of correlation analysis. We also included covariates associated with asthma outcomes and/or hospital charges: age, race (white, black, Hispanic, or other), gender, income, insurance (Medicare, Medicaid, private, self-pay, no charge, or other), hospital region of the country (Northeast, South, Midwest, or West), teaching status of the hospital, and month of admission. We used the median household income quartile for patient's zip code as a proxy for income.
Any pollutant found to be a significant predictor was also categorized by quartiles to examine the effects at different levels of exposure. For air pollutants found to be significant predictors, we ran stratified models for different seasons and different age groups (2-5 years, 6-12 years, 13-17 years). For the linear regressions, we retransformed the results from log10 to the original scale using the smearing factor technique described by Duan.42
The Effect of Chronicity
For pollutants that were significant predictors of hospitalization charges, we examined the effect of increased exposure chronicity on outcomes. We built linear regression models with log10 charges as the outcome and average PM2.5 levels over differing lengths of exposure (2-5 months) as the predictor in four separate models. The strength of the association between PM2.5 levels of increasing duration and log10 total charges were compared.
Appendicitis Outcomes
To validate findings between air pollutants and asthma outcomes, we ran similar multivariable models for appendicitis, a frequent cause of pediatric hospitalization that should have no association with change in air pollutant levels. We hypothesized that there would be no significant relationships found.
Results
Between 1999 and 2007, there were 70,052,217 hospital admissions in the NIS database; 206,562 were for children ages 2-17 with a primary diagnosis of asthma. Hospital admissions with no corresponding air pollutant data were excluded for a final sample of 66,256 hospitalizations. Compared to all pediatric asthma hospitalizations, discharges included in the analysis had more patients who were black or Hispanic, were more likely to be in teaching hospitals, almost exclusively located in urban settings, and less likely to be located in the South (Table 1). These findings reflect the fact that air pollutant monitors are primarily located in urban areas, where air pollutant levels are highest. Race was unreported for a large percentage of patients as many states do not report this data to NIS.43,44
Table 1. Characteristics of Patients and Hospitals among Pediatric Asthma Hospitalizations in the Nationwide Inpatient Sample, 1999-2007.
| All pediatric asthma hospitalizations | Pediatric asthma hospitalizations with corresponding air pollutant data1 | |
|---|---|---|
| Patient Characteristics | weighted % | weighted % |
|
| ||
| Female | 38.7 | 38.9 |
| Race | ||
| White | 28.8 | 18.9 |
| Black | 25.6 | 32.1 |
| Hispanic | 15.3 | 21.4 |
| Asian or Pacific Islander | 1.6 | 2.4 |
| Native American | 0.4 | 0.4 |
| Other | 3.9 | 6.8 |
| Unreported | 24.5 | 18.0 |
| Median household income for patient zip code | ||
| 1st quartile | 26.4 | 28.1 |
| 2nd quartile | 27.1 | 24.8 |
| 3rd quartile | 23.1 | 22.7 |
| 4th quartile | 23.4 | 24.4 |
| Primary Payer | ||
| Medicare | 0.1 | 0.1 |
| Medicaid | 46.7 | 49.1 |
| Private including HMO | 45.1 | 42.9 |
| Self-pay | 5.2 | 6.5 |
| No charge | 0.2 | 0.1 |
| Other | 2.7 | 1.4 |
| Hospital characteristics | ||
| Region | ||
| Northeast | 24.8 | 47.8 |
| Midwest | 19.8 | 16.1 |
| South | 38.4 | 6.4 |
| West | 16.9 | 29.7 |
| Urban location | 86.7 | 99.8 |
| Teaching hospital | 57.5 | 84.0 |
Hospitalizations with data available for all six pollutants were included in bivariate and multivariable analyses
The mean total charges for pediatric asthma hospitalizations was $7,341, with a maximum charge of $994,726. The median charges and interquartile range were $4972 and $5227 respectively. The mean total cost was $3,235, with a maximum cost of $441,001. The median costs and interquartile range were $2338 and $2147 respectively. The descriptive data for air pollutant levels are shown in Table 2, including the current EPA criteria.
