Abstract
OBJECTIVE
Pre-injury functional status has not been prospectively studied as a predictor of risk in trauma patients. We hypothesized that the VES-13, a survey based on functional status that has been validated among uninjured older populations, will predict complications and mortality among injured geriatric patients.
DESIGN
Prospective observational pilot study
SETTING
Level-1 trauma center
PARTICIPANTS
63 geriatric patients (age ≥65 years) with a traumatic injury, who survived and required inpatient care for at least 24 hours.
MEASUREMENTS
Predictor: Pre-injury VES-13 score (0-10 points, higher = greater risk) obtained by interview of patients or proxies. Outcomes: composite outcome of one or more medical complication (e.g., aspiration pneumonia, respiratory failure) or death; discharge destination (home versus nursing home versus death); length of stay; hospital charges. Co-variates: Charlson Comorbidity Index (CCI), Injury Severity Score (ISS), and gender.
RESULTS
Of the 63 patients, four (6%) died, 21 (33%) developed one or more complications, 30 (48%) were discharged to home and 28 (44%) to a nursing facility. In a model that also controlled for ISS and co-morbidity, each additional VES-13 point was associated with increased risk of complication or death (OR 1.53 per point, 95% CI 1.12-2.07).
CONCLUSIONS
These results suggest that the VES-13, in combination with injury severity, may be useful early in the hospital course to predict complications and death among geriatric trauma patients, potentially identifying candidates who may benefit from additional inpatient geriatric services.
Keywords: Trauma, acute surgical care, functional status
INTRODUCTION
With continued aging of the population, traumatic injuries sustained by older patients are increasingly common.1 Death due to traumatic injury is twice as likely at age 75 as at age 45 (20% versus 10%),2 and mortality in older patients is more twice that of younger patients (≥ 65 versus < 65) for motor vehicle accidents, falls, pedestrian accidents, and penetrating injuries.2 Older patients also utilize disproportionate hospital care and suffer greater morbidity and mortality compared with younger patients of similar injury severity.3-5
The geriatric population is heterogeneous in its vulnerability to deterioration in health6 and ability to recover from injury7. Better identification of older patients at the highest risk for death, hospital complications and resource utilization would allow for improved targeting of inpatient interventions, for example, a focused geriatric program to reduce post-operative complications and facilitate discharge planning to post-hospital settings. Of demographic and clinical characteristics (e.g., age, gender, co-morbidity, injury severity, vital signs) tested in older patients, age and injury severity are the strongest predictors of survival. 3, 5, 7-13 The Injury Severity Score (ISS),11 a measure of overall injury severity that includes multiple injuries across anatomic regions, is used universally and predicts survival even in older patients. 5, 8, 12, 13 The Eastern Association for the Surgery of Trauma, however, has recommended against the use of injury severity indices such as ISS in clinical care of individual patients that are not available until after hospital discharge.14
Pre-injury functional status of geriatric patients, or a person’s ability to perform daily activities, has predicted survival and health care utilization in acute15, 16 as well as outpatient settings.17-19 Older individuals with higher functional status may be more resilient to physiologic insult independent of their chronologic age, for example after hip fracture.20 The Vulnerable Elders-13 Survey (VES-13), a simple function-based screening tool, was developed to predict the risk of death and functional decline in geriatric patients.17 This survey can be administered at bedside or over the telephone, by non-clinical personnel, and can be answered by patients or proxy respondents within five minutes. The survey has been validated in ambulatory17, 18, 21 and acute medical care settings.22 The VES-13 does not require knowledge of pre-existing conditions. Because it can be obtained from patients or caregivers, it has the added advantage of being available at the time of admission. Whether or not pre-injury functional status is predictive of hospital outcomes in an inpatient setting following serious traumatic injury has not yet been reported in the literature. We hypothesized that the VES-13, a simple survey based on functional status and age, will predict hospital survival, discharge destination, and hospital complications among geriatric trauma patients independently of traditional risk factors.
METHODS
This analysis was part of a larger study to implement a routine geriatric consultation for older trauma patients at an academic Level-1 Trauma Center (determined by state requirements of availability of trauma, neurosurgery and orthopedic surgeons). The hospital has an annual trauma admission volume of approximately 1000 patients, of which 10% are ≥65 years of age, and three-quarters of these are admitted for inpatient care. This study was approved by the local Institutional Review Board.
