Abstract
The p53 protein has not only important tumor suppressor activity, but also additional immunological and other functions, whose nature and extent are only beginning to be recognized. Here we show that p53 has a novel inflammation-promoting action in the intestinal tract, since loss of p53 or the upstream activating kinase, ATM, protects against acute intestinal inflammation in murine models. Mechanistically, deficiency in p53 leads to increased survival of epithelial cells and lamina propria macrophages, higher IL-6 expression due to enhanced glucose-dependent NF-κB activation, and increased mucosal STAT3 activation. Blockade or loss of IL-6 signaling reverses the protective effects of p53 deficiency. Conversely, IL-6 treatment protects against acute colitis in a manner dependent on STAT3 signaling and induction of cytoprotective factors in epithelial cells. Together, these results indicate that p53 promotes inflammation in the intestinal tract through suppression of epithelium-protective factors, thus significantly expanding the spectrum of physiological and immunological p53 activities unrelated to cancer formation.
Introduction
The multi-functional protein, p53, acts as a tumor suppressor (1) that arrests cell growth at the G1/S check point upon DNA damage, giving DNA repair enzymes time to correct the damage before resumption of DNA replication, and directly activates these enzymes (2). Furthermore, if DNA damage proves irreparable, p53 mediates programmed cell death and thus removal of mutated cells. In the absence of p53, certain forms of DNA damage fail to cause normal cell death of the afflicted cells. P53 also acts as a transcriptional activator or repressor of multiple target genes such as CD95/Fas and Puma (3, 4), and has an impact on cellular glucose metabolism. Tumor cells often switch from aerobic to anaerobic generation of ATP from glucose, which is in part mediated by p53 through differential expression of the key mitochondrial enzyme, cytochrome c oxidase 2, and the glucose transporter, SLC2A3/GLUT3 (5). Enhanced glucose metabolism in the absence of p53 also leads to increased constitutive protein glucosylation, which can enhance signaling through the NF-κB signaling kinase, IKKβ (6). Due to this impact on IKKβ activation and perhaps other mechanisms related to direct transcriptional activation, p53 deficiency is associated with increased NF-κB activation and expression of NF-κB target genes in macrophages (7), although the physiological consequences of these observations are not well understood.
The physiological functions of p53 have been extensively studied in gene-targeted mice. These animals are fertile and healthy when born and are grossly normal for the first few months, but then develop spontaneous tumors in the thymus and other sites. Before tumor formation, the knockout mice are more susceptible to severe joint destruction in collagen-induced arthritis due to increased T cell proliferation and release of cartilage-degrading enzymes (8–10). Mice lacking p53 also develop more severe experimental autoimmune encephalomyelitis (11) and enhanced chronic gastric inflammation upon infection with Helicobacter pylori (12). Together, these data suggest that p53 has anti-inflammatory functions in different organ systems.
Intestinal inflammation, which is associated with increased risk of colorectal cancer (13, 14), leads to p53 activation (15). Conversely, dysfunction of the protein plays a role in inflammation-associated colon cancer formation. For example, p53 mutations occur early in the development of UC-related colorectal cancers, whereas such mutations occur only late in the pathogenesis of sporadic colorectal cancers (16). Mutations of p53 have been identified in non-dysplastic, inflamed tissues of UC patients without cancer (17), suggesting that p53 dysfunction may have an unrecognized physiological impact on mucosal inflammation. Based on the findings in several organs and the role of p53 in inflammation-associated colon cancer, we set out to test the hypothesis that p53 antagonizes inflammatory processes in the intestinal tract. Surprisingly, our studies reveal the opposite, a novel pro-inflammatory function of p53 related to limiting the production of survival factors for the intestinal epithelium. These results not only significantly expand the known physiological functions of p53 unrelated to tumor suppression, but also have important implications for the connection between inflammation and neoplastic transformation under chronic inflammatory conditions in the intestinal tract.
Materials and Methods
Mice
Trp53−/− mice were obtained from The Jackson Laboratory. Atm−/− mice were kindly provided by Y. Xu (18) and Mlh1−/− and Msh1−/− mice by W. Edelman (19, 20). All three strains were backcrossed for at least ten generations to a 129/SVJ background, and wild-type 129/SVJ mice were used as controls. Wild-type C57/BL6 and Il6−/− mice were obtained from The Jackson Laboratory and crossed with Trp53−/− mice (on a C57BL/6J background) to obtain Trp53−/− x Il6−/− mice. Mice with a floxed Trp53 allele, originally acquired from the National Cancer Institute, Bethesda, MD (21), were crossed with Villin-Cre transgenic mice or Lysozyme M-Cre (The Jackson Laboratory) (22, 23) to obtain selective deletion of p53 in intestinal epithelial cells (Trp53IEC-KO) or macrophages and neutrophils (Trp53Mac/PMN-KO), respectively. Epithelial STAT3 knock-out mice were generated as described before (24). Results from males and females are reported together, as no significant differences were detected between the genders in the severity of colitis. Mice were kept under specific pathogen-free conditions. All animal studies were approved by the Institutional Animal Care and Use Committee s of the University of California, San Diego or the Technical University, Munich.
Bone marrow transplantation
Bone marrow chimeric mice were generated by lethally irradiating recipient mice with 9 Gy of γ-irradiation from a cesium source, followed by adoptive transfer of 5 × 106 bone marrow cells from Trp53−/− or wild-type mice. Experiments were conducted 8 weeks after transplantation.
Colitis model
Colitis was induced by administration of 2.5–3% dextran sulfate sodium (DSS3, MP Biomedicals) in the drinking water for 5–6 days, with daily monitoring of body weight. When specified, mice were also given 10 mg/kg of N-acetylcysteine (Sigma Aldrich) in the drinking water one day before and throughout the respective experiment.
Histological analysis
Tissues were fixed with formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Histological inflammation scores (scale 0–12) and extent of epithelial ulceration were determined as we have described before (25). Apoptotic cells were detected by the TUNEL technique using the in situ cell death detection kit (Clontech) and counterstaining with Hoechst 33342 dye.
Myeloperoxidase (MPO) analysis
MPO activity was determined by enzymatic assay as described before (26). Enzyme activity was normalized against wet tissue weight.
Real-time PCR analysis
Total RNA was extracted using Trizol reagent (Invitrogen). Contaminating DNA was removed from the RNA by treatment with Turbo DNase (Ambion). Reverse transcription was performed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) and real-time PCR amplification using the Mesa Green 2x SYBR reaction mixture (Eurogentec). Relative changes in mRNA levels were calculated by the 2ΔΔCt method, with GAPDH as reference standard.
Immunoblotting
Tissues were lysed for 30 min on ice in a buffer containing 150 mM NaCl, 5 mM KCl, 10 mM HEPES, 0.5 mM EDTA, 0.2 mM EGTA, 1 mM sodium fluoride, 1 mM vanadate, 0.05% Nonidet P-40, 1 mM DTT, and a protease inhibitor cocktail (Roche Applied Science). Proteins were size-separated by electrophoresis on a 10% Tris-glycine polyacrylamide gel (Bio-Rad Laboratories) and transferred to a polyvinylidene difluoride membrane. After blocking, specific proteins were detected by staining with one of the following rabbit primary antibodies: Anti-mouse p53 (Santa Cruz), Anti phospho-p53 (Ser15) (Cell Signaling), Anti-mouse STAT3, or Anti-mouse phospho-STAT3 (Ser727) (both Santa Cruz). After washing and staining with HRP-conjugated goat anti-rabbit IgG, specific signals were detected by enhanced chemiluminescence (Pierce).