Table 2. Pollutant levels corresponding to Pediatric Asthma Hospitalizations from the Nationwide Inpatient Sample 1999-2007 and United States Air Quality Standards.
| Mean | Median | Maximum | National Ambient Air QualityStandards2 | ||
|---|---|---|---|---|---|
| Level | Averaging Time | ||||
| Particulate Matter PM2.5 (ug/m3) | 13.72 | 12.93 | 65.6 | 15 35 |
Annual3 24-hour4 |
| Particulate Matter PM10 (ug/m3) | 26.15 | 24.45 | 131.6 | 150 | 24-hour5 |
| Ozone (ppm) | 0.023 | 0.0217 | 3.694 | 0.075 0.12 |
8-hour6 1-hour7 |
| Nitrogen Dioxide (ppm) | 0.049 | 0.022 | 23.7 | 0.053 0.100 |
Annual 1-hour8 |
| Sulfur Dioxide (ppm) | 0.005 | 0.004 | 0.025 | 0.03 0.14 0.075 |
Annual 24-hour9 1-hour10 |
| Carbon Monoxide (ppm) | 0.714 | 0.646 | 9.48 | 9 35 |
8-hour9 1-hour9 |
abbreviations:
PM2.5=particulate matter of diameter<2.5microns;
PM10=particulate matter of diameter<10microns;
ppm=parts per million
To attain this standard, the 3-year average of the weighted annual mean PM2.5 concentrations from single or multiple monitors must not exceed 15.0 μg/m3
To attain this standard, the 3-year average of the 98th percentile of 24-hour concentrations at each monitor within an area must not exceed 35 μg/ m3 (effective December 17, 2006)
Not to be exceeded more than once per year on average over 3 years
To attain this standard, the 3-year average of the fourth-highest daily maximum 8-hour average ozone concentrations measured at each monitor within an area over each year must not exceed 0.075 ppm (effective May 27, 2008)
The standard is attained when the expected number of days per calendar year with maximum hourly average concentrations above 0.12 ppm is ≤ 1
To attain this standard, the 3-year average of the 98th percentile of the daily maximum 1-hour average at each monitor within an area must not exceed 0.1ppm or 100 ppb (effective January 22, 2010)
Not to be exceeded more than once per year
To attain this standard, the 3-year average of the 99th percentile of the daily maximum 1-hour average at each monitor within an area must not exceed 0.075 ppm or 75 ppb (effective June 2, 2010)
In unadjusted analyses, all six pollutants had minimal correlation with the three outcomes: LOS, total charges, and total costs (rho<0.1 and p<0.001 for all analyses). Among the different air pollutants, PM2.5 and PM10 were most highly correlated (rho=0.5). SO2 and CO were negatively correlated with O3 (rho= −0.4), and PM2.5 and CO were positively correlated (rho=0.4). As mentioned above, we prioritized PM2.5 and O3 for inclusion in final models because of known relationships with asthma morbidity. Therefore, PM2.5, O3, and NO2 were further examined in three-pollutant multivariable analyses.
In multivariable analyses, none of the three pollutants were significant predictors of LOS (Table 3). However, PM2.5 remained a significant predictor of both charges and costs. A 1-unit (μg/m3) increase in monthly PM2.5 led to a $123 increase in charges (95% CI: +$40 to +$249) and a $47 increase in costs (95% CI: +$15 to +$93). O3 (+$4797, 95% CI: -$10,111 to +$6,463,445) and NO2 (+$88, 95% CI: -$162 to +$483) were not significant predictors of charges. Other significant predictors of charges included older age, female gender, and hospital location in the west (p<0.0001 for all associations).
Table 3. Multivariable results for Pediatric Asthma Hospitalization Length of Stay, Charges, and Costs as predicted by Changes in Levels of Fine Particulate Matter1.