We approached all patients ≥65 years of age who met criteria for trauma team activation (blunt or penetrating mode of injury with suspicion of traumatic injury) and were hospitalized for ≥24 hours. If an eligible patient was unable to provide consent, we approached possible proxy respondents who knew the patient well enough to answer questions about pre-injury functional status. Our protocol included enrollment and interview within 48 hours of admission.
We interviewed patients using the VES-13,17 which assigns points in four categories: activities of daily living (ADLs), common physical tasks, self-rated health, and age. For ADLs, presence of any of 5 disabilities (shopping, managing money, light housework, bathing, or walking) is assigned four points (zero points for no disabilities). Disability is defined as difficulty requiring help, or inability to perform the activity due to health reasons. For common physical tasks (stooping, crouching, or kneeling; walking one quarter of a mile; lifting 10 pounds; heavy housework; reaching above shoulder level; writing or grasping small objects), 1 point is assigned for each task that a patient had “a lot of difficulty” or was “unable to do”, up to 2 tasks maximum. The patient’s self-rated health is compared to others of the same age, with a response of “fair” or “poor” conferring 1 point. For our study, we modified the initial stem of these questions to inquire pre-injury, rather than current, functional status.16, 22 Last, VES-13 confers points according to age category (75–84 = 1 point, ≥85 = 3 points). The total VES-13 score ranges from 0 (best prognosis) to 10 (worst prognosis). For older ambulatory care patients, scores of ≥3 represent a 4.2 increased 2-year risk of further functional decline and death compared to those with scores of ≤2.17 This survey has been prospectively validated in outpatient populations over 1-year18 and 5-year21 intervals, with higher scores (e.g., ≥6) conferring a ≥50% risk of declining or dying.
Co-variates
The ISS was evaluated by the hospital trauma registry. The ISS11 consists of a sum of squared severity ratings for the three most injured body regions, ranging from 0 to 75, with a scores of 16 and 25 considered moderate and severe overall injury, respectively. The Charlson Co-morbidity Index (CCI) was collected by medical record abstraction for pre-existing diabetes, respiratory disease, coronary artery disease (history of myocardial infarction, coronary artery bypass graft, percutaneous transluminal angioplasty, and angina pectoris), congestive heart failure, chronic kidney disease (moderate to severe), hypertension, cancer (local and disseminated), chronic liver disease, dementia, alcoholism, cerebrovascular accident, and current smoker.23, 24
Outcome variables
We collected death versus survival to discharge from the hospital trauma registry as the primary outcome measure for all patients age ≥65. As secondary measures, we considered development of death or medical complication (versus survival with no complication), discharge to home (versus discharge to nursing or rehabilitation facility), hospital charges (dollars), and length of stay (LOS, in days). Post-trauma complications collected by our registry were: acute renal failure, coma, cardiopulmonary resuscitation, decubitus ulcer, deep venous thrombosis, aspiration pneumonia, pneumonia, pulmonary embolism, respiratory failure, and hyponatremia.
Sensitivity analyses variables
In addition to the ISS, we also calculated a physiologic measure of injury severity, the Revised Trauma Score (RTS), calculated from hospital trauma registry data (blood pressure, respiratory rate, and Glascow coma score).25 We also considered age (in years, rather by age category as in the VES-13) separately from the VES-13; to do so, we recalculated an alternative VES-13 score without points awarded for age. Last, we considered whether or not a patient received a major surgical procedure which we also obtained from the trauma registry.
Analysis
We used ordinary least squares regression to model continuous outcomes (log-transformed LOS and charges), logistic regression for dichotomous outcomes (death versus survival, development of each specific complication versus no complication, and complication/death versus no complications), and ordered logistic regression to evaluate hospital disposition (0=survival to home discharge, the best outcome; 1=survival to nursing facility or rehabilitation hospital discharge; 2= death, the worst outcome).