Isolation of epithelial and lamina propria cells
The colons was cut into 0.5 cm pieces and incubated for 20 min at 37°C with gentle shaking in calcium- and magnesium-free HBSS containing 5 mM EDTA, 5% FCS, and 15 mM HEPES (pH 7.3). Detached cells were passed through a 100 µm pore size nylon mesh strainer, centrifuged, and the pellet was frozen in liquid nitrogen. The remaining tissue pieces were washed and incubated twice at 37°C for 30 min in RPMI 1640 medium containing 1 mg/ml collagenase D (Roche Applied Science) and 100 µg/ml DNase I (Worthington Biochemical). After vortexing for 30 s, cell suspensions were passed through a 40-µm cell strainer, and collected by centrifugation.
Flow cytometry and cell sorting
Suspensions of detached epithelial and lamina propria cells from the colon were stained for 10 min on ice with fluorochrome-conjugated annexin V and/or antibodies against mouse CD45, F4/80, CD11b, CD11c, or EpCAM (eBioscience), and propidium iodide (Molecular Probes). Sorting of viable cells was done on a Beckman Coulter MoFlo high-speed cell sorter. Epithelial cells were identified as CD45−EpCAM+ cells, dendritic cells as CD45+CD11c+ cells, and macrophages as CD45+, CD11b+, CD11c− cells. For analysis of apoptotic macrophages, stained cells were fixed with 2% paraformaldehyde in PBS and analyzed on a BD Biosciences FACSCalibur flow cytometer by gating for CD45 and F4/80 positive cells.
Macrophage and dendritic cell cultures
Bone marrow macrophages were produced by culturing bone marrow suspensions in DMEM, 10% FCS, and 30% L cell-conditioned medium (containing M-CSF) for 7 days. Dendritic cells were generated by culturing bone marrow with 50 ng/ml murine GM-CSF for 7 days. Cells were stimulated with 100 ng/ml LPS from E. coli O127:B8 (Sigma Aldrich, 10 ng/ml IFN-γ, 10 ng/ml TNFα, 10 ng/ml IL-1β (all Pepro Tech), or 200 µM H2O2. Cytokine levels were assayed by ELISA (R&D Systems).
IL-6 reagents
Recombinant murine IL-6, soluble gp130/Fc fusion protein, and monoclonal rat anti-murine IL-6 antibodies were produced as described before (27–30). Rat IgG was used as a control. All reagents were diluted in PBS and injected intraperitoneally.
Statistical analysis
Data were analyzed by t-test, two-way ANOVA, repeated-measures ANOVA, or Wilcoxon rank sum test, as appropriate, with significance levels indicated for p<0.05 (*), p<0.01 (**), or p<0.001 (***). Survival data were plotted as Kaplan-Meier survival curves, and were analyzed for statistical significance by log rank test.
Results
p53 is activated by oxidative stress in acute colitis
Free radicals and oxidative stress can cause DNA damage and lead to p53 activation (31). To assess p53 activity during acute intestinal inflammation, we induced colitis in C57BL/6 mice by oral administration of the irritant, DSS, and determined the levels of total and serine 15-phosphorylated, activated p53 by immunoblotting. Under resting conditions, constitutive p53 activation was observed in the whole colon (Fig. 1A) and in isolated intestinal epithelial cells and in lamina propria cells. DSS administration caused a marked increase in total and phospho-p53 (serine 15) levels by 7 and 10 days, times that coincide with peak inflammatory changes in this colitis model. Increased p53 activity was functionally relevant, since expression of several p53 target genes, including Fas1 and Puma, was increased in colitic mice (Fig. 1B). As expected, induction of these genes was abolished or strongly attenuated in mice lacking p53 (Fig. 1B). In contrast, a p53-independent inflammation-associated gene, Icam1, was induced in colitis but not affected by the absence of p53. Because oxidative stress is a major driver of p53 activation, we determined if it plays a role in colitis-induced p53 activation. Treatment with the antioxidant, N-acetyl cysteine (NAC), prevented the increase in total and phospho-p53 (serine 15) levels after colitis induction (Supplementary Fig. S1). Together, these results show that acute colitis causes marked oxidant-dependent p53 activation in the intestinal mucosa.
FIGURE 1.
Attenuation of acute colitis in the absence of p53. (A) Immunoblots of phospho-p53 (serine 15) and total p53 of whole colon extracts from mice treated with DSS for the indicated times. Whole colon from mice exposed to 4 Gy γ-irradiation and from Trp53−/− mice were used as controls. A second experiment gave similar results. (B) Expression levels of the indicated p53 target genes, and the p53-independent control, ICAM-1, were assayed by real-time PCR in RNA extracted from the colon of wild-type (Trp53+/+) and p53-deficient (Trp53−/−) mice, either treated with DSS (day 7) or left untreated, and are expressed as ratio in DSS-treated over untreated mice, normalized to GAPDH. Data are mean ± SD (n=3; t-test). (C) Trp53−/− (n=5), Trp53+/− (n=16), and Trp53+/+ mice (n=7) were treated with DSS. Body weights are shown as mean ± SD of the percentage of the initial weight. All three groups were significantly different from each other (p<0.01), as calculated by repeated-measures ANOVA for the entire weight curves. (D) Kaplan-Meier survival curves for Trp53−/− (n=45, dotted line), Trp53+/− (n=74, dashed line), and Trp53+/+ mice (n=77, solid line), with p-values calculated by log rank test (*p<0.05). (E) Paraffin sections of the colon from DSS-treated mice (day 7–10) were prepared and stained with hematoxylin and eosin. (F) Histological inflammation scores (range 0–12) and total colon ulcerations were evaluated on the colon sections. Each data point represents one animal, with means shown as horizontal lines (p-values were determined by Wilcoxon rank sum test). (G) MPO levels were assayed in colon lysates on days 0 and 7 of DSS treatment. Data are mean ± SD (n=3/group; p-values were determined by two-way ANOVA and Tukey post-test).
p53 deficiency protects against acute colitis
To define the physiological function of p53 in acute colitis, we employed mice deficient for p53 (Trp53−/−), mice heterozygous for the gene (Trp53+/−), and wild-type littermate controls (Trp53+/+). Controls exhibited maximal body weight loss 10–14 days after initiation of DSS feeding and regained weight over the subsequent 14 days (Fig. 1C). The disease was accompanied by modest mortality (~30%) in the controls over the 4-week period (Fig. 1D). In contrast, Trp53−/− mice showed significantly less weight loss in the acute stage and recovered more rapidly thereafter (Fig. 1C). In parallel, p53-deficient mice experienced significantly lower total mortality (~10%) over the entire observation period (Fig. 1D). Mice heterozygous for Trp53 had an intermediate phenotype, showing weight loss and overall mortality at reduced levels compared to controls, but at higher levels than in Trp53−/− mice (Fig. 1C,D).
