Abstract
Intracellular membrane traffic defines a complex network of pathways that connects many of the membrane-bound organelles of eukaryotic cells. Although each pathway is governed by its own set of factors, they all contain Rab GTPases that serve as master regulators. In this review, we discuss how Rabs can regulate virtually all steps of membrane traffic from the formation of the transport vesicle at the donor membrane to its fusion at the target membrane. Some of the many regulatory functions performed by Rabs include interacting with diverse effector proteins that select cargo, promoting vesicle movement, and verifying the correct site of fusion. We describe cascade mechanisms that may define directionality in traffic and ensure that different Rabs do not overlap in the pathways that they regulate. Throughout this review we highlight how Rab dysfunction leads to a variety of disease states ranging from infectious diseases to cancer.
I. INTRODUCTION
The cytoplasm of a typical eukaryotic cell is populated with a variety of membranous organelles, and a vast array of factors traffic between these organelles by vesicular transport. Despite the complexity of interconnected pathways and the large flux and diversity of transported components, appropriate and accurate delivery of cargo is stringently maintained. The molecular mechanisms by which this traffic is regulated to ensure both the fidelity and efficiency of transport has been, and will continue to be, a significant focus of research. Contributions from a multitude of laboratories have described mechanisms of cargo selection, the budding and scission of vesicles from their donor membranes, the assortment of coats that associate with these vesicles, the mechanism by which these vesicles are transported along cytoskeletal components such as actin filaments or microtubules, the association of the vesicles with the correct target membrane through diverse “tethering” complexes, and finally the mechanism of vesicle fusion with the target membrane through the action of soluble NSF attachment protein receptors (SNAREs) and their associated regulatory machinery. Each step requires a specific set of components to control not only the process itself, but also the transition from one step to the next. Many questions remain concerning the details relating to each of the above steps, for example: How is the transport cargo identified? How do vesicle coats associate and dissociate in a manner consistent with transport? How do vesicles move along cytoskeletal elements? What is the molecular mechanism of a “tethering” complex? What factors ensure appropriate SNARE-mediated membrane fusion? Perhaps the most perplexing question is how the identity of each organelle is maintained such that their assigned functions and the directionality of transport are not lost as cargo is actively exchanged with other organelles. The Rab GTPases have come to the forefront as key regulatory factors that impinge on all of the steps listed above. Specific Rabs are physically associated with each organelle as well as their associated transport vesicles (Fig. 1, Table 1). This review comprehensively describes how Rab proteins act as molecular “switches” to regulate the formation, transport, tethering, and fusion of transport vesicles as a general mechanism for regulating traffic between organelles. The proteins recruited by a specific Rab, so-called effectors, carry out the diverse functions needed at each step on their respective membrane transport pathways. We discuss how altering the functions of Rabs or their interaction partners can result in a range of disease states and how intracellular pathogens can exploit the Rab regulatory system to evade host defenses and reproduce. Finally, we review the mechanisms by which different Rabs communicate with one another in regulatory circuits that help to define each organelle and to establish the direction of membrane traffic.
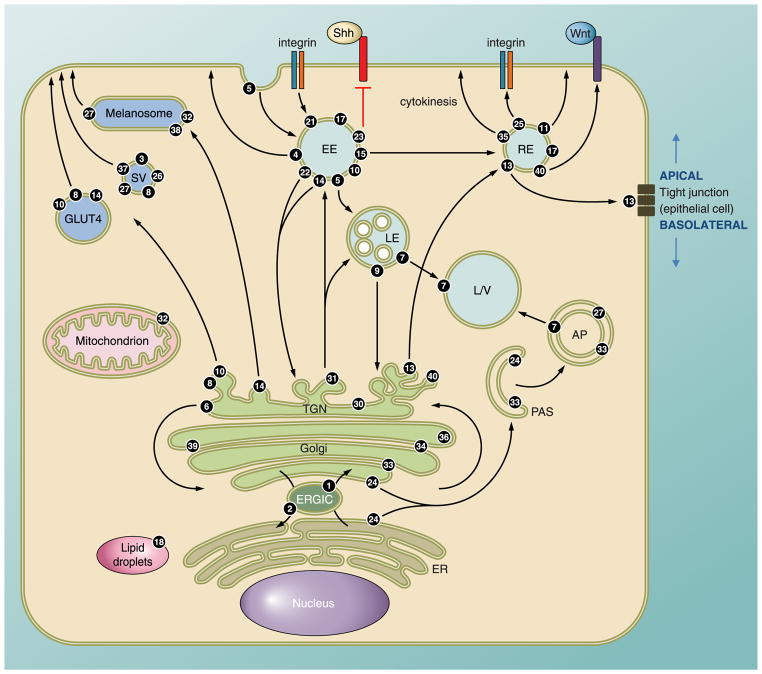
FIG. 1.
The intracellular localization of Rabs. A typical cell showing the intracellular localization and associated vesicle transport pathway(s) of several Rab GTPases. Rab1 regulates ER-Golgi traffic while Rab2 is involved in recycling, or retrograde traffic, from Golgi and the ERGIC back to the ER. Rab6 regulates intra-Golgi traffic. Several Rabs including Rab8, -10, and -14 regulate biosynthetic traffic from the trans-Golgi network (TGN) to the plasma membrane. The glucose transporter GLUT4 is found in vesicles that use these Rabs to reach the plasma membrane. Several secretory vesicles and granules use Rab3, -26, -27, and -37 to exocytose their cargo. Rab27 is well-studied in the melanosome transport that also relies on Rabs 32 and 38. There are numerous Rabs associated with endosomal traffic, and the most active site of localization is the early endosome. Most early endocytic steps rely on Rab5, which mediates fusion of endocytic vesicles to form the early endosome. Traffic can be directed towards the lysosome for degradation, which relies on action of Rab7, or to various recycling compartments to return factors to the plasma membrane. Rab15 directs membrane traffic from the early endosome to the recycling endosome. Rab4 and Rab11 regulate fast and slow endocytic recycling, respectively. Specialized Rab functions include Rab18-mediated regulation of lipid droplets, intracellular lipid storage sites. Rab24 and Rab33 mediate formation of the preautophagosomal structure that engulfs cellular components to form the autophagosome that is subsequently targeted to the lysosome/vacuole. Rab21 and Rab25 regulate transport of integrins to control cell adhesion and cytokinesis. Rab13 directs traffic to and regulates formation of tight junctions in polarized epithelial cells. Tight junctions define the boundary between the apical and basolateral regions of the polarized cell. Mutations in the mouse Rab23 gene lead to a severe developmental defect, open brain, because Rab23 acts downstream to negatively regulate Sonic hedgehog (Shh) signaling during dorsoventral development of the mouse spinal cord. It potentially interacts with the transcription factors activated by the Shh pathway. Rab40 also acts in a signaling pathway; it recruits components of the ubiquitination machinery to regulate Wnt signaling. There are several poorly characterized Rabs, such as Rab35. It controls plasma membrane recycling of an essential factor in cytokinesis. Rab34 and Rab39 are found on the Golgi, but it is unclear what role they play. AP, autophagosome; ERGIC, ER-Golgi intermediate compartment; ER, endoplasmic reticulum; EE, early endosome; LD, lipid droplet; LE, late endosome (multivesicular body); L/V, lysosome/vacuole; PAS, preautophagosomal structure; RE, recycling endosome; SV, secretory vesicle/granule.
TABLE 1.