We first performed all analyses using the VES-13 without adjustment, then performed multivariable analyses adjusting for ISS, CCI, and gender. The first sensitivity analysis compared RTS to VES-13 in predicting outcomes. Second, because age alone might be predictive of outcomes, we tested the modified VES-13 score that did not account for age and added age as a separate variable to the models. Third, we stratified our final analysis by whether or not the patient received surgery. Last, we considered the VES-13 as a series of dummy variables to consider non-linear effects.
Area under the receiver-operating-curves (AUCs) were calculated for logistic regression models with and without VES-13 (p<.05). All analyses were performed using STATA 10 (StataCorp LP).
RESULTS
From December 1, 2007 to July 31, 2009, 63 of 87 (72.4%) eligible patients were enrolled for participation. The mean age was 78 years, two-thirds were male, and nearly all were Caucasian and suffered from blunt injuries. The mean VES-13 score was 2.8 (SD 2.8), mean ISS was 14.0 (SD 9.2). One-third of the interviews were collected by proxy respondent. Patients who we did not enroll (unable to identify appropriate proxy for 6, enrollment refused by 18) did not differ from the enrolled group with respect to age (p=.4), gender (p=.4), or ISS (p=.9). However, non-white patients were less likely to enroll than white patients (40.0% versus 78.2%, p=.002), due to inability to locate an appropriate proxy (20.0% versus 4.2%, p=.03) and refusal to participate (40.0% versus 16.7%, p=.04).
Of the 63 patients, four died (6.4%), and 21 suffered complications (33.3%). All 4 patients who died also suffered from at least 1 complication. Complications (Table 1) included pneumonia (19.0%), respiratory failure (9.5%), aspiration pneumonia (6.3%), decubitus ulcer (3.2%), acute renal failure (3.2%), coma (3.2%); and pulmonary embolism, deep venous thrombosis, cardiopulmonary resuscitation, hyponatremia, or urinary tract infection (1.3% each).
Table 1. Relationship between VES-13, Death, Charges, LOS, and Specific Complications.
| Outcome | Prevalence (categorical variables) or mean (continuous variables) |
Mean VES-13 among those | Effect of each additional VES-13 point: OR or β (95% CI) |
|||
|---|---|---|---|---|---|---|
| …with outcome |
…without outcome |
Unadjusted | Adjusted* | |||
| Specific complications |
Coma | 3.2% | 5.5 | 2.8 | 1.33 (.86-2.05) | |
| Acute Renal Failure |
3.2% | 3.5 | 2.8 | 1.09 (.69-1.72) | ||
| Decubitus Ulcer | 3.2% | 2.0 | 2.8 | 0.88 (.48-1.63) | ||
| Aspiration pneumonia |
6.3% | 5.8 | 2.6 | 1.39 (1.00-1.93) | 1.97 (1.03-3.78) | |
| Respiratory Failure |
9.5% | 4.2 | 2.6 | 1.19 (.90-1.55) | 1.36 (.95-1.94) | |
| Pneumonia | 19.0% | 2.6 | 2.8 | 0.97 (.84-1.28) | 1.18 (.88-1.59) | |
| Death | 6.3% | 2.8 | 2.8 | 1.0 (.69-1.43) | 1.07 (.66-1.73) | |
| Composite Complication |
Any complication†, including death |
33.3% | 3.7 | 2.3 | 1.19 (1.01-1.48) | 1.53 (1.12-2.07) |
| Discharge Destination‡ |
Home | 47.6% | 2.4 | 1.09 (.92-1.29)‡ | 1.21 (.99-1.48)‡ | |
| Nursing or rehabilitation facility |
44.4% | 3.0 | ||||
| Death or hospice | 7.9% | 3.4 | ||||
| Charges over hospitalization, dollars |
$134K (SD=$146K) |
β=1.8% ¶ (−8.2%, + 12.0%) |
β=3.9% ¶ (−2.8%, + 10.6%) |
|||
| LOS, days | 9.8 days (SD= 8.5) | β=4.8% ¶ (−3.0%, + 12.6%) |
β=6.5% ¶ (−0.05%, + 13.0%) |
|||
OR = Odds Ratio
ISS = Injury Severity Index
VES-13 = Vulnerable Elders-13 Survey
LOS = Length of stay
SD = Standard Deviation
Adjusted for ISS, gender, and Charlson Co-morbidity Index. Results of the adjusted models for specific complications should be interpreted with caution because there were fewer than 5 events per predictor variable.