| PM2.5 level2 | Increment Change (95% CI) in Length of Stay3 | Increment Change (95% CI) in Total Charges4 | Increment Change (95% CI) in Total Costs4 |
|---|---|---|---|
| 1 month avg5 | +0.6% (-0.3-1.5) | +$123 (40-249)** | +$47 (15-93)** |
| 2 month avg | +0.9% (-0.2-2.1)* | +$170 (67-321)*** | +$55 (20-110)** |
| 3 month avg | +1.1% (-0.2-2.4)* | +$195 (82-362)*** | +$62 (24-121)*** |
| 4 month avg | +1.2% (-0.1-2.6)* | +$210 (93-386)*** | +$67 (29-126)*** |
| 5 month avg | +1.1% (-0.3-2.6)* | +$222 (100-404)*** | +$69 (30-127)*** |
Increment changes for 1-unit (ug/m3) increase in particulate matter of diameter <2.5ug (PM2.5)
Average level of PM2.5 within a 10-mile radius of the hospital
Increment percent change calculated from exponentiating Poisson beta coefficient
Increment change in dollars calculated using Duan transformation for linear regression
p-value ≤ 0.1
p-value ≤ 0.001
p-value ≤ 0.0001
avg=average
When the model for costs was run with PM2.5 as a variable categorized by quartile, the lowest quartile of exposure led to an average decrease of $918 (95% CI: +$559 to +$1105) in costs compared to the highest quartile of exposure. Stratified models examining PM2.5 and total charges showed evidence of effect modification by season and age (Table 4). The relationship was strongest in the summer and weakest in the winter. The oldest age group had the strongest association between PM2.5 and charges (+$240, 95% CI: +$29 to +$658).
Table 4. Pediatric Asthma Hospitalization Charges in Association with Fine Particulate Matter Levels, Stratified by Age and Season.
| Increment changes in Total Charges (95% CI)1 | |
|---|---|
| Age group (years) | |
| 2-5 | $118 (42-227)*** |
| 6-12 | $132 (36-280)** |
| 13-17 | $240 (29-658)* |
| Season | |
| Winter | $30 (−70-195) |
| Spring | $223 (72-485)*** |
| Summer | $226 (111-406)*** |
| Fall | $103 (24-222)* |
Increment change in hospitalization charges for 1-unit (ug/m3) increase in particulate matter of diameter <2.5ug (PM2.5) calculated using Duan transformation for linear regression
p-value<0.05
p-value<0.005
p-value<0.0005
The Effect of Chronicity
When increasing durations of PM2.5 exposure were used as the predictor in the linear regression model for asthma total charges, the relationship between PM2.5 and charges continued to become stronger and more significant, with average PM2.5 during the 5 months preceding hospitalization having the strongest association (+$222, 95% CI: +$100 to +$404, Figure 1).
Figure 1.
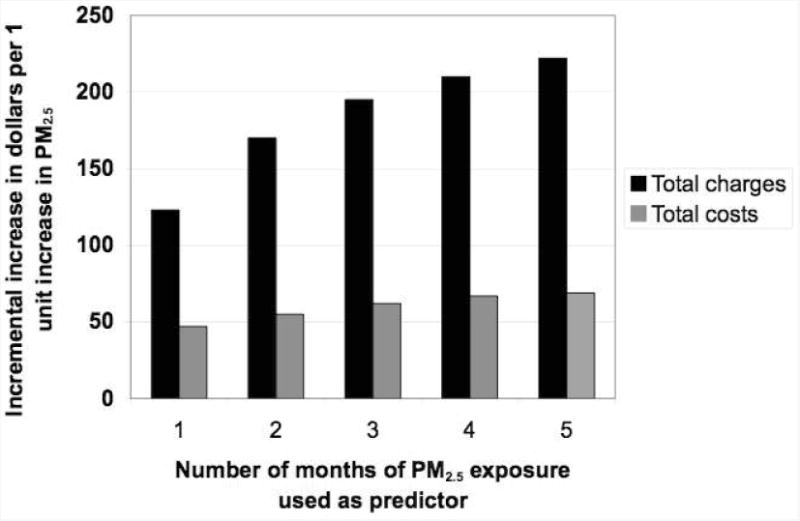
Total charges and costs of pediatrics asthma hospitalizations for increasing durations of particulate matter exposure. PM2.5 is particulate matter with diameter less than 2.5 micrometers.
Appendicitis Outcomes
Linear regression models with the outcomes of total charges and costs for appendicitis hospitalizations including the same predictors as in the other models did not show a significant association with PM2.5. Although some variables were significant predictors, demonstrating the potential variability in appendicitis charges, PM2.5 was not significantly associated with the outcome, contrary to the findings with asthma hospitalizations.
Discussion
In this national study, we found a significant association between average PM2.5 levels around hospitals and pediatric asthma hospitalization total charges and costs, but not LOS. The relationship was found to be the strongest in the summer and among older children. The association was stronger with an increasing chronicity of exposure. We found no association between PM2.5 and appendicitis outcomes.