The Rab GTPase family: intracellular localization, known pathways, effectors, and related diseases
| Rab Protein | Localization | Membrane Traffic Pathway/Function | Effector Proteins; Related Diseases | Reference Nos. |
|---|---|---|---|---|
| Rab1A | ER, Golgi | ER to Golgi, intra-Golgi | p115/Uso1, GM130, giantin, golgin-84, GCC185, MICAL-1, MICAL COOH-terminal like, JRAB/MICAL-L2, OCRL1, INPP5B, Cog6, GBF1 (Arf1 GEF), Iporin, Chlamydia pneumoniae Inc protein Cpn 0585, Trypanosoma brucei golgin Tbg63 | Localization (19, 322, 373) |
| Rab1B | ER, Golgi | ER to Golgi, intra-Golgi | Effectors (6, 27, 28, 86, 106, 124, 135, 194, 284, 333, 346, 361, 460, 461, 466) | |
| Rab2A | ER, ER-Golgi intermediate compartment, Golgi | ER to Golgi | INPP5B, golgin-45, RIC-19 (Caenorhabditis elegans ortholog of ICA69), PKC iota/lambda, GM130, GAPDH, Drosophila melanogaster germ cell-less homolog 1, GARI, Fam71f2, Fam71b | Localization (66) |
| Rab2B | ER, ER-Golgi intermediate compartment, Golgi | ER to Golgi | Effectors (45, 135, 385, 413, 426, 427, 466) | |
| Rab3A | Secretory vesicles, plasma membrane | Exocytosis, neurotransmitter release | rabin3, RIM1α, RIM2α, granuphilin, Noc2, Munc18-1, rabphilin, INPP5B, SNAP-29, synapsin, polymeric IgA receptor (Rab3b), Gas8 (Rab3b), Zwint-1 (Rab3c), OCRL1; Warburg Micro/Martsolf syndromes (Rab3GAP) | Localization (125) |
| Rab3B | Secretory vesicles, plasma membrane | Exocytosis, neurotransmitter release | ||
| Rab3C | Secretory vesicles, plasma membrane | Exocytosis, neurotransmitter release | Effectors (42, 83, 132, 135, 136, 144, 147, 157, 178, 297, 362, 383, 439, 440, 454, 466) | |
| Rab3D (Rab16) (317) | Secretory vesicles, plasma membrane | Exocytosis, regulated Exocytosis in nonneuronal cells | Disease (4, 5) | |
| Rab4A | Early endosome | Protein recycling/transport to plasma membrane | CD2AP, D-AKAP2, Rabip4, Rabip4=, Rabaptin-5α, Rabaptin-5, Syntaxin 4, Dynein LIC-1, Rab coupling protein (RCP), Rabenosyn-5 | Localization (436, 437) |
| Rab4B | Early endosome | Protein recycling/transport to plasma membrane | Effectors (32, 84, 85, 97, 118, 128, 237, 245, 291, 351, 448) | |
| Rab5A | PM, CCVs, early endosome | Early endosome fusion | EEA1, Rabaptin-5/5β, Rabex-5, Rabenosyn-5, INPP5B, OCRL1, PI3 kinases (hVPS34-p150, p110β-p85α), Rabankyrin-5, APPL1, APPL2, Huntingtin-HAP40, caveolin-1, angiotensin II type 1A receptor, Rabi6p4’ | Localization (66) |
| Rab5B | PM, CCVs, early endosome | Early endosome fusion | ||
| Rab5C | PM, CCVs, early endosome | Early endosome fusion | Effectors (55, 76, 77, 115, 128, 135, 156, 168, 194, 248, 272, 296, 310, 363, 368, 379, 407, 448) | |
| Rab6A | Golgi | Endosome to Golgi, intra-Golgi transport, Golgi to ER | Rab6 interacting protein 1/2A/2B, Cog6, kinesin Rab6-KIFL, GCC185, giantin, OCRL1, ELKS, INPP5B, golgin SCYL1BP1, golgin-97, golgin-245, hVps52 (GARP/VFT complex), dynein light chain DYNLRB1, p150 (Glued) subunit of dynein/dynactin complex, mint3 adaptor protein, Bicaudal-D1/2, VFT complex, golgin Sgm1 (TMF/ARA160); Gerodermia osteodysplastica (golgin SCYL1BP1) | Localization (155, 262) |
| Rab6A′ | Golgi | Endosome to Golgi, intra-Golgi transport, Golgi to ER | ||
| Rab6B | Golgi | Intra-Golgi transport, preferentially expressed in neuronal cells | Effectors (25, 86, 116, 129, 135, 180, 194, 206, 217, 244, 264, 280, 346, 386, 391, 393, 420, 455) | |
| Rab7A | Late endosomes, lysosomes/vacuole, melanosomes, phagosomes | Late endosome to lysosome | Vps 35/29/26 complex (retromer), Rabring7, proteasome alpha-subunit PSMA7, Vps34/p150 PI3-kinase complex, oxysterol binding protein related protein 1, RILP; Charcot-Marie-Tooth | Localization (66) |
| Rab7B | Lysosomes | Late endosome to lysosome | Effectors (57, 107, 108, 113, 212, 277, 292, 405) Disease (447) |
|
| Rab8A | Cell membrane, vesicles, primary cilia | Exocytosis, TGN/RE to plasma membrane | Rabphilin, MICAL-1, MICAL COOH-terminal like, MICAL-L1, JRAB/MICAL-L2, TRIP8b (Rab8b), FIP-2, optineurin, otoferlin, RIM1, RIM2, Noc2, OCRL1, Sro7 (Sec4), cenexin3; Bardet-Biedel syndrome (BBSome), Huntington’s disease | Localization (191) |
| Rab8B | Cell membrane, vesicles | Exocytosis, TGN/RE to plasma membrane | Effectors (69, 132, 135, 136, 159, 175, 179, 355, 475, 480) Disease (290) |
|
| Rab9A | Late endosomes | Endosome to TGN | TIP47, INPP5B, GCC185, PI3P PIKfyve kinase associated protein p40, NdeI, 14-3-3 protein theta, HPS4 | Localization (250, 480) |
| Rab9B | Late endosomes, Golgi | Endosome to TGN | Effectors (1, 60, 114, 135, 172, 199, 224, 334) | |
| Rab10 | Golgi, basolateral sorting endosomes, GLUT4 vesicles | Exocytosis, TGN/RE to plasma membrane | Rim1, MICAL-1, MICAL COOH-terminal like, MICAL-L1, JRAB/MICAL-L2, Chlamydia pneumoniae Inc protein Cpn 0585 | Localization (18, 235, 358, 364) Effectors (86, 132, 135) |
| Rab11A | Golgi, RE, early endosomes | TGN/RE to plasma membrane | Sec15, Rab11-FIP1 to FIP5 [FIP3 3 eferin/arfophilin, FIP5 3 Rip11, FIP1c 3 Rab coupling protein(RCP)], D. melanogaster nuclear fallout, arfophilin-2, myosin Vb, PI4-kinase β, rabphilin-11, Rab6 interacting protein 1, Rabin3, Chlamydia pneumoniae Inc protein Cpn 0585, Sec2 (Ypt31/32), Gyp1 (Ypt32); Huntington’s disease | Localization (336, 429, 432) |
| Rab11B | Golgi, RE, early endosomes | TGN/RE to plasma membrane | Effectors (86, 94, 135, 170, 182, 230, 245, 246, 260, 276, 304, 323, 324, 341, 462, 486, 490) | |
| Rab12 | Golgi, secretory vesicles | Exocytosis | Rab interacting lysosomal protein-like 1 (RILP-L1) | Localization (198, 301) Effectors (135) |
| Rab13 | Cell/tight junctions, TGN, RE | TGN/RE to plasma membrane | MICAL-1, MICAL COOH-terminal like, MICAL-L1, JRAB/MICAL-L2, protein kinase A, INPP5B, OCRL1 | Localization (484) Effectors (135, 229, 421, 475) |
| Rab14 | Golgi, early endosome, GLUT4 vesicles | TGN/RE to plasma membrane; apical membrane targeting | FIP2, RCP, Rip11, D-AKAP2 | Localization (215, 231) Effectors (135, 221) |
| Rab15 | Early/sorting endosome, RE | Sorting endosome/RE to plasma membrane | MICAL-1, MICAL COOH-terminal like, MICAL-L1, JRAB/MICAL-L2, Rab15 effector protein | Localization (496) Effectors (135, 408) |
| Rab17 | RE | Transcytosis | Localization (193, 253, 483) | |
| Rab18 | Golgi, lipid droplets | Lipid droplet formation | Localization (98, 210, 255, 309) | |
| Rab19 | Golgi | Unknown | D-AKAP2, ddGCC88, dGolgin97, Wdr38, oxidative stress-induced growth inhibitor family member 2 (D. melanogaster GRIP domain proteins) | Localization (254, 393) Effectors (135, 393) |
| Rab20 | Golgi, endosome | Apical membrane recycling | INPP5E | Localization (9, 255) Effectors (135) |
| Rab21 | Early endosome | Endosomal transport | α-Integrin subunit | Localization (389) Effectors (314) |
| Rab22A | Early endosome | Endosomal transport, protein recycling to plasma membrane | Rabex-5, EEA1, rabenosyn-5, RAD51, INPP5B, OCRL1, rKIAA1055 | Localization (301) Effectors (115, 135, 218, 220, 278, 493) |
| Rab23 | PM, endosome | Protein recycling/transport to plasma membrane | Carpenter syndrome | Localization (120) Disease (211) |
| Rab24 | ER | Autophagosome formation | COOH-terminal binding protein 1 | Localization (301) Effectors (135) |
| Rab25 | RE | RE (apical) to plasma membrane | Integrin β-1 subunit, FIP2, Rip11; epithelial cancers | Localization (61) Effectors (63, 135) Disease (72, 73) |
| Rab26 | Secretory granules | Exocytosis | RIM1 | Localization (479) Effectors (132) |
| Rab27A | Melanosomes | Exocytosis | Slp1-5, Slac2-a (melanophilin), Slac2-b, granuphilin, MyRIP(Slac2-c), Rim2, Rabphilin, Noc2, Munc13-4, Golga4/p230; Griscelli syndrome | Localization (192, 471, 478) |
| Rab27B | Melanosomes | Exocytosis | Effectors (74, 132, 133, 135–138, 227, 228, 381, 410) | |
| Rab28 | Unknown | Unknown | Unknown | |
| Rab28L | Unknown | Unknown | Unknown | |
| Rab30 | ER, Golgi | Unknown | Cog4, Golga4/p230, dGCC88, dGolgin97, dGolgin245 (D. melanogaster GRIP domain proteins) | Localization (95, 393, 424) Effectors (135, 393) |
| Rab31 (Rab22B)(317) | TGN, endosome | M6P receptor transport to endosome | OCRL1 | Localization (343) Effectors (344) |
| Rab32 | Mitochondria, melanosomes | TGN to melanosome, mitochondrial fission | Varp/Ankrd27, PKA | Localization (8, 78, 456) Effectors (8, 313, 419) |
| Rab33A | Golgi, dense-core vesicles | Autophagosome formation | ATG16L, GM130, rabaptin-5, rabex-5 | Localization (492) |
| Rab33B | Golgi | Autophagosome formation | Effectors (134, 200, 435) | |
| Rab34 | Golgi, macropinosomes | Intra-Golgi transport, peri-Golgi positioning of lysosome | Hmunc13, RILP, RILP-L1 | Localization (414, 450) Effectors (135, 398, 450) |
| Rab35 | PM, endosome | RE to plasma membrane, actin assembly | MICAL COOH-terminal like, MICAL-L1, MICAL-1, OCRL1, fascin, Centaurin β2 | Localization (225) Effectors (135, 218, 488) |
| Rab36 | Golgi | Unknown | MICAL-1, MICAL-L1, RILP, RILP-L1, GAPCenA, Leprecan | Localization (68) Effectors (135, 218) |
| Rab37 | Secretory granules | Exocytosis | RIM1 | Localization (263) Effectors (132) |
| Rab38 | Melanosomes | TGN to melanosome | Varp/Ankrd27 | Localization (216, 249) Effectors (419) |
| Rab39 | Golgi | Unknown | Caspase-1 | Localization (70) Effectors (29) |
| Rab40A | Golgi, RE | Endosome/intracellular transport | Elongin B/C, Cullin5, D-AKAP2, RILP-L1, RME-8 | Localization (236) |
| Rab40B | Golgi, RE | |||
| Rab40C | Golgi, RE | Effectors (135, 218, 236) | ||
| Rab41 | Golgi | Unknown | Cog6, Golga4/p230, D-AKAP2 | Localization (167) Effectors (135) |
| Rab42 | Unknown | Unknown | Unknown | |
| Rab43 | ER, Golgi | ER to Golgi, Shiga toxin transport | Unknown | Localization (98, 131) |
| Rab44 | Unknown | Unknown | Unknown | |
| Rab45 | Perinuclear region | Unknown | Unknown | Localization (380) |
ER, endoplasmic reticulum; CCV, clathrin-coated vesicles; TGN, trans-Golgi network; PM, plasma membrane; RE, recycling endosome.