Patients could have more than one specific complication.
Discharge destination was an ordinal categorical outcome, with home considered as the best outcome, nursing/rehabilitation facility as the next worse outcome, and death/hospice as the poorest outcome. The OR reported for this ordinal logistic regression represents the increase in odds of a poorer outcome (versus one better level of outcome) associated with each additional VES-13 point.
LOS and charges were log-transformed for these linear regressions, so results displayed are the percent increase in dollars or days (multiplicative rather than additive) associated with each additional VES-13 point.
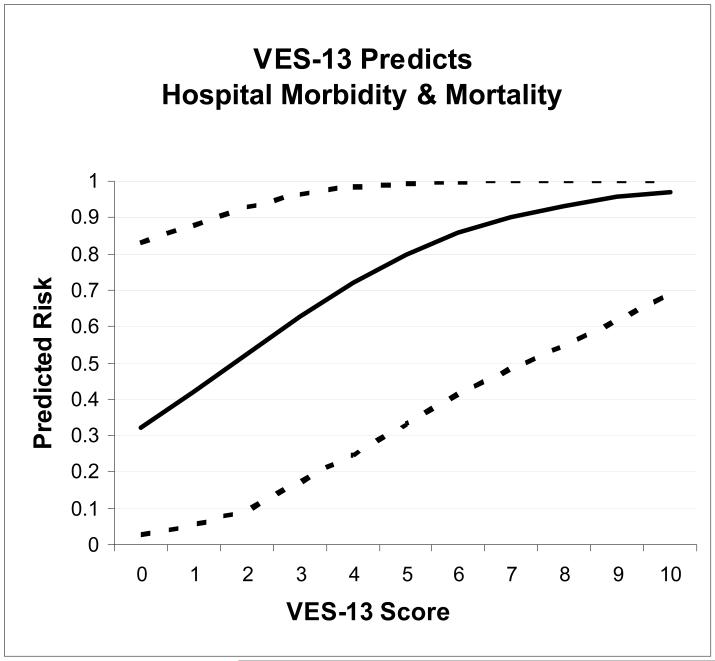
The relationship of VES-13 and clinical outcomes is described in Table 1. Analysis of the individual specific complications was limited by small sample size, with higher VES-13 scores only predicting development of aspiration pneumonia (unadjusted OR 1.39, 95% CI 1.00-1.93). However, the VES-13 predicted the composite outcome (any complication or death) in both adjusted and unadjusted models. Each VES-13 point increased odds of complication by 1.53 (95% CI 1.12-2.07). In the same model, ISS also predicted complication or death (OR 1.2 per point). To put these odds into clinical perspective, a severely injured male with ISS score of 25 would be expected to have a 32% risk of complication or death if he had a pre-injury VES-13 score of 0, 63% risk for a VES-13 score of 3, and 97% for a VES-13 score of 10 (Fig 1).
Figure 1. VES-13 Predicts Hospital Morbidity and Mortality.
Explanation: The predicted risks of the composite outcome (development of a hospital complication and/or death) are plotted against the VES-13 score (solid line). Higher VES-13 scores represent greater risk. The dotted lines represent 95% confidence intervals (obtained by bootstrapping 1000 times, percentile method). The model was adjusted for Injury Severity Score (ISS), Charlson Comorbidity Index, and gender. The displayed predicted risks are for a male with ISS score of 25 (severe injury), and no co-morbidities.
VES-13= Vulnerable Elders-13 Survey
The AUC for the composite outcome model was excellent: 88.0% versus 82.7% for the model with and without the VES-13, respectively, a substantial improvement in outcome discrimination by 5.3 percentage points. The Hosmer-Lemeshow goodness-of-fit test did not suggest lack of fit (X2 = .6). The results were robust to exclusion of four individuals with the highest leverage (dbeta <.05, OR 1.8, p=.011) and residuals (OR 2.1, p=.004).
