Abstract
Everyone ages, but only some will acquire a neurodegenerative disorder in the process. Disease might occur when cells fail to respond adaptively to age-related increases in oxidative, metabolic and ionic stress resulting in excessive accumulation of damaged proteins, DNA and membranes. Determinants of neuronal vulnerability might include cell size and location, metabolism of disease-specific proteins, and repertoire of signal transduction pathways and stress resistance mechanisms. Emerging evidence on protein interaction networks that monitor and respond to the normal aging process suggests that successful neural aging is possible for most, but also cautions that cures for neurodegenerative disorders are unlikely in the near future.
Occasional problems with short-term memory, shakiness and muscle weakness – are these just unavoidable changes that occur during normal aging, or are they prodromal to a fatal neurodegenerative disorder? Cells in all regions of the nervous system are affected by aging as indicated by the decline of sensory, motor and cognitive functions with time1. However, there is considerable variability among individuals in the apparent rate of aging, the neural systems most affected, and if and how age-related deficits are compensated. There is a dramatic increase in the probability of developing a neurodegenerative disorder during the sixth, seventh and eighth decades of life. A person who lives to the age of 85 years is as likely as not to suffer from Alzheimer's disease (AD; www.alz.org); Parkinson's disease (PD) is most common in those above the age of 70 (www.parkinson.org); and the probability of developing amyotrophic lateral sclerosis (ALS) rises sharply above the age of 40 (www.alsa.org)2-6. There is growing evidence that aging has an important role in the occurrence of such diseases, and the relationship between ageing and neurodegenerative disorders is the focus of this article. It is conceivable that the initiation of nerve cell death is programmed to occur after a given period of time independently of the cell modifications caused by aging. However, a purely genetically programmed fate of neurons seems unlikely given that late onset neurodegenerative disorders are sporadic within families and that some individuals live for a century or more with little or no evidence of neuronal degeneration.
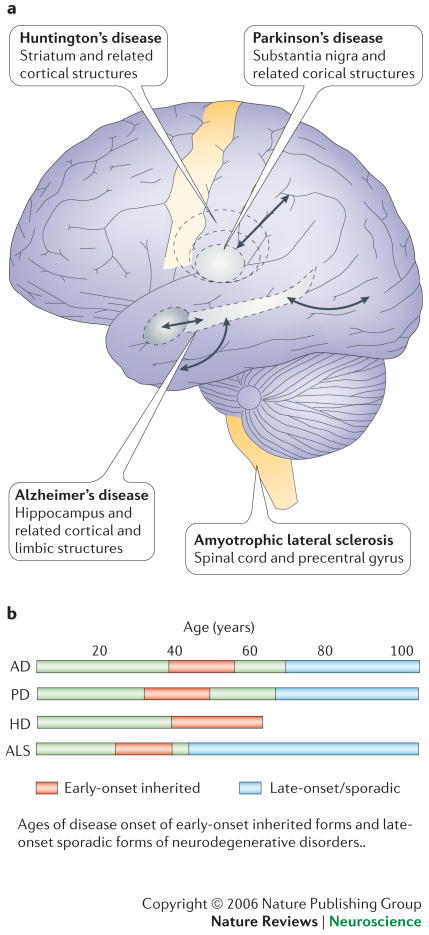
Why is the hippocampus primarily affected in AD, the substantia nigra in PD, the striatum in HD and the spinal cord and primary motor cortex in ALS (Fig. 1)? Why is it that, although certain neuronal populations of neurons are affected early and most severely, neuronal death also occurs in other brain regions as the disease progresses? Despite recent advances in understanding the molecular genetics and pathophysiology of neurodegenerative disorders, the problem of selective neuronal vulnerability (SNV) has proven difficult to solve. However, recent progress has begun to show how cellular and molecular changes that occur during normal aging render neurons vulnerable to degeneration, and how disease-specific genetic and environmental factors determine which neurons succumb. Rare cases of AD, PD and ALS are caused by mutations in specific genes and have an early age of disease onset (30s, 40s and 50s), up to 40 years earlier than the more common sporadic forms of these diseases1-6. The clinical presentation and histopathological findings are essentially indistinguishable in familial and sporadic forms of the diseases, suggesting that the genetic mutations accelerate the same molecular and cellular cascades that occur in late-onset disease. Thus, mutations in presenilin-1 and the amyloid precursor protein (APP) that cause early-onset AD enhance production of neurotoxic forms of the amyloid β-peptide (Aβ)2, mutations in α-synuclein, Parkin and DJ-1 that cause PD result in impaired proteasome-mediated proteolysis4 and mutations in copper/zinc-superoxide dismutase (Cu/Zn-SOD) that cause early-onset ALS exacerbate oxidative stress in motor neurons6.
Figure 1. The who, where and when of neuronal death in age-related neurodegenerative disorders.
a. Different neurodegenerative diseases such as ALS, Parkinson's disease (PD), Huntington's disease (HD), and Alzheimer's disease (AD) affect different areas of the adult brain. Each starts in specific regions and later affects other regions. Even within these early affected regions a selective injury of neuron subclasses can be observed; for example the dopaminergic neurons in PD, the motor neurons in ALS, or the cholinergic and glutmatergic neurons in AD. b. Ages of disease onset of early-onset inherited forms and late-onset sporadic forms of neurodegenerative disorders. For further information visit: www.alz.org; www.parkinson.org; www.alsa.org; www.hdfoundation.org.
Here we describe cellular and molecular changes that occur during normal aging, and how those changes might interact with genes and the environment to determine whether neurons age successfully or degenerate.
Aging: setting the stage for a neurocatastrophe
Cells in the nervous system are affected by, and respond to, aging much as do cells in other organ systems. Thus, cells in the brain experience increased amounts of oxidative stress7, 8, perturbed energy homeostasis9, accumulation of damaged proteins1, 11 and lesions in their nucleic acids12, 13. These changes during normal aging are exacerbated in vulnerable populations of neurons in neurodegenerative disorders. So, whether or not an individual succumbs to a neurodegenerative disorder during aging is determined by genetic and environmental factors that counteract or facilitate fundamental molecular and cellular mechanisms of aging (Fig. 2). Molecular genetic studies support the existence of evolutionarily conserved genes associated with successful neural aging14, as well as genes that cause or increase the risk of a neurodegenerative disorder2-6. Among the genes that are believed to play important roles in aging are those that encode proteins involved in insulin signaling15, DNA and protein methylation and acetylation16, DNA repair12 and lipid metabolism17.
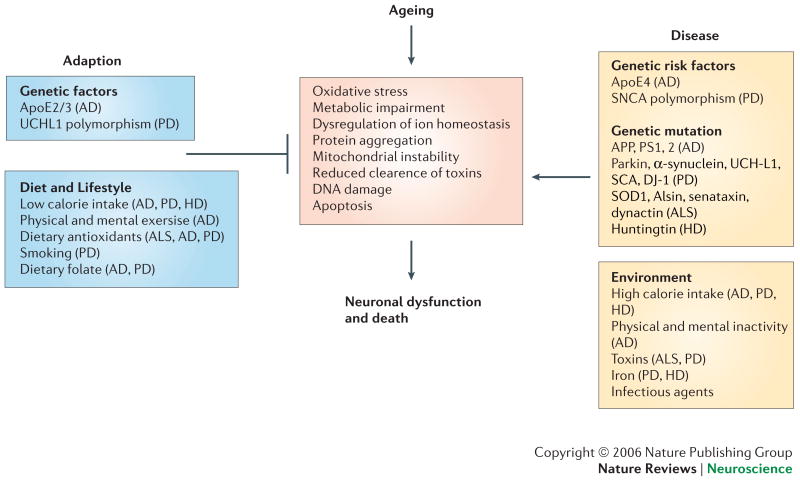
Figure 2. The nervous system may respond adaptively, or may succumb, to ageing.
In aging and neurodegenerative diseases, neuronal death may be triggered by specific genetic mutations (for example mutations in huntingtin, presenilins, α-synuclein, and Cu/Zn-SOD) and/or environmental factors such as toxins or dietary components. Initiating factors promote cellular alterations including increased oxyradical production, perturbed energy and calcium homeostasis, and activation of apoptotic cascades. However, each factor cooperates with age-related increases in oxidative stress, metabolic compromise, DNA instability, and ion homeostasis dysregulation to disrupt neuronal integrity resulting in synaptic dysfunction and cell death. In addition, changes in glial cell homeostasis occur and contribute to inflammatory processes and white matter damage in neurodegenerative disorders. AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; Apo E2/3, apolipoprotein isoforms 2 and 3; APP, amyloid precursor protein; DJ-1, HD, Huntington's disease; PD, Parkinson's disease; PS1, 2, presenilins 1 and 2; SCA, spinocerebellar ataxia; SCNA, ; SOD1, superoxide dismutase 1; UCHL1, ubiquitin c-terminal hydrolase 1.
Molecular alterations that are qualitatively similar to those that occur in the nervous system during normal aging are amplified in vulnerable neuronal populations by disease-related processes resulting in their dysfunction and death. Several of these processes are illustrated in Box 1. For example, during normal aging there are progressive increases in the amounts of oxidatively modified DNA bases, proteins and lipids in the brain. Specific age-related modifications of proteins include carbonylation, nitration and covalent binding of the lipid peroxidation product 4-hydroxynonenal18. Such protein modifications are dramatically increased in vulnerable neurons in AD, PD, HD and ALS2, 5, 19, 20. Similarly, the accumulations of Aβ in AD, α-synuclein in dopaminergic neurons in PD and Cu/Zn-superoxide dismutase in motor neurons in ALS occur to a lesser extent during normal aging11, 21. Such protein aggregates might arise, in part, as a consequence of impaired proteasomal and/or autophagic removal of the (oxidatively) damaged proteins10, 22. Alterations in numerous neurotransmitter and neurotrophic factor signaling pathways occur during normal aging, and many such changes are amplified in neurodegenerative disease. Examples include depletion of dopamine in substantia nigra neurons in normal aging and PD23, and lower levels of brain-derived neurotrophic factor (BDNF) in aging, AD and HD2, 24.
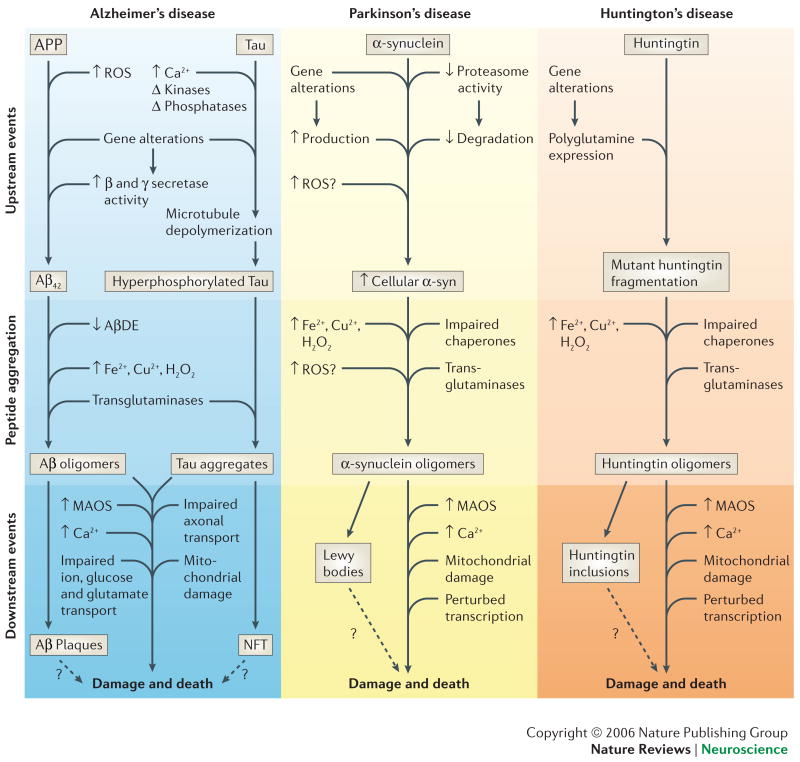
Box 1. Mechanisms of abnormal protein accumulation in neurodegenerative disorders.