II. THE CONSERVED STRUCTURE OF Rabs
Rabs constitute the largest family of small Ras-like GTPases with 11 identified in yeast and more than 60 members in humans that can be classified in several phylogenetic and functional groups (316, 367). The structures of at least 16 different Rab proteins in either their active (GTP-bound) or inactive (GDP-bound) state have been solved (140, 160). Almost every group has at least one member represented as a crystal structure, allowing for some generalization regarding the specific structural features that contribute to Rab function (319). Rabs generally possess the GTPase fold, composed of a six-stranded β-sheet flanked by five α-helices, common to all members of the Ras superfamily. COOH-terminal to the GTPase fold is the hypervariable region of the Rab followed by the CAAX boxes that normally contains two cysteine residues to which geranylgeranyl moieties are covalently attached. These geranylgeranyl tails allow for regulated membrane insertion of the Rab that will be discussed in greater detail below. Because of the overall structural conservation, the differences between the active and inactive states must define the regions that determine the specific functions of each Rab. The switch I and II regions of Rabs are the primary determinants of nucleotide-dependent Rab function, and both switch regions make contact with the γ phosphate of GTP. When GDP-bound, the switch regions tend to be disordered and undergo major changes to adopt a structurally well-ordered state upon binding GTP (140). Superimpositions of Rab structures in their active state show the greatest structural heterogeneity in their switch domains and the α3/β5 loop (a loop that connects α helix 3 with β sheet 5) that lies adjacent to the switch II domain, with little change elsewhere in the structure. These structural differences explain how different Rab proteins recruit specific sets of effectors to regulate their respective pathways (115, 140, 319).
There are additional features of Rabs that contribute to their interactions with effector proteins and their mechanism of targeting to specific membranes. A multiple sequence alignment of all known Rabs led to the identification of conserved stretches of amino acids, named F1–F5, that distinguishes Rabs from other members of the Ras superfamily (317). The analysis also led to the identification of Rab subfamily-conserved sequences, named SF1– 4, that allowed for grouping of Rabs into various subfamilies and were predicted to define the sites of interactions with their respective effectors (317). The cocrystal structure of Rab3A with its effector, Rabphilin, identified three complementarity-determining regions (CDRs) of Rab3A that made contact with Rabphilin, and these CDRs essentially overlap three of the four SF motifs (305). It should be noted that the switch domains contain F1, F3, and F4 and the α3/β5 loop is equivalent to CDR2, which has also been called SF3.
At the COOH terminus of Rab proteins, upstream of the CAAX box, is the hypervariable region of ~35– 40 amino acids. As the name implies, this portion of the Rab shows the greatest divergence in primary sequence among the different phylogenetic groups. This region has been shown to play a role in targeting of the Rab to specific membranes. Replacement of the Rab5 hypervariable region with that of Rab7 targets the chimera to late endosomes that are normally marked by Rab7 (65). In yeast, a similar chimera of Sec4 containing the hypervariable region of Ypt1 localized to Golgi structures that normally contain Ypt1 (40). Chimeras of Rab1 or Rab5 with the hypervariable region of Rab9 (that interacts with TIP47) can be relocated from the Golgi (normal Rab1 localization) or the early endosome (normal Rab5 localization) to the late endosome (normal Rab9 localization) upon overexpression of TIP47 (1). More recent studies in mammalian cells show that certain F and SF regions of Rabs are more important than their hypervariable domains for membrane targeting and implicate interactions with effector proteins for proper localization (3, 307). It is important to note that the hypervariable region contains a motif that interacts with proteins that regulate the membrane-bound state of the Rab (see below). Therefore, the conflict in targeting mechanisms may reflect the different pathways being studied and the overall contribution of multiple Rab motifs and interacting partners to membrane localization.
III. Rab PROTEINS AS MOLECULAR SWITCHES
Rab proteins cycle between the cytosol and the membrane of its respective transport compartment (Fig. 1, Table 1). The nucleotide-bound state of the Rab influences its localization and activity (Fig. 2). Once the Rab protein is first translated, it associates with Rab escort protein (REP), which presents the Rab to Rab geranylgeranyl transferase (RabGGT) that catalyzes the addition of one or, in most cases, two geranylgeranyl lipid groups to the COOH terminus of the Rab (2, 14, 102). In its GDP-bound or “inactive” state, it is subsequently inserted into its respective membrane. A GDP dissociation inhibitor (GDI) dissociation factor (GDF) may assist in targeting and inserting the Rab in the appropriate membrane (80, 396). A guanine nucleotide exchange factor (GEF) acts on the membrane-inserted Rab to convert it to a GTP-bound or “active” state. The active Rab now interacts with effector proteins that specifically facilitate traffic in its respective pathway. A GTPase accelerating protein (GAP) binds to the Rab to catalyze hydrolysis of the bound GTP to GDP and thereby convert the Rab back to its inactive state (318, 372). The inactive Rab is then a substrate for GDI, which is able to extract the Rab in its GDP-bound conformation from the membrane (80, 360, 430, 470). REP and GDI, both of which bind to GDP-bound Rab proteins in the cytosol, are related proteins that are part of the GDI superfamily (7, 470). The Rab, bound to GDI, is now ready to be reinserted into a membrane and begin the cycle again.
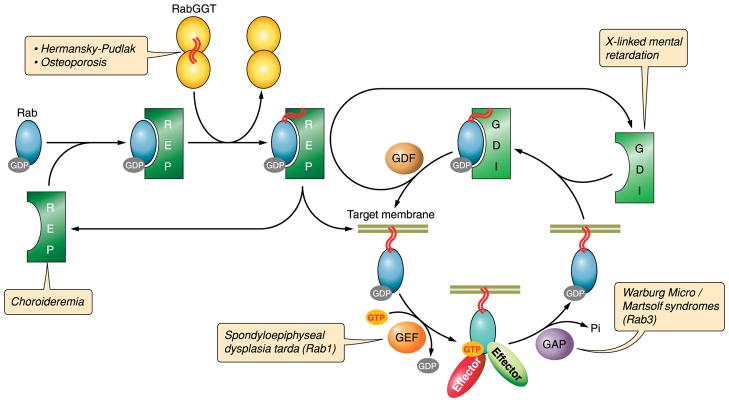
FIG. 2.
The Rab cycle. The newly synthesized Rab protein associates with Rab escort protein (REP) that directs it to Rab geranylgeranyl transferase (RabGGT) to receive its prenyl tails (red wavy lines). REP delivers the Rab to its target membrane. Throughout this process, the Rab is GDP-bound. A guanine nucleotide exchange factor (GEF) catalyzes exchange of GDP for GTP to activate the Rab. The GTP-bound Rab interacts with effector proteins that mediate membrane traffic in the pathway regulated by its associated Rab. The Rab then interacts with its associated GTPase activating protein (GAP) that catalyzes hydrolysis of GTP to GDP by the Rab. The Rab is then removed from the membrane by guanine nucleotide dissociation inhibitor (GDI) in preparation for the next cycle. The insertion of the Rab into the target membrane is mediated by a GDI dissociation factor (GDF) that releases the Rab from GDI. Loss-of-function mutations at each of the above steps produce disease phenotypes as indicated by the red text boxes.
The Rab cycle is critical for regulating traffic to and from particular organelles and thus helps to define their identity. Any perturbation in the steps described above can result in a variety of disease states (Fig. 2). Mutations in the human REP-1 gene lead to choroideremia, a disease characterized by progressive atrophy of the choroid, retinal pigment epithelium, and retina that lead to eventual blindness (365). The cause of the disease is most likely due to loss of Rab27A function, which accumulates in an unprenylated form in retinal tissue samples from patients with the disease. Although there is a second REP gene, REP-2, it apparently cannot compensate for loss of REP-1 in the prenylation of Rab27A (89, 366).
Modulation of RabGGT function has also been shown to play a role in several diseases. The mouse gunmetal mutant is a RabGGTα loss-of-function mutant that is phenotypically similar to patients with Hermansky-Pudlak syndrome, a disease marked by albinism, prolonged bleeding, and lysosomal defects (35, 104, 367). Bisphosphonate drugs that inhibit geranylgeranylation of Rab proteins have been used to remedy bone diseases characterized by excessive resorption, such as osteoporosis (88, 350). These drugs have also been shown to induce apoptosis in certain types of cancers (234). These data correlate well with the identification of several Rab proteins as cancer markers. This is discussed in detail below in this article.