In this sample, there was a trend toward poorer discharge condition and greater length of hospitalization with higher VES-13 scores, but confidence intervals were broad, and none were statistically significant at p <.05 (Table 1). The ISS, however, predicted nearly all other outcomes of interest. Each ISS point was also related (p<.05) to greater odds of death (OR 3.3), poorer discharge condition (OR 1.2), increase in charges (8.1%), and increased LOS (4.8%). The RTS predicted death but not any of the other outcomes.
Sensitivity analysis results
The effect of the VES-13 on complications was unaffected by adding RTS or age in years to the model, and both RTS and age variables were not significant predictors in the sensitivity analyses (p=.3, p=.7). When VES-13 was rescored without age category, similar results were seen. When we considered if the effect of VES-13 on complications could be non-linear, we found no increase in risk between scores of 0 and 1 (44% risk for both), but the risk increased linearly between 2 to 10 (70% risk for score of 2 to 95% risk for score of 10). Using the originally published VES-13 cutoff score for vulnerability of ≥3 and an ISS cutoff score of ≥16, the predicted risks of complication ranged from 4% for a non-vulnerable woman with non-severe injury to 75% for a vulnerable woman with severe injury (Table 2).
Table 2.
Predicted Risk of Hospital Complication by Gender, Vulnerability, and Injury Severity
| Men: | ||
|---|---|---|
| Risk of complication or death (95% CI) | ||
| Low ISS (≤ 15) | High ISS (≥ 16) | |
| Not Vulnerable (VES-13 ≤ 2) |
8.6% (0.2-18.8%) |
40.7% (10.4-70.0%) |
| Vulnerable (VES-13 ≥ 3) |
29.0% (3.6-59.3%) |
74.9% (44.3-97.8%) |
| Women: | ||
|---|---|---|
| Probability of complication or death (95% CI) | ||
| Low ISS (≤ 15) | High ISS (≥ 16) | |
| Not Vulnerable (VES-13 ≤ 2) |
3.8% (0%-13.5%) |
22.7% (.4-66.0%) |
| Vulnerable (VES-13 ≥ 3) |
14.8% (.4-41.0%) |
56.0% (10.5-93.7%) |
VES-13 = Vulnerable Elders-13 Survey, cutoff for vulnerability of ≥3 from Saliba et al17 ISS = Injury Severity Score
Predicted risks are based on logistic regression model using dichotomous VES-13, dichotomous ISS, gender, and co-morbidity. Actual number of non-vulnerable subjects with low ISS, vulnerable with low ISS, non-vulnerable with high ISS, and vulnerable with high ISS were n=12, 15, 13, and 13, respectively. Predicted risks were obtained by setting the sample to male and female for genderspecific estimates. 95% confidence intervals were obtained via bootstrapping with replacement.
Of the 21 patients (33.3%) who underwent operations, ten (47.6%) developed ≥ 1 complications. Despite the reduced sample size, the VES-13 predicted complications (adjusted for ISS, CCI, and gender) among the 21 surgery patients (OR 3.3, 95% CI 1.1-10.2).
DISCUSSION
In this pilot study, we found that the VES-13, originally developed for outpatient use, can potentially be used in conjunction with injury severity to predict inpatient complications in hospitalized geriatric trauma patients. Small sample size limited analysis of specific complications, but the VES-13 predicted composite hospital complications independently of age, gender, co-morbidity, and injury severity. With validation on a larger sample, the VES-13 may be useful as an important and practical tool shortly after hospital admission to help differentiate risk and target hospital services toward those geriatric trauma patients with the greatest risk of specific post-trauma complications.