Differences among neuronal populations in the production and/or clearance of abnormal proteins might be determinants of age-related neuronal vulnerability in AD, PD and HD. The pathogenic proteins are Aβ and tau in AD, α-synuclein in PD and huntingtin in HD. Genetic and age-related factors that can increase the amounts of pathogenic proteins (upstream events) include: Aβ42 in AD - mutations in APP or presenilins (γ-secretase), reactive oxygen species (ROS) and reductions in Aβ-degrading enzymes (AβDE) such as neprilysin and insulin-degrading enzyme. Tau in AD – ROS, phosphorylation and calcium. α-synuclein in PD – mutations in α-synuclein, parkin, DJ-1, UCH-L1, PINK1 or LRKK-2, ROS and proteasome impairment. HD – polyglutamine expansions in huntingtin (Htt), ROS and DNA damage and repair. The protein aggregation process itself is enhanced by increasing protein concentration, posttranslational alterations such as oxidative modifications (induced by hydrogen peroxide, iron and copper, for example) and phosphorylation, the actions of calcium and transglutaminases and/or protein chaperone insufficiency. Although the proteins involved may differ, there is considerable overlap in mechanisms by which they damage and kill neurons. Oligomers of Aβ, α-synuclein and Htt might damage and kill neurons by inducing membrane-associated oxidative stress (MAOS), impairing mitochondrial function and disrupting calcium homeostasis. See references 2-11.
If the processes of aging are central to all neurodegenerative disorders (Fig. 3), then it would be expected that an intervention that slows this process will also guard against neurodegenerative disorders. Studies of the effects of dietary energy restriction (DR), a manipulation that can retard aging processes in rodents, monkeys and humans, indicate that this might be so25-28. Low calorie diets and intermittent fasting retard the physiological manifestations of aging and extend the average and maximum lifespan of rodents by up to 40 percent25, and might also increase the lifespan of primates, including humans27, 28,. Age-related deficits in cognitive and motor function, and increases in oxidative stress and DNA damage, are lessened in animals maintained on DR compared to ad libitum diets29. In addition, DR protects neurons against dysfunction and degeneration in animal models relevant to AD30, PD31, HD32 and stroke33. Moreover, DR enhances BDNF production and neurogenesis, processes that might, in part, counteract age-related dysfunction and degeneration of neuronal circuits34.
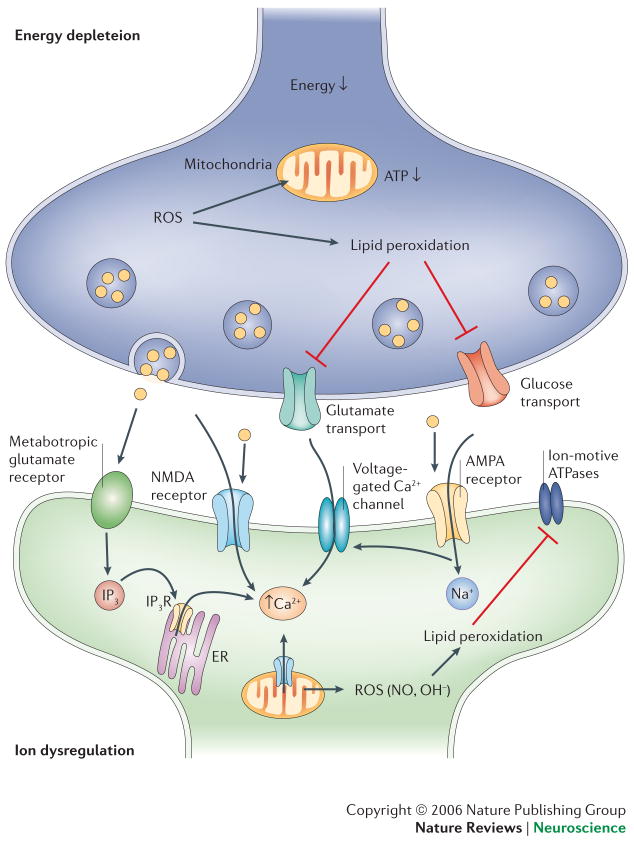
Figure 3. The sensitive synapse.
Age- and disease-related stressors promote the activation of biochemical cascades that result in the ion dysregulation and energy depletion in synaptic terminals and neurites. One example is the stimulation of glutamate receptors which, under conditions of reduced energy availability or increased oxidative stress leads to Ca2+ influx into postsynaptic regions of dendrites. This in turn can trigger apoptosis (see figure 4). In addition, among other processes, ROS can induce lipid peroxidation resulting in the dysfunction of ion-motive ATPases and glucose and glutamate transporters. This leads to further ion dysregulation, energy depletion and excitotoxcity.
One mechanism responsible for anti-aging effects of DR on the nervous system might be decreased production of reactive oxygen species (ROS) in mitochondria as a result of lower amounts of glucose metabolism35. Less superoxide anion radical is produced, resulting in lower levels of hydrogen peroxide, hydroxyl radical (formed by the interaction of hydrogen peroxide with Fe2+ or Cu+) and peroxynitrite (formed by the interaction of superoxide with nitric oxide). Accordingly, cumulative damage to proteins, lipids and DNA is lessened35. Because ROS are involved in the dysfunction and death of neurons in neurodegenerative disorders, suppression of ROS production by DR might protect brain cells against age-related diseases. A second major mechanism by which DR might promote neural cell plasticity and survival is hormesis. Like vigorous exercise or cognitive stimulation, DR seems to impose a mild beneficial stress on neurons that “conditions” them such that they are more resistant to aging and disease34, 36, 37. At the molecular level the cellular stress response involves upregulation of the expression of neurotrophic factors, heat-shock proteins, sirtuins and mitochondrial uncoupling proteins34, 38, 39.
Selective neuronal vulnerability
Why do hippocampal and frontal lobe pyramidal neurons die in AD, whereas dentate gyrus granule neurons and cortical interneurons are spared? Why do dopaminergic neurons in the substantia nigra, medium spiny neurons in the striatum and lower motor neurons in the spinal cord succumb in PD, HD and ALS, respectively? Once the most vulnerable neurons are affected, what determines which neuronal populations degenerate later in the course of the disease (upper motor neurons in ALS and cortical neurons in PD, for example)? Unfortunately, in no case is the mechanism responsible for SNV in an age-related neurodegenerative disorder known. Although many different genetic abnormalities have been identified that can cause a neurodegenerative disorder (Fig. 2), in no case is it known why the mutant gene causes SNV. Nevertheless, a rapidly growing literature provides many clues as to the molecular and cellular factors that determine whether a particular neuron succumbs to or resists an age-related disease. The physical and molecular characteristics of neurons, their functional properties and their location within neural circuits are all likely to influence their fate during aging40. Vulnerable neurons are typically large with myelinated axons that extend relatively long distances, from one region of the nervous system to another or from the CNS to peripheral targets. This is true for the hippocampal and cortical pyramidal neurons affected in AD, upper and lower motor neurons in ALS and striatal medium spiny neurons in HD2-6. The dopaminergic neurons in the substantia that succumb to PD, though smaller than the aforementioned neurons, are also projection neurons with relatively long axons41. There are several reasons why large projection neurons might be particularly vulnerable to aging including a high energy requirement, reliance on axonal transport (anterograde and retrograde) for sustained function and trophic support, and a large cell surface area which increases exposure of the cells to toxic environmental conditions. The cytoskeleton of large neurons might be particularly prone to dysfunction as suggested by the aggregation and displacement of axonal neurofilaments and the microtubule-associated protein tau in motor neurons of ALS cases5 and pyramidal neurons of AD cases42.
Degeneration is often limited to subpopulations of neurons with a particular neurotransmitter phenotype. For example, ALS strikes cholinergic motor neurons, GABAergic striatal neurons are most vulnerable in HD and dopaminergic neurons in PD. However, if one considers the cumulative neurodegenerative topography among disorders, it is clear that aging endangers neurons of all the major neurotransmitter phenotypes (Table 1). Among the different neurotransmitters, glutamate might play an active and essential role in neuronal damage and death in all neurodegenerative disorders (see section on excitotoxicity below). On the other hand, dopamine might itself contribute to the demise of the neurons that produce it in PD by inducing oxidative stress in presynaptic terminals3. The signaling pathways of various neuropeptides, including corticotropin releasing hormone43, vasopressin and oxytocin44, might also be disrupted, particularly in later stages of neurodegenerative disorders.
Table 1.
Phenotypes of neurons vulnerable to age-related neurodegenerative disorders.
| Disorder | Affected Regions | Phenotypes |
|---|---|---|
| Alzheimer | EC, H, FC, BF, P, O, AM, LC*, RN* | Projection neurons; multiple transmitters (glutamate, acetylcholine, norepinephrine, serotonin); low CBPs |
| Parkinson | SN, FC, LC*, RN* | Projection neurons; primarily dopaminergic neurons; low CBPs |
| Huntington | ST, FC, LC* | Projection neurons; GABAergic and glutamatergic; |
| ALS | MC, SC | Projection neurons; cholinergic and glutamatergic neurons; low CBPs. |
| Stroke | most CNS regions | Large neurons; low CBPs |
these neuronal populations are typically affected late in the disease process. AM, amygdala; BF, basal forebrain; CBPs, calcium-binding proteins; EC, entorhinal cortex; FC, frontal cortex; H, hippocampus; LC, locus coeruleus; MN, motor cortex; P, parietal; O, occipital; RN, raphe nucleus; SC, spinal cord; SN, substantia nigra; ST, striatum.
Dysfunction and death of neurons adversely affects both the pre- and post-synaptic neurons with which they communicate. Therefore, patterns of neuronal degeneration are often domino-like. In the case of AD, neurons in the entorhinal cortex that provide input to the hippocampus degenerate early in the course of the disease, followed by hippocampal neurons and then cortical neurons that communicate with hippocampal neurons45. Although substantia nigra dopaminergic neurons have been the primary focus of PD research, they might not be the first affected. Instead, neurons in the dorsal motor nuclei of the medulla oblongata, and raphe nucleus and locus coeruleus of the brainstem, succumb first46. Substantia nigra damage is followed by degeneration of neurons in the trans-entorhinal region, motor and sensory cortex and prefrontal cortex. The degeneration of motor neurons in ALS often follows a progression from lower to upper spinal cord, followed by loss of upper motor neurons in the cerebral cortex, although there is considerable variability among patients47. It is increasingly appreciated that synapses are the most vulnerable regions of neurons (Fig. 3). Differences among synapses in their structure, metabolism and signaling mechanisms might therefore be determinants of neuronal vulnerability. Finally, changes that occur in the cellular milieu in which neurons reside, including phenotypes of astrocytes, oligodendrocytes, microglia and vascular cells, likely influence the fate of neurons during aging.