Mutations in the human GDI1 gene lead to X-linked nonspecific mental retardation (91). Mice carrying a deletion of the Gdi1 gene have defects in short-term memory formation and social interaction patterns that is phenotypically similar to humans carrying GDI1 mutations (92). Analysis of brain extracts from mutant mice revealed an accumulation of membrane-bound Rab proteins, but Rab4 and Rab5, both of which regulate endosomal traffic, were more significantly affected than other Rab proteins analyzed (92).
Mutations in the genes encoding the regulatory and catalytic subunits of the Rab3GAP lead to Warburg Micro and Martsolf syndromes, diseases characterized by developmental abnormalities of the eye, nervous system, and genitalia (4, 5). Rab3A is the most abundant Rab found in the brain and regulates exocytosis of synaptic vesicles (144, 145, 267). A Rab GEF has also been implicated in human disease. Mutations in the human SEDL gene, the homolog of the yeast TRAPP subunit Trs20, lead to spon-dyloepiphyseal dysplasia tarda, an X-linked disorder characterized by disproportionately short stature, a short neck and trunk, and degeneration of the spine and hips (142, 143, 162, 258, 425). Mutations in the human TRAPPC9 gene, the homolog of the yeast TRAPP subunit Trs120, lead to nonsyndromic autosomal-recessive mental retardation, intellectual disability, and postnatal microcephaly (275, 279). The TRAPP complex is a GEF for Rab1/Ypt1 that performs a regulatory “tethering” step for endoplasmic reticulum (ER)-derived vesicles targeted to the Golgi (353). These are clear examples of physiological disorders that arise from disrupting the Rab cycle. Additional examples below highlight how interacting partners of Rab proteins are involved in diseases ranging from Huntington’s to cancer and how intracellular pathogens manipulate Rab-regulated pathways to their advantage.
IV. STRUCTURAL DATA OF Rab REGULATORS
Although Rabs in general are strikingly similar in their overall structure, the proteins that interact with them, to either regulate their activity or carry out their downstream functions are not. Recent crystal structures illustrate several distinct mechanisms by which GAPs and GEFs regulate the nucleotide-bound state of Rab proteins. The structures of GDI and REP cocrystallized with Rabs show how they associate with Rab proteins and their hydrophobic geranylgeranyl tails that mediate membrane insertion.
A. GDI and REP
Several features distinguish the functions of GDI and REP and thereby allow them to play different roles in the life cycle of a Rab protein. Although both GDI and REP are members of the GDI superfamily, REP associates with RabGGT to facilitate the addition of geranylgeranyl lipid moieties to the COOH termini of Rabs while GDI extracts inactive, prenylated Rabs from membranes. They are structurally similar and related in function by their affinity for the GDP-bound form of Rabs and their ability to interact with the Rab geranylgeranyl tails. However, REP binds with high affinity to the GDP-bound Rab protein either prenylated or unprenylated, while GDI binds tightly to the Rab with its prenyl groups and binds poorly to the unprenylated Rab protein (326, 473). The interaction of REP with unprenylated Rabs is consistent with its role in facilitating Rab prenylation by RabGGT, while the main function of GDI is to extract Rabs from membranes as part of the Rab cycle. The structures of GDI bound to mono- and di-geranylgeranylated Ypt1 and REP bound to mono-geranylated Rab7 help to distinguish their functions (7, 11, 140, 326, 330 –332). Both GDI and REP are composed of two domains: domain I interacts with the GDP-bound Rab, while domain 2 contains the pocket that binds the geranylgeranyl motifs. The structures show strong conservation in their interaction with the switch and interswitch domains of their associated Rab, maintaining it in a GDP-bound state. Domain I also contains the binding site for the aliphatic-X (polar)-aliphatic (AXA) motif in the Rab hypervariable region while in domain 2, both geranylgeranyl motifs bind in the same prenyl-binding pocket. REP binds RabGGT exclusively through domain 2 and contains two critical amino acid substitutions in domain 2 that mediate its interaction with RabGGT, differentiating it from GDI. Insight into the differences in their functions comes from binding studies of REP and GDI with prenylated forms of Rab7 (473). REP binds with high affinity to unprenylated Rab7 (Kd = 0.22 nM) and even higher affinity to monoprenylated Rab7 (Kd = 0.061 nM). It binds with less affinity to diprenylated Rab7 (Kd = 1.3 nM) compared with monoprenylated Rab7. The more constricted prenyl-binding pocket of REP compared with GDI suggests the second prenyl group may bind outside of the pocket and partially displace the first geranylgeranyl moiety to reduce its overall affinity for REP. The higher affinity for monoprenylated Rab7 may ensure a second geranylgeranyl group is attached to the Rab as Rabs with only one prenyl group tend to be retained at the ER and do not move to their normal intracellular location (152). In the case of GDI, it binds poorly to unprenylated Rabs but with high affinity to mono- and diprenylated Rab7. There is little difference in the affinity of GDI for mono-versus diprenylated Rab7, unlike REP. The structure of GDI with di-geranylgeranylated Ypt1 shows both groups in an overlapped arrangement in the prenyl-binding pocket (326). Although the above data describe the interactions of prenylated Rabs with GDI or REP, it remains unclear how GDI extracts Rab proteins from membranes or how GDFs dissociate GDI or REP to insert Rabs into membranes. The ~1,000-fold higher affinity of GDI for prenylated versus unprenylated Rabs provides a potential explanation for how GDI might remove a membrane-bound Rab by masking its hydrophobic prenyl tails from the aqueous environment (473). This implies that the opposite reaction would require additional factors, such as a GDF or the molecular chaperone Hsp90, to efficiently break the stable Rab-GDI interaction (67, 140, 153, 197). The GDF Yip3, an integral membrane protein found on endosomes, has been shown to catalyze dissociation of GDI from Rab9 through an as yet uncharacterized mechanism (110, 396). The opposing GDI-mediated Rab extraction and GDF-mediated Rab insertion mechanisms are undoubtedly related, and uncovering one mechanism will likely shed light on the other.
B. Rab GAP Proteins
All characterized Rab GAP proteins to date contain a conserved TBC (Tre2/Bub2/Cdc16) domain that confers GAP activity (338). The crystal structure of the TBC domain of Gyp1, the GAP for Ypt1, revealed the mechanism to be dependent on a conserved arginine finger that interfaces with the Rab nucleotide binding pocket to stimulate GTP hydrolysis (114, 329). On the basis of the crystal structure, the fundamental GAP mechanism of Gyp1 was expected to be the same as that of GAP proteins for Ras or Cdc42, despite significant overall structural differences. However, the more recent crystal structures of Gyp1 with several different Rab proteins revealed an additional glutamine “finger” which substitutes for a glutamine from the Rab to mediate GTP hydrolysis (311, 329). This GAP mechanism is likely to be conserved among all Rab-GAP combinations, but additional structures will be needed to test this prediction.
C. Rab GEF Proteins
Unlike Rab GAP proteins, there are, to date, no clear motifs that define Rab GEF proteins. However, the structures of several GEF proteins indicate that they directly insert into, or indirectly alter, the Rab nucleotide or magnesium-binding site to cause displacement of the bound nucleotide (38). The recent crystal structures of Sec2, the GEF for Sec4, and the TRAPP complex, the GEF for Ypt1, highlight the diversity in mechanisms of Rab nucleotide exchange compared with the structures of other Rab GEF proteins: Rabex5, the GEF for Rab5, Rab21 and Rab22 and Mss4, a protein that stimulates nucleotide dissociation from Rab8 (54, 101, 112, 202, 354).
1. Sec2
Sec2 is the GEF for Sec4 and is recruited to secretory vesicles as an effector of the Rab GTPase Ypt32 (304, 449). The crystal structure of Sec2-Sec4 complex was recently solved and revealed the mechanism by which the coiled coil Sec2 dimer facilitates nucleotide exchange on the Rab Sec4 (112). Sec2 interacts with residues in the switch I and switch II domains of Sec4 to induce structural changes in the nucleotide binding pocket that reduce its affinity for nucleotide. No part of Sec2 directly inserts into the nucleotide binding pocket of Sec4, unlike the Rabex5 mechanism of nucleotide exchange (101). Sec4 is the closest yeast homolog of Rab8, and Rabin8 is a GEF for Rab8 that shares a region of homology with the catalytic site of Sec2p (174).
2. Mss4 and Dss4
Sec4 and Rab8 interact with two other related proteins, Dss4 and Mss4, respectively. These are much less efficient than Sec2 and Rabin8 in catalyzing exchange (203), and in the case of Dss4, it only stimulates dissociation of GDP, not the subsequent binding of GTP (283). The structure of Rab8 complexed with Mss4 indicates that Mss4 forms a stable binary association with Rab8 through its switch I and interswitch domains, resulting in an intermolecular β-sheet (202). Mss4 has also been shown to form a stable association with other Rab proteins on both exocytic and endocytic pathways, and this activity of Mss4 may relate to its proposed function as a general chaperone for misfolded Rab proteins rather than a specific GEF (47– 49, 283, 299, 409).
3. TRAPP complex
The TRAPP protein complex is interesting in that it is a multisubunit vesicle tethering complex (see below), yet it is also a GEF for Ypt1 (353, 452). The crystal structure of the TRAPP complex with and without bound Ypt1 revealed how the interplay of several TRAPP subunits facilitates the exchange of GDP for GTP in the Rab GTPase (54, 223). The crystallized complex contains two copies of Bet3 and one copy each of Bet5, Trs23, and Trs31. Within the complex, Bet5, Trs23, and both copies of Bet3 interact with regions of Ypt1 that include the switch I, II, and P-loop domains. These interactions of TRAPP with Ypt1 stabilize the open form of its nucleotide binding pocket, i.e., nucleotide-free form, in preparation for binding GTP. Although the COOH terminus of one of the Bet3 subunits inserts into the Ypt1 nucleotide binding pocket, it is mechanistically different from the Rabex 5 “aspartate finger” that wedges into the magnesium binding site of Rab21 to catalyze nucleotide release (101). Other subunits in TRAPP do not make contact with Ypt1 but are important for allosteric regulation of the TRAPP subunits that directly interact with Ypt1.
