Summary
1. Acute kidney injury (AKI) puts a major burden on health systems that may arise from multiple initiating insults, including ischemia-reperfusion injury, cardiovascular surgery, radio-contrast administration as well as sepsis. Similarly, the incidence and prevalence of chronic kidney disease (CKD) continues to increase with significant morbidity and mortality. Moreover, an increasing number of AKI patients survive to develop CKD and end-stage kidney disease (ESRD).
2. Although the mechanisms for development of AKI and progression of CKD remain poorly understood, initial impairment of oxygen balance is likely to constitute a common pathway, causing renal tissue hypoxia and ATP starvation that will in turn induce extracellular matrix production, collagen deposition and fibrosis. Thus, possible future strategies for one or both conditions may involve dopamine, loop-diuretics, inducible nitric oxide synthase inhibitors and atrial natriuretic peptide, substances that target kidney oxygen consumption and regulators of renal oxygenation such as nitric oxide and heme oxygenase-1.
Keywords: AKI, CKD, glomerular filtration rate, nitric oxide, oxygenation, oxygen consumption, renal blood flow
Introduction
Acute kidney injury (AKI) may result from a variety of insults, e.g. ischemia-reperfusion (IR) injury, cardiovascular surgery, radio-contrast administration and sepsis. It has been estimated that AKI develops in ~5% of hospitalized patients1, and the risk is increased to 15-30% in high-risk post-operative patients.2, 3 Specifically, IR-induced AKI affects 5-20% of patients admitted to the intensive care unit and can be a major complication after kidney transplantation.4-6 Chronic kidney disease (CKD) is also associated with significant morbidity, mortality and health care expenditure. It has a high prevalence worldwide and the prevalence of CKD continues to increase with increasing incidence of its precursors such as diabetes, hypertension, cardiovascular disease, and AKI.
To compound the problem, therapeutic options for the treatment of AKI and CKD remain inadequate. Treatments targeting currently known mechanisms of disease have not been very successful in improving outcomes. Lately, alteration in renal oxygenation has been identified as a common pathway to the development and progression of disease in both acute and chronic renal injury. In this review, we outline the available data on renal oxygenation in AKI and CKD and discuss possible future treatment strategies.
Kidney oxygenation
For optimal function of intracellular organelles, cells require continuous ATP generation. Both oxidative and glycolytic pathways produce ATP, but oxidative phosphorylation is more efficient (38 molecules of ATP per mole of glucose vs. 2 molecules of ATP with glycolysis). Hence, most cells have an incessant need for oxygen to generate ATP via mitochondrial oxidative phosphorylation. While lack of oxygen supply is a definite cellular stress, excess oxygen can also lead to oxidant damage.7 Hence, tight regulation of tissue oxygenation to prevent hypoxia or hyperoxia is critical for survival.
At the whole body level, the cardiovascular system controls the blood flow and hence oxygen delivery to individual organs, but all organs possess local mechanisms to regulate blood flow.8, 9 Local autoregulation is one such mechanism and refers to the stabilization of blood flow in the presence of altered arterial or perfusion pressure. Autoregulation occurs in nearly all vascular beds, but is prominent in the kidney and brain. In the kidney, myogenic and tubuloglomerular feedback mediate autoregulation of renal blood flow (RBF).10 In addition, metabolic regulation of blood flow also occurs in tissues. This refers to regulation of blood flow by vasoactive end products of metabolism when increased metabolic activity augments oxygen demand.11 Unlike other organs where increments in blood flow uniformly improves oxygenation by increasing oxygen supply, increased RBF augments glomerular filtration rate (GFR) and the filtered reabsorptive load. This further increases oxygen demand, due to a linear relationship between tubular transport of filtered sodium (TNa) and oxygen consumption (QO2).12 So, in the kidneys, an increased oxygen supply simultaneously increases oxygen demand and may not improve net oxygenation, unless GFR and RBF are dissociated.
Determinants of kidney tissue oxygen tension (pO2) include oxygen in arterial blood, oxygen consumed by the cells and arterial-to-venous oxygen shunting, which describes the diffusion of oxygen from preglomerular arteries to post-glomerular veins without being available to the cell for consumption.13 The kidney enjoys a high blood flow, nearly 25% of the cardiac output, which is needed to sustain GFR. Compared to other major body organs, renal QO2 per gram of tissue is high, second only to the heart (2.7 mmol/kg/min vs. 4.3 mmol/kg/min for the heart).14 This is largely driven by the high RBF, since renal oxygen extraction (RO2Ex) is low, presumably to prevent hyperoxia. It has been hypothesized that renal arterial-to-venous O2 shunting is an adaptation to prevent hyperoxia due to high renal perfusion needed to sustain GFR.13
A peculiarity in the kidney is the inhomogeneous blood supply. The cortex receives nearly 20% of the cardiac output with a tissue pO2 of 50-60 mmHg, while the blood supply to the medulla is limited to just 5-10% of the total RBF with a tissue pO2 of 10-20 mmHg. (38) The low blood flow is necessary to preserve medullary osmotic gradients for urinary concentration, but leaves the medullary tissue at the brink of hypoxia. The high metabolic requirements of the medullary thick ascending limbs (mTAL) also contribute to the low tissue pO2 in this region. The oxygen costs of sodium (Na) reabsorption increase along the nephron with the proximal tubule being most metabolically efficient. Although the bulk of Na transport occurs in the proximal tubule, only 60% of this is active transport. The mTALs are the site of significant active Na transport, needed to generate the osmotic gradients and have higher Na/K-ATPase activity than the proximal tubules. The metabolic energy used per milligram of protein is 50% higher in the TAL than in proximal tubules, and is further increased in the distal tubule.15 Increase in Na transport in the TAL without an increase in oxygen supply can aggravate medullary hypoxia. Brezis et al have clearly demonstrated that inhibition of Na transport in the TAL and proximal tubule by specific diuretics increased pO2 in the medulla and cortex respectively.16 Hence, tubular transport and QO2 are the principal determinants of intrarenal oxygenation.