Histopathological analysis of colon sections confirmed and extended the clinical observations. Wild-type mice had marked ulcerations in the acute stage of colitis characterized by a large areas of crypt loss and heavy infiltration of mucosa and submucosa with inflammatory cells, whereas Trp53−/− mice showed markedly less inflammation, as evidenced by significantly lower histological inflammation scores and smaller ulcerative lesions (Fig. 1E,F). Heterozygous mice exhibited an intermediate phenotype. Validation of the histological findings came from enzymatic assays of the neutrophil marker, MPO, in total colon extracts. MPO levels were significantly lower in Trp53−/− and Trp53+/− mice compared to controls (Fig. 1G). Thus, the clinical, histological, and biochemical data demonstrate that p53 deficiency markedly attenuates inflammation and mortality in the acute colitis model.
Because p53 plays a key role in protecting genome integrity, we questioned whether its loss would lead to increased DNA damage during acute intestinal inflammation. To assess DNA damage and repair processes, we assayed expression of the DNA repair enzyme, OGG1, which recognizes and excises 8-hydroxyl-deoxyguanosines that form in response to oxidative stress in inflammation and is an early marker of DNA damage. Ogg1 mRNA was increased by 9.1-fold in the colon of wild-type mice by 7 days of DSS administration, consistent with other observations that oxidant-related DNA damage activates expression of this enzyme (32). By comparison, Ogg1 mRNA levels were only 2.9-fold increased in the colon of DSS-treated Trp53−/− mice, suggesting that attenuated inflammation in the absence of p53 is associated with reduced production of DNA-damaging agents and is not associated with increased acute DNA damage.
ATM dependence of p53 activation in acute colitis
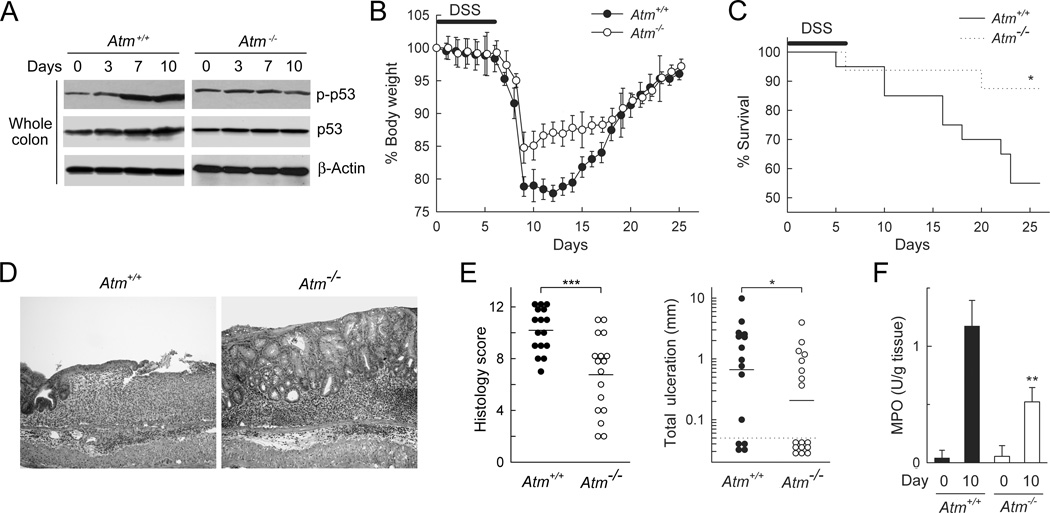
Activation of p53 requires phosphorylation of its N-terminal domain, which can occur upon activation of several signaling pathways. One of the major p53-activating kinases is the serine/threonine-specific protein kinase, ATM, encoded by the Ataxia Telangiectasia Mutated (Atm) gene. ATM phosphorylates p53 at the serine 15 position (33). To determine whether ATM is important for p53 activation in acute colitis, we administered DSS to ATM-deficient (Atm−/−) and wild-type (Atm+/+) mice and examined the levels of total and serine 15-phosphorylated p53 in the colon by immunoblotting. Atm−/− mice exhibited constitutive p53 activation (presumable due to ATM-independent p53 phosphorylation by kinases such as DNA-PK and ATR), but failed to induce further p53 activation and induction upon colitis induction, whereas wild-type littermates showed the expected p53 activation at 7 and 10 days after DSS administration (Fig. 2A). Furthermore, Atm−/− mice exhibited a similar colitis phenotype compared to Trp53−/− mice, as evidenced by less body weight loss in the acute stages of the disease (Fig. 2B), reduced mortality (Fig. 2C), decreased colon inflammation and ulceration (Fig. 2D,E), and lower MPO levels in colon extracts (Fig. 2F) compared with wild-type littermates. Together, these findings indicate that ATM is required for inflammation-induced p53 activation and that its loss is protective against acute colitis, which, taken together, suggests that ATM and p53 are located in the same signaling pathway relevant to acute colon inflammation.
FIGURE 2.
Function of ATM in acute colitis. Wild-type (Atm+/+) and ATM-deficient (Atm−/−) mice were treated with 3% DSS in the drinking water for 6 days and returned to regular water. (A) Lysates of colon tissue were prepared at the indicated times and examined by immunoblotting for phospho-p53 (serine 15) and total p53. The results are representative of three separate experiments. (B) Body weights were determined daily and are shown as mean ± SD of the percentage of initial weight (n=9–10 mice/group). The curves were significantly different (p<0.05) as determined by repeated-measures ANOVA. (C) Kaplan-Meier survival curves after DSS treatment (n=20 for Atm+/+ and n=16 for Atm−/− mice); *p<0.05 by log rank test. (D) Colon sections were prepared after 10 days and stained with hematoxylin and eosin. (E) Histological inflammation scores and total mucosal ulcerations were determined on H&E stained colon sections of DSS-treated mice (day 7–10). Each data point represents one animal, with means shown as horizontal lines (p-values were determined by rank sum test). (F) MPO levels were assayed in colon lysates on days 0 and 10 of DSS treatment. Data are mean ± SD (n=3/group; p-values were calculated by t-test).
DNA repair is not important in modulating acute colitis
One of the major functions of p53 is sensing DNA damage and initiating DNA repair processes (34). Because inflammation-associated production of oxidants may cause DNA damage, we asked whether the DNA repair-related functions of p53 are important in mediating its activity in acute colitis. For this purpose, we utilized mice deficient for one of two critical DNA repair enzymes, MLH1 and MSH1, and subjected them to DSS challenge. In contrast to Trp53−/− mice, we observed no differences in body weight loss, colon ulceration, or MPO levels in the colon of MLH1- or MSH1-deficient mice compared to their wild-type littermate controls (Supplementary Fig. S2). These data show that DNA repair processes mediated by MLH1 or MSH1 play no role in determining the extent of acute colitis injury or in the recovery from this injury. Thus, the observed inflammatory functions of p53 appear to be independent of DNA repair.