V. EFFECTORS OF Rab PROTEINS
Rab proteins regulate their respective pathways by interacting with various effector proteins. Effectors are generally defined as proteins that preferentially interact with the GTP-bound form of their respective Rab, although there are examples, such as protrudin, that interact preferentially with the GDP-bound form of Rab11 (382). Different Rab effectors act during vesicle formation, movement, tethering, and fusion, with each pathway having its own unique set of effectors (Fig. 3). We begin by highlighting some of the best-characterized Rab effectors and their specific functions in membrane traffic.
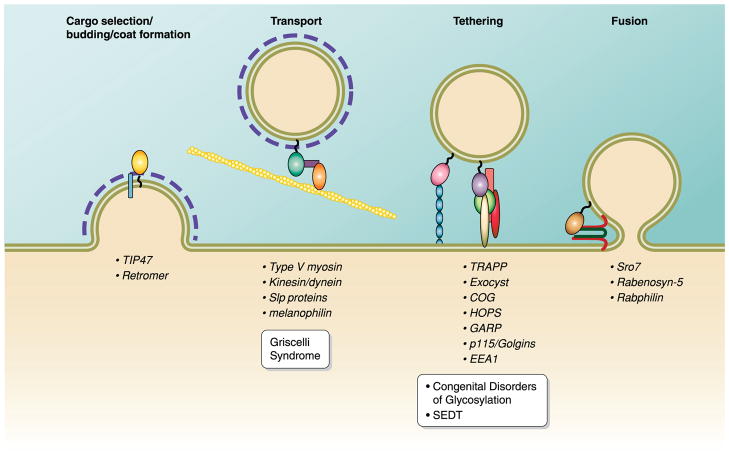
FIG. 3.
Rab effectors. Rabs perform their regulatory function by recruiting a variety of effectors to mediate different functions in membrane transport. These functions are as follows: 1) cargo selection/budding/coat formation, 2) vesicle transport, 3) vesicle uncoating/tethering, and 4) vesicle fusion. Below each function are examples of Rab effectors that perform said function. Mutations in Rab effectors also lead to disease phenotypes: Griscelli Syndrome is caused by mutations in either Rab27A, the Rab27A effector protein melanophilin, or myosin VA, while congenital disorders of glycosylation and spondyloepiphyseal dysplasia tarda (SEDT) are caused by mutations in several COG subunits (COG1, COG7, and COG8) and the TRAPP subunit Trs20, respectively.
A. Rab Proteins and Cargo Selection/Vesicle Formation
A significant portion of intracellular membrane traffic utilizes coated vesicles of the COPI, COPII, or clathrin variety. Vesicle cargo selection is determined by components of each coat complex that recognize specific elements of the cargo to be transported. The Sar/Arf family of GTPases plays a major role in recruiting the coat complexes as well as additional effectors that facilitate vesicle formation. However, several Rab proteins also have been shown to participate in this process.
The best example of this involves Rab9, which regulates membrane traffic between late endosomes and the trans-Golgi network (TGN) (250). TIP47 is a Rab9 effector that interacts with the cytoplasmic domain of mannose-6-phosphate receptors and is required for them to be recycled from endosomes to the TGN (1, 60, 107). The interaction of Rab9 with TIP47 enhances the interaction between the mannose-6-phosphate receptor and TIP47 during the formation of the transport vesicle.
Another example of a complex that acts to appropriately select cargo is the retromer complex. It is required for retrieval of transmembrane proteins from endosomes to the TGN (36, 370). The retromer is composed of a dimer of sorting nexins (SNXs; Vps5 and Vps17 in yeast) associated with the Vps26-Vps29-Vps35 trimer (187, 370). The SNXs contain a PX (phox homology) domain that interacts with phosphoinositides and a BAR domain that can serve as a multimerization interface to induce membrane curvature (58, 130). The Vps26-Vps29-Vps35 trimer is responsible for cargo binding, and the sequential actions of Rab5 and Rab7 are required for recruiting this trimer complex (345). Rab5 is important for phosphoino-sitide regulation through its effector, phosphatidylinositol 3-kinase, while the retromer trimer is an effector of Rab7. It is not known if Rab7 influences the interaction of retromer with cargo. Traffic in the opposing direction relies on the AP-3 pathway that is required for the movement of alkaline phosphatase from the Golgi to the vacuole/lysosome (87). The protein Vps41, a component of the HOPS complex that is an effector of Ypt7, binds to the AP-3 subunit Apl5, and this step is essential for AP-3-dependent traffic (50, 335, 339, 369).
B. Rab Proteins and Vesicle Movement
In addition to selecting cargo, Rab proteins recruit effectors that are critical for vesicle movement along actin- or microtubule-based cytoskeletal structures. There are several outstanding examples of such effectors. Ypt31/32, yeast homologs of Rab11, recruit the type V myosin Myo2 as an effector to transport secretory vesicles to sites of secretion (30, 62, 247). Rab11 in mammalian cells interacts with myosin Vb through its effector, Rab11 family interacting protein 2 (Rab11-FIP2), to regulate plasma membrane recycling (171). Rab27a regulates transport of melanosomes, melanin-containing organelles found in melanocytes, to the plasma membrane through recruitment of its effector melanophilin/Slac2-a that couples it to myosin Va (20, 192, 265, 410, 471, 472). This tripartite complex is physiologically important because mutations in any one member lead to the rare autosomal recessive disorder Griscelli syndrome (GS), first identified by the mouse mutants dilute, leaden, and ashen (Myo5a, Rab27a, and melanophilin, respectively) (438). These patients display a range of symptoms from hypopigmentation (GS3, melanophilin mutation) to immunological defects (GS2, Rab27a mutations) and neurological impairments (GS1, MyoVa mutations).
Rab proteins are also involved in movement of organelles. Yeast cells utilize different pathways, some of which share factors in common, to ensure that the daughter cell acquires the full complement of organelles necessary for survival. Organelles generally utilize the actin cytoskeleton, appropriately polarized through the action of formin proteins, as a track for transport by a type V myosin from the mother cell to the bud (123). The Rab Ypt11 has been shown to regulate the inheritance of both mitochondria and Golgi in yeast by recruiting the type V myosin Myo2 as an effector (17, 34, 201). Although Golgi appear to travel by associating with Myo2, mitochondrial movement may be powered in part by actin polymerization, and the recruitment of Myo2 by Ypt11 is necessary for retaining mitochondria at the poles of mother and daughter cell during the cell cycle (34).
In animal cells, many membrane traffic pathways rely on microtubules, and Rabs have been shown to interact with microtubule-based motors to regulate these pathways. Microtubules are generally organized with their minus ends at microtubule organizing centers, such as the centrosome, and direct their plus ends into the cytoplasm and towards the cell periphery. Rab proteins can regulate traffic in either direction by interacting with members of the kinesin (plus-end directed motors) or dynein (minus-end directed motors) family. Dynein is normally in a complex with dynactin that couples the motor to and stimulates vesicle motility along microtubules (219, 268, 445, 458). Rab6 localizes to the Golgi and primarily regulates retrograde traffic between endosomes, Golgi, and the ER but has recently been shown to also be involved in exocytic traffic to the plasma membrane (99, 148, 158, 207, 259, 261, 262, 303, 433, 463). Rab6 interacts directly with Rabkinesin-6 (kinesin family member 20A) to facilitate intra-Golgi transport (116, 262). Rab6 also indirectly regulates microtubule motors through the effector proteins Bicaudal D1/D2 that link Rab6-containing vesicles to the dynein-dynactin motor complex and, more recently, kinesin for exocytosis (116, 158, 184, 264, 481). Rab7, which coordinates traffic between late endosomes and the lysosome, interacts with Rab-interacting lysosomal protein (RILP) to recruit the dynein-dynactin motor complex to transport late endosomes towards centrosomes and the lysosome (213, 214). This particular Rab-effector interaction is of interest because it is manipulated by several intracellular pathogens. The Salmonella SifA protein prevents the recruitment of RILP by Rab7 to facilitate growth of the membrane-bound compartment in which the bacterium can replicate (163, 173). Heliobacter pylori takes advantage of this interaction to create a bacterium-containing vacuolar compartment that requires Rab7 and RILP to direct endosomal traffic to it (242, 423).
C. Rab Proteins and Vesicle Uncoating
Most membrane traffic pathways utilize coated vesicles of one sort or another, and these coats must be shed to allow the vesicles to fuse with their target membrane. In addition to playing a role in coat formation, Rabs may also play a role in uncoating. Rab5 regulates the early endocytic pathway and is found on clathrin-coated vesicles (CCVs). Recruitment of clathrin to newly forming endocytic vesicles is primarily through the assembly polypeptide 2 (AP-2) clathrin adaptor complex that recognizes and binds to both cargo (i.e., transferrin receptor) destined for internalization and clathrin triskelions to facilitate coat formation (31, 308, 397). The μ2 subunit of AP-2 recognizes cargo, and it must be phosphorylated by μ2 kinase, which is recruited by clathrin, to perform this function (204). Phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2], a phosphoinositide that is normally found at the plasma membrane, is also a significant component for recruiting AP-2 during clathrin-mediated endocytosis (195, 495). Rab5 regulates CCV uncoating in two ways: 1) it induces displacement of the μ2 kinase (through the action of the Rab5 GAP GAPVD1) from AP-2 to prevent it from phosphorylating the μ2 subunit, and 2) it mediates PI(4,5)P2 turnover (374). Modulation of PI(4,5)P2 levels by Rab5 may occur through recruitment of effectors such as PI(3)P kinases or PI phosphatases (77, 379).