Substantial health care dollars are spent each year on asthma,1,3 and controlling levels of PM2.5 may result in considerable savings. We found that a 1-unit increase in PM2.5 increased pediatric asthma hospitalization costs on average by $47. There are approximately 200,000 pediatric asthma hospitalizations in the US annually, about 85% of which are in urban areas.45 Extrapolating our findings to all urban pediatric asthma hospitalizations, reducing the average level of PM2.5 by 1 unit could save about $8 million annually. We also found that decreasing PM2.5 from the highest to lowest quartile decreased costs on average by $918 per hospitalization. Extrapolating this to the 25% of urban hospitals in the highest quartile, decreasing PM2.5 to the lowest quartile could save about $39 million. This amount represents only a small fraction of potential healthcare savings resulting from reduced PM2.5 levels, especially given other well-known adverse health outcomes associated with this pollutant, such as cardiac mortality.46,47 These figures are also conservative as they represent total costs, not charges.
Interestingly, increased charges were not found with other air pollutants, such as O3, known to have adverse effects on asthma outcomes. One explanation is that, as shown in previous studies, ambient O3 levels measured at central monitoring sites may not be representative of personal exposure, in contrast to PM2.5 for which ambient levels are highly correlated with personal exposure.48-50 Inaccurate represention of personal exposure biases the results towards the null; perhaps this is why no relationship is seen with other pollutants. Another potential explanation is that the effects of sub-chronic exposure to O3 are different than acute exposure. Although the acute effects of O3 on asthma are well known, sub-chronic exposure to O3 may not play a significant role in adverse asthma outcomes, at least not enough to translate into increased hospitalization costs and charges.
We hypothesized that higher PM2.5 levels lead to increased asthma severity and thus increased charges, in part mediated by increased LOS. However, we found no significant association with LOS. Because LOS is measured in units of whole days, it would take quite a significant increase in severity to result in another full day of hospitalization and as mentioned above, the narrow distribution of LOS limited our analytic capacity.
The associations between charges and PM2.5 showed effect modification by season and age, with results most significant for summer months and older children. Previous studies have shown that ambient PM2.5 concentrations are most highly correlated to personal exposure during summer months when people spend the most time outdoors.48 Older children also tend to spend more time outdoors. In both cases, the stronger associations found may be because personal exposure is being more closely approximated by ambient PM2.5 levels during warmer months and in older children. Although asthma severity and overall hospitalization charges may be highest in the winter, PM2.5 levels may not be as strongly associated with charges because children have relatively lower exposure compared to warmer seasons.
There is little known about sub-chronic effects of PM2.5 on asthma outcomes. We found that the chronicity of PM2.5 exposure played an important role in the association with costs and charges: an increasing duration of exposure showed stronger and more significant associations. That is, long-term exposure to PM2.5 had more of an effect than short-term exposure. These results are supported by a recent study showing that children with higher exposure to PM2.5 for one year were more likely to be diagnosed with asthma and have asthma attacks.24 One plausible explanation is that similar to its role in cardiac mortality,46 PM2.5 leads to increased inflammation over time and consequently increased severity when children have asthma attacks. Our findings raise some interesting questions and highlight the importance of continued research into the associations between sub-chronic PM2.5 exposure and asthma morbidity.
An important limitation of this study is that the exposure measurement is crude. We have used ambient pollutant concentrations near the hospitals because information about personal exposures is unavailable. Prior studies have shown that ambient PM2.5 levels measured at central monitoring sites are highly correlated with average personal PM2.5 exposure. 48 In contrast, ambient O3, NO2, and SO2 concentrations are weakly correlated with personal exposures to these gases.48-50 This supports the fact that our PM2.5 exposure measure represents personal exposure fairly accurately, provided that the majority of patients live in the vicinity of the hospital. There are inevitably some incorrect exposure assessments; however, these misclassifications would tend to bias the results towards the null, and we found clear and consistent relationships with PM2.5 in spite of these misclassifications. The lack of association with the other pollutants may be due to incorrect exposure assessment. An alternate explanation, as mentioned above, is that sub-chronic exposure to the other pollutants may not play a role in asthma severity.
It is important to note that all four regions of the country were not equally represented. Although the South represented 38% of all hospitalizations, but only 6% of included hospitalizations. Because fewer hospitalizations in the South occur in urban areas (75%) compared with other regions (>=85%), a greater proportion did not have corresponding pollutant data, limiting the sample of Southern hospitalizations compared with other regions. Correspondingly, the Northeast and West are over-represented in our analyses.