These results extend prior research on hospital outcomes of older trauma patients. The effect of age is well-understood in observational data: mortality due to injury increases with age3 and age-related risk accelerates in the fourth decade.7 Certain pre-existing conditions have been found to predict mortality.8 Co-morbidity scales have been used to predict outcomes in mildly injured older trauma patients,9 but added little value in other samples.10 Injury severity can be measured as degree of physiologic compromise (e.g., respiratory rate, blood pressure, and level of consciousness as measured by the RTS25) or degree of trauma involvement by anatomical body regions (e.g., ISS11), but the ISS is used most universally to predict survival among older patients. 5, 8, 12 To our knowledge, this is the first study to prospectively collect an ADL-based measure upon admission to study hospital outcomes in geriatric trauma patients. Our findings are in agreement with a study of older inpatients on a medical ward where VES-13 was predictive of post-discharge survival.22
Self-reported functional status is attractive as a predictor in geriatric patients because it has predicted outcomes in other clinical settings17-19 and is consistent with the concept that physical and functional reserve may protect older individuals during traumatic injury. 20, 26 One widely-used measure of function, the Functional Independence Measure (FIM) 27 is typically measured by trained personnel during the hospital stay (i.e., post-injury function) and has been found to predict nursing home admission after trauma.5 The benefit of the VES-13 over the FIM is its brevity, thus decreasing burden on the patient or their family during the trauma admission. It can also be completed by any member of the care team, including clerical personnel.
VES-13 was associated with but was not statistically significant in predicting discharge destination. One explanation might be discharge destination is not a good surrogate for health status at discharge.28 We assumed that discharge to home represented a better outcome than discharge to a facility, but discharge location is influenced by factors external to the patient’s health (e.g., availability of facilities28 or family caregivers, patient preference). Some individuals with poor functional outcome may have been sent home because they were deemed poor rehabilitation candidates. Given the borderline significance of this finding, however, the most likely explanation is inadequate sample size.
We found that the ISS was consistently the strongest predictor of all hospital outcomes we tested in our sample. This is consistent with other literature of the ISS in older patients.5, 8, 12 Our results suggest that the VES-13 is more useful at differentiating risk in conjunction with the ISS, however ISS is typically collected after hospital discharge. Therefore, future work to validate the VES-13 as an early predictor of hospital complications should utilize an alternative simpler estimate of injury severity (rather than ISS) that can be collected upon admission.
Complications of injury and surgery are highly relevant to this population. We believe that the VES-13 can be used in an inpatient protocol to target inpatient services to prevent complications and mortality, for example geriatric consultation, geriatric case management, multidisciplinary team care, and quality improvement efforts. Our results may also be useful for identifying which older patients are likely to suffer specific complications, for example aspiration pneumonia, a recognized complication among older inpatients that has been studied for possible preventive interventions.29 Despite the small size of our subsample of patients requiring surgery, the VES-13 was predictive of development of post-surgery complications. While a larger study among surgical patients is necessary, our pilot study suggests that the VES-13 can be potentially helpful with targeting post- and peri-operative hospital services to prevent post-operative complications.
An important strength of this study is that we prospectively collected data before hospital outcomes and complications were known. Thus, we were able to minimize potential recall bias regarding functional status. Our results should also be interpreted in the context of a few limitations. First, the VES-13 was developed to predict both death and functional decline over a longer 1-5 year time frame.17, 18, 21 However, this study was underpowered to detect the previously-validated death outcome, and we did not collect discharge functional status. Other authors have hypothesized that hospital complications are precursors of the worst hospital outcome, death.30 Our data suggests that over a shorter and more acute time frame, the VES-13 score may be related to this continuum of post-injury complications to death. Second, we were unable to adequately test specific complications due to low event rates. Third, eligible participants at our trauma center were predominantly white. Refusals may have been due to language barriers or historical reluctance of minorities to participate in research, which further compounded the lack of diversity in our sample.
There are several future directions for this study. A larger study is needed to validate risk for specific complications that we had pooled in our study. Since ISS scores are not readily available upon admission, a larger study of the VES-13 stratified by broad categories of injury severity (e.g., head versus non-head injury, with high, mid, and low severity classifications on admission) would be helpful as part of a clinically-feasible algorithm that combines VES-13 with injury severity. Natural cutoff values of the VES-13 predict risk among large samples of older outpatients,17 so a future direction would be to explore various cutoff scores in a larger older trauma patient population. Last, we would like to test the VES-13 in a population with higher minority group representation.
In conclusion, the VES-13 predicted inpatient outcomes in this pilot study of older trauma patients independent of traditional trauma risk indices. The VES-13 should be explored as part of an early risk assessment to target geriatric-specific hospital services aimed at preventing complications and death.