Pathways to neuronal death
Apoptosis
The fact that mutations in specific genes can cause one neurodegenerative disorder, but not others, is evidence for multiple mechanisms of neuronal death. However, the development and analyses of animal and cell culture models of neurodegenerative disorders, based upon the expression of disease-causing mutant human genes, suggest a convergence of disease-specific upstream factors on well-known cell death cascades. The most widely studied type of programmed cell death in the nervous system is apoptosis, a process regulated by specific cysteine proteases called caspases (Fig. 4)48. Many different triggers of neuronal apoptosis have been documented including oxidative stress, overactivation of glutamate receptors, trophic factor insufficiency, DNA damage and accumulation of damaged proteins48-51. In this section we briefly review key subcellular molecular cascades in apoptosis and then describe evidence supporting the involvement of such cascades in age-related neuronal death.
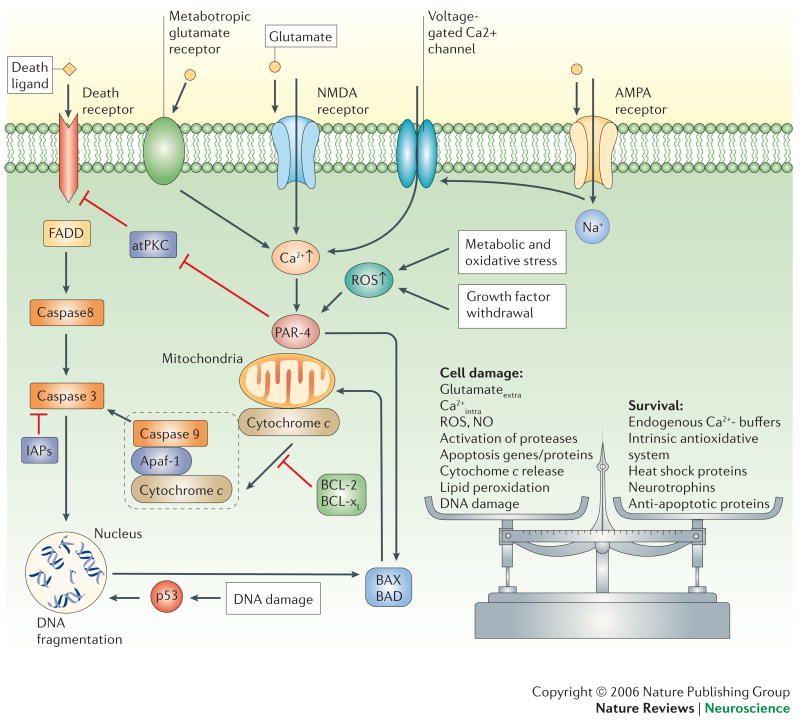
Figure 4. Once triggered, the death of neurons is programmed.
Death signals activate intracellular cascades involving increased levels of ROS and Ca2+, production of Par-4 (prostate apoptosis response-4) and p53, and translocation of pro-apoptotic Bcl-2 family members (Bax and Bad) to the mitochondrial membrane. These events are followed by increased mitochondrial dysregulation and release of cytochrome c into the cytosol. Cytochrome c forms a complex with apoptotic protease-activating factor 1 (Apaf-1) and caspase-9. Activated caspase-9 cleaves and activates caspase-3 which, in turn cleaves protein substrates that effect changes in the plasma membrane, cytoskeleton and nucleus. Certain caspases (caspase-8, for example) can also be directly activated through death ligands and can act independently of mitochondrial changes. The process of apoptosis can be inhibited at different stages through anti-apoptotic mechanisms such as IAPs (inhibitor of apoptosis proteins) or Bcl2 and Bcl-xl. In general, cell fate is decided by a balance between survival factors and potentially harmful or destructive factors. atPKC, atypical protein kinase C; FADD, Fas-associated death domain protein.
Two major groups of proteins in the Bcl-2 family play pivotal roles in most types of apoptosis: pro-apoptotic proteins such as Bax and Bad, and anti-apoptotic proteins such as Bcl-2 and Bcl-xL52. These proteins control the fate of cells by interacting with membranes of mitochondria and the endoplasmic reticulum. Bax and Bad increase mitochondrial membrane permeability and release of apoptotic factors, while Bcl-2 and Bcl-xL stabilize the membranes48, 53. Proteases such as caspases and calpains execute the death process by degrading various structural proteins54, 55. Both caspases and calpains have been implicated in the death of neurons that occurs in AD48, 56, PD57, 58, HD59, 60 and ALS60. Mitochondrial changes that occur in most instances of neuronal death include Ca2+ uptake, formation of permeability transition pores and release of cytochrome c and apoptosis inducing factor (AIF)61. The endoplasmic reticulum is also actively involved in many cases of neuronal death by releasing Ca2+ and factors that modulate the expression of pro- and anti-apoptotic genes62, 63. Interactions of mitochondria and endoplasmic reticulum in apoptosis are being established, including cytochrome c-mediated Ca2+ release from the endoplasmic reticulum64.
Oxidative stress can trigger apoptosis by several different mechanisms. ROS produced in the mitochondria promote Ca2+ uptake and increased membrane permeability resulting in the release of cytochrome c and apoptosis61. Hydroxyl radicals can directly attack DNA bases, and if the damage is extensive, a cell death pathway is activated which involves ATM kinase, p53 and Bax translocation to the mitochondria65. Membrane-associated oxidative stress can trigger apoptosis by several mechanisms. For example, lipid peroxidation generates the aldehyde 4-hydroxynonenal which can induce apoptosis by perturbing ion homeostasis and inducing mitochondrial permeability transition; this mechanism might mediate neuronal death resulting from neurotrophic factor deprivation, AD or ALS 20, 66, 67. Oxidative stress also activates sphingomyelinases resulting in the release of ceramides from membrane sphingomyelin; ceramides can trigger apoptosis by activating kinases and by interactions with mitochondrial membranes68. Increased ceramide production has been linked to neuronal death in AD, HIV dementia and ALS69-71.
After the period of developmental cell death, when large numbers of brain cells undergo apoptosis, the rate of apoptosis is relatively low during adult life72. However, apoptosis might accelerate during late life, and the involvement of apoptotic cascades in age-related neuronal death is suggested by studies of normal aging, and of neurodegenerative disorders in humans and animal models. The activities of the pro-apoptotic proteins caspase-3 and PARP is increased in brain cells during normal aging, but might be counteracted by the upregulation of anti-apoptotic proteins such as XIAP and NGF; dietary energy restriction can suppress the age-related increase in caspase-3 and PARP, while enhancing the expression of anti-apoptotic proteins73. Old neurons might die during aging and in neurodegenerative disorders, and neurons arising from stem cells might also succumb. For example, hippocampal neurogenesis is reduced during aging, apparently as the result of reduced stem cell proliferation and increased apoptosis of newly generated neurons74, 75. Neurogenesis might be impaired in AD, possibly as a result of increased death of newly generated neurons76, although evidence for a compensatory increase in neurogenesis in AD has also been reported77. Whether mature or young, neurons seem to become more vulnerable to death with aging and the cell death cascades of aging are exacerbated in age-related neurodegenerative disorders.
Excitotoxicity
Glutamate is the major excitatory neurotransmitter in the CNS, with most neurons receiving synaptic inputs from glutamatergic neurons. Glutamate therefore plays essential roles in the synaptic transmission and plasticity underlying all behaviors including learning and memory, emotions, and sensory and motor activities. These actions of glutamate are mediated by cell surface receptors that flux Na+ and Ca2+, of which AMPA and NMDA receptors are the most abundant. Excessive activation of glutamate receptors can cause damage to dendrites, and even cell death, by excitotoxicity, which results from sustained Ca2+ influx and ROS production78. Molecular and cellular changes that occur during aging are known to render neurons vulnerable to excitotoxicity. For example, by impairing the function of ion-motive ATPases, and glutamate and glucose transporters, oxidative and metabolic stress and impaired cellular energy metabolism render neurons vulnerable to excitotoxicity. Disease-specific abnormalities might compound the adverse effects of glutamate on neurons. Indeed, Aβ, dopamine, mutant huntingtin and mutant Cu/Zn-SOD have each been shown to sensitize neurons to excitotoxic death. Additional studies of mouse models of AD, PD, HD and ALS support a role for excitotoxicity in these disorders2-6. Characteristics of neurons that make them particularly prone to excitotoxicity include high amounts of NMDA and AMPA receptors, and low levels of protective calcium-binding proteins78.
The discovery and study of environmental neurotoxins, and the involvement of an excitotoxic mechanism in their cell death-inducing actions, has been a valuable contribution to neurodegenerative disorder research (Box 2) Several such toxins bind and activate glutamate receptors directly. Kainic acid and domoic acid, which are potent agonists of the kainic acid subtype of glutamate receptor, can induce epileptic seizures resulting in damage to hippocampal pyramidal neurons and cognitive deficits reminiscent of AD79, 80. BMAA, an excitotoxin that can kill spinal cord motor neurons, has been implicated in the pathogenesis of some cases of ALS81. Other disease-relevant neurotoxins render neurons vulnerable to excitotoxicity by impairing mitochondrial function. For example, exposure of humans, monkeys and/or rodents to MPTP or rotenone causes PD-like pathology and symptoms, while exposure to 3-nitropropionic acid can cause HD-like damage to striatal neurons82-84. The MPTP metabolite MPP+ and rotenone are potent inhibitors of mitochondrial complex I, while 3-nitropropionic acid inhibits succinate dehydrogenase.
Box 2. The human experience: environmental neurotoxins and age-related neurodegenerative disorders.
An incident where several drug users in California rapidly developed a PD-like syndrome led to the discovery of the dopaminergic neurotoxin MPTP82. In 1987 more than 100 individuals who had recently eaten shellfish at restaurants in Canada became ill with 25% of them suffering short term memory loss clinically similar to AD. Subsequent investigations established that the shellfish contained unusually high amounts of domoic acid, aneurotoxin excitotoxin produced by the algal food source of the shellfish220. Studies of several children in China who rapidly developed symptoms similar to those of HD led to the discovery of 3-nitropropionic acid (3-NP), a potent inhibitor of mitochondrial complex II84. An ALS-like syndrome was discovered in native populations in islands of Guam and, based on epidemiological and genetic studies, appears to have had an environmental cause. Evidence suggests that the cycad seed, a staple of the natives' diet, is a source of the putative toxin called BMAA81. In rodents and monkeys MPTP, domoic acid, 3-NP and BMAA can destroy the same populations of neurons that die in PD, AD, HD and ALS, respectively. Moreover, the vulnerability of neurons to excitotoxins and mitochondrial toxins described above increases with advancing age, suggesting that they may act on neurodegenerative pathways similar to those involved in age-related neurodegenerative disorders.
Neurotoxin-based models have provided important evidence supporting the involvement of mitochondrial compromise and excitotoxicity in neurodegenerative disorders. This information has led to the search for novel environmental toxins that may determine whether or not individuals develop a neurodegenerative disorder as they age. Several such toxins have been identified, but their contributions to disease are not yet unclear. For example, the widely used pesticide rotenone and the herbicide paraquat, which epidemiological data suggest play a role in some cases of PD, are capable of inducing PD-like pathology in rodents222. It is likely that multiple genetic and environmental factors determine whether or not exposures to a neurotoxin results in disease. Indeed, when rats or mice are maintained on DR regimens, they exhibit increased resistance to several different neurotoxins including kainic acid, MPTP and 3-NP116, 117, data consistent with epidemiological findings suggesting that high calorie diets are associated with an increased risk of PD and AD213-215.