Another limitation is that the NIS does not contain details regarding management during hospitalization, e.g. medications used and intensive care. As discussed above, the proposed mechanism of the association is increased asthma severity resulting in increased use of hospital resources. Without detailed information about hospital stays, we are unable to examine the specific etiologies of the increased charges.
An additional potential limitation is residual confounding by socioeconomic status. It is known that asthma severity is worse in urban, inner-city areas, which are also the areas where PM2.5 levels tend to be the highest. In multivariable analyses, we did our best to control for income and insurance as markers of socioeconomic status. Also, urban areas are where other air pollutant levels are highest, and no associations were found with pollutants besides PM2.5. The clear and consistent associations found between increasing durations of PM2.5 and hospital charges make it likely that the relationships seen are specific to PM2.5 and not due to confounding by socioeconomic status.
A major strength is that our results are based on a large amount of data nationally representative of urban area hospitals. Although these results cannot be translated to non-urban areas, as stated above, 85% of pediatric asthma hospitalizations do occur in urban areas.45 Another important strength is that secondary analysis examining the relationship between PM2.5 and appendicitis hospitalization charges showed no significant association, validating our results that the association is for a diagnosis with a biologically plausible connection with PM2.5 and not a spurious finding. We also have results in costs, not just charges. It is a strength that our study provides results in actual healthcare dollars spent.
Our results should be interpreted with caution because the exposure assessment is inherently with limitations. Other studies have employed more complex methods to assess personal exposure to air pollutants, such as modeling multiple point source data into an aggregate exposure metric. However, no other studies have examined healthcare costs associated with specific air pollutants or the health effects of sub-chronic air pollutant exposure on pediatric asthma – two important areas of future research. In spite of the deficiencies noted, our findings merit further study using more rigorous exposure assessment and datasets containing detailed information regarding healthcare utilization.
Sub-chronic PM2.5 exposure is associated with increased costs for pediatric asthma hospitalizations. Studies such as this one should stimulate further research in this area and incite regulatory agencies such as the EPA to consider sub-chronic pollutant levels when setting standards for air pollutants. Our results provide economic data to reinforce the need for ongoing efforts to reduce levels of air pollutants in this country. Policy changes to improve air quality may lead to improved asthma outcomes as well as substantial savings in healthcare spending.
Acknowledgments
The study was funded by the National Institutes of Health Research Training Grant in Environmental Pediatrics [NIH 5T32 HD049311] and the Mount Sinai Children's Environmental Health Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang LY, Zhong Y, Wheeler L. Direct and indirect costs of asthma in school-age children. Prev Chronic Dis. 2005;2(1):A11. [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami L. Asthma prevalence, health care use and mortality, 2003-05. [Accessed 09/10, 2008]; Available at: http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm.
- 3.Landrigan PJ, Schechter CB, Lipton JM, et al. Environmental pollutants and disease in American children: estimates of morbidity, mortality, and costs for lead poisoning, asthma, cancer, and developmental disabilities. Environ Health Perspect. 2002 Jul;110(7):721–728. doi: 10.1289/ehp.02110721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moorman J, Rudd R, Johnson C. National Surveillance for Asthma-United States, 1980-2004. 2007 Oct 19;56(SS08):1-14–18-54. [PubMed] [Google Scholar]
- 5.Togias A, Fenton MJ, Gergen PJ, et al. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010 Mar;125(3):540–544. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Webber MP, Carpiniello KE, Oruwariye T, et al. Prevalence of asthma and asthma-like symptoms in inner-city elementary schoolchildren. Pediatr Pulmonol. 2002;34(2):105–111. doi: 10.1002/ppul.10146. [DOI] [PubMed] [Google Scholar]
- 7.Aligne A. Risk factors for pediatric asthma. Contributions of poverty, race, and urban residence. American Journal of Respiratory and Critical Care Medicine. 2000;162(3):873. doi: 10.1164/ajrccm.162.3.9908085. [DOI] [PubMed] [Google Scholar]