Importance of p53 for colitis-associated apoptosis of epithelial and lamina propria cells
Besides initiation of DNA repair, another key function of p53 is induction of cell cycle arrest and apoptosis (35–37). Based on this, we hypothesized that apoptosis control may be important in mediating the functions of p53 in acute intestinal inflammation. Induction of colitis by DSS feeding caused apoptosis in the colon of wild-type mice, as evidenced by increased levels of the cleaved, activated form of caspase 3, the central effector caspase responsible for integrating different pro-apoptotic signals and initiating a program of apoptotic cell death (Fig. 3A). By comparison, Trp53−/− mice had reduced levels of cleaved caspase 3 after DSS administration (Fig. 3A). TUNEL staining of histological sections confirmed that wild-type mice had greatly increased numbers of apoptotic cells in the epithelium and lamina propria of the colon at 7 days, whereas Trp53−/− mice showed only a mild increase in apoptotic cells (Fig. 3B). Furthermore, we found by flow cytometry of isolated lamina propria cells that the number of F4/80-positive macrophages undergoing apoptosis (as defined by positive staining for annexin V and negative staining for propidium iodide) was 6-fold increased in colitic wild-type mice, but <2.5-fold increased in Trp53−/− mice (Fig. 3C). These results indicate that p53 deficiency protects against apoptotic cell death of epithelial cells and macrophages in the course of acute colitis, which may be partly responsible for protection against colitis-associated tissue damage.
FIGURE 3.
p53 deficiency attenuates apoptosis in acute colitis. Wild-type (Trp53+/+) and Trp53−/− mice were left untreated (day 0) or were treated with 3% DSS for 6 days followed by one day of regular drinking water. (A) Levels of total and cleaved caspase 3 in total colon lysates were determined by immunoblotting. Blots are from one representative out of two independent experiments. (B) Apoptotic cells were identified in paraffin sections of the colon by TUNEL staining. Representative images from one out of three independent experiments are shown. Apoptotic cells were quantitated per high-power field (HPF) with a fluorescence microscope using a 20x lens. Data are mean + SD (n=5; p-value was determined by t-test). (C) Flow cytometric analysis of CD45+ F4/80+ lamina propria macrophages isolated from the colon and stained with fluorochrome-labeled annexin V and propidium iodide. The percentage of cells in each quadrant is shown in the corners of each dot plot. Data are representative of three independent experiments.
Intestinal epithelial cells are not responsible for p53 functions in colitis
Because epithelial cells were protected against apoptosis in the absence of p53 and since these cells are important in colitis induction (38), we next investigated whether they are responsible for the actions of p53 in acute colitis. To generate a model of selective p53 deficiency in intestinal epithelial cells, we crossed mice carrying floxed Trp53 alleles with Villin-Cre transgenic mice that express Cre recombinase specifically in the intestinal epithelium (22). The resulting Trp53IEC-KO mice, which were fertile and grossly normal, showed selective p53 ablation in the colon epithelium (Fig. 4A), but not in intestinal lamina propria cells or in the spleen. DSS challenge of the Trp53IEC-KO mice revealed that they were equally susceptible to acute colitis as controls, with no significant differences in body weight, histological colitis scores or extent of colon ulceration between the groups (Fig. 4B,C). Furthermore, quantitation of apoptotic cells in the colon showed no significant difference between the groups (Fig. 4D). These data strongly imply that any direct epithelial functions of p53, such as epithelial restitution or regeneration, are not likely to account for the overall activity of p53 in acute colitis.
FIGURE 4.
Lamina propria cells mediate protection against acute colitis in the absence of p53. Conditional knock-out mice for p53 in the intestinal epithelium (Trp53IEC-KO, open circles) and controls (Trp53IEC-WT, closed circles) were generated by crossing floxed p53 mice with villin-Cre transgenic mice. (A) Total p53 expression was examined by immunoblotting in intestinal epithelial cells (IEC) isolated from the colon. (B) Mice were treated with DSS. Body weights were recorded daily and are displayed as mean ± SD of the percentage of the initial weight (n=13 to 15 mice/group; analysis by repeated-measures ANOVA showed no significant difference between the groups). (C) Histological inflammation scores and total mucosal ulcerations were determined on colon sections on day 7. Each data point represents one animal, with means shown as horizontal lines (n= 13 to 15 mice/group); analysis by t-test or rank sum test yield no significant (N.S.) difference. (D) Numbers of apoptotic cells were determined by TUNEL staining and counting per high-power field (HPF) under a fluorescence microscope using a 20x lens. Data are mean + SD (significance evaluated by t-test).
p53 deficiency leads to increased IL-6 and IL-22 expression and STAT3 activation in the colon
The studies above had shown that p53 deficiency protects epithelial cells against apoptotic death in colitis (Fig. 3). At the same time, these cells were not directly responsible for mediating the physiological functions of p53 (Fig. 4), suggesting that they may be protected by indirect means. Because maintenance of an intact epithelial barrier is important in protection against acute colitis, we reasoned that loss of p53 might lead to increased production of cytokines that protect the epithelium. For example, IL-22 promotes intestinal epithelial wound healing (39), IL-11 protects against acute intestinal injury (40), and IL-6 is anti-apoptotic in intestinal epithelial cells and protective against acute colon injury (26). We therefore assayed expression of these cytokines in the colon of Trp53−/− and Trp53+/+ mice before and after induction of acute colitis by DSS administration. Trp53−/− mice had significantly increased IL-6 and IL-22 mRNA levels relative to wild-type controls (Fig. 5A). In parallel, the levels of these cytokines in whole colon lysates were significantly elevated in Trp53−/− mice compared to wild-type littermates (Fig. 5A). By comparison, other inflammatory cytokines were differentially affected by p53 loss, with significantly increased mucosal levels for IL-1β, but not TNFα, IL-11, or CXCL1 (Fig. 5A). Cell sorting experiments showed that increased expression of IL-6 mostly occurred in CD11C+ dendritic cells and to a lesser degree in CD11b+ macrophages in the inflamed colon, while no induction was observed in epithelial cells (Fig. 5B). For IL-11, induction was only seen in dendritic cells (Fig. 5B).
FIGURE 5.
Increased production of cytoprotective cytokines in acute colitis in p53-deficient mice. Wild-type (Trp53+/+, closed bars) and p53-deficient (Trp53−/−, open bars) mice were treated with DSS or were left untreated. (A) Left: Expression levels were assayed by real-time PCR in whole colon RNA, and are expressed as ratio in DSS-treated (day 7) over untreated mice, normalized to β-actin. Middle and right: Cytokine levels in whole colon lysates were determined by ELISA. All expression data are mean + SD (n=3; p-values relative to p53-proficient controls were determined by t-test). (B) Single cell suspensions of epithelium and lamina propria from the colon of untreated and DSS-treated wild-type mice were FACS-sorted into epithelial cells (Epi), macrophages (Mac), and dendritic cells (DC). Cytokine mRNA levels were analyzed by real-time PCR, and are expressed relative to the levels in cells from untreated mice. Data are mean + SD (n=3). (C) Activation of STAT3 was examined in lysates from isolated colon epithelial cells (IEC) and whole colon by immunoblotting for phospho-STAT3 and, as a control, total STAT3. (D) None marrow-derived macrophages and dendritic cells were stimulated with the indicated agonists for 8 hours. Supernatants were analyzed by ELISA. Data are mean + SD from three mice in one experiment out of five independent experiments (p-values were determined by t-test). (E) Wild-type and p53 deficient bone marrow-derived macrophages were stimulated with LPS in the absence or presence of 2-Deoxy-glucose (2-DG), and mRNA levels were determined by real-time PCR and IL-6 levels in the supernatants were assayed by ELISA. Data are mean + SD (n=6; p-values relative to controls were calculated by t-test). (F) Macrophages were stimulated with LPS for 4 h, with and without 2-DG, and NF-κB/p65 DNA-binding activity was determined by ELISA. Data are mean + SD (n=3; p-values relative to controls and relative to 2-DG treatment were determined by t-test).