Regulation of renal oxygenation
Nitric oxide (NO) is a major regulator of microvascular oxygen supply and QO2. NO increases RBF via vasodilation and hence oxygen delivery. Laycock et al have also showed that non-specific NOS blockade reduces GFR and TNa, and augments renal QO2 in dogs. Inhibition of neuronal NO synthase (nNOS/NOS-1) increases QO2 both in vivo and in freshly harvested proximal tubules in vitro.17, 18 These studies and other contemporary work suggest that NO acts as a “brake” on oxidative metabolism at various sites, including direct competition with oxygen for mitochondrial respiration and inhibition of cytochrome c oxidase.18-21 This implies a basal modulatory role for NO on QO2. Decreased NO bioavailability is thought to contribute to the high renal QO2 in various experimental pathophysiological conditions e.g. diabetes, hypertension and progressive renal disease.17, 22-24 Angiotensin II (Ang II) also significantly impacts renal oxygenation and appears to be antagonistic to the effects of NO. It induces renal vasoconstriction, lowering oxygen delivery and increases QO2 as observed in various high Ang II states such as 2-kidney, 1-clip Goldblatt hypertensive rats, Ang II infusion and in subtotal nephrectomy (STN), a classic CKD model of nephron mass reduction.24-28 Inhibition of Ang II with ACE-inhibitors and receptor blockers lower the elevated renal QO2. In addition, kidney ischemia may itself play a key role in activation of the renin-Ang II system,29 which may further implicate it in the pathogenesis of CKD.
Recently, the role of hypoxia inducible factor-1α (HIF-1α), a master regulator of cellular hypoxia response, has received significant attention. Downstream HIF-1 target proteins have important functions in renal physiology including vasomotor regulation (inducible iNOS/NOS-2 and heme oxygenase-1 (HO-1), angiogenic growth (vascular endothelial growth factor), energy metabolism (glucose transporters and key glycolytic enzymes) and cell proliferation and survival.30 HIF-1α also has significant effects on mitochondrial metabolism.31 It suppresses mitochondrial QO2 by diminishing NADH supply to the electron transport chain (ETC).32, 33 It induces a subunit switch in complex IV of ETC to optimize its efficiency in hypoxia.33 Finally, it represses mitochondrial biogenesis and induces mitochondrial autophagy as an adaptive metabolic response to prevent increased levels of reactive oxygen species and cell death in hypoxia.34 Inherent HIF-1α expression has been observed in early STN 35 and diabetic kidney.36, 37 but activation appears to be sub-maximal, as the activity can be further augmented by various measures. Pharmacological HIF induction by cobalt chloride and dimethyloxalyglycine (DMOG) significantly improved the high QO2/TNa ratio in early STN kidney along with significant increments in RBF and GFR.38 The specific mechanisms underlying this response remain to be investigated. Thus, the HIF transcription complex is expressed in the kidney in hypoxic conditions and induces several target proteins that impact O2 delivery and consumption. The significance of the regulation of renal oxygenation by HIF in pathophysiological conditions is a novel area of investigation and is being increasingly explored.
Acute kidney injury and renal oxygenation in cardiovascular surgery
In patients undergoing major cardiovascular surgery, even minor changes in serum creatinine are associated with increased in-patient mortality.39 Postoperative AKI in this group of patients is considered a consequence of impaired renal oxygen delivery, in turn caused by intra-operative hypotension and hemodilution-induced anaemia,40 as well as perioperative low cardiac output.3 Particularly the outer portion of the medulla is sensitive to ischemia, which is in turn explained by the high QO2 of the renal medulla due to concentration mechanisms.41
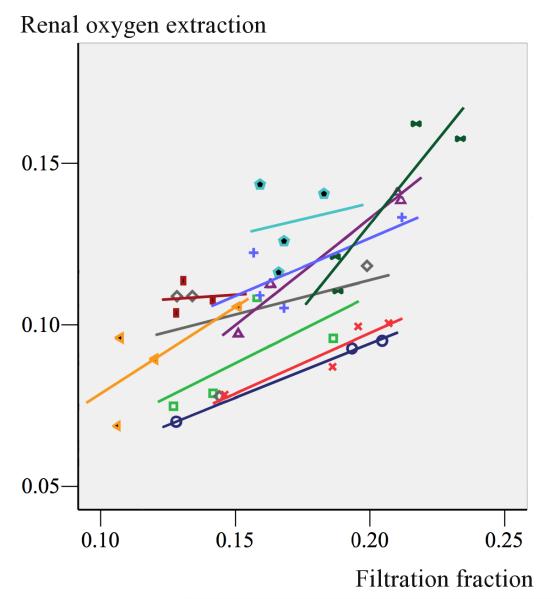
In patients undergoing cardiovascular surgery, it is possible to perform rapid bedside estimations of RBF, GFR, QO2 and renal oxygenation (renal oxygen supply/demand) by the use of the retrograde renal vein thermodilution technique and renal extraction of 51chromium–ethylenediaminetetraacetic acid (51Cr–EDTA).(39-41) From these studies it has been shown that TNa stays as the major determinant of renal QO2 as under normal conditions,42 and in postoperative patients, there is a close correlation between GFR, TNa and QO2,42-44 Thus, manoeuvres that increase GFR and sodium load to the tubules may potentially impair medullary oxygenation and vice versa. In uncomplicated postoperative patients, agents like atrial natriuretic peptide (ANP),43 mannitol,42 and vasopressin44 augment renal filtration fraction and RO2Ex. But the increase in GFR and consequently renal QO2 is not matched by a proportional increase in RBF. The close positive correlation between filtration fraction and renal oxygen extraction after administration of mannitol in post-cardiac surgery patients is shown in Figure 1.
Figure 1.