Another possible example is Ypt1-mediated regulation of traffic between the ER and Golgi that relies on COPII-coated vesicles. A subunit of the COPII coat, Sec23, has been shown to interact with Bet3, a subunit of the TRAPP complex that is a GEF for Ypt1 and tethers ER-derived vesicles to the Golgi prior to fusion (52). Ypt1, or Rab1, is required for ER-to-Golgi traffic and presumably recruits factors that facilitate uncoating of COPII vesicles in preparation for fusion (209, 243, 284, 321, 371).
D. Rabs and Vesicle Tethering
To ensure fidelity of transport, most membrane transport pathways require factors that “tether” the vesicles to the target membrane before they fuse. These tethering factors fall into two categories: long coiled-coil tethers or multiprotein complexes. Members of both categories of tethers are Rab effectors, and some also regulate the nucleotide-bound state of their associated Rabs (such as the TRAPP complex described above). Despite differences in structure and organization, all of these tethering factors ensure fidelity in transport as they regulate SNARE-mediated fusion of their respective vesicles to the target membrane.
1. Coiled-coil tethers
The Golgins are a family of coiled-coil tether proteins with members that include p115 (Uso1 in yeast), giantin, and GM130 (384). p115 was first discovered as a peripheral membrane protein required for an in vitro inter-Golgi transport assay (459). Sequence analysis suggested an evolutionary relationship of p115 with Uso1, a protein previously defined as an essential factor in ER to Golgi transport in yeast (293, 359, 375, 376). Both Uso1 and p115 are homodimers that consist of a long coiled-coil tail that binds to factors such as the cis-Golgi-localized GM130 and the COPI vesicle factor giantin and a globular head that binds to Rab1 (6, 10, 284, 294, 384, 396a). GM130 associates with the cis-Golgi through its interaction with GRASP65, also an effector of Rab1, and this interaction regulates fusion of COPII vesicles with the cis-Golgi (26, 284). p115 has also been shown to bind to and regulate SNARE proteins; it can interact directly with both syn-taxin 5 and Sly1 and binds to GM130 to disrupt its interaction with both Rab1 and syntaxin 5 (6, 105). Rab1 is the essential regulatory factor in this process that is recruited by its GEF, TRAPP I, to COPII-coated ER-derived vesicles (see above) to assemble its accessory factors (p115, GM130, etc.) that tether the incoming vesicles to the Golgi membrane for SNARE-mediated fusion. More recent data demonstrate that golgins containing a GRIP domain, such as golgin-97, GCC88, and GCC185, contain binding sites for multiple Rabs (177, 393). The GRIP domain mediates an interaction with the Arf-like protein Arl1 to participate in trans-Golgi recruitment of the golgin (312, 468), unlike the above golgins normally found at the cis-Golgi. These golgins would therefore potentially serve as scaffolds to recruit traffic from multiple Rab-regulated pathways to the correct side of the Golgi. Although the significant players in the process have been identified, defining how they interact at a molecular level to regulate ER-to-Golgi and intra-Golgi traffic still requires more work.
Another coiled-coil tether protein is early endosome antigen 1 (EEA1), an effector of Rab5 that is involved in tethering and fusion of early endosomes (285, 388, 406). As a dimer, EEA1 is thought to bridge endosomes through its FYVE domain, an evolutionarily conserved phosphati-dylinositol 3-phosphate [PI(3)P] binding motif, and through its interaction with the SNARE protein syntaxin 6 to mediate homotypic endosomal fusion (55, 56, 176, 233, 273, 387). Therefore, similar to Rab1, Rab5 interacts with coiled-coil tethers to connect membranes and specific SNARE proteins that mediate fusion in their respective pathways.
2. Multisubunit tethers
In most cases, vesicle tethering is performed by multisubunit complexes. There are eight known complexes: TRAPP I and TRAPP II (ER-Golgi and intra-Golgi/endosome-late Golgi, respectively), the exocyst (Golgi-PM), the COG complex (endosome-Golgi, intra-Golgi), the Dsl complex (Golgi-ER), the HOPS complex (vacuole-vacuole and endosome-vacuole), the CORVET complex (endosome-Golgi), and the GARP/VFT complex (membrane protein recycling from endosome to Golgi). From recent structural data, an emerging theme is the structural similarity of several tethering complexes and their interface with components of the SNARE machinery as a mechanism of regulating fusion.
3. The TRAPP complexes
The TRAPP complexes are multisubunit tethers that regulate traffic at different parts of the Golgi (51). Unlike the tethers listed above, the TRAPP complexes are not recruited by a Rab but act as GEFs for Rab1, allowing it to interact with effectors to coordinate membrane traffic. In yeast, the TRAPP I complex functions in ER-to-Golgi traffic while the TRAPP II complex (that contains all TRAPP I subunits and an additional three subunits) regulates intra-Golgi and endosome-to-late Golgi traffic (53, 353). In mammalian cells, there appears to be only one TRAPP complex (271, 482). In addition to TRAPP I and TRAPP II, a recent discovery indicates there is a third TRAPP complex that is required for activating Ypt1 during autophagy in yeast (252). How does TRAPP act as a GEF and a tether? A recent discovery proved insightful: the TRAPP subunit Bet3 binds to the COPII subunit Sec23 (51, 482). Bet3 also has genetic interactions with Bet1, Sed5, and Sec22, all SNARE proteins that function in ER-to-Golgi traffic (347, 353). In studies of mammalian TRAPP, mBet3 is required for homotypic tethering of COPII-coated vesicles from vesiculotubular clusters, an intermediate compartment between the ER and Golgi (482). Following activation of Rab1/Ypt1, known effectors such as Uso1/p115 and giantin (see above) can tether these intermediate vesicles to the Golgi. TRAPP may perform its function in regulating intra-Golgi and endosome-to-late Golgi traffic through its interaction with the COP I coat (476).
4. The exocyst
The exocyst is an octameric complex that tethers secretory vesicles to the plasma membrane in preparation for fusion (286, 422). The vesicle-associated Rab Sec4 recruits the exocyst by interacting with one of its subunits, Sec15, as an effector protein (164). The exocyst is unique in that some of its subunits are also effectors of Rho proteins found on the plasma membrane. This arrangement presumably ensures efficient and accurate tethering to sites marked by these polarity determinants (165, 467, 489). Furthermore, the exocyst has both direct and indirect interactions with components of the SNARE machinery. The exocyst subunit Sec6 has been shown to interact with Sec9, a t-SNARE and SNAP25 homolog found at the plasma membrane (395), while Exo84 interacts with the SNARE regulatory protein Sro7 (491). Pull downs of the exocyst coprecipitate Sec1, a SM (Sec1/mUnc18) protein that binds to and promotes membrane fusion by assembled SNARE complexes (59, 161, 377, 465). It is unclear how exactly Sec4, Rho proteins, and SNAREs interact with the exocyst to control the fusion of secretory vesicles at the plasma membrane. However, some insight comes from recent crystal structures of Exo70, Exo84, and Sec6 from yeast, Sec15 from Drosophila, and mammalian Exo70 that reveal long, rod-shaped proteins composed of bundled α-helices (111, 281, 394, 469). These structures are consistent with quick-freeze/deep-etch micrographs of purified mammalian exocyst complexes that depict sets of “arms” ~10 –30 nm long, consistent with the length of the Exo70 structure (190). Exocyst subunits, as rods, can potentially bundle together in a side-by-side fashion and perhaps in parallel to the two opposed vesicular and plasma membranes. This would bring together the SNARE proteins found on the opposing membranes, as well as Sro7 and Sec1 to regulate their assembly and function, and this process is controlled by the concurrent interactions of several exocyst subunits with Sec4 on vesicles and Rho proteins at the plasma membrane (286).
5. The COG complex
The conserved oligomeric Golgi (COG) complex is composed of eight subunits and regulates retrograde traffic within the Golgi as well as between the endosome and the Golgi (431). COG is an effector of Ypt1 and acts as a tether by interacting with the COPI coat, the SNARE protein Sed5, and the SM protein Sly1 (232, 300, 378, 415, 494). COG plays a role in recycling Golgi resident proteins, highlighted by the observation that mutations in subunits of the COG complex produce defects in glycosylation that lead to severe congenital disease phenotypes (127, 485). The crystal structure of the COG subunit COG4 revealed that a disease-causing mutation in the protein disrupts a COOH-terminal domain that is important for the role of COG complex in glycosylation (337). In addition to being structurally similar to the COG2 subunit, COG4 is remarkably similar in structure to Sec6 as well as the other solved structures of exocyst proteins (64). This observation is discussed further below.
6. GARP/VFT
The GARP/VFT complex is composed of four subunits (Vps51/52/53/54) that function in the recycling of membrane proteins from late endosomes to Golgi (81). In yeast, the GARP complex is recruited by Ypt6/Rab6 to the late Golgi and also associates with Tlg1, a Golgi SNARE protein (392). Subunits of the GARP/VFT complex have regions of sequence similarity to subunits of the COG and exocyst tethering complexes and furthermore share the functional similarities of interacting with Rabs and SNARE proteins (464). The recent crystal structures of the COOH-terminal fragments of Vps53 and Vps54 confirm that these two subunits of the GARP complex are structurally similar to subunits of the exocyst and COG complexes (327, 444). The structure of Vps54 revealed that the mutation responsible for the wobbler mouse phenotype, which leads to spinal muscular atrophy and serves as an animal model for amyotrophic lateral sclerosis, destabilizes Vps54 and results in reduced levels of the protein and of the GARP complex (327). Functionally, the GARP/VFT complex overlaps with the retromer in the transport of cargo between endosomal compartments and the Golgi despite the different components and Rab regulation. A potential link may be Rab6 interacting protein 1, a protein that interacts with Rab6, Rab11, and the retromer (276, 457).