Because both IL-6 and IL-22 activate STAT3 signaling upon binding of their respective cognate receptors, we next examined whether their increased production in Trp53−/− mice led to enhanced STAT3 activation. Immunoblot analysis of extracts from whole colon and isolated epithelial cells showed that the phosphorylated, active form of STAT3 was markedly increased in Trp53−/− mice on days 7 and 10 after colitis induction, whereas phospho-STAT3 levels were only minimally elevated in wild-type littermates (Fig. 5C). Together, these results indicate that the absence of p53 leads to increased expression of the cytoprotective cytokines, IL-6 and IL-22, and activation of STAT3 in epithelial and other cells in response to acute colitis.
Importance of glucose-dependent NF-κB activation and IL-6 expression in p53-deficient macrophages
Because macrophages are a major source of many inflammatory cytokines, we next investigated how p53 deficiency might enhance expression of selected cytokines in these cells. Bone marrow-derived macrophages were generated from Trp53−/− and Trp53+/+ mice and tested for IL-6 expression in response to different stimuli. Macrophages lacking p53 secreted significantly more IL-6 after stimulation with LPS, TNFα, and H2O2 than p53-proficient cells (Fig. 5D). Similarly, p53-deficient bone marrow-derived dendritic cells also secreted significantly more IL-6 than control cells after LPS stimulation (Fig. 5D). Enhanced IL-6 secretion was paralleled by increased mRNA levels in LPS-stimulated p53-deficient macrophages, whereas mRNA levels for the cytokines, IL-10 and CCL5, were not altered by p53 loss (Fig. 5E). These results indicate that p53 deficiency enhances macrophage IL-6 production in a relatively selective manner. The data further suggest that increased colon expression of IL-6 and perhaps other cytoprotective cytokines in the absence of p53 can be explained, at least in part, by a cell-autonomous function in hematopoietic cells rather than changes in the number of cells producing such cytokines (although the latter also occurs and may synergize with increased cellular cytokine production by increasing the number of cytokine-producing cells).
Because IL-6 is a target gene of NF-κB, whereas IL-10 and CCL5 are not, we explored the role of p53 in regulating NF-κB function in our macrophage system. As reported before in other cell models (7), p53 deficiency was associated with increased NF-κB activation upon agonist stimulation of macrophages (Fig. 5F). Several mechanisms have been proposed that may account for enhanced NF-κB activation under these circumstances. For example, increased glucose uptake and metabolism in p53-deficient cells have been suggested to promote glucosylation of the central signaling kinase, IKKβ, which leads to increased cumulative NF-κB activity by preventing the normal delayed inactivation of the kinase upon sustained stimulation (6). Consistent with this notion, we observed that p53 loss in macrophages was associated with increased glucose consumption after LPS stimulation (181 nmol glucose/106 cells/h in p53−/− cells vs. 132 in p53+/+ cells). Importantly, glucose consumption was critical for mediating the impact of p53 deficiency on cytokine expression, since addition of the glycolysis inhibitor, 2-deoxyglucose, completed abrogated the increase in IL-6 secretion and mRNA expression and NF-κB activation in p53-deficient macrophages (Fig. 5E). The inhibitor had no effect on LPS-stimulated mRNA expression of IL-10 and CCL5, proving that cell viability and general responsiveness were not compromised. Complementary studies revealed that addition of high levels of glucose to wild-type macrophages enhanced IL-6 secretion in response to LPS (315 pg/ml IL-6 with 6 g/L glucose vs. 218 pg/ml in 1 g/L glucose after 4 h of LPS stimulation). Taken together, these data support the concept that increased glucose consumption and metabolism, and secondarily protein glucosylation, in the absence of p53 can enhance NF-κB activation and IL-6 secretion in macrophages.
Bone marrow-derived cells mediate the functions of p53 in regulating colitis
Macrophages are central inflammatory cells and display increased cytokine production in the absence of p53, which prompted us to explore their role in mediating the protective effects of p53 deficiency. We crossed mice with floxed Trp53 alleles to LysM-Cre transgenic mice, which express Cre recombinase specifically in macrophages and neutrophils (23), to obtain mice with selective loss of p53 in these cells. Induction of colitis by DSS feeding showed that the conditional p53 knock-out mice were not significantly different from p53-proficient controls in the severity of mucosal inflammation (Fig. 6A). These data suggest that p53 in macrophages (and neutrophils) is not sufficient to mediate the overall p53 functions in colitis.
FIGURE 6.
Hematopoietic cells are responsible for colitis protection due to p53 deficiency. (A) Mice lacking p53 in macrophages and polymorphonuclear neutrophils (Mac/PMN) were generated by crossing floxed Trp53 mice with LysM-Cre mice, and subjected to DSS-induced colitis. Histological inflammation scores were determined after 7 days. Analysis by rank sum test showed no significant (N.S.) difference. (B–D) Bone marrow chimeric mice were generated with wild-type (Trp53+/+) recipients and bone marrow from wild-type (closed circles/bars) or p53-deficient donors (Trp53−/−, open circles/bars). After 8 weeks, mice were subjected to DSS treatment. (B) Body weights are shown as mean ± SD of the percentage of the initial weight (n=10 mice/group). The curves were significantly different (p<0.05) as determined by repeated-measures ANOVA. (C) Histological inflammation scores and total ulcerations were determined on colon sections on day 7–10. The horizontal lines represent the means (n=16 to 17 mice/group), significances were calculated by rank sum test (histology scores) or t-test (ulcerations). (D) MPO levels were determined in colon lysates on days 0 and 10 of DSS treatment. Data are mean + SD (n=3, p-value was determined by t-test).
Given that neither epithelial cells (Fig. 4) nor macrophages/neutrophils (Fig. 6A) were responsible for the protection that resulted from p53 deficiency, we asked more broadly whether any cells of hematopoietic origin were involved. To this end, we generated bone marrow chimeric mice, in which wild-type recipients were reconstituted with either p53-deficient or wild-type bone marrow. We could not test the reverse situation, since Trp53−/− mice are relatively radioresistant and thus not suitable as bone marrow recipients. Evaluation of acute DSS-induced colitis in the bone marrow chimeric mice showed that mice reconstituted with p53-deficient bone marrow lost less weight, had significantly lower histological inflammation scores, and showed reduced colon ulceration and mucosal MPO levels compared to mice reconstituted with wild-type bone marrow (Fig. 6B–D). The extent of these changes was comparable to those seen in total p53-deficient mice (Fig. 1C,F,G), suggesting that hematopoietic cells are responsible for mediating the overall functions of p53 in acute colitis, while non-hematopoietic cells in the lamina propria (such as endothelial cells and myofibroblasts) are unlikely to play a major role under these conditions.
IL-6 mediates protection due to p53 deficiency
Because IL-6 was increased in Trp53−/− mice upon colitis induction, and in stimulated macrophages and dendritic cells from these mice, and this cytokine can be mucosa-protective (26), we next examined its physiological role in protection against colitis in the absence of p53. Mice double-deficient for p53 and IL-6 were not significantly different from p53-proficient mice in regard to histological inflammation scores and extent of colon ulceration, whereas mice lacking only p53 showed the expected protection against colitis (Fig. 7A). Similarly, administration of neutralizing antibodies against IL-6 exacerbated colitis in Trp53−/− mice and made them not significantly different from p53-proficient mice (Fig. 7B). These findings indicate that IL-6 is necessary to mediate the protective functions of p53 deficiency in acute colitis.