Shows the close correlation between renal filtration fraction and renal oxygen extraction in individual uncomplicated post-cardiac surgery patients treated with mannitol. Mannitol induced a de-swelling effect of renal tubules which increased GFR and renal QO2 with no effect on RBF. By permission from Redfors et al. 42
Renal oxygenation is impaired in postoperative ischemic acute kidney injury
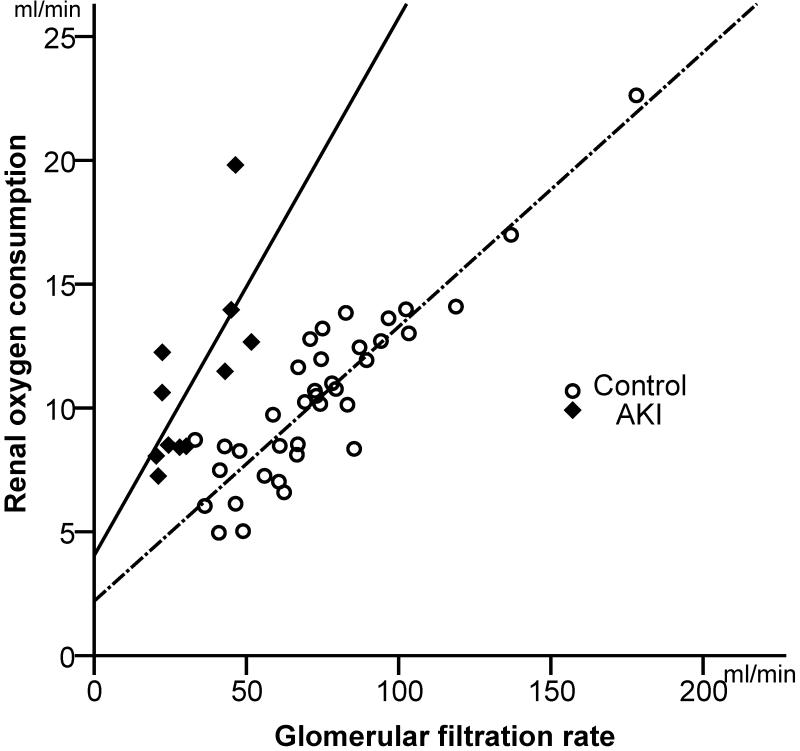
In patients with AKI, data on renal QO2, RBF, GFR and renal oxygenation, i.e. the renal oxygen supply-demand relationship, are lacking and current views on renal oxygenation are presumptive and largely based on experimental studies. Recently, it was shown that renal QO2 is approximately 10-12 ml/min in the postoperative sedated, mechanically ventilated patients,45 a value, which is slightly lower than previously reported in conscious healthy volunteers.46 These patients consumed a mean of 0.82 ml/mmol QO2/TNa, which is similar to findings from animal studies.47 In contrast, in high-risk cardiac surgery complicated by AKI, patients consumed 1.9 ml/mmol QO2/TNa. Thus, the AKI group consumed 2.4 times more oxygen than the non-AKI group to reabsorb the same amount of sodium. Figure 2 shows the close correlation between GFR and renal QO2 in patients with early AKI after cardiac surgery vs. those undergoing uncomplicated surgery. It also demonstrates that the resetting of the relationship between GFR and QO2 in clinical ischemic AKI is accompanied by a severe impairment of the renal oxygen demand/supply relationship, as demonstrated by 70% higher RO2Ex, in the presence of pronounced vasoconstriction and hypoperfusion but a similar QO2, compared to controls (Table 1).45 One can only speculate about the mechanisms underlying the increased QO2/TNa in AKI patients. A potential explanation could be the loss of epithelial cell-polarisation and tight junction integrity in AKI, as has been shown in experimental studies and after human renal transplantation,47, 48 making TNa less efficient.49 Another explanation may be diminished renal NO generation due to endothelial damage and down-regulation of endothelial NOS (eNOS/NOS-3) impairing basal NO modulation of QO2 18, 50
Figure 2.
Shows the close correlation between GFR and renal QO2 in uncomplicated post-cardiac surgery patients (controls, n= 37)) and in patients with postoperative acute kidney injury (AKI). Note that the slope of the regression line is steeper in the AKI group. Thus filtration and reabsorption of sodium consumes approximately 2.5 times more oxygen in the AKI group. By permission from Redfors et al.96
Table 1.
Renal perfusion, filtration and oxygenation in postoperative AKI.
| Control group (n=37) |
AKI group (n=12) |
p-value | |
|---|---|---|---|
| Mean arterial pressure (mmHg) | 73.9 ± 1.1 | 73.5 ± 0.7 | ns |
| Cardiac index (L/min/m2) | 2.63 ± 0.08 | 2.77 ± 0.16 | ns |
| RBF (ml/min) | 758 ± 40 | 477 ± 54 | <0.001 |
| RVR (mmHg/ml/min) | 0.097 ± 0.005 | 0.146 ± 0.015 | <0.01 |
| GFR (ml/min) | 74.7 ± 4.7 | 32.3 ± 3.6 | <0.001 |
|
Sodium reabsorption
(mmol/min) |
9.7 ± 0.7 | 4.0 ± 0.4 | <0.001 |
| Renal QO2 (ml/min) | 10.4 ± 0.6 | 11.0 ± 1.1 | ns |
| RO2Ex | 0.097 ± 0.004 | 0.163 ± 0.009 | <0.001 |
RBF, renal blood flow assessed by the thermodilution technique; RVR, renal vascular resistance; GFR, glomerular filtration rate; QO2, oxygen consumption; RO2Ex, renal oxygen extraction. Values are means±SEM.