7. The Dsl complex: similarities in the structure of tethering complexes
The Dsl complex, composed of Dsl1, Tip20, and Sec39, regulates retrograde traffic from the Golgi to the ER. It does so by interacting with COPI-coated vesicles (Dsl1 interacts with the subunits of the COPI coat) originating from the Golgi and stabilizing the assembly of SNARE proteins required for this pathway (12, 13, 226, 443). No known Rab has been shown to participate in this process. However, the crystal structures of Dsl1 and Tip20 show both proteins to be structurally similar to COG4 and COG2 of the COG complex, Vps53 and Vps54 of the GARP complex, as well as subunits of the exocyst despite little, if any, sequence similarity (327, 337, 428, 444). All of these complexes interact directly with SNARE proteins: 1) Tip20 and Sec39 of the Dsl complex interact with the SNAREs Sec20 and Use1, respectively; 2) the COG complex interacts with multiple v- and t-SNAREs found at the Golgi; 3) GARP complex interacts with the SNARE Tlg1 at the Golgi and Vps53 and Vps54 interact with the SNARES syntaxin 6, syntaxin 16, and Vamp4; and 4) the exocyst subunit Sec6 interacts with the SNAP-25 homolog Sec9 (222, 226, 328, 378, 392, 395, 415, 416, 428, 441, 442). How these common structural features contribute to the tethering process and SNARE function are undoubtedly a critical focus of future research.
8. HOPS and CORVET
The HOPS and CORVET complexes regulate traffic at the level of the endosome and the lysosome/vacuole and share certain subunits in common (369). The core of both complexes is composed of the class C Vps proteins (Vps11, Vps18, Vps16, and Vps33), first identified in yeast through the isolation of mutants that produce no identifiable vacuoles (22, 23, 342, 348, 349). The HOPS (homo-typic fusion and vacuole protein sorting) complex, in addition to the class C Vps proteins, also contains the subunits Vps39 and Vps41 that impart Ypt7 effector and GEF function to the HOPS complex. The HOPS complex and Ypt7 are required for efficient and accurate homotypic fusion of vacuolar membranes (79, 119, 325, 369, 402, 474). Vps41 interacts directly with Ypt7 to allow the HOPS complex to be a Ypt7 effector (41, 306). The HOPS complex is able to perform its tethering function through its interaction with phosphoinositides and the SNARE protein Vam7 found on vacuolar membranes (411). Furthermore, the class C protein Vps33 is a member of the SM family of proteins that regulates SNARE-mediated membrane fusion by binding to trans-SNARE complexes (169, 411a). The more recently discovered CORVET (class C core vacuole/endosome tethering) complex contains Vps3 and Vps8, instead of Vps39 and Vps41 found in the HOPS complex, and is an effector of Vps21, the yeast homolog of Rab5 (315). Both Vps3 and Vps8 are members of the Vps class D proteins, identified through the isolation of mutants with enlarged vacuoles, and are implicated in sorting of proteins to endosomes (71, 186). Vps21 is also a class D protein, and these data suggest that the CORVET complex is involved in recycling factors from late endosomal compartments marked by Rab7/Ypt7 (and interacting with HOPS) to those containing Vps21/Rab5. Thus the interchangeable nature of the HOPS and CORVET complexes facilitates regulation of traffic in both directions between endosomes and the vacuole/lysosome through their interaction with Rabs that define specific compartments in the pathway (81, 276, 392, 457, 464).
E. Rabs and Membrane Fusion
In addition to recruiting tethers that ensure the proper association of cargo and target membranes, Rab proteins also regulate the SNARE-dependent fusion of transport and target membranes. Rabs can either interact directly with SNARE proteins or with proteins that regulate SNARE function, such as SM or Lgl proteins, to perform this regulatory function.
1. Sec4 and Sro7
The Rab Sec4 is a yeast homolog of Rab8 and regulates the final stage of the secretory pathway in yeast. A recently discovered effector of Sec4 is Sro7, a member of the lethal giant larvae (lgl) family of proteins that interacts with Sec9 and regulates SNARE function (159). Several mutations that disrupt the secretory pathway can be rescued by overexpression of Sec4, and this mechanism of rescue requires the function of Sro7 (159, 465).
2. Rab5 interacts with rabenosyn-5 and EEA1
Rab5 is found on early endosomes and plays a critical role in targeting endosomal traffic towards lysosomes through the function of its numerous effectors. EEA1 and rabenosyn-5 are Rab5 effectors that interact with the SNARE protein syntaxin-6 and the SM protein VPS45, respectively (296, 387). Both EEA1 and rabenosyn-5 also possess a FYVE domain that binds to the phosphoinositide PI(3)P, which is normally found on early endosomal membranes (103, 233, 296). PI(3)P is enriched on early endosomal membranes through the action of the PI 3-OH kinase Vps34 and PI(4)- and PI(5)-phosphatases, all of them being effectors of Rab5 (77, 379). Recruitment of effectors using this dual mechanism is physiologically important because in the absence of Vps34 function, recruitment of both EEA1 and rabenosyn-5 is prevented and fusion of early endosomes is blocked (273, 274, 296, 388).
VI. Rab CASCADES: TRANSITIONING FROM ONE Rab TO ANOTHER
As membrane flows from one organelle to another, it must transition through different Rab-defined compartments. To what extent the compartment defines the Rab, or vice versa, has been an open question, which has been framed primarily by studies of specific pathways, the Rab proteins that are involved, and how they are each activated and inactivated to generate a programmed transition from one Rab to the next. How does this process occur? What mechanisms ensure the directionality of the switch and that the compartment is ready to receive the next Rab and its set of effectors? In several specific cases, recent evidence supports a maturation model whereby the compartment transitions from an upstream Rab to a downstream Rab by recruiting as effectors the GAP and GEF for the upstream and downstream Rabs, respectively (Fig. 4). The countercurrent cascades of GAPs and GEFs not only ensure that the appropriate downstream Rab is recruited but that the upstream Rab is concomitantly inactivated to delineate one compartment from another.
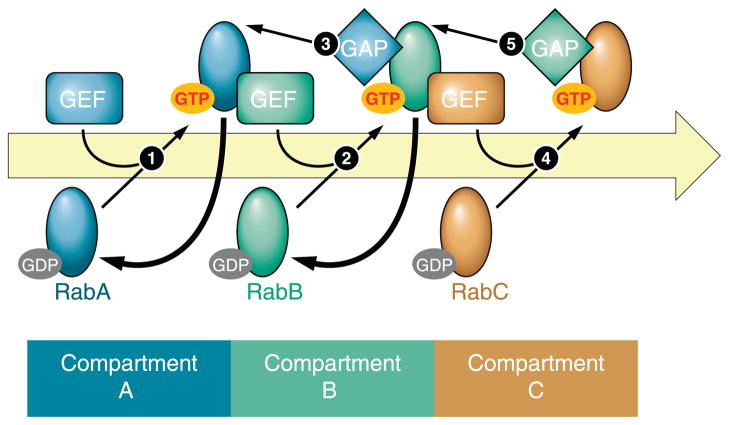
FIG. 4.
The Rab GEF and GAP cascade. Once RabA inserts into its target membrane, it is activated by its respective GEF (step 1). Activated RabA recruits the GEF for the downstream Rab in the pathway RabB (step 2). GTP-bound RabB performs two functions: it recruits the GAP to inactivate RabA (step 3) as well as the GEF for the downstream Rab, RabC (step 4). Activated RabC now recruits the GAP that inactivates RabB (step 5). The concomitant action of GAPs and GEFs ensures the boundaries of each membrane compartment, determined by the actions of their associated Rab, are well-defined.
A. A Transition From Ypt31/32 to Sec4
Ypt31/32, two yeast paralogs homologous to Rab11, are found on late Golgi compartments destined to generate the secretory vesicles, marked with Sec4 (homolog of Rab8), that will go on to fuse with the plasma membrane (30, 154, 208, 356). To initiate this Rab switch, Ypt31/32, in its GTP-bound state, recruits the Sec4 GEF, Sec2. By activating Sec4, Sec2 promotes the stable association of Sec4 with secretory vesicles that ensures their delivery to and fusion with the plasma membrane (304). In this manner, Sec4 is assured of its involvement in the correct pathway because its association with secretory vesicles is dependent on the Rab directly upstream of it. Furthermore, Sec2 also associates with Sec15, a component of the exocyst and an effector of Sec4 as an additional mechanism to recruit Sec4 to secretory vesicles (164, 269, 357). A similar mechanism is in play for Rabex5, the GEF for Rab5. Rabex5 interacts with Rabaptin5, an effector of Rab5, to ensure proper spatiotemporal activation of Rab5 (188).
A somewhat related example of an effector playing a role in targeting a Rab to a specific membrane involves the interaction of Rab9 with its effector TIP47. Several chimeras of Rab1 or Rab5 with the hypervariable region of Rab9 (that interacts with TIP47) can be relocated from the Golgi (normal Rab1 localization) or the early endosome (normal Rab5 localization) to the late endosome (normal Rab9 localization) upon overexpression of TIP47 (1). This result indicates the importance of the Rab-effector interaction in determining the proper localization of the Rab of interest.