Acknowledgements
We would like to acknowledge Dr. Heather McCreath from the UCLA Claude D. Pepper Older American Independence Center for her assistance with data management on this project.
Drs. Min and Tillou were each supported by the Mentored Clinical Scientist Development Program in Geriatrics Awards sponsored by the UCLA Claude D. Pepper Older American Independence Center. Dr. Min is now supported by a Research Career Development Core Career Development Award from the University of Michigan Pepper Center. Dr. Tillou is supported by an American Geriatrics Society Dennis W. Jahnigen Career Development Award. Dr. Kelley-Quon is a Robert Wood Johnson Foundation Clinical Scholar at UCLA. Mr. Ubhayakar was a 2009 Medical Student Training in Aging Research Scholar at UCLA. These results were presented at the 2010 American Geriatric Society Meeting in Orlando, Florida.
Sponsor’s Role: Indicate sponsor’s role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Footnotes
Conflict of Interest Disclosures: Below is a checklist for all authors to complete and attach to their papers during submission.
| Elements of Financial/Personal Conflicts |
*Author 1 Lillian Min |
Author 2 Nitin Ubhayakar |
Author 3 Debra Saliba |
Author 4 Lorraine Kelley- Quon |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts |
*Author 5 Eric Morley |
Author 6 Jonathan Hiatt |
Author 7 Henry Cryer |
Author 8 Areti Tillou |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
Author Contributions: Indicate authors’ role in study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
Lillian Min: study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript.
Nitin Ubhayakar: study concept and design, acquisition of data, analysis and interpretation of data, and preparation of manuscript
Debra Saliba: study concept and design, analysis and interpretation of data and preparation of manuscript.
Lorraine Kelley-Quon: acquisition of subjects and data, preparation of manuscript.
Eric Morley: acquisition of subjects and/or data and preparation of manuscript.
Jonathan Hiatt: study concept and design and preparation of manuscript.
Henry Cryer: study concept and design and preparation of manuscript.
Areti Tillou: study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Soreide K, Kruger AJ, Vardal AL, et al. Epidemiology and contemporary patterns of trauma deaths: changing place, similar pace, older face. World J Surg. 2007;31:2092–2103. doi: 10.1007/s00268-007-9226-9. [DOI] [PubMed] [Google Scholar]
- 2.Finelli FC, Jonsson J, Champion HR, et al. A case control study for major trauma in geriatric patients. J Trauma. 1989;29:541–548. doi: 10.1097/00005373-198905000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Champion HR, Copes WS, Buyer D, et al. Major trauma in geriatric patients. Am J Public Health. 1989;79:1278–1282. doi: 10.2105/ajph.79.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKenzie EJ, Morris JA, Jr., Smith GS, et al. Acute hospital costs of trauma in the United States: implications for regionalized systems of care. J Trauma. 1990;30:1096–1101. doi: 10.1097/00005373-199009000-00005. discussion 1101-1093. [DOI] [PubMed] [Google Scholar]
- 5.Richmond TS, Kauder D, Strumpf N, et al. Characteristics and outcomes of serious traumatic injury in older adults. J Am Geriatr Soc. 2002;50:215–222. doi: 10.1046/j.1532-5415.2002.50051.x. [DOI] [PubMed] [Google Scholar]
- 6.Seeman TE, Charpentier PA, Berkman LF, et al. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol. 1994;49:M97–108. doi: 10.1093/geronj/49.3.m97. [DOI] [PubMed] [Google Scholar]
- 7.Morris JA, Jr., MacKenzie EJ, Damiano AM, et al. Mortality in trauma patients: the interaction between host factors and severity. J Trauma. 1990;30:1476–1482. [PubMed] [Google Scholar]
- 8.Morris JA, Jr., MacKenzie EJ, Edelstein SL. The effect of preexisting conditions on mortality in trauma patients. JAMA. 1990;263:1942–1946. [PubMed] [Google Scholar]
- 9.Camilloni L, Farchi S, Giorgi Rossi P, et al. Mortality in elderly injured patients: the role of comorbidities. Int J Inj Contr Saf Promot. 2008;15:25–31. doi: 10.1080/17457300701800118. [DOI] [PubMed] [Google Scholar]