Acute kidney injury and renal oxygenation in ischemia-reperfusion
IR injury is the leading cause of AKI, affects 5-20% of patients admitted to the intensive care unit and brings in its trail up to 50% mortality.4, 5 It constitutes a major setback after kidney transplantation, and can also develop from congestive heart failure, sepsis, renal artery stenosis or following shock from any cause.6, 51, 52
In 2010, Oostendorp et al. used MRI to demonstrate reduced oxygenation accompanied by impaired RBF and GFR after IR.53 In kidney transplantation, during the ischemic phase, renal oxygen delivery is completely disrupted and the kidney becomes anoxic. However, especially in the medulla, renal oxygen delivery is also reduced during the subsequent reperfusion phase, due to vasoconstriction, endothelial congestion, oedema and capillary obstruction.52, 54 QO2 may increase due to ATP depletion, mitochondrial damage and loss of tubular integrity and in the long-term, post-AKI, capillary density is reduced, leading to hypoxia.52, 55, 56 In addition, interstitial fibrosis can increase diffusion distances between blood and tissue.54 All in all, structural and functional changes induce tissue hypoxia and cause a vicious cycle by further worsening kidney damage and hypoxia, and can lead to the development of CKD.55, 56
As with other AKI, hypoxia is believed to be the key pathogenic feature in renal transplantation as well as other causes of IR injury.53, 54, 57, 58 Injury originates from oxygen deficiency, but paradoxically also from restoration of RBF.51 During reperfusion, oxygen shunting and renal arterial resistance may increase, whereas RBF and QO2 appear to decrease.59
Diabetes increases susceptibility to IR injury.6, 60, 61 A possible explanation for this is the renal tissue hypoxia seen in diabetes,23, 62 likely caused by a combination of oxidative stress and glomerular hyperfiltration increasing tubular electrolyte load, resulting in increased TNa and QO2, but without a compensatory increment in oxygen delivery.23, 63, 64 This is also supported by reports that reduction in QO2 protects against IR injury,5 and by reports such as that by Hu et al., proposing crucial involvement of increased oxidative stress in renal IR injury.65
Acute kidney injury as a result of sepsis
In critically ill patients, sepsis-induced kidney injury is the most common form of AKI and is manifested by hemodynamic alterations, inflammation, as well as endothelial and epithelial cell injury.66, 67 The traditional concept that septic AKI is initiated by renal ischemia and vasoconstriction68 has been questioned, as it has been shown that RBF may be increased in early, volume-resuscitated, hyperdynamic septic animals with AKI.69 Thus, it has been hypothesized that other factors than renal oxygenation might contribute to the pathophysiology of septic AKI, e.g. a combination of immunologic, toxic, inflammatory factors and apoptosis that may affect the microvasculature and the tubular cells.70 There is still no data available on RBF, GFR, or renal oxygenation in early clinical sepsis, but recent observations suggest alterations in vasoconstrictor tone of the afferent and efferent arterioles may be crucial in inducing loss of GFR, contributing to pathogenesis of septic AKI.71
Acute kidney injury after radio-contrast administration
Radio-contrast administration can influence renal hemodynamics, subsequently reduce renal tissue oxygenation and renal function. It is known that radio-contrast media increase the risk of perioperative AKI, especially in diabetic patients, and hence should be avoided in conjunction with high-risk procedures such as cardiac surgery.72 It may well be that hypoxia of the renal medulla is the underlying cause of contrast-induced AKI: predisposing conditions such as tubulointerstitial disease and diabetes are distinguished by chronic medullary hypoxia and upregulation of hypoxia-inducible factors, and when Hofmann et al. used blood oxygenation level-dependent magnetic resonance imaging (BOLD-MRI) to study acute alterations in renal tissue oxygenation induced by iodinated radio-contrast media, they were able to demonstrate a significant decrease in medullary oxygenation after iopromide injection.73 No alterations were observed in renal cortex.
Kidney oxygenation in chronic kidney disease
Today, it is estimated that more than 35% of the adult US population with diabetes and more than 20% of the adult population with hypertension suffer from CKD.74 In addition, AKI mortality has increased over the past few decades, and as a consequence, an increasing number of AKI patients now survive to develop CKD and end-stage renal disease. In total, CKD afflicts over 10 million North Americans and inexorably progresses to ESRD. Its impact extends well beyond being a precursor to ESRD, given the associated morbidity, mortality and high risk of cardiovascular disease.75 The notion that disease progression in CKD results from certain common pathways irrespective of underlying aetiology of CKD is well accepted. Lately, experimental evidence suggests that oxygen demand-supply mismatch may be the common final pathway of progression in CKD.76 According to this hypothesis, oxygen imbalances leading to renal tissue hypoxia in turn promote extracellular matrix production, collagen deposition and fibrosis. This chronic hypoxia hypothesis proposed by Norman and Fine and expounded by Nangaku and others has gained significant traction and has been validated by several investigators in experimental and human CKD.76-78 Hence, it is imperative to understand the factors that regulate kidney oxygenation in health and disease.
The major focus of research in validating the chronic hypoxia hypothesis has been on the compromised oxygen supply due to functional and structural changes in CKD impairing blood flow. Functional reduction in RBF due to intrarenal vasoconstriction as a result of increased local Ang II generation and/or low NO activity can contribute to intrarenal hypoxia. Development of anaemia in CKD can also impair oxygen delivery and contribute to hypoxia. Structural lesions that can impair oxygen delivery to the tubules include arteriolar disease due to underlying aetiology of CKD (diabetes, hypertension) and intraglomerular lesions (glomerulonephritis).79 Since peritubular capillaries originate from post-glomerular efferent arteriole, any impairment in glomerular blood flow will impact peritubular blood flow. Finally, density of peritubular capillaries is decreased in several experimental models of kidney disease - glomerulonephritis, remnant kidney, ureteral obstruction and renal artery stenosis.80-82 Human biopsy specimens from progressive kidney disease also demonstrate rarefaction of peritubular capillaries.83, 84 Development of hypoxia due to these structural changes can set-off downstream fibrogenic pathways, leading to interstitial fibrosis, which can further impair oxygen diffusion from capillaries to the tubular cells, thus, aggravating the hypoxia.
Recent findings from experimental models in diabetes, hypertension and CKD suggest that metabolic abnormalities and alterations in oxygen utilization may precede and aggravate decrements in GFR and nephron loss.23, 24, 28 Tubular hypoxia has been demonstrated to precede any structural changes which occur late in the disease.85 In early stages, hemodynamic and metabolic adaptations may increase oxygen demand leading to early hypoxia, which can instigate the above-mentioned structural changes and perpetuate hypoxia. Hence, targeting the determinants and regulators of QO2 at the early stages offers the benefit of developing potential therapeutics to alter the course of disease before any permanent damage sets in.
In experimental CKD, nephron loss provokes hyperfiltration in the remaining nephrons. Hyperfiltration begets hyper-reabsorption, which likely occurs at an increased metabolic price. An increased QO2 factored for TNa or nephron number has been observed in STN.17, 86, 87 Due to the linear relationship between TNa and QO2, a majority of increase in QO2 is presumed to be driven by the increase in TNa, and QO2/TNa has been described as an index of transport efficiency. But, it is possible that the slope of the linear relationship between TNa and QO2 is altered in a diseased kidney. In an isolated perfusion kidney model, Harris et al. found that the 40% increase in TNa could not completely account for all the nearly 3-fold increase in the QO2 per nephron observed in STN at 4 weeks 86. Nath et al. performed in vivo QO2 studies and found similar results in a 3-week STN kidney.87 Increased QO2/TNa has also been observed in even earlier stages in a 1-week STN kidney.85
The lower amounts of TNa for a given amount of oxygen utilized may suggest that TNa is more energetically expensive in the STN kidney, perhaps due to a disproportionate increase in transport in the distal segments with typically higher energy expenditure for TNa compared to the proximal segments. Alternatively, other energy consuming processes in STN, especially cellular hypertrophy, which is prominent in STN, gluconeogenesis or activation of oxidases, have to be invoked to explain the increased QO288, 89. Lastly, a dissociation of oxygen utilization with ATP generation in the mitochondria could also account for some of the increase in QO2.