- 8.Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol. 2005;115(4):689–699. doi: 10.1016/j.jaci.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Brunekreef B, Holgate ST. Air pollution and health. The Lancet. 2002;360(9341):1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 10.Slaughter JC, Lumley T, Sheppard L, et al. Effects of ambient air pollution on symptom severity and medication use in children with asthma. Annals of Allergy Asthma and Immunology. 2003;91(4):346–353. doi: 10.1016/S1081-1206(10)61681-X. [DOI] [PubMed] [Google Scholar]
- 11.Just J, Segala C, Sahraoui F, et al. Short-term health effects of particulate and photochemical air pollution in asthmatic children. European respiratory journal. 2002;20(4):899. doi: 10.1183/09031936.02.00236902. [DOI] [PubMed] [Google Scholar]
- 12.Ostro B, Lipsett M, Mann J, et al. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001:200–208. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Thurston GD, Lippmann M, Scott MB, et al. Summertime haze air pollution and children with asthma. American journal of respiratory and critical care medicine. 1997;155(2):654. doi: 10.1164/ajrccm.155.2.9032209. [DOI] [PubMed] [Google Scholar]
- 14.Peel JL, Tolbert PE, Klein M, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16(2):164. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- 15.Wilson AM, Wake CP, Kelly T, et al. Air pollution, weather, and respiratory emergency room visits in two northern New England cities: an ecological time-series study. Environ Res. 2005;97(3):312–321. doi: 10.1016/j.envres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Tolbert PE, Mulholland JA, Macintosh DL, et al. Air Quality and Pediatric Emergency Room Visits for Asthma and Atlanta, Georgia. Am J Epidemiol. 2000;151(8):798. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- 17.Norris G, YoungPong SN, Koenig JQ, et al. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect. 1999;107(6):489–493. doi: 10.1289/ehp.99107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White MC, Etzel RA, Wilcox WD, et al. Exacerbations of childhood asthma and ozone pollution in Atlanta. Environ Res. 1994;65(1):56–68. doi: 10.1006/enrs.1994.1021. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor GT, Neas L, Vaughn B, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008 May;121(5):1133–1139.e1. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Dales R, Chen L, Frescura AM, et al. Acute effects of outdoor air pollution on FEV1: a panel study of schoolchildren with asthma. European Respiratory Journal 2009. 2009 Aug;34:316. doi: 10.1183/09031936.00138908. [DOI] [PubMed] [Google Scholar]
- 21.Linn WS, Shamoo DA, Anderson KR, et al. Short-term air pollution exposures and responses in Los Angeles area schoolchildren. J Expo Anal Environ Epidemiol. 1996 Oct-Dec;6(4):449–472. [PubMed] [Google Scholar]
- 22.Lin S, Liu X, Le LH, et al. Chronic Exposure to Ambient Ozone and Asthma Hospital Admissions among Children. Environ Health Perspect. 2008;116(12):1725. doi: 10.1289/ehp.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babin SM, Burkom HS, Holtry RS, et al. Pediatric patient asthma-related emergency department visits and admissions in Washington, DC, from 2001-2004, and associations with air quality, socio-economic status and age group. Environ Health. 2007 Mar 21;6:9. doi: 10.1186/1476-069X-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akinbami LJ, Lynch CD, Parker JD, et al. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001-2004. Environ Res. 2010 Jan 29; doi: 10.1016/j.envres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Zanobetti A, Schwartz J. The Effect of Fine and Coarse Particulate Air Pollution on Mortality: A National Analysis. Environ Health Perspect. 2009;117(6):899. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schildcrout JS, Sheppard L, Lumley T, et al. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164(6):505. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 27.Vedal S, Petkau J, White R, et al. Acute effects of ambient inhalable particles in asthmatic and nonasthmatic children. American Journal of Respiratory and Critical Care Medicine. 1998;157(4):1034. doi: 10.1164/ajrccm.157.4.9609008. [DOI] [PubMed] [Google Scholar]
- 28.Yu O, Sheppard L, Lumley T, et al. Effects of ambient air pollution on symptoms of asthma in Seattle-area children enrolled in the CAMP study. Environ Health Perspect. 2000;108(12):1209. doi: 10.1289/ehp.001081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortimer KM, Neas LM, Dockery DW, et al. The effect of air pollution on inner-city children with asthma. European respiratory journal. 2002;19(4):699–705. doi: 10.1183/09031936.02.00247102. [DOI] [PubMed] [Google Scholar]
- 30.Agency for Toxic Substances and Disease Registry. Glossary of terms. [Accessed 06/23,2010]; Available at: http://www.atsdr.cdc.gov/glossary.html#Intermediate%20Duration%20Exposure.