FIGURE 7.
Role of IL-6 in mediating protection against acute colitis in the absence of p53. (A,B) Double knock-out mice for p53 and IL-6 (Trp53−/− x IL-6−/−), single knock-out mice for p53 (Trp53−/− x IL-6+/+), and/or IL-6 (Trp53+/+− x IL-6−/−), and wild-type (Trp53+/+ x IL-6+/+) were subjected to DSS colitis. Some mice were treated i.p. with 3 µg of a monoclonal rat antibody against IL-6, soluble gp130/Fc fusion protein, or rat IgG, as indicated, every 3 days during DSS treatment for 6 days. As a control, Trp53+/+ mice were given rat IgG. Histological inflammation scores and total ulceration were determined on colon sections on day 7–10. Each data point represents one animal, with means shown as horizontal lines. P-values were calculated by rank sum test (histology scores) or t-test (ulcerations). (C–E) DSS colitis was induced in wild-type (Trp53+/+) mice and conditional knock-out mice for STAT3 in the intestinal epithelium (STAT3IEC-KO) and their controls (STAT3IEC-WT), with or without parallel injection with recombinant IL-6 (5 µg i.p.) or vehicle every 3 days from days 0–6 (C,D,E) or days 7–13 (E). Body weights (C) are expressed as means ± SD (n=5) of the percentages of the initial weight. The weight curves were significantly different (p<0.01) as determined by repeated-measures ANOVA. (D) Colon sections were prepared on days 7 or 14, stained with hematoxylin/eosin, and (E) analyzed for histological inflammation scores and total ulcerations. Each data point represents one animal, with means shown as horizontal lines. P-values were calculated by rank sum test (histology scores) or t-test (ulcerations); N.S. not significant. (F) Intestinal epithelial cells (IEC) were isolated from the colon of the indicated mice at 12 h after i.p. injection of 5 µg recombinant IL-6 or PBS as a control. Levels of the indicated gene products were examined by immunoblotting. The blots are representative of two separate experiments. Expression levels were determined by real-time PCR in IEC RNA obtained 2 h after IL-6 injection, and are expressed as mean + SD (n=3) of the ratio of mRNA in treated over untreated cells (x Control); p-values were determined by t-test.
Certain functions of IL-6 can be mediated by cell-independent association of the cytokine with the soluble form of the IL-6 receptor α chain (IL-6R) followed by binding of the complex to the cell-bound co-receptor, gp130 (28). We therefore tested whether IL-6 trans-signaling had a role in colitis protection. A recombinant form of soluble gp130 (sgp130/Fc) interferes with binding of the IL-6/IL-6R to membrane gp130 and thereby blocks IL-6 trans-signaling, whereas direct binding of IL-6 to cell-bound IL-6R and gp130 (leading to classic IL-6 signaling) is not affected (28). Treatment of Trp53−/− mice with sgp130/Fc exacerbated colitis and made it indistinguishable from colitis in Trp53+/+ mice (Fig. 7B). Thus, IL-6 trans-signaling contributes to the protection against acute inflammation.
IL-6 administration protects against acute colitis through epithelial STAT3 activation
Although IL-6 was necessary for protection against colitis, other cytoprotective cytokines were increased in the absence of p53 (Fig. 5A) and might contribute to protection under these conditions. To further address the importance of IL-6, we administered recombinant IL-6 to wild-type mice and assessed the impact on acute colitis. Mice treated with IL-6 from the beginning of DSS administration lost significantly less body weight than vehicle-treated mice (Fig. 7C) and exhibited strikingly reduced mucosal inflammation and colon ulceration on day 7 (Fig. 7D,E). By comparison, delayed IL-6 administration after establishment of acute colitis had no significant effect on inflammation on day 14 (Fig. 7E). Thus, IL-6 was not only necessary but also sufficient for protection against acute colitis in the early stages.
We next explored the mechanism by which IL-6 exerts protection. A major signaling pathway utilized by this cytokine involves STAT3, whose activation in the intestinal epithelium helps to maintain the mucosal barrier (24). Therefore, we administered IL-6 to mice lacking STAT3 selectively in intestinal epithelial cells (STAT3IEC-KO) and their littermate controls. Treatment of STAT3IEC-KO with recombinant IL-6 failed to attenuate acute colitis, whereas IL-6 treatment of STAT3-proficient littermates showed the anticipated protection (Fig. 7E). Furthermore, IL-6 administration induced expression of two cytoprotective factors, HSP70 and Bcl-XL, in the colon epithelium of STAT3-proficient controls, but not in their STAT3-deficient counterparts (Fig. 7F). These data suggest that IL-6 protects against acute colitis through activation of STAT3 signaling and expression of cytoprotective factors in the intestinal epithelium.
Discussion
The findings reported herein indicate that p53 has a novel inflammation-promoting function in the intestinal tract, which is opposed to its inflammation-limiting activity in the joints and central nervous system (8, 11). The underlying mechanism of intestinal p53 function is primarily indirect as it could be explained by a suppression of factors that protect the epithelium. Direct p53-dependent induction of pro-inflammatory cytokines may also occur (9), but is not likely to be primarily responsible for p53 function in acute colitis. Furthermore, the pro-inflammatory action of p53 is mediated by leukocytes in the lamina propria, which is in contrast to the importance of p53 in epithelial cells in protecting against neoplastic transformation (41). Nonetheless, our data suggest that leukocytic p53 can contribute to protection against the development of intestinal neoplasms by limiting the production of paracrine factors that promote survival and outgrowth of transformed cells.
Chronic intestinal inflammation is associated with accumulation of p53 mutations in the mucosa even in the absence of adenomas or cancers (17). Our findings raise the intriguing possibility that functional p53 loss might act as a delayed mechanism of limiting inflammation severity in the intestinal tract. This notion may have evolutionary implications, as colon cancer formation involving p53 inactivation is a late consequence of long-standing disease, whereas acute inflammatory damage can be life-threatening in acute flare-ups in inflammatory bowel disease. In any case, the present data provide further support for the concept that p53 has physiologically important inflammation-controlling functions that are independent of its role in DNA damage repair and prevention of cancer formation (9).
Activation of p53 can occur in response to DNA double-strand breaks, but also by mechanisms not involving DNA damage. In particular, reactive oxygen radicals lead to p53 phosphorylation and activation (42). Consistent with this, we observed serine 15 phosphorylation of p53 in acute colitis, a condition associated with radical production (43), and administration of the radical scavenger, NAC, prevented this phosphorylation event. Oxygen radicals activate the upstream serine kinase, ATM, which can phosphorylate p53 at serine 15 (33). Loss of ATM prevented serine 15-p53 phosphorylation in acute colitis, the physiological importance of which is suggested by the finding that ATM deficiency caused a similar colitis phenotype as p53 deficiency. However, it must be noted that p53 activation is a complex process that involves multiple phosphorylation sites and protein stabilization, depending on the specific cellular stresses (44). Nonetheless, our data suggest that p53 activation may be a new mechanism by which reactive oxygen radicals can promote inflammation (45). In variance to our findings, another study found that ATM deficient mice had more severe acute colitis (46). The reasons for this discrepancy are not immediately apparent, but the genetic mouse background may be important or the approach to disease assessment, where we relied on actual histological changes in the colon rather than a more indirect disease activity index (46).