Treatment and prevention of acute kidney injury and’ chronic kidney disease
Dialysis-dependent AKI causes a three-fold increased risk for ultimately requiring chronic dialysis and AKI should therefore be considered as a risk factor for CKD.90 Furthermore, in cardiac surgery, the risk for CKD progression is linked to the severity of AKI.91 However, the mechanisms underlying AKI and CKD progression remain partially understood, at best, and despite scientific and medical advances, the outcomes have not improved satisfactorily. Treatment strategies which can efficiently prevent or reverse AKI and CKD and their progression to end stage renal disease remain a paramount therapeutic challenge. Existing therapies targeting known mechanisms of disease progression have been inadequate in significantly improving the outcomes, and transplantation aside, supportive, life-long renal replacement therapy remains the obvious conventional therapy, though at tremendous financial costs.
The chronic hypoxia hypothesis offers a promising target for future therapy strategies in AKI and CKD. If hypoxia resulting from oxygen demand-supply mismatch promotes kidney injury, this may well be the common final pathway of progression of CKD.76 In prevention of ischemic AKI, it would seem logical to decrease renal QO2 and/or to increase RBF to improve renal oxygenation.
A common treatment target in patients involves Ang II inhibition. In rats, seven day treatment with Ang II receptor blocker telmisartan prevented IR-induced AKI, possibly by decreasing renal QO2.27, 28, 92 However, for late renal recuperation from ischemia Ang II receptor blockade does not offer long-term protection from IR injury. Moreover, ACE inhibition initially improves renal function, but causes long-term aggravation of hypoxia and oxidative stress.60, 93 Thus, treatment options targeting pathways other that the renin-Ang II system require attention.
Loop diuretics have been shown to decrease tubular metabolic demand and maintain medullary oxygenation.16 Indeed, there are several reports demonstrating the renoprotective effect of furosemide in experimental ischemic AKI,94 and recently, Briguori and colleagues were able to demonstrate effects on prevention of contrast-induced acute kidney injury, as well.95 In post-cardiac surgery patients, Swärd et al. showed that furosemide improved renal oxygenation through a 20-30% decrease in TNa, renal QO2 and RO2Ex with no change in RBF.43 Redfors et al. recently demonstrated that in post-cardiac surgery patients, low-dose dopamine induced a 40-50% increase in RBF with no significant change in GFR or renal QO2.96 Thus, dopamine decreased RO2Ex, which suggests that this agent can be used prophylactically to increase the tolerance to ischemia during perioperative renal hypoperfusion. The use of furosemide or low-dose dopamine for treatment of acute renal dysfunction of various aetiologies is not supported by recent meta-analyses.97, 98 However, the potential preventive effects of furosemide or dopamine on renal outcome have, so far, not been studied in high-risk cardiac surgery patients in a prospective randomised controlled trial.
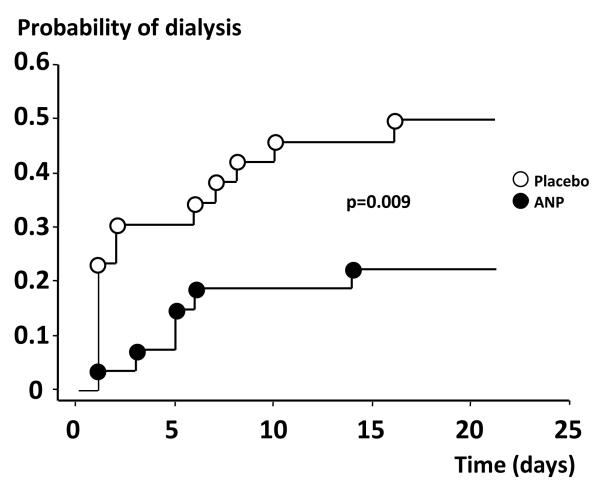
An ideal agent to treat most patients with AKI would be one that increases both RBF and GFR, i.e. an agent that induces a vasodilation of preferentially the preglomerular resistance vessels. Such an agent will not only augment GFR, but also meet the increased renal metabolic demand of the medulla by increases in renal oxygen delivery. In patients with postoperative ischemic AKI, it has been shown that ANP induces a selective dilation of renal afferent arterioles with an increase in both RBF and GFR by approximately 40%,99, 100 in contrast to postoperative uncomplicated patients with no AKI, in whom ANP increased only GFR.43 In addition, in a prospective, randomized, blinded trial, Swärd et al. showed that infusion of ANP at an infusion rate of 50 ng/kg/min, enhanced renal excretory function, decreased the probability of dialysis and improved dialysis-free survival in early, ischemic AKI after complicated cardiac surgery (Fig. 3).101 As of today, clinical treatment of AKI and CKD is still inadequate. It is clear that maintaining GFR is of utmost importance for reducing long-term morbidity and development of ESRD in AKI and CKD, but what mechanisms to target remain largely obscure. However, recent data increasingly supports the hypothesis that early oxygen demand/supply mismatch leading to renal tissue hypoxia is a major culprit in the development of AKI and CKD, and that targeting early events have better odds at succeeding. Thus, preservation of GFR by the prevention or reversal of kidney hypoxia could be considered a major therapeutic avenue for treatment and prevention of AKI.
Figure 3.
Shows the effects of treatment with ANP (50 ng/kg/min) vs. placebo on the probability of dialysis in patients with acute kidney injury after complicated cardiac surgery, By permission from Sward et al.101
Acknowledgments
Sources of Funding This work was supported by the Swedish Research Council, the Jeansson Foundations, NIDDK K08-DK084305 and O’Brien Kidney Center for Acute Kidney Injury P30DK079337 pilot and feasibility grant.