The fact that such toxins are capable of causing AD-, PD-, HD- and ALS-like syndromes in animals, together with the reports of exposures to high levels of these toxins in humans80-84, suggests that they might act on mechanisms operative in these diseases, and that neurotoxins might have a role in some cases of age-related neurodegeneration.
Calcium dysregulation
The concentration of Ca2+ in the cytosol is tightly regulated: under resting conditions the Ca2+ concentration is typically in the range of 75-200 nM, and transiently increases to 1-10 uM in response to membrane depolarization and opening of voltage-dependent Ca2+ and NMDA receptor channels85. In addition, Ca2+ is released from IP3- and ryanodine-sensitive stores in response to extracellular signals or increased cytosolic Ca2+ levels (Ca2+-induced Ca2+ release). Mitochondria are also capable of sequestering Ca2+ and then releasing it into the cytosol. Ca2+ is removed from the cytosol by the activities of plasma membrane and endoplasmic reticulum Ca2+ ATPases, and by sequestration by Ca2+-binding proteins. Perturbations of neuronal Ca2+ homeostasis have been documented during normal aging including increased Ca2+-dependent afterhyperpolarizations in hippocampal CA1 neurons, and alterations in the Ca2+-handling properties of mitochondria and the endoplasmic reticulum86. It is now well established that sustained elevations of intracellular Ca2+ levels can cause neuritic degeneration and cell death by activating proteases and inducing ROS production. Studies of mutations in the genes that cause HD and familial cases of AD, PD and ALS, suggest that perturbed neuronal Ca2+ homeostasis is a consequence of those mutations that contribute to the degeneration of neurons. For example, mutations in presenilin-1 and APP promote neuronal Ca2+ overload and cell death under conditions of oxidative and metabolic stress87, 88, and mutations in α-synuclein and huntingtin have been associated with perturbed Ca2+ regulation in PD and HD, respectively89, 90. Spinal cord motor neurons might die in ALS as the result of overactivation of glutamate receptors or autoimmune attack on voltage-dependent Ca2+ channels91, 92.
Alterations in cellular Ca2+ homeostasis might play a role in SNV during aging. In this regard, studies of the hippocampus have been particularly informative. CA1 and CA3 pyramidal neurons are vulnerable in AD, severe epileptic seizures and ischemia, whereas dentate granule neurons are relatively invulnerable. This differential neuronal vulnerability might be explained, in part, by the fact that dentate granule neurons express very high levels of the neuroprotective Ca2+-binding protein calbindin, whereas pyramidal neurons contain little or no calbindin93, 94. Age-related reductions in calbindin expression have been implicated in the SNV of basal forebrain cholinergic neurons95 and entorhinal cortex layer II neurons96 in AD, dopaminergic neurons in PD, and striatal neurons in HD97. Spinal cord motor neurons with low levels of calbindin and parvalbumin are vulnerable in ALS, whereas cranial nerve motor neurons, which express high levels of these calcium-binding proteins, are relatively resistant98. Neurons expressing high levels of NMDA receptors (such as CA1 hippocampal neurons in AD99) and/or Ca2+-permeable AMPA receptors (such as spinal cord motor neurons in ALS)100 might be prone to age-related degeneration.
Mitochondrial perturbations
Decrements in mitochondrial function have often been associated with aging in general, and aging of the nervous system in particular101. Positron emission tomography imaging of radiolabeled glucose uptake in the brains of normal human subjects, aged 20 to 67 years indicated widespread age-dependent reductions in glucose utilization in most brain regions, with the exception of the cerebellum and occipital cortex102. Similar analyses of AD, PD and HD patients reveal dramatic reductions in glucose utilization in the brain regions most severely affected, abnormalities that can be detected prior to the onset of clinical disease103-105. Measurements of the activities of mitochondrial enzyme activities in brain tissue samples revealed significant decreases in the activities of the pyruvate dehydrogenase complex, isocitrate dehydrogenase and the α-ketoglutarate dehydrogenase complex in AD patients compared to control subjects106. Mitochondrial complex I activity declines in the brain during normal aging and much more so in PD107. HD patients lose weight progressively, despite maintaining a high caloric intake, an abnormality that might result from impaired mitochondrial function108. Deficits in mitochondrial function might also occur early in the course of ALS, perhaps first in the axons and presynaptic terminals of the motor neurons109.
Studies of normal aging and animal models of neurodegenerative disorders have provided further insight into the nature of perturbed neuronal energy metabolism that might predispose to SNV. Analyses of mitochondria isolated from different brain regions of young, middle age and old rats revealed that mitochondria from cerebral cortex of old rats exhibit enhanced ROS production and mitochondrial swelling in response to increasing Ca2+ loads compared to cortical mitochondria from younger rats110. In contrast, the sensitivity of cerebellar mitochondria to Ca2+ was unaffected by aging. The ability of mitochondria to respond appropriately to excitation might be impaired during aging as suggested by reduced buffering of voltage-gated Ca2+ influx in basal forebrain neurons from aged rats111. Moreover, aging increases the vulnerability of mitochondria to toxins such as 3-nitropropionic acid112. In addition to alterations in mitochondria, neurons also exhibit impaired glucose uptake during normal aging113, further compromising their ability to maintain ion homeostasis and other energy-dependent cellular processes. Many of the age-related deficits in energy metabolism might be a consequence of oxidative stress. As evidence, neurons in mice deficient in glutathione peroxidase are more vulnerable to being killed by 3-nitropropionic acid and MPTP114. Similarly, mitochondrial manganese superoxide dismutase protects neurons against oxidative damage115. Finally, caloric restriction can preserve mitochondrial function during aging, apparently by reducing ROS production38 and can protect neurons from being killed by mitochondrial toxins116, 117.
Several abnormalities in mitochondrial function and energy homeostasis have been observed in mice expressing mutant forms of APP and/or presenilin-1 that cause AD in humans. APP mutant mice exhibit reduced cerebral glucose utilization and cerebral blood flow which is correlated with Aβ accumulation118. Aβ has been shown to impair mitochondrial function, and studies of APP mutant mice suggest a key role for an Aβ-binding alcohol dehydrogenase in this pathogenic action of Aβ119. Mitochondria in neurons of presenilin-1 mutant knockin mice exhibit increased sensitivity to toxins120 and cellular Ca2+ overload121. Huntingtin mutant mice also manifest alterations in mitochondrial function and energy metabolism. For example, Panov et al.122 found that mitochondria from huntingtin mutant mice maintain an abnormally low resting membrane potential and are hypersensitive to Ca2+. Mutant huntingtin might also perturb energy metabolism indirectly by impairing the transport of mitochondria along axons123. ALS (Cu/Zn-SOD mutant) mice exhibit impaired mitochondrial function in spinal cord neurons109, and treatment of the mice with creatine suppresses the neurodegenerative process and improves survival in ALS mice124. Administration of agents that enhance cellular energy metabolism (creatine, coenzyme Q10 and nicotinamide, for example) delays disease onset and progression in mouse models of neurodegenerative disorders suggesting a key role for cellular energy deficits in the disease process125. Collectively, the available data suggest that the aging process is associated with impaired mitochondrial function and energy metabolism in neurons, and that environmental and genetic factors can exacerbate or protect against these adverse effects of aging.
Accumulation of damaged molecules
One of the most widely documented and obvious alterations that occur in neurons during aging, is the accumulation of damaged molecules within the cells. A conspicuous example is lipofuscin, an autofluorescent material consisting of oxidatively damaged proteins and lipids that may accumulate as a result of impaired mechanisms for their removal10, 126, 127. In addition, aging is associated with increased amounts of damaged DNA in neurons which may result from impaired DNA repair systems13. Mutations in DNA repair proteins can cause premature aging syndromes with neurodegenerative phenotypes12, consistent with a role for impairment of these systems in normal aging of the nervous system.
A major problem neurons encounter during aging that is strongly linked to neurodegenerative disorders is the accumulation of damaged proteins which form insoluble aggregates that accumulate within and/or outside of the cells (Box 1). Damaged proteins are removed by enzymatic degradation by cytosolic proteases, lysosomes and the proteasome, and there is evidence that alterations in each of these three mechanisms occur in neurons during aging128-130. Proteasome activity decreases with advancing age in the cerebral cortex, hippocampus and spinal cord, but not in the cerebellum or brainstem, of rats131. Analyses of brain tissue samples suggest a much more severe malfunction of the proteasomal system in AD132 and PD133. Several of the adverse consequences of aging on neuronal function and survival can be mimicked by pharmacological inhibition of proteasomes134 or lysosomes135. In addition, increasing evidence suggests an important role for impaired autophagy in neuronal dysfunction and death in aging and age-related disease136. Autophagy is impaired during normal aging and can be restored by DR, consistent with a possible role for impaired removal of damaged organelles in neuropathologies of aging137.
Four major neuronal proteins that are prone to aggregation and contribute to neuronal dysfunction and death in neurodegenerative disorders are Aβ, tau, α-synuclein and huntingtin. Variations in the amino acid sequence of Aβ have a major impact on its self-aggregating properties; for example, sequence differences among species are associated with either the presence (humans and dogs, for example) or absence (rats and mice, for example) of Aβ deposits in the brains of old animals of those species, and with the propensity of the peptides to self aggregate138. In addition, subtle variations in peptide structure influence the ability of Aβ to recruit soluble Aβ into fibrillar aggregates139. Aβ can be degraded by several enzymes including neprilysin, insulin-degrading enzyme and the proteasome130, 140. Impairments of one or more of these protein degradation systems by age-related increases in oxidative stress and protein damage might contribute to the formation of intracellular and extracellular Aβ aggregates in aging and AD. Oxidative processes involving hydrogen peroxide, Fe2+ and Cu+ might promote the aggregation of Aβ and toxic effects on neurons2.
Tau forms fibrillar aggregates within neurons (neurofibrillary tangles) during normal aging and more so in AD, frontotemporal lobe dementia and related neurodegenerative disorders141. Different isoforms of tau are produced by neurons and studies of mutations that cause tangle disorders, such as fronto-temporal lobe dementia with Parkinsonism linked to chromosome 17 (FTDP-17), suggest a critical role for the ratio of three-repeat isoforms to four-repeat isoforms in the aggregation of tau to form neurofibrillary tangles141. Age-related alterations in tau kinases and phosphatases might result in the accumulation of aggregation-prone hyperphosphorylated forms of tau142. In addition, oxidative stress and impaired protein clearance mechanisms likely contribute to the accumulation of tau142, 143. Tau normally plays a key role in neuronal plasticity and axonal transport by regulating the polymerization of microtubules. Hyperphosphorylation and aggregation of tau therefore disrupts microtubule functions which might play a major role in the death of neurons in tauopathies and, to a lesser extent, in neuronal degeneration associated with normal aging.