Acute inflammation causes DNA damage, which can lead to cell loss by apoptosis. Because epithelial cell damage is a key pathogenetic feature of acute ulcerating colitis, we had initially pursued the idea that adequate DNA repair is necessary for preventing epithelial loss and thus limiting mucosal damage in acute colitis. Our data argue against this notion. Neither total deficiency in MLH1 or MSH1, key components of the DNA repair complex, nor epithelium-selective ablation of p53, which plays a role in initiating DNA repair in a cell-autonomous manner in epithelial cells (47), had a significant impact on epithelial ulceration or the severity of mucosal inflammation. Although it remains possible that other DNA repair processes independent of MLH1 and MSH1 have a role in attenuating acute inflammation, our results are consistent with the notion that any inflammation-associated DNA damage during acute colitis is not sufficiently extensive to interfere with survival and proliferation in the vast majority of epithelial cells and perhaps other cells required for limiting and overcoming acute mucosal damage. However, the long-term consequences of loss of DNA repair under chronic inflammatory conditions are likely to be detrimental, since accumulation of DNA damaged cells can contribute to tumorigenesis.
Several prototypic target genes of p53, including Fas1 and Puma, were induced in acute colitis and could contribute to its overall pro-inflammatory activity, although little is known in this regard. Loss of Fas1 is associated with the development of autoimmune diseases (48), and Fas ligand deficiency causes more severe colitis (49), so decreased Fas expression in the absence of p53 might be expected to increase colitis susceptibility, but the opposite was observed. PUMA deficiency blocks epithelial apoptosis following radiation (50), but an impact on acute inflammation has not been reported. It is possible that one or several of the hundreds of direct target genes of p53 (3) may mediate its overall function in acute colitis, or that loss of p53 dependent transcriptional repression of particular target genes, such as IL-6 (51), may be important. However, our data suggest that p53 acts indirectly during colitis by regulating other transcriptional circuits. In particular, p53 attenuates the transcriptional activity of NF-κB (52), which controls expression of multiple inflammation-associated and cytoprotective factors. Although NF-κB can promote inflammation under certain circumstances (53), it also protects against cell death (54). The latter is the dominant activity in acute colitis, since inhibition of total NF-κB in the mucosa causes exacerbation of inflammation (38). Our data suggest that the same overall concept applies, albeit in reverse, in the context of p53 deficiency, since the increase in NF-κB activity in the absence of p53 is consistent with the observed colitis-associated enhanced expression of selected NF-κB target genes, such as IL-6 and IL-1β, both of which can protect against acute inflammation injury in the intestine (26, 55). Importantly, neutralization or loss of IL-6 reversed the protection, strongly arguing that the impact of p53 deficiency on this NF-κB dependent pathway is responsible for protection, rather than any effects on classical p53 target genes.
Consistent with the IL-6 mediated protection of p53 deficient mice against acute colitis, direct IL-6 administration to wild-type mice also conferred mucosal protection when given in the early stages. This effect was dependent on STAT3 signaling in epithelial cells and was likely due to cytoprotection against epithelial apoptosis and ulceration (56). Although IL-6 played a central role in our acute colitis model, other cytoprotective factors were increased in the absence of p53 and could contribute to mucosal protection (39, 57). IL-6 is a multi-functional cytokine that can suppress or stimulate inflammation, depending on the underlying pathophysiological mechanisms. For example, IL-6 protects against mucosal inflammation upon infection with an epithelium-adherent enteric pathogen and after DSS challenge (26). IL-6 trans-signaling is an important mechanism responsible for promoting wound healing via trefoil factor activation (58), which is consistent with the observation that intestinal epithelial cells express only low levels of membrane-bound IL-6 receptors and require soluble IL-6 receptors for effective IL-6 signaling (59). On the other hand, IL-6 signaling promotes activation and survival of T cells, and can thereby promote the development of adaptive immune responses (29). Consequently, in models of chronic inflammation in the intestine and other organs that are driven by aberrant T cell responses, IL-6 blockade can attenuate disease (29). Furthermore, IL-6 may not have any major functions under particular conditions, as suggested by our observations in mice treated with IL-6 only late in established colitis. The dual, seemingly opposing roles of IL-6 in protecting against acute mucosal injury but also promoting potentially colitogenic T cell responses may explain why therapeutic IL-6 blockade has not been effective in inflammatory bowel disease (60). Nonetheless, the efficacy of exogenously administered IL-6 in protecting against acute, T cell-independent colitis in the present study suggests that this cytokine, or others with similar cytoprotective actions in the mucosa, may have therapeutic potential for ameliorating acute inflammatory bowel disease upon initial presentation or on relapses with acute disease exacerbation.
Supplementary Material
Acknowledgements
The authors thank Lucia Hall for expert technical support.
Footnotes
Disclosures: The authors have no financial conflicts of interest.
This work was supported by NIH grants DK80506, CA58320, and DK35108. M.E.S. was supported by fellowship grants from MFG Educative Science, Hamburg, Germany and Deutsche Forschungsgemeinschaft (DFG). J.S. and S.R.-J. were supported by the DFG (SFB415-project B5) and the cluster of excellence ‘Inflammation at interfaces’.