Abbreviations
- AKI
acute kidney injury
- Ang II
angiotensin II
- ANP
atrial natriuretic peptide
- BOLD-MRI
blood oxygenation level-dependent magnetic resonance imaging
- CKD
chronic kidney disease
- DMOG
dimethyloxalyglycine
- ESRD
end-stage kidney disease
- GFR
glomerular filtration rate
- HIF-1α
hypoxia inducible factor-1α
- HO-1
heme oxygenase-1
- IR
ischemia-reperfusion
- NO
nitric oxide
- NOS
nitric oxide synthase
- QO2
oxygen consumption
- pO2
oxygen tension
- RBF
renal blood flow
- RO2Ex
renal oxygen extraction
- RVR
renal vascular resistance
- STN
subtotal nephrectomy
Footnotes
Conflict of Interest Disclosure The authors have no conflict of interest to disclose.
References
- 1.Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J. Am. Soc. Nephrol. 2003;14:2178–87. doi: 10.1097/01.asn.0000079042.13465.1a. [DOI] [PubMed] [Google Scholar]
- 2.Heringlake M, Knappe M, Vargas Hein O, et al. Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol. 2006;72:645–54. [PubMed] [Google Scholar]
- 3.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann. Intern. Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: an update and primer for the intensivist. Crit. Care Med. 2010;38:261–75. doi: 10.1097/CCM.0b013e3181bfb0b5. [DOI] [PubMed] [Google Scholar]
- 5.Brady HR, Singer GG. Acute renal failure. Lancet. 1995;346:1533–40. doi: 10.1016/s0140-6736(95)92057-9. [DOI] [PubMed] [Google Scholar]
- 6.Charbonney E, Saudan P, Triverio PA, Quinn K, Mentha G, Martin PY. Prognosis of acute kidney injury requiring renal replacement therapy in solid organ transplanted patients. Transpl. Int. 2009;22:1058–63. doi: 10.1111/j.1432-2277.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- 7.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J. Biol. Chem. 1981;256:10986–92. [PubMed] [Google Scholar]
- 8.Davis MJHM, Kuo L. Local regulation of microvascular perfusion. In: Tuma RFDW, Ley K, editors. Handbook of Physiology: Microcirculation. 2nd edn Elsevier; San Diego, CA: 2008. [Google Scholar]
- 9.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 10.Cupples WA, Braam B. Assessment of renal autoregulation. Am. J. Physiol. Renal Physiol. 2007;292:F1105–23. doi: 10.1152/ajprenal.00194.2006. [DOI] [PubMed] [Google Scholar]
- 11.Pittman RN. Regulation of Tissue Oxygenation. 1st edn. Morgan & Claypool Life Science; San Rafael: 2011. [PubMed] [Google Scholar]
- 12.Blantz RC, Deng A, Miracle CM, Thomson SC. Regulation of kidney function and metabolism: a question of supply and demand. Trans. Am. Clin. Climatol. Assoc. 2007;118:23–43. [PMC free article] [PubMed] [Google Scholar]
- 13.Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am. J. Physiol. Renal Physiol. 2008;295:F1259–70. doi: 10.1152/ajprenal.90230.2008. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JJ, Damm KE. Renal metabolism: relation to renal function. In: Brenner BM, Rector BA, editors. The Kidney. 2nd edn Saunders; Philadelphia: 1986. [Google Scholar]
- 15.Mandel LJ. Primary active sodium transport, oxygen consumption, and ATP: coupling and regulation. Kidney Int. 1986;29:3–9. doi: 10.1038/ki.1986.2. [DOI] [PubMed] [Google Scholar]
- 16.Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am. J. Physiol. 1994;267:F1059–62. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 17.Deng A, Tang T, Singh P, et al. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int. 2009;75:197–204. doi: 10.1038/ki.2008.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laycock SK, Vogel T, Forfia PR, et al. Role of nitric oxide in the control of renal oxygen consumption and the regulation of chemical work in the kidney. Circ. Res. 1998;82:1263–71. doi: 10.1161/01.res.82.12.1263. [DOI] [PubMed] [Google Scholar]
- 19.Borutaite V, Brown GC. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem. J. 1996;315:295–9. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–8. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 21.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–4. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 22.Miloradovic Z, Mihailovic-Stanojevic N, Grujic-Milanovic J, et al. Comparative effects of L-arginine and vitamin C pretreatment in SHR with induced postischemic acute renal failure. Gen. Physiol. Biophys. 2009;28:105–11. [PubMed] [Google Scholar]
- 23.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–60. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 24.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int. 2003;63:202–8. doi: 10.1046/j.1523-1755.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- 25.Palm F, Onozato M, Welch WJ, Wilcox CS. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: effects of tempol and Angiotensin blockade. Hypertension. 2010;55:298–304. doi: 10.1161/HYPERTENSIONAHA.109.135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension. 2008;51:345–51. doi: 10.1161/HYPERTENSIONAHA.107.097832. [DOI] [PubMed] [Google Scholar]
- 27.Schachinger H, Klarhofer M, Linder L, Drewe J, Scheffler K. Angiotensin II decreases the renal MRI blood oxygenation level-dependent signal. Hypertension. 2006;47:1062–6. doi: 10.1161/01.HYP.0000220109.98142.a3. [DOI] [PubMed] [Google Scholar]
- 28.Deng A, Miracle CM, Suarez JM, et al. Oxygen consumption in the kidney: effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int. 2005;68:723–30. doi: 10.1111/j.1523-1755.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 29.Kontogiannis J, Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am. J. Physiol. 1998;274:F79–90. doi: 10.1152/ajprenal.1998.274.1.F79. [DOI] [PubMed] [Google Scholar]
- 30.Gunaratnam L, Bonventre JV. HIF in kidney disease and development. J. Am. Soc. Nephrol. 2009;20:1877–87. doi: 10.1681/ASN.2008070804. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 32.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell. Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 2010;300:C385–93. doi: 10.1152/ajpcell.00485.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Bosch-Marce M, Shimoda LA, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Singh P, Blantz RC, Rosenberger C, Gabbai FB, Schoeb TR, Thomson SC. Aberrant tubuloglomerular feedback and HIF-1alpha confer resistance to ischemia after subtotal nephrectomy. J. Am. Soc. Nephrol. 2012;23:483–93. doi: 10.1681/ASN.2011020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katavetin P, Miyata T, Inagi R, et al. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J. Am. Soc. Nephrol. 2006;17:1405–13. doi: 10.1681/ASN.2005090918. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberger C, Khamaisi M, Abassi Z, et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 2008;73:34–42. doi: 10.1038/sj.ki.5002567. [DOI] [PubMed] [Google Scholar]