Cytoplasmic and intranuclear inclusions containing α-synuclein are a prominent feature of PD and might also occur in normal aging. The identification of genetic aberrancies responsible for early-onset inherited forms of PD, and elucidation of their metabolism and normal functions, has resulted in strong evidence for impaired proteolytic clearance of α-synuclein as a fundamental abnormality that occurs in dopaminergic neurons in PD3,144. Mutations in α-synuclein, parkin (a ubiquitin E3 ligase) and ubiquitin C-terminal hydrolase-1 result in reduced degradation of α-synuclein. Moreover, a triplication of the α-synuclein gene is sufficient to cause PD, further supporting an impaired ability of dopaminergic neurons to degrade α-synuclein during aging145. Although the mechanism(s) by which impaired clearance of α-synuclein promotes neuronal degeneration is unknown, emerging evidence suggests the involvement of perturbed dopamine storage and release from presynaptic terminals146.
Polyglutamine expansions in the huntingtin protein cause HD, and those pathogenic proteins self-aggregate and are neurotoxic147. Although HD has a genetic cause, those affected typically develop symptoms after the age of fifty, suggesting that changes that occur during aging might facilitate the aggregation and neurotoxicity of mutant huntingtin. Indeed, two age-related processes, oxidative stress and perturbations in protein chaperones can promote aggregation and cytotoxicity of mutant huntingtin148, 149. Moreover, when huntingtin mutant mice are maintained on DR the formation of intraneuronal huntingtin inclusions and the degeneration of striatal and cortical neurons is retarded, and lifespan is extended32. DR is known to reduce oxidative stress and increase the production of protein chaperones, suggesting mechanisms by which DR might counteract pathogenic processes in HD. Protein chaperones such as heat-shock protein 70 (HSP70) and glucose-regulated protein-78 (GRP78) can protect neurons against death in cell culture and animal models of neurodegenerative disorders150. Abnormalities in protein chaperone mechanisms have been documented in studies of several neurodegenerative disorders in addition to HD, AD, PD and ALS151-153. In the case of ALS, an impaired ability of motor neurons to upregulate HSP-70 in response to stress might play a role in their selective vulnerability154.
Neurotrophic factors
Cells of the nervous system, as well as peripheral targets such as muscle cells, produce neurotrophic factors that promote neuronal survival, neurite outgrowth and synaptic plasticity. Here we focus on neurotrophic factor signaling pathways that might play roles in determining whether neurons resist or succumb to a neurodegenerative disorder. Neuronal populations vulnerable to age-related disease are believed to be protected by one or more neurotrophic factors. For example: hippocampal pyramidal neurons (AD) respond to BDNF, nerve growth factor (NGF), insulin-like growth factors (IGF) 1 and 2 and basic fibroblast growth factor (bFGF); basal forebrain cholinergic neurons (AD) respond to NGF and bFGF; substantia nigra dopaminergic neurons (PD) respond to glial cell line derived neurotrophic factor (GDNF) and BDNF; striatal medium spiny neurons (HD) respond to BDNF and NGF; and motor neurons (ALS) respond IGF-1 and BDNF155-160. Declining production of a neurotrophic factor(s) or impaired signal transduction during aging could play a role in SNV. Age-related decreases in the expression of BDNF in the hippocampus have been reported161 and might contribute to age-related cognitive impairment162. The responsiveness of BDNF signaling to environmental stimuli might be compromised during aging. For example, the abilities of cognitive challenges163, exercise164 and brain injury165 to upregulate BDNF signaling are impaired in aged rats. Although levels of receptors for IGF-1 or IGF-2 were not different in aged memory-impaired rats compared to unimpaired rats166, the ability of IGF-1 to stimulate protein synthesis in the cerebral cortex is diminished167 and deafferentation-induced IGF-1 expression is attenuated168 in old rats. Moreover, infusion of IGF-1 into the brain can restore cognitive function in old rats169. Age-related decrements in motor function were improved, and stimulus-evoked dopamine release was increased, by GDNF infusion in aged monkeys170. Considerable evidence suggests a role for deficits in NGF signaling in age-related atrophy of basal forebrain cholinergic neurons and, indeed, age-dependent cognitive deficits in rats can be reversed by transplantation of NGF-secreting fibroblasts171. In addition to direct actions on neurons themselves, neurotrophic factors might affect other cell types including glia and vascular cells172.
Data from patients and animal models suggest roles for compromised neurotrophic factor signaling in age-related neurodegeneration. BDNF signaling might be compromised early in the course of AD173, while the role of impaired NGF signaling in AD appears more complex174. Studies of HD patients and huntingtin mutant mice have revealed reduced levels of BDNF in the striatum and cortex175, and some manipulations that suppress the disease process in HD mice (DR and paroxetine) also increase BDNF expression29, 172. Mechanisms by which neurotrophic factors might prevent age- and disease-related neuronal degeneration have been reviewed in detail previously and include suppression of oxidative and metabolic stress, excitotoxicity and calcium overload, and protein and DNA damage78. The neuroprotective signal transduction pathways for neurotrophic factors often involve receptor tyrosine kinases, phosphatidylinositol-3-kinase, Akt kinase, mitogen-activated protein kinases, and transcription factors such as CREB (cyclic AMP response element binding protein) and NF-κB78. Examples of neuroprotective genes upregulated by the latter signaling pathways include Bcl-2, inhibitor of apoptosis proteins (IAP), Mn-SOD and calbindin78.
Cytoskeletal disruption
The complex morphologies of neurons, with long axons and elaborate dendritic arbors, are maintained and modified by the cytoskeleton (microtubules, microfilaments and intermediate filaments) and associated proteins (microtubule-associated proteins and actin-binding proteins, for example)177, 178. The shafts of axons and dendrites contain large numbers of microtubules, whereas the more dynamic growth cones and synaptic terminals are actin-rich; neurofilaments are concentrated in axons. The cytoskeletal organization is disrupted in neurons that degenerate during aging and in neurodegenerative disorders. In particular, microtubules depolymerize and the microtubule-associated protein tau, which is normally present only in axons, accumulates in the cell body179. Axonal neurofilament pathology is prominent in motor neurons in ALS, but also occurs in hippocampal neurons in AD and dopaminergic neurons in PD178. Hyperphosphorylation of tau on specific amino acid residues occurs in vulnerable neurons in AD as the result of alterations in tau kinases and phosphatases; data suggest that such alterations decrease the affinity of tau for microtubules and promote its self-aggregation179. Events upstream of cytoskeletal abnormalities in aging and disease might include oxidative stress and perturbed calcium homeostasis180, 181. In AD, Aβ abnormalities are believed to cause the tau pathology. Many of the cytoskeletal abnormalities present in human neurodegenerative disorders are also manifest in animal models. For example, hippocampal and cortical neurons exhibit neurofibrillary tangle-like tau pathology in a mouse model of AD182, impaired axonal transport of BDNF in cortical neurons from huntingtin mutant knockin mice183 and motor neurons suffer severe neurofilament pathology in Cu/Zn-SOD mutant mice184.
Although cytoskeletal abnormalities are prominent in vulnerable neuronal populations in a range of neurodegenerative disorders, until recently it was unclear whether the alterations were pivotal in the cell death process. The identification of tau mutations as the causal genetic factor in subjects with the inherited disorder FTDP-17 firmly established the sufficiency of tau pathology for SNV185. Transgenic mice engineered to mimic the FTDP-17 defect exhibit age-dependent filamentous tau pathology in cortical, brainstem and spinal cord neurons186. Why pyramidal neurons in the frontal cortex are particularly vulnerable in FTDP-17 is unknown, but could involve the ratios of different tau isoforms or the mechanisms of tau degradation or aggregation in the vulnerable neurons187. However, the determinants of SNV to cytoskeletal pathology during aging are likely to be the same as those that determine whether a neuron lives or dies. For example, aberrant processing of APP in AD results in the production of neurotoxic forms of Aβ which induce oxidative stress and Ca2+ dysregulation in neurons resulting in microtubule depolymerization and tau pathology (Box 1). Axonal microtubule and neurofilament pathologies in motor neurons in ALS might also be secondary to oxidative stress and neurotrophic insufficiency.
Inflammation
There is considerable evidence for both local and humoral inflammatory and immune responses in aging and neurodegenerative disorders. This has been perhaps best studied in regards to AD where activated microglia and astrocytes are associated with Aβ plaques and neurofibrillary pathology188. Pro-inflammatory cytokines are produced by the activated glial cells and might contribute to the neurodegenerative process189. In addition, complement factors that can damage cells are localized to Aβ plaques190. Epidemiological findings, and studies of the effects of anti-inflammatory agents in cell culture and animal models of AD suggest a role for inflammatory processes in neuronal degeneration and disease progression188. Humoral immune responses to the neuronal and glial pathologies in the brain appear to occur in AD as indicated by increased levels of leukocyte adhesion molecules and the presence of cells that express lymphocyte markers in association with amyloid pathology190, 191. Interestingly, antibodies against Aβ have been detected in association with Aβ deposits and circulating in the blood of AD patients; such antibodies might either function adaptively, removing Aβ from the brain or they might contribute to the neurodegenerative process192-194. Other neurodegenerative disorders also manifest inflammatory processes in association with the pathology including PD195 and ALS196. In addition, it has been proposed that autoantibodies directed against motor neuron antigens play a role in the pathogenesis of ALS197. Altogether, the available data suggest the possibility that immune-based processes can be targeted for therapeutic intervention to promote healthy brain aging and treat neurodegenerative disease.
Therapeutic implications
There are currently no treatments available for those suffering from a neurodegenerative disorder that will halt the disease process. However, a few treatments have been shown to slow the course of the disease including riluzole in ALS patients198 and possibly memantine in AD patients199. Recent advances in early diagnosis and preclinical studies suggest that effective treatments that slow the disease course in different neurodegenerative disorders will be found. Based upon their site of action in the aging process and/or neurodegenerative cascade (Fig. 2), treatments might be either disorder-specific or might be useful for more than one disorder (Table 2). For example, γ- and β-secretase inhibitors selectively target a mechanism (APP processing) that is abnormal in AD, but might not be operative in other disorders (PD, HD or ALS). On the other hand, antioxidants and anti-inflammatory agents target a pathogenic process common to all neurodegenerative disorders. Because extensive neuronal degeneration and death occurs prior to diagnosis, treatments that can restore function are unlikely. Instead, there is a great potential for preventing, or at least delaying, disease onset in those at risk due to genetic and/or environmental factors. Indeed, everyone is at a high risk for AD and PD as they enter their 7th and 8th decades of life.
Table 2.
Examples of approaches for preventing and treating age-related neurodegeneration.
| Approach | Mechanism/Target | Disorders |
|---|---|---|
| Dietary restriction | decreased ROS, hormesis*, NTFS | AD, PD, HD |
| Exercise | hormesis, NTFS | AD, PD |
| Cognitive stimulation | NTFS, hormesis | AD, PD |
| Dietary phytochemicals | antioxidants, hormesis | AD, PD, HD, ALS |
| Dietary lipid modification | decreased ROS, membrane homeostasis | AD, PD |
| Antioxidants | decreased ROS | AD, PD, HD, ALS |
| Anti-inflammatory agents | decreased ROS and neurotoxic cytokines | AD, PD, HD, ALS |
| Anti-excitotoxic agents | decreased calcium influx and ROS | AD, PD |
| Calcium-stabilizing agents | prevention of cellular calcium oveload | AD, PD, HD, ALS |
| Aβ modulating agents | β- or γ-secretase inhibitors, Aβ degrading agents | AD |
| Energy modulation | increased cellular energy availability | AD, PD, HD, ALS |
| Inhibitors of apoptosis | caspases, Bcl-2 proteins, mito/ER membranes | AD, PD, HD, ALS |
| Neurotrophic factors | neuronal survival and plasticity, neurogenesis | AD, PD, HD, ALS |
| Immunotherapy | Active/passive immune attack on diseased protein | AD, others? |
| RNA interference | suppressed production of diseased protein | HD, familial disease |
Hormesis is a general term used to describe a mechanism in which subjection of a cell or organism to a mild stress activates stress-response pathways that increase the ability of the cell/organism to resist disease and death.