Abbreviations used in this paper: DSS. dextran sulfate sodium; MPO, myeloperoxidase
References
- 1.Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 2.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Wu Q, Qiu P, Mirza A, McGuirk M, Kirschmeier P, Greene JR, Wang Y, Pickett CB, Liu S. Analyses of p53 target genes in the human genome by bioinformatic and microarray approaches. J Biol Chem. 2001;276:43604–43610. doi: 10.1074/jbc.M106570200. [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Xiao Z, Ko HL, Ren EC. The p53 response element and transcriptional repression. Cell Cycle. 2010;9:870–879. doi: 10.4161/cc.9.5.10825. [DOI] [PubMed] [Google Scholar]
- 5.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawauchi K, Araki K, Tobiume K, Tanaka N. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci U S A. 2009;106:3431–3436. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. p53 Attenuates lipopolysaccharide-induced NF-kappaB activation and acute lung injury. J Immunol. 2009;182:5063–5071. doi: 10.4049/jimmunol.0803526. [DOI] [PubMed] [Google Scholar]
- 8.Yamanishi Y, Boyle DL, Pinkoski MJ, Mahboubi A, Lin T, Han Z, Zvaifler NJ, Green DR, Firestein GS. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol. 2002;160:123–130. doi: 10.1016/S0002-9440(10)64356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simelyte E, Rosengren S, Boyle DL, Corr M, Green DR, Firestein GS. Regulation of arthritis by p53: critical role of adaptive immunity. Arthritis Rheum. 2005;52:1876–1884. doi: 10.1002/art.21099. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Zeng XR, Wenger L, Firestein GS, Cheung HS. P53 down-regulates matrix metalloproteinase-1 by targeting the communications between AP-1 and the basal transcription complex. J Cell Biochem. 2004;92:258–269. doi: 10.1002/jcb.20044. [DOI] [PubMed] [Google Scholar]
- 11.Okuda Y, Okuda M, Bernard CC. Regulatory role of p53 in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2003;135:29–37. doi: 10.1016/s0165-5728(02)00428-9. [DOI] [PubMed] [Google Scholar]
- 12.Nagata J, Kijima H, Takagi A, Ito M, Goto K, Yamazaki H, Nakamura M, Mine T, Ueyama Y. Helicobacter pylori induces chronic active gastritis in p53-knockout mice. Int J Mol Med. 2004;13:773–777. [PubMed] [Google Scholar]
- 13.Fujii S, Fujimori T, Kawamata H, Takeda J, Kitajima K, Omotehara F, Kaihara T, Kusaka T, Ichikawa K, Ohkura Y, Ono Y, Imura J, Yamaoka S, Sakamoto C, Ueda Y, Chiba T. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–716. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Mitsuhashi J, Mikami T, Saigenji K, Okayasu I. Significant correlation of morphological remodeling in ulcerative colitis with disease duration and between elevated p53 and p21 expression in rectal mucosa and neoplastic development. Pathol Int. 2005;55:113–121. doi: 10.1111/j.1440-1827.2005.01802.x. [DOI] [PubMed] [Google Scholar]
- 16.Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, Burmer GC. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 17.Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 18.Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 19.Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, Pollard JW, Kolodner RD, Kucherlapati R. Meiotic pachytene arrest in MLH1-deficient mice. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- 20.Smits R, Hofland N, Edelmann W, Geugien M, Jagmohan-Changur S, Albuquerque C, Breukel C, Kucherlapati R, Kielman MF, Fodde R. Somatic Apc mutations are selected upon their capacity to inactivate the beta-catenin downregulating activity. Genes Chromosomes Cancer. 2000;29:229–239. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1033>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 22.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 23.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic research. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 24.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ, Hanson E, Eckmann L. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dam M, Mullberg J, Schooltink H, Stoyan T, Brakenhoff JP, Graeve L, Heinrich PC, Rose-John S. Structure-function analysis of interleukin-6 utilizing human/murine chimeric molecules. Involvement of two separate domains in receptor binding. J Biol Chem. 1993;268:15285–15290. [PubMed] [Google Scholar]
- 28.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 29.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 30.Heremans H, Dillen C, Put W, Van Damme J, Billiau A. Protective effect of anti-interleukin (IL)-6 antibody against endotoxin, associated with paradoxically increased IL-6 levels. Eur J Immunol. 1992;22:2395–2401. doi: 10.1002/eji.1830220932. [DOI] [PubMed] [Google Scholar]
- 31.Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, Jhappan C, Higashimoto Y, He P, Linke SP, Quezado MM, Zurer I, Rotter V, Wink DA, Appella E, Harris CC. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A. 2003;100:143–148. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bancroft LK, Lupton JR, Davidson LA, Taddeo SS, Murphy ME, Carroll RJ, Chapkin RS. Dietary fish oil reduces oxidative DNA damage in rat colonocytes. Free radical biology & medicine. 2003;35:149–159. doi: 10.1016/s0891-5849(03)00240-5. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Inageda K, Nishitai G, Matsuoka M. Phosphorylation of p53 at serine 15 in A549 pulmonary epithelial cells exposed to vanadate: involvement of ATM pathway. Toxicol Appl Pharmacol. 2007;220:83–91. doi: 10.1016/j.taap.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Wagner J, Rice JJ, Hu W, Levine AJ, Stolovitzky GA. A plausible model for the digital response of p53 to DNA damage. Proc Natl Acad Sci U S A. 2005;102:14266–14271. doi: 10.1073/pnas.0501352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Levine AJ. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci U S A. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inagaki-Ohara K, Yada S, Takamura N, Reaves M, Yu X, Liu E, Rooney I, Nicholas S, Castro A, Ware CF, Green DR, Lin T. p53-dependent radiation-induced crypt intestinal epithelial cells apoptosis is mediated in part through TNF-TNFR1 system. Oncogene. 2001;20:812–818. doi: 10.1038/sj.onc.1204172. [DOI] [PubMed] [Google Scholar]
- 38.Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J, Lang R, Robine S, Kagnoff MF, Schmid RM, Karin M, Arkan MC, Greten FR. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358–1368. doi: 10.1016/s0016-5085(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 41.Fujii S, Fujimori T, Kawamata H, Takeda J, Kitajima K, Omotehara F, Kaihara T, Kusaka T, Ichikawa K, Ohkura Y, Ono Y, Imura J, Yamaoka S, Sakamoto C, Ueda Y, Chiba T. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–716. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, Harris CC. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci U S A. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rachmilewitz D, Karmeli F, Okon E, Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–255. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakaya N, Lowe SW, Taya Y, Chenchik A, Enikolopov G. Specific pattern of p53 phosphorylation during nitric oxide-induced cell cycle arrest. Oncogene. 2000;19:6369–6375. doi: 10.1038/sj.onc.1204100. [DOI] [PubMed] [Google Scholar]
- 45.Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371–2384. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westbrook AM, Schiestl RH. Atm-deficient mice exhibit increased sensitivity to dextran sulfate sodium-induced colitis characterized by elevated DNA damage and persistent immune activation. Cancer Res. 2010;70:1875–1884. doi: 10.1158/0008-5472.CAN-09-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keimling M, Wiesmuller L. DNA double-strand break repair activities in mammary epithelial cells--influence of endogenous p53 variants. Carcinogenesis. 2009;30:1260–1268. doi: 10.1093/carcin/bgp117. [DOI] [PubMed] [Google Scholar]
- 48.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sayani FA, Keenan CM, Van Sickle MD, Amundson KR, Parr EJ, Mathison RD, MacNaughton WK, Braun JE, Sharkey KA. The expression and role of Fas ligand in intestinal inflammation. Neurogastroenterol Motil. 2004;16:61–74. doi: 10.1046/j.1365-2982.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 50.Shao L, Sun Y, Zhang Z, Feng W, Gao Y, Cai Z, Wang ZZ, Look AT, Wu WS. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010;115:4707–4714. doi: 10.1182/blood-2009-10-248872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santhanam U, Ray A, Sehgal PB. Repression of the interleukin 6 gene promoter by p53 and the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1991;88:7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawauchi K, Araki K, Tobiume K, Tanaka N. Activated p53 induces NF-kappaB DNA binding but suppresses its transcriptional activation. Biochem Biophys Res Commun. 2008;372:137–141. doi: 10.1016/j.bbrc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 54.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: a question of life or death. J Biochem Mol Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Navajas JM, Law J, Nguyen KP, Bhargava M, Corr MP, Varki N, Eckmann L, Hoffman HM, Lee J, Raz E. Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production. J Exp Med. 2010;207:2799–2807. doi: 10.1084/jem.20101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka K, Namba T, Arai Y, Fujimoto M, Adachi H, Sobue G, Takeuchi K, Nakai A, Mizushima T. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem. 2007;282:23240–23252. doi: 10.1074/jbc.M704081200. [DOI] [PubMed] [Google Scholar]
- 57.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, Heath JK, Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 59.Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S, Takada T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543–1551. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 60.Ding C, Jones G. Anti-interleukin-6 receptor antibody treatment in inflammatory autoimmune diseases. Rev Recent Clin Trials. 2006;1:193–200. doi: 10.2174/157488706778250168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.