Abstract
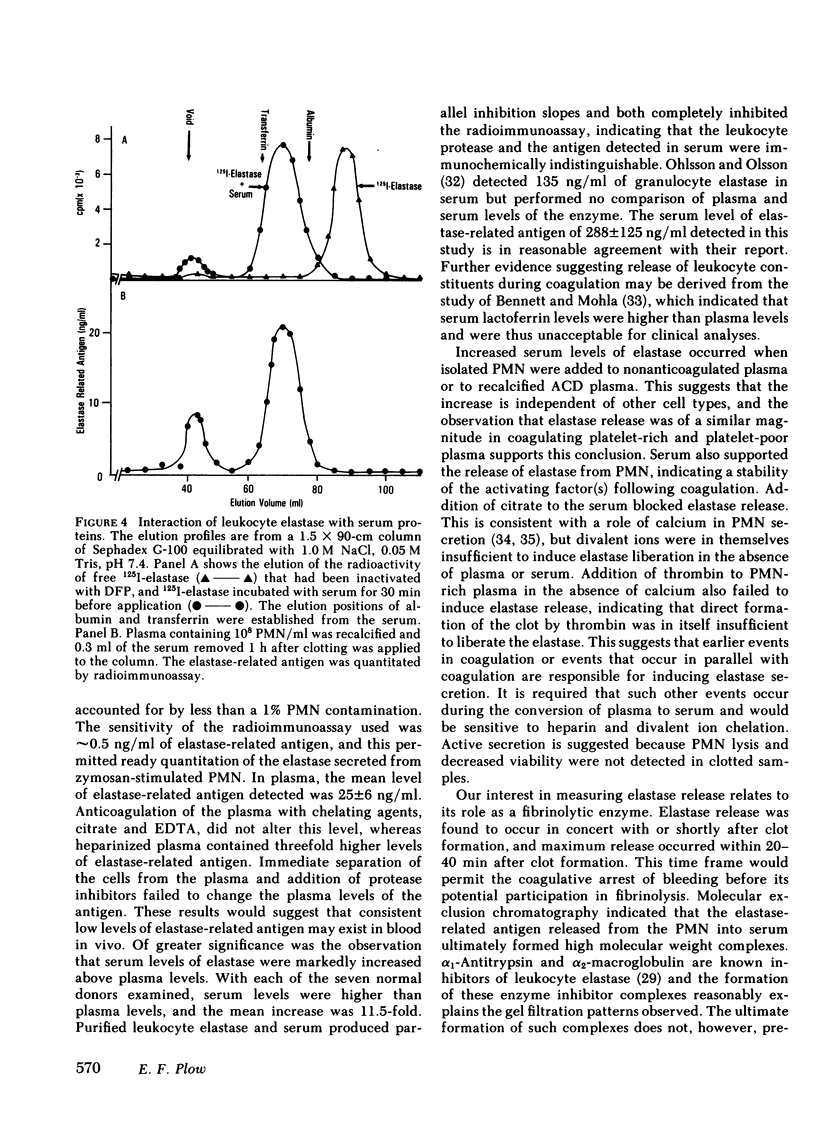
Immunological detection of elastase, an enzyme present within leukocyte granules, has been used as a marker for polymorphonuclear leukocyte activation. Polymorphonuclear leukocytes contained 4.6 μg/107 cells, whereas erythrocytes, mononuclear cells, and platelets contained <1% of this level. In plasma that was separated from blood cells after 1 h at 22°C, the mean level of elastase-related antigen in seven normal donors was 25±6 ng/ml. This level was unaltered by immediate separation of the plasma from the cells, by inclusion of protease inhibitors, or by anticoagulation of the plasma with either EDTA or acidcitrate-dextrose (the level in heparinized plasma was approximately threefold higher). In serum, the level of elastase-related antigen was 288±125 ng/ml, representing an 11.5-fold increase above plasma levels. The antigen detected in serum was immunochemically indistinguishable from the leukocyte enzyme. Release of elastase was observed when isolated polymorphonuclear leukocytes were added to nonanticoagulated platelet-rich or platelet-poor plasma, recalcified plasma, or to serum. Addition of a chelating agent to serum prevented elastase release, but calcium or magnesium did not induce release in the absence of plasma. Coagulation induced by addition of thrombin to plasma also failed to induce release. In whole blood or in anticoagulated plasma reconstituted with polymorphonuclear leukocytes and then recalcified, initial release of elastase occurred concomitantly with or slightly after clotting and reached maximal levels within 20-40 min after clot formation. The data indicates that early events in coagulation or other pathways that occur in parallel with coagulation induce leukocyte release. The release of elastase, a major fibrinolytic protease of leukocytes, from the cells provides a mechanism for this enzyme or other granule proteases to participate in physiological events.