Evidence is emerging that age-related neuronal dysfunction can be delayed — that the risk of neurodegenerative disorders can be modified by diet and lifestyle (Fig. 5). For example, DR extends brain longevity and suppresses the disease process in animal models of AD, PD, HD and stroke30-33, 200. Exercise and cognitive stimulation slow the pathogenic cascades in AD mice201. However, although studies with animal models have been promising, the extent to which such approaches will counteract ageing and neurodegenerative disease in humans remainst to be determined. Dietary and pharmacological manipulations of lipid metabolism have proven effective in preclinical studies of AD, findings supported by epidemiological data202, 203. Other approaches include glutamate receptor modulating agents for AD204, anti-apoptotic agents for PD and HD205, 206, agents that enhance energy metabolism for HD207, and neurotrophic factor therapies for PD, HD and ALS208-210. Particularly exciting are the possibilities that pathogenic proteins such as Aβ, tau and polyglutamine repeat proteins such as huntingtin can be targeted by active or passive immunization211 or by RNA interference technology212.
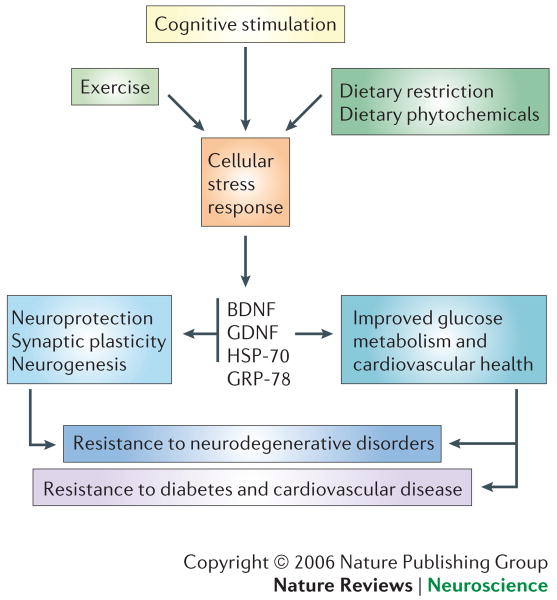
Figure 5. Counteracting aging by stimulating beneficial cellular stress responses.
Exercise, dietary restriction (DR) and cognitive stimulation have all been shown to protect neurons against dysfunction and death in animal models of neurodegenerative disorders. This occurs, in part, by induction of a mild stress response which induces the production of neurotrophic factors such as BDNF and GDNF, as well as protein chaperones such as HSP-70 and GRP-78. In addition, exercise and DR improve energy metabolism (increased insulin sensitivity) and cardiovascular health (decreased blood pressure and enhanced cardiovascular stress adaptation). This model is based largely on the results of studies in animals and, although such studies have been promising, it's not yet clear that exercise, DR and cognitive stimulation can protect against neurodegeneration in humans
Conclusions and perspectives
A major goal of aging research is to extend “healthspan” by identifying approaches for delaying or preventing age-related diseases. The fact that many individuals maintain a well-functioning nervous system and continue productive lives through their seventies, eighties and even nineties is encouraging. The implication is that, if the cellular and molecular mechanisms that determine whether nervous systems adapt positively or develop a disease during aging can be identified, then disease processes can be averted. In this regard, oxidative and metabolic stress, and impaired cellular stress adaptation are mechanisms of aging that render neurons vulnerable to degeneration. On this background of age-related endangerment, genetic and environmental factors determine whether or not a disease process develops. These include causal mutations, more subtle genetic risk factors, and environmental factors including aspects of diet and lifestyle. Because of the cellular and molecular complexity of the nervous system, and the signaling mechanisms that influence neuronal plasticity and survival, the basis of SNV remains elusive. Nevertheless, the proposed mechanisms of age-related neuronal vulnerability described above are apparently operative in multiple neurodegenerative disorders. Disorder-specific differences in the phenotypes of neurons determine which neurons succumb. For example, in AD the amounts and location of APP within neurons, levels of α- and β-secretase activities, and factors that affect APP processing (oxidative stress, lipid metabolism and calcium dynamics) might determine SNV.
Currently, most efforts to prevent and treat neurodegenerative disorders focus on diet and lifestyle modification and drugs that target disease processes. Although the evidence in humans is still limited, the emerging evidence that DR, exercise and cognitive stimulation can bolster neuroprotective mechanisms suggests that diet and lifestyle changes could reduce the risk of neurodegenerative disorders213-221. An understanding of the mechanisms of action of such environmental risk-reduction factors has led to efforts to develop dietary supplements and drugs that mimic their action. Together with advances in the development of drugs that target specific molecular events in neurodegenerative cascades, it seems likely that extension of neural healthspan is possible for most individuals.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Glossary
- Selective neuronal vulnerability
The susceptibility of specific populations of neurons that is limited to a region or regions of the nervous system
- Proteasome
A protein complex responsible for degrading intracellular proteins that have been tagged for destruction by the addition of ubiquitin
- Autophagy
A process whereby damaged organelles are degraded within membrane-bound organelles
- Dietary energy restriction
A decrease in the amount of food consumed over time (caloric restriction) and/or the frequency of meals (intermittent fasting)
- Reactive oxygen species
Highly reactive oxygen-based molecules with an unpaired electron in their outer orbital that are capable of damaging proteins, lipids and nucleic acids
- Hormesis
A process in which exposure of a cell or organism to a sublethal level of stress increases the resistance of that cell or organism to a subsequent higher and otherwise lethal level of the same or different stress
- Sirtuins
A family of histone deacetylases that play important roles in cellular stress responses and energy metabolism
- Mitochondrial uncoupling proteins
A family of proteins that reside in the mitochondrial inner membrane that promote a proton leak across the membrane, thereby decreasing oxidative phosphorylation and reactive oxygen species production
- Trans-entorhinal region
An area of the brain, located between association cortices and the hippocampus, which plays important roles in the integration of information and learning and memory processes
- Caspases
A family of intracellular cysteine endopeptidases that have a key role in inflammation and mammalian apoptosis. They cleave proteins at specific aspartate residues
- Bcl-2 family
A family of proteins that promote the survival of neurons by stabilizing mitochondrial membranes and decreasing oxidative stress
- Calpains
Cysteine proteases activated by calcium that cleave a variety of substrates including cytoskeletal proteins
- Permeability transition pores
Pores in the mitochondrial membranes formed by proteins in response to signals that trigger apoptosis
- Lipid peroxidation
An autocatalytic process in which free radicals attack double bonds in membrane lipids resulting in structural damage to membranes and to the liberation of toxic aldehydes such as 4-hydroxynonenal
- Ceramides
Membrane lipids that are incorporated into sphinomyelin and are released in response to the activation of sphingomyelinases
- Ion-motive ATPases
Energy-dependent ion pumps in membranes that are essential for the restoration and maintenance of the sodium and calcium gradients
- Afterhyperpolarization
The membrane hyperpolarization that follows the occurrence of an action potential
- Glutathione peroxidase
An antioxidant enzyme that converts hydrogen peroxide to water
- Mitochondrial manganese superoxide dismutase
An antioxidant enzyme located within mitochondria that converts superoxide anion radical to hydrogen peroxide
- Lysosome
A membrane-bound organelle with a low pH containing high concentrations of enzymes that degrade proteins
- Frontotemporal dementia
A neurodegenerative disorder resulting from the degeneration of neurons in the frontal lobe
- Repeat isoform
An isoform of the microtubule-associated protein tau which contains either 3 or 4 microtubule-binding domains
- Cytokines
A large class of intercellular signaling proteins that play important roles in neural-immune interactions and inflammatory processes
- Complement factors
Proteins that function in innate immunity, often forming pores in membranes that kill cells
- Leukocyte adhesion molecules
Proteins located on the surface of vascular endothelial cells that bind to leukocytes, thereby facilitating the passage of the leukocytes across the blood-brain barrier
References
- 1.Hofer SM, Berg S, Era P. Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychol Aging. 2003;18:285–305. doi: 10.1037/0882-7974.18.2.285. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review article integrates information on the molecular pathogenesis of Alzheimer's disease and efforts to prevent and treat this disorder.
- 3.Cookson MR. The biochemistry of Parkinson's disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 4.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;27:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]; The involvement of mitochondrial dysfunction and perturbed ubiquitin-mediated proteolysis in Parkinson's disease is reviewed.
- 5.Sieradzan KA, Mann DM. The selective vulnerability of nerve cells in Huntington's disease. Neuropathol Appl Neurobiol. 2001;27:1–21. doi: 10.1046/j.0305-1846.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 7.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 9.Ames BN. Delaying the mitochondrial decay of aging. Ann N Y Acad Sci. 2004;1019:406–411. doi: 10.1196/annals.1297.073. [DOI] [PubMed] [Google Scholar]
- 10.Gray DA, Tsirigotis M, Woulfe J. Ubiquitin, proteasomes, and the aging brain. Sci Aging Knowledge Environ. 2003 Aug 27;:RE6. doi: 10.1126/sageke.2003.34.re6. [DOI] [PubMed] [Google Scholar]
- 11.Trojanowski JQ, Mattson MP. Overview of protein aggregation in single, double, and triple neurodegenerative brain amyloidoses. Neuromolecular Med. 2003;4:1–6. doi: 10.1385/NMM:4:1-2:1. [DOI] [PubMed] [Google Scholar]
- 12.Kyng KJ, Bohr VA. Gene expression and DNA repair in progeroid syndromes and human aging. Ageing Res Rev. 2005;4:579–602. doi: 10.1016/j.arr.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 14.Butler RN, et al. Longevity genes: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci. 2003;58:581–584. doi: 10.1093/gerona/58.7.b581. [DOI] [PubMed] [Google Scholar]
- 15.Wolkow CA. Life span: getting the signal from the nervous system. Trends Neurosci. 2002;25:212–216. doi: 10.1016/s0166-2236(02)02133-1. [DOI] [PubMed] [Google Scholar]; This article describes how insulin-like signaling in the nervous system may regulate aging and determine lifespan.
- 16.Mattson MP. Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res Rev. 2003;2:329–342. doi: 10.1016/s1568-1637(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 17.Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23:843–853. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 18.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 19.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53:S26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen WA, et al. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann Neurol. 1998;44:819–824. doi: 10.1002/ana.410440518. [DOI] [PubMed] [Google Scholar]
- 21.Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J Biol Chem. 2004;279:15499–15504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- 22.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]; Experimental evidence that impaired autophagy contributes to α-synuclein pathology in Parkinson's disease.