- 38.Deng A, Arndt MA, Satriano J, et al. Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade. Am. J. Physiol. Renal Physiol. 2010;299:F1365–73. doi: 10.1152/ajprenal.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J. Am. Soc. Nephrol. 2004;15:1597–605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 40.Kanji HD, Schulze CJ, Hervas-Malo M, et al. Difference between pre-operative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac surgery-associated acute kidney injury. J. Cardiothorac. Surg. 2010;5:71. doi: 10.1186/1749-8090-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brezis M, Rosen S. Hypoxia of the renal medulla-its implications for disease. N. Engl. J. Med. 1995;332:647–55. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 42.Redfors B, Sward K, Sellgren J, Ricksten SE. Effects of mannitol alone and mannitol plus furosemide on renal oxygen consumption, blood flow and glomerular filtration after cardiac surgery. Intensive Care Med. 2009;35:115–22. doi: 10.1007/s00134-008-1206-5. [DOI] [PubMed] [Google Scholar]
- 43.Sward K, Valsson F, Sellgren J, Ricksten SE. Differential effects of human atrial natriuretic peptide and furosemide on glomerular filtration rate and renal oxygen consumption in humans. Intensive Care Med. 2005;31:79–85. doi: 10.1007/s00134-004-2490-3. [DOI] [PubMed] [Google Scholar]
- 44.Bragadottir G, Redfors B, Nygren A, Sellgren J, Ricksten SE. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anaesthesiol. Scand. 2009;53:1052–9. doi: 10.1111/j.1399-6576.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 45.Redfors B, Bragadottir G, Sellgren J, Sward K, Ricksten SE. Acute renal failure is NOT an “acute renal success”-a clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit. Care Med. 2010;38:1695–701. doi: 10.1097/CCM.0b013e3181e61911. [DOI] [PubMed] [Google Scholar]
- 46.Clark JK, Barker HG. Studies of renal oxygen consumption in man. I. The effect of tubular loading (PAH), water diuresis and osmotic (mannitol) diuresis. J. Clin. Invest. 1951;30:745–50. doi: 10.1172/JCI102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lassen NA, Munck O, Thaysen JH. Oxygen consumption and sodium reabsorption in the kidney. Acta Physiol. Scand. 1961;51:371–84. doi: 10.1111/j.1748-1716.1961.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 48.Molitoris BA, Falk SA, Dahl RH. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J. Clin. Invest. 1989;84:1334–9. doi: 10.1172/JCI114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molitoris BA. Na(+)-K(+)-ATPase that redistributes to apical membrane during ATP depletion remains functional. Am. J. Physiol. 1993;265:F693–7. doi: 10.1152/ajprenal.1993.265.5.F693. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R913–35. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 51.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N. Engl. J. Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 52.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 2006;17:1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 53.Oostendorp M, de Vries EE, Slenter JM, et al. MRI of renal oxygenation and function after normothermic ischemia-reperfusion injury. NMR Biomed. 2010;24:194–200. doi: 10.1002/nbm.1572. [DOI] [PubMed] [Google Scholar]
- 54.Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol. Med. 2008;14:502–16. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 2001;281:F887–99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 56.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–6. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 57.Hattori R, Ono Y, Kato M, Komatsu T, Matsukawa Y, Yamamoto T. Direct visualization of cortical peritubular capillary of transplanted human kidney with reperfusion injury using a magnifying endoscopy. Transplantation. 2005;79:1190–4. doi: 10.1097/01.tp.0000160760.70984.25. [DOI] [PubMed] [Google Scholar]
- 58.Kwon O, Hong SM, Sutton TA, Temm CJ. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am. J. Physiol. Renal Physiol. 2008;295:F351–9. doi: 10.1152/ajprenal.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Legrand M, Almac E, Mik EG, et al. L-NIL prevents renal microvascular hypoxia and increase of renal oxygen consumption after ischemia-reperfusion in rats. Am. J. Physiol. Renal Physiol. 2009;296:F1109–17. doi: 10.1152/ajprenal.90371.2008. [DOI] [PubMed] [Google Scholar]
- 60.Melin J. Renal Ischemia/reperfusion in Diabetes. Acta Upsaliensis, Uppsala university; Uppsala: 2002. [Google Scholar]
- 61.Melin J, Hellberg O, Akyurek LM, Kallskog O, Larsson E, Fellstrom BC. Ischemia causes rapidly progressive nephropathy in the diabetic rat. Kidney Int. 1997;52:985–91. doi: 10.1038/ki.1997.420. [DOI] [PubMed] [Google Scholar]
- 62.Ries M, Basseau F, Tyndal B, et al. Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J. Magn. Reson. Imaging. 2003;17:104–13. doi: 10.1002/jmri.10224. [DOI] [PubMed] [Google Scholar]
- 63.Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43:629–33. doi: 10.2337/diab.43.5.629. [DOI] [PubMed] [Google Scholar]
- 64.Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia. 2004;47:1223–31. doi: 10.1007/s00125-004-1434-3. [DOI] [PubMed] [Google Scholar]
- 65.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–60. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 66.Hu H, Batteux F, Chereau C, et al. Clopidogrel protects from cell apoptosis and oxidative damage in a mouse model of renal ischaemia-reperfusion injury. J. Pathol. 2011;225:265–75. doi: 10.1002/path.2916. [DOI] [PubMed] [Google Scholar]
- 67.Lopes JA, Jorge S, Resina C, et al. Acute renal failure in patients with sepsis. Crit. Care. 2007;11:411. doi: 10.1186/cc5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. J. Am. Med. Assoc. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 69.Schrier RW, Wang W. Acute renal failure and sepsis. N. Engl. J. Med. 2004;351:159–69. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 70.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69:1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 71.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit. Care Med. 2008;36:S198–203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 72.Bellomo R, Wan L, Langenberg C, Ishikawa K, May CN. Septic acute kidney injury: the glomerular arterioles. Contrib. Nephrol. 2011;174:98–107. doi: 10.1159/000329246. [DOI] [PubMed] [Google Scholar]