Full text
PDF

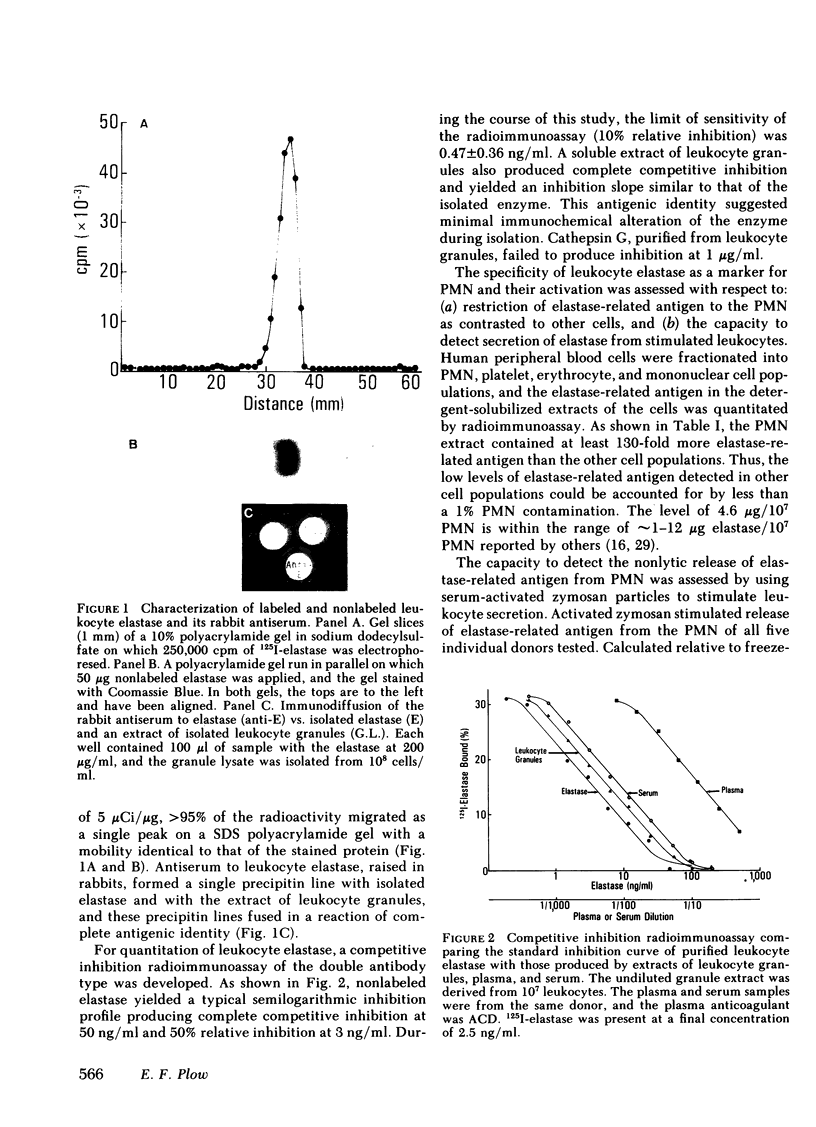



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart M. I. Importance of neutrophilic leukocytes in the resolution of fibrin. Fed Proc. 1965 Jul-Aug;24(4):846–853. [PubMed] [Google Scholar]
- Bennett R. M., Mohla C. A solid-phase radioimmunoassay for the measurement of lactoferrin in human plasma: variations with age, sex, and disease. J Lab Clin Med. 1976 Jul;88(1):156–166. [PubMed] [Google Scholar]
- Bilezikian S. B., Nossel H. L. Unique pattern of fibrinogen cleavage by human leukocyte proteases. Blood. 1977 Jul;50(1):21–28. [PubMed] [Google Scholar]
- CAVIEZEL O., VOLLERY M., VANNOTTI A. FIBRINOLYSE LEUCOCYTAIRE. Schweiz Med Wochenschr. 1964 Jul 18;94:1016–1020. [PubMed] [Google Scholar]
- Chisari F. V., Gerin J. L., Edgington T. S. Immunochemistry of the hepatitis B virus: 125I HB Ag ligand. J Immunol. 1974 Aug;113(2):543–553. [PubMed] [Google Scholar]
- GANS H. FIBRINOLYTIC PROPERTIES OF PROTEASES DERIVED FROM HUMAN, DOG AND RABBIT LEUKOCYTES. Thromb Diath Haemorrh. 1964 Jan 1;10:379–389. [PubMed] [Google Scholar]
- Gay R. J., McComb R. B., Bowers G. N., Jr Optimum reaction conditions for human lactate dehydrogenase isoenzymes as they affect total lactate dehydrogenase activity. Clin Chem. 1968 Aug;14(8):740–753. [PubMed] [Google Scholar]
- Gramse M., Bingenheimer C., Schmidt W., Egbring R., Havemann K. Degradation products of fibrinogen by elastase-like neutral protease from human granulocytes. Characterization and effects on blood coagulation in vitro. J Clin Invest. 1978 Apr;61(4):1027–1033. doi: 10.1172/JCI109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRY R. L. LEUKOCYTES AND THROMBOSIS. Thromb Diath Haemorrh. 1965 Mar 15;13:35–46. [PubMed] [Google Scholar]
- Hermann G., Miescher P. A. Differentiation of leukocytic fibrinolytic enzymes from plasmin by the use of plasmatic proteolytic inhibitors. Int Arch Allergy Appl Immunol. 1965;27(6):346–354. doi: 10.1159/000229618. [DOI] [PubMed] [Google Scholar]
- Hornebeck W., Starkey P. M., Gordon J. L., Legrand Y., Pignaud G., Robert L., Caen J. P., Ehrlich H. P., Barrett A. J. The elastase-like enzyme of platelets. Thromb Haemost. 1980 Feb 29;42(5):1681–1683. [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Moroz L. A. Mini-plasminogen: a mechanism for leukocyte modulation of plasminogen activation by urokinase. Blood. 1981 Jul;58(1):97–104. [PubMed] [Google Scholar]
- Nachman R. L., Ferris B. Studies on human platelet protease activity. J Clin Invest. 1969 Nov;47(11):2530–2540. doi: 10.1172/JCI105935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Olsson A. S. Immunoreactive granulocyte elastase in human serum. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1531–1539. doi: 10.1515/bchm2.1978.359.2.1531. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of two granulocyte collagenases. Eur J Biochem. 1973 Jul 16;36(2):473–481. doi: 10.1111/j.1432-1033.1973.tb02932.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K. Properties of leucocytic protease. Clin Chim Acta. 1971 May;32(3):399–405. doi: 10.1016/0009-8981(71)90441-4. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. An alternative pathway for fibrinolysis. I. The cleavage of fibrinogen by leukocyte proteases at physiologic pH. J Clin Invest. 1975 Jul;56(1):30–38. doi: 10.1172/JCI108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. Comparative immunochemical characterization of products of plasmin and leukocyte protease cleavage of human fibrinogen. Thromb Res. 1978 Apr;12(4):653–665. doi: 10.1016/0049-3848(78)90255-4. [DOI] [PubMed] [Google Scholar]
- Plow E. F. The major fibrinolytic proteases of human leukocytes. Biochim Biophys Acta. 1980 Jun 5;630(1):47–56. doi: 10.1016/0304-4165(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Plow E., Edgington T. S. Immunobiology of fibrinogen. Emergence of neoantigenic expressions during physiologic cleavage in vitro and in vivo. J Clin Invest. 1973 Feb;52(2):273–282. doi: 10.1172/JCI107183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C. F., Travis J. The degradation of human lung elastin by neutrophil proteinases. Biochim Biophys Acta. 1980 Jan 24;621(1):147–157. doi: 10.1016/0005-2795(80)90070-7. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Barrett A. J. Identification of proteinases in rheumatoid synovium. Detection of leukocyte elastase cathepsin G and another serine proteinase. Biochim Biophys Acta. 1980 Sep 9;615(1):167–177. doi: 10.1016/0005-2744(80)90020-0. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Egbring R., Havemann K. Effect of elastase-like and chymotrypsin-like neutral proteases from human granulocytes on isolated clotting factors. Thromb Res. 1975 Apr;6(4):315–329. doi: 10.1016/0049-3848(75)90081-x. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. The effects of extracellular K+, Na+ and Ca++ on lysosomal enzyme secretion from polymorphonuclear leukocytes. J Immunol. 1977 Sep;119(3):804–811. [PubMed] [Google Scholar]
- Taylor J. C., Crawford I. P., Hugli T. E. Limited degradation of the third component (C3) of human complement by human leukocyte elastase (HLE): partial characterization of C3 fragments. Biochemistry. 1977 Jul 26;16(15):3390–3396. doi: 10.1021/bi00634a016. [DOI] [PubMed] [Google Scholar]
- Taylor J. C., Crawford I. P. Purification and preliminary characterization of human leukocyte elastasel. Arch Biochem Biophys. 1975 Jul;169(1):91–101. doi: 10.1016/0003-9861(75)90320-3. [DOI] [PubMed] [Google Scholar]
- Visser L., Blout E. R. The use of p-nitrophenyl N-tert-butyloxycarbonyl-L-alaninate as substrate for elastase. Biochim Biophys Acta. 1972 Apr 7;268(1):257–260. doi: 10.1016/0005-2744(72)90223-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weissmann G., Korchak H. M., Perez H. D., Smolen J. E., Goldstein I. M., Hoffstein S. T. The secretory code of the neutrophil. J Reticuloendothel Soc. 1979 Dec;26(Suppl):687–700. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintroub B. U., Coblyn J. S., Kaempfer C. E., Austen K. F. Cleavage of fibrinogen by the human neutrophil neutral peptide-generating protease. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5448–5452. doi: 10.1073/pnas.77.9.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]