- 23.Grondin R, et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- 24.Zuccato C, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- 25.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 27.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 28.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- 30.Patel NV, et al. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Maswood N, et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc Natl Acad Sci USA. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan W, et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 34.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 37.Spires TL. Environmental enrichment rescues protein deficits in a mouse model of Huntington's disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D, et al. Mitochondrial UCP4 induces a glycolytic shift in energy metabolism and increases the resistance of neurons to oxidative stress. Neuromolecular Med. 2006 doi: 10.1385/NMM:8:3:389. In press. [DOI] [PubMed] [Google Scholar]
- 39.Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 40.Morrison BM, Hof PR, Morrison JH. Determinants of neuronal vulnerability in neurodegenerative diseases. Ann Neurol. 1998;44:S32–44. doi: 10.1002/ana.410440706. [DOI] [PubMed] [Google Scholar]
- 41.Smith DE, Saji M, Joh TH, Reis DJ, Pickel VM. Ibotenic acid-induced lesions of striatal target and projection neurons: ultrastructural manifestations in dopaminergic and non-dopaminergic neurons and in glia. Histol Histopathol. 1987;2:251–263. [PubMed] [Google Scholar]
- 42.Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Bayatti N, Behl C. The neuroprotective actions of corticotropin releasing hormone. Ageing Res Rev. 2005;4:258–70. doi: 10.1016/j.arr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Ishunina TA, Swaab DF. Neurohypophyseal peptides in aging and Alzheimer's disease. Ageing Res Rev. 2002;1:537–558. doi: 10.1016/s1568-1637(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 46.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 47.Mills KR. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain. 2003;126:2558–2566. doi: 10.1093/brain/awg260. [DOI] [PubMed] [Google Scholar]
- 48.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]; Reviews evidence supporting a role for apoptosis in the pathogenesis of neurodegenerative disorders.
- 49.Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 50.Miller FD, Pozniak CD, Walsh GS. Neuronal life and death: an essential role for the p53 family. Cell Death Differ. 2000;7:880–888. doi: 10.1038/sj.cdd.4400736. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka Y, et al. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- 52.Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004;1644:189–203. doi: 10.1016/j.bbamcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Cheung EC, et al. Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci. 2005;25:1324–1334. doi: 10.1523/JNEUROSCI.4261-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- 55.Stefanis L. Caspase-dependent and -independent neuronal death: two distinct pathways to neuronal injury. Neuroscientist. 2005;11:50–62. doi: 10.1177/1073858404271087. [DOI] [PubMed] [Google Scholar]
- 56.Chan SL, et al. Presenilin-1 mutations sensitize neurons to DNA damage-induced death by a mechanism involving perturbed calcium homeostasis and activation of calpains and caspase-12. Neurobiol Dis. 2002;11:2–19. doi: 10.1006/nbdi.2002.0542. [DOI] [PubMed] [Google Scholar]
- 57.Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann Neurol. 2003;53(3):S61–70. doi: 10.1002/ana.10489. [DOI] [PubMed] [Google Scholar]
- 58.Crocker SJ, et al. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gafni J, Ellerby LM. Calpain activation in Huntington's disease. J Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 61.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 62.Glazner GW, et al. Endoplasmic reticulum D-myo-inositol 1,4,5-trisphosphate-sensitive stores regulate nuclear factor-kappaB binding activity in a calcium-independent manner. J Biol Chem. 2001;276:22461–22467. doi: 10.1074/jbc.M101315200. [DOI] [PubMed] [Google Scholar]
- 63.Milhavet O, et al. Involvement of Gadd153 in the pathogenic action of presenilin-1 mutations. J Neurochem. 2002;83:673–681. doi: 10.1046/j.1471-4159.2002.01165.x. [DOI] [PubMed] [Google Scholar]
- 64.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 65.Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 66.Kruman I, et al. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruckner SR, Perry G, Estus S. 4-hydroxynonenal contributes to NGF withdrawal-induced neuronal apoptosis. J Neurochem. 2003;85:999–1005. doi: 10.1046/j.1471-4159.2003.01749.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cutler RG, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Links age-related alterations in the metabolism of membrane lipids to oxidative stress and neuronal degeneration in Alzheimer's disease.
- 70.Haughey NJ, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 71.Cutler RG, et al. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- 72.White LD, Barone S., Jr Qualitative and quantitative estimates of apoptosis from birth to senescence in the rat brain. Cell Death Differ. 2001;8:345–356. doi: 10.1038/sj.cdd.4400816. [DOI] [PubMed] [Google Scholar]
- 73.Hiona A, Leeuwenburgh C. Life-long caloric restriction counteracts apoptotic effects of aging in the brain and bolsters the action of apoptosis inhibitors. Neurobiol Aging. Sep 26; doi: 10.1016/j.neurobiolaging.2005.08.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 74.Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that age-related declines in hippocampal neurogenesis can be counteracted by cognitive stimulation.
- 75.Sun W, et al. Programmed cell death of adult-generated hippocampal neurons is mediated by the proapoptotic gene Bax. J Neurosci. 2004;24:11205–11213. doi: 10.1523/JNEUROSCI.1436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haughey NJ, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 77.Jin K, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 79.Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 80.Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990;323:1631–1633. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- 81.Kisby GE, Ellison M, Spencer PS. Content of the neurotoxins cycasin (methylazoxymethanol beta-D-glucoside) and BMAA (beta-N-methylamino-L-alanine) in cycad flour prepared by Guam Chamorros. Neurology. 1992;42:1336–1340. doi: 10.1212/wnl.42.7.1336. [DOI] [PubMed] [Google Scholar]
- 82.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]; First direct evidence that an environmental neurotoxin can cause Parkinson's disease.
- 83.Panov A, et al. Rotenone model of Parkinson Disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005;280:42026–42035. doi: 10.1074/jbc.M508628200. [DOI] [PubMed] [Google Scholar]
- 84.Ludolph AC, He F, Spencer PS, Hammerstad J, Sabri M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can J Neurol Sci. 1991;18:492–498. doi: 10.1017/s0317167100032212. [DOI] [PubMed] [Google Scholar]
- 85.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 86.Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Guo Q, et al. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 88.Schneider I, et al. Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J Biol Chem. 2001;276:11539–11544. doi: 10.1074/jbc.M010977200. [DOI] [PubMed] [Google Scholar]
- 89.Martinez J, Moeller I, Erdjument-Bromage H, Tempst P, Lauring B. Parkinson's disease-associated alpha-synuclein is a calmodulin substrate. J Biol Chem. 2003;278:17379–17387. doi: 10.1074/jbc.M209020200. [DOI] [PubMed] [Google Scholar]
- 90.Tang TS, et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci USA. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith RG, Kimura F, Harati Y, McKinley K, Stefani E, Appel SH. Altered muscle calcium channel binding kinetics in autoimmune motoneuron disease. Muscle Nerve. 1995;18:620–627. doi: 10.1002/mus.880180609. [DOI] [PubMed] [Google Scholar]
- 92.Von Lewinski F, Keller BU. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 2005;28:494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Mattson MP, Rychlik B, Chu C, Christakos S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 94.Magloczky Z, Freund TF. Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience. 1993;56:317–335. doi: 10.1016/0306-4522(93)90334-c. [DOI] [PubMed] [Google Scholar]
- 95.Guela C, et al. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003;455:249–259. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- 96.Thorns V, Licastro F, Masliah E. Locally reduced levels of acidic FGF lead to decreased expression of 28-kda calbindin and contribute to the selective vulnerability of the neurons in the entorhinal cortex in Alzheimer's disease. Neuropathology. 2001;21:203–211. doi: 10.1046/j.1440-1789.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 97.Iacopino AM, Christakos S. Specific reduction of calcium-binding protein (28-kilodalton calbindin-D) gene expression in aging and neurodegenerative diseases. Proc Natl Acad Sci U S A. 1990;87:4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that age- and neuron-specific changes in calcium-binding proteins are associated with selective neuronal vulnerability.
- 98.Alexianu ME, et al. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- 99.Ikonomovic MD, et al. Distribution of glutamate receptor subunit NMDAR1 in the hippocampus of normal elderly and patients with Alzheimer's disease. Exp Neurol. 1999;160:194–204. doi: 10.1006/exnr.1999.7196. [DOI] [PubMed] [Google Scholar]
- 100.Williams TL, Day NC, Ince PG, Kamboj RK, Shaw PJ. Calcium-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors: a molecular determinant of selective vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1997;42:200–207. doi: 10.1002/ana.410420211. [DOI] [PubMed] [Google Scholar]
- 101.Melov S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 2004;27:601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Petit-Taboue MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- 103.Masconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 104.Dagher A. Functional imaging in Parkinson's disease. Semin Neurol. 2001;21:23–32. doi: 10.1055/s-2001-13116. [DOI] [PubMed] [Google Scholar]
- 105.Feigin A, et al. Metabolic network abnormalities in early Huntington's disease: an [(18)F]FDG PET study. J Nucl Med. 2001;42:1591–1595. [PubMed] [Google Scholar]
- 106.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 107.Smigrodzki R, Parks J, Parker WD. High frequency of mitochondrial complex I mutations in Parkinson's disease and aging. Neurobiol Aging. 2004;25:1273–1281. doi: 10.1016/j.neurobiolaging.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 108.Seong IS, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- 109.Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5:77–87. doi: 10.1016/j.mito.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Brown MR, Geddes JW, Sullivan PG. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J Bioenerg Biomembr. 2004;36:401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- 111.Murchison D, Zawieja DC, Griffith WH. Reduced mitochondrial buffering of voltage-gated calcium influx in aged rat basal forebrain neurons. Cell Calcium. 2004;36:61–75. doi: 10.1016/j.ceca.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 112.Kim GW, Chan PH. Oxidative stress and neuronal DNA fragmentation mediate age-dependent vulnerability to the mitochondrial toxin, 3-nitropropionic acid, in the mouse striatum. Neurobiol Dis. 2001;8:114–126. doi: 10.1006/nbdi.2000.0327. [DOI] [PubMed] [Google Scholar]; Evidence that neuronal vulnerability to mitochondrial toxins increases with advancing age.
- 113.Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J Neurosci Res. 2003;72:527–536. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- 114.Klivenyi P, et al. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keller JN, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 117.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 118.Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- 119.Lustbader JW, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 120.Keller JN, et al. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci. 1998;18:4439–4450. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci U S A. 1998;95:3227–3232. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Panov AV, et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 123.Trushina E, et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klivenyi P, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 125.Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol. 2001;49:561–574. [PubMed] [Google Scholar]; Reviews evidence that a cellular energy deficit plays a key role in the degeneration of motor neurons in HD and ALS.