- 10.Sampalis JS, Nathanson R, Vaillancourt J, et al. Assessment of mortality in older trauma patients sustaining injuries from falls or motor vehicle collisions treated in regional level I trauma centers. Ann Surg. 2009;249:488–495. doi: 10.1097/SLA.0b013e31819a8b4f. [DOI] [PubMed] [Google Scholar]
- 11.Baker SP, O’Neill B, Haddon W, Jr., et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 12.vanderSluis CK, Klasen HJ, Eisma WH, et al. Major trauma in young and old: What is the difference? Journal of Trauma-Injury Infection and Critical Care. 1996;40:78–82. doi: 10.1097/00005373-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Knudson MM, Lieberman J, Morris JA, Jr., et al. Mortality factors in geriatric blunt trauma patients. Arch Surg. 1994;129:448–453. doi: 10.1001/archsurg.1994.01420280126017. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs DG, Plaisier BR, Barie PS, et al. Practice management guidelines for geriatric trauma: the EAST Practice Management Guidelines Work Group. J Trauma. 2003;54:391–416. doi: 10.1097/01.TA.0000042015.54022.BE. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SE, Seymour DG, Primrose WR. A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing. 2004;33:110–115. doi: 10.1093/ageing/afh036. [DOI] [PubMed] [Google Scholar]
- 16.Covinsky KE, Palmer RM, Counsell SR, et al. Functional status before hospitalization in acutely ill older adults: validity and clinical importance of retrospective reports. J Am Geriatr Soc. 2000;48:164–169. doi: 10.1111/j.1532-5415.2000.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 17.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 18.Min LC, Elliott MN, Wenger NS, et al. Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc. 2006;54:507–511. doi: 10.1111/j.1532-5415.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 19.McGee HM, O’Hanlon A, Barker M, et al. Vulnerable older people in the community: relationship between the Vulnerable Elders Survey and health service use. J Am Geriatr Soc. 2008;56:8–15. doi: 10.1111/j.1532-5415.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 20.Svensson O, Stromberg L, Ohlen G, et al. Prediction of the outcome after hip fracture in elderly patients. J Bone Joint Surg Br. 1996;78:115–118. [PubMed] [Google Scholar]
- 21.Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57:2070–2076. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora VM, Fish M, Basu A, et al. Relationship between quality of care of hospitalized vulnerable elders and postdischarge mortality. J Am Geriatr Soc. 2010;58:1642–1648. doi: 10.1111/j.1532-5415.2010.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23:30–34. doi: 10.1016/s1010-7940(02)00721-2. [DOI] [PubMed] [Google Scholar]
- 25.Gilpin DA, Nelson PG. Revised trauma score: a triage tool in the accident and emergency department. Injury. 1991;22:35–37. doi: 10.1016/0020-1383(91)90158-b. [DOI] [PubMed] [Google Scholar]
- 26.Maggi S, Siviero P, Wetle T, et al. A multicenter survey on profile of care for hip fracture: predictors of mortality and disability. Osteoporos Int. 21:223–231. doi: 10.1007/s00198-009-0936-8. [DOI] [PubMed] [Google Scholar]
- 27.Gabbe BJ, Simpson PM, Sutherland AM, et al. Functional measures at discharge - Are they useful predictors of longer term outcomes for trauma registries? Annals of Surgery. 2008;247:854–859. doi: 10.1097/SLA.0b013e3181656d1e. [DOI] [PubMed] [Google Scholar]
- 28.Buntin MB, Garten AD, Paddock S, et al. How much is postacute care use affected by its availability? Health Serv Res. 2005;40:413–434. doi: 10.1111/j.1475-6773.2005.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarin J, Balasubramaniam R, Corcoran AM, et al. Reducing the risk of aspiration pneumonia among elderly patients in long-term care facilities through oral health interventions. J Am Med Dir Assoc. 2008;9:128–135. doi: 10.1016/j.jamda.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Pellicane JV, Byrne K, DeMaria EJ. Preventable complications and death from multiple organ failure among geriatric trauma victims. J Trauma. 1992;33:440–444. doi: 10.1097/00005373-199209000-00018. [DOI] [PubMed] [Google Scholar]