- 31.Karr C, Lumley T, Schreuder A, et al. Effects of subchronic and chronic exposure to ambient air pollutants on infant bronchiolitis. Am J Epidemiol. 2007;165(5):553. doi: 10.1093/aje/kwk032. [DOI] [PubMed] [Google Scholar]
- 32.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332(3):133. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 33.Agency for Healthcare Quality and Research. Overview of the Nationwide Inpatient Sample (NIS) [Accessed 09/11, 2009];2009 Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 34.United States Environmental Protection Agency. Aerometric Information Retrieval System (AIRS) [Accessed 09/11, 2009];2009 Available at: http://www.epa.gov/enviro/html/airs/
- 35.US EPA Technology Transfer Network. Download Detailed AQS Data. [Accessed 02/23, 2010];2010 Available at: http://www.epa.gov/ttn/airs/airsaqs/detaildata/downloadaqsdata.htm.
- 36.United States Department of Labor. Bureau of Labor Statistics. Consumer Price Index. [Accessed 09/11, 2009]; Available at: http://data.bls.gov/cgi-bin/surveymost?cu.
- 37.Agency for Healthcare Research and Quality. Cost-to-Charge Ratio Files. Healthcare Cost and Utilization Project (HCUP) [Accessed 09/11, 2009];2009 Available at: http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp.
- 38.Morse S. Batch Conversions of Address to Latitude/Longitude. [Accessed 02/23, 2010]; Available at: http://stevemorse.org/jcal/latlonbatch.html?direction=forward.
- 39.US EPA. Monitor Values Report - Criteria Pollutants. [Accessed 02/23, 2010]; Available at: http://www.epa.gov/air/data/monvals.html.
- 40.Kim EE. Spatial Variability of Fine Particle Mass, Components, and Source Contributions during the Regional Air Pollution Study in St. Louis. Environ Sci Technol. 2005;39(11):4172–4179. doi: 10.1021/es049824x. [DOI] [PubMed] [Google Scholar]
- 41.Morawska L, Thomas S, Bofinger N, et al. Comprehensive characterization of aerosols in a subtropical urban atmosphere particle size distribution and correlation with gaseous pollutants. Atmos Environ. 1998;32(14-15):2467–2478. [Google Scholar]
- 42.Duan N. Smearing estimate: a nonparametric retransformation method. Journal of the American Statistical Association. 1983;78(383):605–610. [Google Scholar]
- 43.Trasande L, Lee M, Liu Y, et al. Incremental charges, costs, and length of stay associated with obesity as a secondary diagnosis among pregnant women. Med Care. 2009;47:1046–1052. doi: 10.1097/MLR.0b013e31819c94b8. [DOI] [PubMed] [Google Scholar]
- 44.Trasande L, Liu Y, Fryer G, et al. Effects of childhood obesity on hospital care and costs, 1999-2005. Health Aff (Millwood) 2009 Jul-Aug;28(4):w751–60. doi: 10.1377/hlthaff.28.4.w751. [DOI] [PubMed] [Google Scholar]
- 45.Agency for Healthcare Research and Quality. HCUP Net. [Accessed 06/10, 2010]; Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp.
- 46.Belleudi V, Faustini A, Stafoggia M, et al. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology. 2010 May;21(3):414–423. doi: 10.1097/EDE.0b013e3181d5c021. [DOI] [PubMed] [Google Scholar]
- 47.Simkhovich BZ, Kleinman MT, Kloner RA. Particulate air pollution and coronary heart disease. Curr Opin Cardiol. 2009 Nov;24(6):604–609. doi: 10.1097/HCO.0b013e32833161e5. [DOI] [PubMed] [Google Scholar]
- 48.Sarnat JA, Brown KW, Schwartz J, et al. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16(3):385–95. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- 49.Liu LJS, Box M, Kalman D, et al. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ Health Perspect. 2003;111(7):909. doi: 10.1289/ehp.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarnat JA, Koutrakis P, Suh HH. Assessing the relationship between personal particulate and gaseous exposures of senior citizens living in Baltimore, MD. J Air Waste Manag Assoc. 2000;50(7):1184–99. doi: 10.1080/10473289.2000.10464165. [DOI] [PubMed] [Google Scholar]