- 73.Lonnemann G. Diabetic nephropathy and heart surgery--prevention of perioperative acute renal failure. Clin. Res. Cardiol. 2006;95(Suppl 1):i54–8. doi: 10.1007/s00392-006-1102-3. [DOI] [PubMed] [Google Scholar]
- 74.Hofmann L, Simon-Zoula S, Nowak A, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int. 2006;70:144–50. doi: 10.1038/sj.ki.5000418. [DOI] [PubMed] [Google Scholar]
- 75.United States Renal Data System (USRDS) Annual Data Report. USRDS Coordinating Center; Minneapolis: 2011. [Google Scholar]
- 76.Menon V, Sarnak MJ. The epidemiology of chronic kidney disease stages 1 to 4 and cardiovascular disease: a high-risk combination. Am. J. Kidney Dis. 2005;45:223–32. doi: 10.1053/j.ajkd.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 77.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 78.Norman JT, Fine LG. Intrarenal oxygenation in chronic renal failure. Clin. Exp. Pharmacol. Physiol. 2006;33:989–96. doi: 10.1111/j.1440-1681.2006.04476.x. [DOI] [PubMed] [Google Scholar]
- 79.Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am. J. Nephrol. 2008;28:998–1006. doi: 10.1159/000146075. [DOI] [PubMed] [Google Scholar]
- 80.Kang DH, Kanellis J, Hugo C, et al. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 2002;13:806–16. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 81.Kang DH, Joly AH, Oh SW, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J. Am. Soc. Nephrol. 2001;12:1434–47. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 82.Kitamura H, Shimizu A, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N. Apoptosis in glomerular endothelial cells during the development of glomerulosclerosis in the remnant-kidney model. Exp. Nephrol. 1998;6:328–36. doi: 10.1159/000020540. [DOI] [PubMed] [Google Scholar]
- 83.Ohashi R, Kitamura H, Yamanaka N. Peritubular capillary injury during the progression of experimental glomerulonephritis in rats. J. Am. Soc. Nephrol. 2000;11:47–56. doi: 10.1681/ASN.V11147. [DOI] [PubMed] [Google Scholar]
- 84.Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press. Res. 1996;19:191–5. doi: 10.1159/000174072. [DOI] [PubMed] [Google Scholar]
- 85.Choi YJCS, Nguyen V, Nguyen C, Kim BK, Shim SI, Suki WN, Truong LD. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: Altered expression of vascular endothelial growth factor. Hum. Pathol. 2000;31:1491–7. doi: 10.1053/hupa.2000.20373. [DOI] [PubMed] [Google Scholar]
- 86.Manotham K, Tanaka T, Matsumoto M, et al. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J. Am. Soc. Nephrol. 2004;15:1277–88. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- 87.Harris DC, Chan L, Schrier RW. Remnant kidney hypermetabolism and progression of chronic renal failure. Am. J. Physiol. 1988;254:F267–76. doi: 10.1152/ajprenal.1988.254.2.F267. [DOI] [PubMed] [Google Scholar]
- 88.Nath KA, Croatt AJ. Hostetter TH. Oxygen consumption and oxidant stress in surviving nephrons. Am. J. Physiol. 1990;258:F1354–62. doi: 10.1152/ajprenal.1990.258.5.F1354. [DOI] [PubMed] [Google Scholar]
- 89.Silva P, Hallac R, Spokes K, Epstein FH. Relationship among gluconeogenesis, QO2, and Na+ transport in the perfused rat kidney. Am. J. Physiol. 1982;242:F508–13. doi: 10.1152/ajprenal.1982.242.5.F508. [DOI] [PubMed] [Google Scholar]
- 90.Cohen JJ. Relationship between energy requirements for Na+ reabsorption and other renal functions. Kidney Int. 1986;29:32–40. doi: 10.1038/ki.1986.5. [DOI] [PubMed] [Google Scholar]
- 91.Cohen SD, Kimmel PL. Long-term sequelae of acute kidney injury in the ICU. Curr. Opin. Crit. Care. 2012;18:623–8. doi: 10.1097/MCC.0b013e328358d3f5. [DOI] [PubMed] [Google Scholar]
- 92.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch. Intern. Med. 2011;171:226–33. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 93.Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Al-Melhim WN. Nephroprotective effect of telmisartan in rats with ischemia/reperfusion renal injury. Pharmacology. 2010;85:158–67. doi: 10.1159/000269779. [DOI] [PubMed] [Google Scholar]
- 94.Efrati S, Berman S, Hamad RA, et al. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol. Dial. Transplant. 2011;27:136–45. doi: 10.1093/ndt/gfr256. [DOI] [PubMed] [Google Scholar]
- 95.Bayati A, Nygren K, Kallskog O, Wolgast M. The effect of loop diuretics on the long-term outcome of post-ischaemic acute renal failure in the rat. Acta Physiol. Scand. 1990;139:271–9. doi: 10.1111/j.1748-1716.1990.tb08924.x. [DOI] [PubMed] [Google Scholar]
- 96.Briguori C, Visconti G, Focaccio A, et al. Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II): RenalGuard System in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124:1260–9. doi: 10.1161/CIRCULATIONAHA.111.030759. [DOI] [PubMed] [Google Scholar]
- 97.Redfors B, Bragadottir G, Sellgren J, Sward K, Ricksten SE. Dopamine increases renal oxygenation: a clinical study in post-cardiac surgery patients. Acta Anaesthesiol. Scand. 2010;54:183–90. doi: 10.1111/j.1399-6576.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 98.Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann. Intern. Med. 2005;142:510–24. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- 99.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333:420. doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sward K, Valson F, Ricksten SE. Long-term infusion of atrial natriuretic peptide (ANP) improves renal blood flow and glomerular filtration rate in clinical acute renal failure. Acta Anaesthesiol. Scand. 2001;45:536–42. doi: 10.1034/j.1399-6576.2001.045005536.x. [DOI] [PubMed] [Google Scholar]
- 101.Valsson F, Ricksten SE, Hedner T, Lundin S. Effects of atrial natriuretic peptide on acute renal impairment in patients with heart failure after cardiac surgery. Intensive Care Med. 1996;22:230–6. doi: 10.1007/BF01712242. [DOI] [PubMed] [Google Scholar]
- 102.Sward K, Valsson F, Odencrants P, Samuelsson O, Ricksten SE. Recombinant human atrial natriuretic peptide in ischemic acute renal failure: a randomized placebo-controlled trial. Crit. Care Med. 2004;32:1310–5. doi: 10.1097/01.ccm.0000128560.57111.cd. [DOI] [PubMed] [Google Scholar]