- 126.Szweda PA, Camouse M, Lundberg KC, Oberley TD, Szweda LI. Aging, lipofuscin formation, and free radical-mediated inhibition of cellular proteolytic systems. Ageing Res Rev. 2003;2:383–405. doi: 10.1016/s1568-1637(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 127.Gray DA, Woulfe J. Lipofuscin and aging: a matter of toxic waste. Sci Aging Knowledge Environ. 2005 Feb 2;(5):re1. doi: 10.1126/sageke.2005.5.re1. [DOI] [PubMed] [Google Scholar]
- 128.Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 129.Bi X, Yong AP, Zhou J, Gall CM, Lynch G. Regionally selective changes in brain lysosomes occur in the transition from young adulthood to middle age in rats. Neuroscience. 2000;97:395–404. doi: 10.1016/s0306-4522(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 130.Keller JN, Gee J, Ding Q. The proteasome in brain aging. Ageing Res Rev. 2002;1:279–293. doi: 10.1016/s1568-1637(01)00006-x. [DOI] [PubMed] [Google Scholar]
- 131.Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev. 2000;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 132.Lam YA, et al. Inhibition of the ubiquitin-proteasome system in Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:9902–9906. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. Failure of the ubiquitin-proteasome system in Parkinson's disease. Nat Rev Neurosci. 2001;2:589–594. doi: 10.1038/35086067. [DOI] [PubMed] [Google Scholar]
- 134.Sullivan PG, et al. Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J Biol Chem. 2004;279:20699–20707. doi: 10.1074/jbc.M313579200. [DOI] [PubMed] [Google Scholar]
- 135.Bednarski E, Ribak CE, Lynch G. Suppression of cathepsins B and L causes a proliferation of lysosomes and the formation of meganeurites in hippocampus. J Neurosci. 1997;17:4006–4021. doi: 10.1523/JNEUROSCI.17-11-04006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 137.Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57:203–208. doi: 10.1016/s0753-3322(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 138.Maggio JE, et al. Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc Natl Acad Sci U S A. 1992;89:5462–5466. doi: 10.1073/pnas.89.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer's beta-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]; Demonstration that subtle variations in the environment of pathogenic peptides modifies their self-propagating and neurotoxic properties.
- 140.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 141.Goedert M. Tau gene mutations and their effects. Mov Disord. 2005;12:S45–S52. doi: 10.1002/mds.20539. [DOI] [PubMed] [Google Scholar]
- 142.Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739:280–297. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 143.Montine TJ, Amarnath V, Martin ME, Strittmatter WJ, Graham DG. E-4-hydroxy-2-nonenal is cytotoxic and cross-links cytoskeletal proteins in P19 neuroglial cultures. Am J Pathol. 1996;148:89–93. [PMC free article] [PubMed] [Google Scholar]
- 144.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 145.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]; The first evidence that a modest increase in the production of α-synuclein is sufficient to cause Parkinson's disease.
- 146.Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bates G. Huntingtin aggregation and toxicity in Huntington's disease. Lancet. 2003;361:1642–1644. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- 148.Fox JH, et al. Cystamine increases L-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J Neurochem. 2004;91:413–422. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- 149.Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 150.Giffard RG, et al. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004;207:3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- 151.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 152.Zhao L, Longo-Guess C, Harris BS, Lee JW, Ackerman SL. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet. 2005;37:974–979. doi: 10.1038/ng1620. [DOI] [PubMed] [Google Scholar]
- 153.Rousseau E, Dehay B, Ben-Haiem L, Trottier Y, Morange M, Bertolotti A. Targeting expression of expanded polyglutamine proteins to the endoplasmic reticulum or mitochondria prevents their aggregation. Proc Natl Acad Sci U S A. 2004;101:9648–9653. doi: 10.1073/pnas.0403015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kieran D, et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]; Pharmacological induction of protein chaperones results in a therapeutic benefit in a mouse model of ALS.
- 155.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 156.Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94:4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005;64:263–272. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- 158.Gash DM, Zhang Z, Gerhardt G. Neuroprotective and neurorestorative properties of GDNF. Ann Neurol. 1998;44:S121–125. doi: 10.1002/ana.410440718. [DOI] [PubMed] [Google Scholar]
- 159.Ross CA. Huntington's disease: new paths to pathogenesis. Cell. 2004;118:4–7. doi: 10.1016/j.cell.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 160.Wilczak N, de Keyser J. Insulin-like growth factor system in amyotrophic lateral sclerosis. Endocr Dev. 2005;9:160–169. doi: 10.1159/000085764. [DOI] [PubMed] [Google Scholar]
- 161.Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 162.Gooney M, Messaudi E, Maher FO, Bramham CR, Lynch MA. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiol Aging. 2004;25:1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 163.Monti B, Berteotti C, Contestabile A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus. 2005;15:1041–1049. doi: 10.1002/hipo.20099. [DOI] [PubMed] [Google Scholar]
- 164.Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 165.Yurek DM, Fletcher-Turner A. Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp Neurol. 2000;161:392–396. doi: 10.1006/exnr.1999.7274. [DOI] [PubMed] [Google Scholar]
- 166.Dore S, Kar S, Rowe W, Quirion R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience. 1997;80:1033–1040. doi: 10.1016/s0306-4522(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 167.D'Costa AP, Xu X, Ingram RL, Sonntag WE. Insulin-like growth factor-1 stimulation of protein synthesis is attenuated in cerebral cortex of aging rats. Neuroscience. 1995;65:805–813. doi: 10.1016/0306-4522(94)00495-q. [DOI] [PubMed] [Google Scholar]
- 168.Woods AG, Guthrie KM, Kurlawalla MA, Gall CM. Deafferentation-induced increases in hippocampal insulin-like growth factor-1 messenger RNA expression are severely attenuated in middle aged and aged rats. Neuroscience. 1998;83:663–668. doi: 10.1016/s0306-4522(97)00539-3. [DOI] [PubMed] [Google Scholar]; Evidence that the ability of brain cells to increase neurotrophic factor signaling is impaired during aging.
- 169.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 170.Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Martinez-Serrano A, Fischer W, Bjorklund A. Reversal of age-dependent cognitive impairments and cholinergic neuron atrophy by NGF-secreting neural progenitors grafted to the basal forebrain. Neuron. 1995;15:473–484. doi: 10.1016/0896-6273(95)90051-9. [DOI] [PubMed] [Google Scholar]; Demonstration that it is possible to recover age-dependent cognitive deficits by transplantation of neurotrophic factor-producing cells into the basal forebrain.
- 172.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 174.Scott SA, Crutcher KA. Nerve growth factor and Alzheimer's disease. Rev Neurosci. 1994;5:179–211. doi: 10.1515/revneuro.1994.5.3.179. [DOI] [PubMed] [Google Scholar]
- 175.Zuccato C, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]; First evidence that mutant huntingtin perturbs transcriptional regulation of neurotrophic factor production.
- 176.Duan W, et al. Paroxetine retards disease onset and progression in Huntingtin mutant mice. Ann Neurol. 2004;55:590–594. doi: 10.1002/ana.20075. [DOI] [PubMed] [Google Scholar]
- 177.Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6(1):204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Al-Chalabi A, Miller CC. Neurofilaments and neurological disease. Bioessays. 2003;25:346–355. doi: 10.1002/bies.10251. [DOI] [PubMed] [Google Scholar]
- 179.Trojanowski JQ, Lee VM. The role of tau in Alzheimer's disease. Med Clin North Am. 2002;86:615–627. doi: 10.1016/s0025-7125(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 180.Norris EH, Giasson BI. Role of oxidative damage in protein aggregation associated with Parkinson's disease and related disorders. Antioxid Redox Signal. 2005;7:672–684. doi: 10.1089/ars.2005.7.672. [DOI] [PubMed] [Google Scholar]
- 181.O'Neill C, et al. Dysfunctional intracellular calcium homoeostasis: a central cause of neurodegeneration in Alzheimer's disease. Biochem Soc Symp. 2001;67:177–194. doi: 10.1042/bss0670177. [DOI] [PubMed] [Google Scholar]
- 182.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 183.Gauthier LR, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 184.Zhang B, Tu P, Abtahian F, Trojanowski JQ. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]; Direct evidence that a mutation that causes ALS impairs axonal transport in motor neurons.
- 185.Hutton M. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 186.Ishihara T, et al. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 187.Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta. 2005;1739:331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 188.McGeer PL, McGeer EG. Local neuroinflammation and the progression of Alzheimer's disease. J Neurovirol. 2002;8:529–538. doi: 10.1080/13550280290100969. [DOI] [PubMed] [Google Scholar]
- 189.Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 190.Eikelenboom P, Hack CE, Rozemuller JM, Stam FC. Complement activation in amyloid plaques in Alzhiemer's dementia. Virchows Arch. 1989;56:259–262. doi: 10.1007/BF02890024. [DOI] [PubMed] [Google Scholar]
- 191.Itagaki S, McGeer PL, Akiyama H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer's disease brain tissue. Neurosci Lett. 1988;91:259–264. doi: 10.1016/0304-3940(88)90690-8. [DOI] [PubMed] [Google Scholar]
- 192.Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol (Berl) 1982;57:239–242. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- 193.Nath A, et al. Autoantibodies to amyloid beta-peptide (Abeta) are increased in Alzheimer's disease patients and Abeta antibodies can enhance Abeta neurotoxicity: implications for disease pathogenesis and vaccine development. Neuromolecular Med. 2003;3:29–39. doi: 10.1385/nmm:3:1:29. [DOI] [PubMed] [Google Scholar]
- 194.Schenk D, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]; First evidence that the immune system can be activated so as to remove amyloid from the brain, suggesting a potential for immunization therapies in the treatment of AD.
- 195.Teismann P, Schulz JB. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–161. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- 196.Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241–253. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- 197.Smith RG, Alexianu ME, Crawford G, Nyormoi O, Stefani E, Appel SH. Cytotoxicity of immunoglobulins from amyotrophic lateral sclerosis patients on a hybrid motoneuron cell line. Proc Natl Acad Sci U S A. 1994;91:3393–3397. doi: 10.1073/pnas.91.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:191–206. [PubMed] [Google Scholar]
- 199.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 200.Wang J, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- 201.Lazarov O, et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]; First evidence that exercise/cognitive stimulation can enhance clearance of Aβ from the brain.
- 202.Lim GP, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.DeKosky ST. Statin therapy in the treatment of Alzheimer disease: what is the rationale? Am J Med. 2005;118:S48–53. doi: 10.1016/j.amjmed.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 204.Lipton SA. Paradigm shift in NMDA receptor antagonist drug development: molecular mechanism of uncompetitive inhibition by memantine in the treatment of Alzheimer's disease and other neurologic disorders. J Alzheimers Dis. 2004;6:S61–74. doi: 10.3233/jad-2004-6s610. [DOI] [PubMed] [Google Scholar]
- 205.Duan W, et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 206.Bae BI, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 207.Ellis AC, Rosenfeld J, Ellis AC, Rosenfeld J. The role of creatine in the management of amyotrophic lateral sclerosis and other neurodegenerative disorders. CNS Drugs. 2004;18:967–980. doi: 10.2165/00023210-200418140-00002. [DOI] [PubMed] [Google Scholar]
- 208.Kordower JH, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]; Demonstration of efficacy of gene therapy in a non-human primate model of Parkinson's disease.
- 209.Dobrowolny G, et al. Muscle expression of a local IGF-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Levy YS, Gilgun-Sherki Y, Melamed E, Offen D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. BioDrugs. 2005;19:97–127. doi: 10.2165/00063030-200519020-00003. [DOI] [PubMed] [Google Scholar]
- 211.Gelinas DS, DaSilva K, Fenili D, St Georger-Hyslop P, McLaurin J. Immunotherapy for Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:S14657–14662. doi: 10.1073/pnas.0404866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Harper SQ, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann Neurol. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- 214.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 215.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol. 2004;3:579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 216.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 217.Carro E, Trejo JL, Busiguina S, Torres-Aleman J. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neuro sci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Laurin D, Verrault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 219.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 220.Jeffery B, Barlow T, Moizer K, Paul S, Boyle C. Amnestic shellfish poison. Food Chem Toxicol. 2004;42:545–557. doi: 10.1016/j.fct.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 221.Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 222.Di Monte DA. The environment and Parkinson's disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]