B. From Rab5 to Rab7
Endocytic cargo is initially found in Rab5-containing early endosomal compartments that can undergo maturation to become Rab7-containing late endosomal compartments targeted for lysosomes (340). In a Rab GEF cascade similar to that described above, among the effectors of Rab5 is the HOPS complex (potentially through an interaction with the HOPS subunit Vps41), which contains as one of its subunits the Vps39 protein, a GEF for Rab7 (50, 339, 474). Additionally, the HOPS complex is also an effector of Rab7 (369). Thus Rab5-mediated recruitment of the HOPS complex in turn promotes the association of Rab7 with this membrane thereby initiating maturation towards the lysosome/vacuole. This process of Rab conversion has been visualized in mammalian cells and appears to progress in several steps: 1) a dynamically fluctuating association of Rab5 with early endosomes, 2) an association of Rab5 with progressively fewer and larger endosomal compartments (that form through homotypic fusion) that move from the cell periphery towards the cell center, 3) a transient overlap with Rab7 that is dependent on the HOPS complex, and 4) a rapid conversion to a Rab7 compartment destined for the lysosome. These data suggest a maturation model whereby each transport compartment gains the necessary factors to move forward along the pathway while losing those that define the previous compartment (340). Additional support for the maturation model comes from elegant studies of the Golgi in Saccharomyces cerevisiae. Both studies show specific Golgi cisternae transitioning from being marked with an early Golgi marker to a late Golgi marker at a rate consistent with that seen for cargo transitioning through the secretory pathway (251, 266).
In the early endocytic pathway, the early endosome serves as a hub for traffic directed in several different directions through the action of various Rabs. Rab5 can recruit the HOPS complex that mediates a conversion to a Rab7-positive compartment and directs traffic towards the lysosome/vacuole. Another Rab5 effector, rabenosyn-5, has a binding site for and is an effector of Rab4 that is involved in targeting proteins to the Rab11-positive recycling endosome (97). Overexpression of rabenosyn-5 leads to prolonged overlap of Rab5 and Rab4 and shows how a divalent effector can influence Rab conversion and target traffic appropriately from a compartment that serves multiple pathways.
C. A Rab GAP Cascade
The GEF cascades above describe how a Rab conversion can be initiated. However, to avoid an extended period of overlap of Rab domains within a compartment, it is also important to inactivate the upstream Rab once the downstream Rab has been recruited and activated. GAPs are the primary players in this process. For example, the GAP for Rab5 (RabGAP-5) has been shown to play a role in regulating endosomal traffic; either overexpression or loss of RabGAP-5 in HeLa cells blocked trafficking of substrates from early endosomes to the lysosome (167). Therefore, to counterbalance the activating GEF cascade, recent evidence supports a countercurrent GAP cascade whereby the downstream Rab recruits the GAP that inactivates the upstream Rab. In yeast, compartments marked with Ypt1 at the early Golgi mature to contain Ypt32 at the late Golgi. A key step in this process is the recruitment of Gyp1, the GAP for Ypt1, by Ypt32 to inactivate Ypt1 and promote its removal from membranes (341). Loss of Gyp1, which is normally found at the Golgi as an effector of Ypt32, results in the prolonged overlap of Ypt1 and Ypt32 in a Golgi compartment. Ypt1 had previously been shown to recruit the GEF for Ypt32, but the identity of this GEF remains unclear (451).
VII. Rabs AND CANCER
The role of Ras, Ral, and Rho GTPases in oncogenesis is well-documented. However, several Rabs have also been implicated in the progression of multiple cancers (75) as membrane traffic plays a significant role in cancer biology, primarily in the loss of cell polarity and in the metastatic transformation of tumor cells (282). This includes the upregulation of Rab5 in malignant and met-static human lung cell adenocarcinoma, Rab1 in tongue squamous cell carcinomas, and Rab3 in cancers of the nervous system (447). Rab5 is an appropriate target due to its role in receptor-mediated endocytosis. Modulating Rab5 function can significantly alter signaling from growth factors to promote tumorigenesis, and both up-and downregulation of Rab5a is associated with cancer in different tissues (90, 139, 241). The best characterized example of a Rab implicated in cancer is Rab25, a Rab closely related to Rab11 that regulates apical endocytosis and transcytosis in epithelial cells (61, 151, 453). Rab25 is upregulated in certain ovarian and breast cancers due to amplification of a chromosomal region containing the Rab25 gene. The resulting overexpression of Rab25 is associated with more aggressive forms of the associated cancer and a lower patient survival rate (73). Recent studies have demonstrated an interaction between Rab25 and the β1-integrin subunit, and this interaction is required for promoting invasiveness of tumor cells into a three-dimensional extracellular environment (63). Rab25 appears to retain a pool of α5β1-integrin heterodimers at the tip of the invasive pseudopod to facilitate efficient integrin recycling and maintain a stable association of the pseudopod with the extracellular environment. Therefore, Rab25 does not play a role in tumor initiation but facilitates its progression by allowing it to be invasive.
VIII. Rabs AND NEUROLOGICAL DISEASE
Recent discoveries implicate Rabs in several prevalent neurological diseases. Neurons may be more sensitive to perturbations in membrane traffic because of their unique polarized structure and function. Specialized functions of Rabs are critically important for synaptic function (Rab3), neurite growth and remodeling (Rab11 and Rab13), and general nervous system development (Rab23) (211). The section below highlights the connection between Parkinson’s disease and Rab1, Huntington’s disease with Rabs in post-Golgi trafficking, and neuropathies due to activating mutations of Rab7.
A. Parkinson’s Disease
Parkinson’s disease (PD) is the most prevalent neurological disorder of movement due primarily to loss of dopaminergic nerve cells in the substantia nigra (126). The gene encoding the α-synuclein protein, when mutated, causes an autosomally dominant inherited form of PD with several identified missense point mutations (141). α-Synuclein (αsyn) is a major constituent of Lewy bodies, an intracellular protein aggregate found in neurons that are the hallmark pathological feature of PD, one of several neurological diseases commonly referred to as synucleinopathies (399, 400). The connection between Rabs and Parkinson’s disease first came from studies of one of the αsyn point mutations, A30P, expressed in a transgenic mouse model. Rab3a, Rab5, and Rab8 interacted with αsyn in brain extracts from mutant mice and not those containing the wild-type control αsyn (93). In addition to point mutations in αsyn, additional copies of the gene can also lead to PD (196, 390). When overexpressed in yeast, α-synuclein disrupts ER-to-Golgi transport, and this phenotype can be rescued by overexpression of Ypt1, the yeast Rab1 homolog (82). Animal models of PD and mammalian dopaminergic cells also showed reduced α-synuclein-induced toxicity when overexpressing Rab1. Further studies implicated Rab3 and Rab8, suggesting that α-synuclein may affect several membrane traffic pathways (149).
B. Huntington’s Disease
Huntington’s disease (HD) is an autosomal inherited neurological disorder caused by expansion of a trinucleotide repeat in the gene encoding huntingtin (htt) protein (150). The resulting mutation produces an NH2-terminal polyglutamine repeat, and the length of the expansion, and subsequently the polyQ repeat, correlates inversely with age of onset (146, 185). It is unclear how the polyQ repeat produces a disease state, but htt is normally associated with membranes and plays a role in membrane traffic (109, 446). A recent study shows that mutant htt disrupts clathrin-dependent post-Golgi traffic targeted for lysosomes (100). Mutant htt prevents the association of Rab8 with optineurin at the Golgi and results in reduced AP-1- and clathrin-mediated traffic to lysosomes. Htt interacts with the optineurin protein and FIP-2 that are both effectors of Rab8 at the Golgi (121, 175, 355). Rab8 and FIP-2 recruit htt to the Golgi, and the interaction of optineurin with myosin VI is important for maintaining Golgi structure (355). However, it is not known what role htt normally plays in its association with Rab8, FIP-2, and optineurin at the Golgi. In addition to its interaction with Rab8, based on studies of a mouse model for HD, Htt may interact with a GEF for Rab11 (238, 239). Membrane fractions from mutant mouse brains did not catalyze nucleotide exchange on Rab11, and a Rab11 dominant-negative mutant expressed in normal adult brains led to neurodegeneration similar to the HD mutant mouse model. Very recent data show delayed recycling of transferrin to the plasma membrane and impaired Rab11-dependent vesicle formation from recycling endosomes in fibroblasts from Huntington patients compared with healthy individuals (240). Both Rab8 and Rab11 localize to the recycling endosome (RE) and target proteins for the plasma membrane, although it is unclear how the two Rabs are differentiated in function (15, 16, 429, 487). It will be interesting to see how the interplay between Rab8, Rab11, and htt become altered during the onset of HD and how that contributes to the pathophysiology of the disease.
C. Carpenter Syndrome and Rab23, Charcot-Marie-Tooth Disease and Rab7
Carpenter syndrome is an autosomal recessive disorder with symptoms that include skull abnormalities, poly-dactyly, brachydactyly (shortness of fingers and toes), obesity, congenital heart disease, and mental retardation (183). Mutations in Rab23 have been identified as the causative agent of the disease, and surprisingly, the associated phenotypes differ quite dramatically from the mouse Rab23 open brain (opb) mutant that is embryonically lethal (117, 166, 211). Rab23 acts as a negative regulator of sonic hedgehog (shh) signaling during dorsal-ventral axis formation of the neural tube. By activating Rab23, dorsal neural cells can prevent shh signaling that is required for ventral cells of the spinal cord (117). Rab23 signaling through shh is more than likely the cause of symptoms seen in Carpenter syndrome as mutations in shh signaling components also produce phenotypes such as polydactyly and brachydactyly (211). However, Rab23 was first cloned as a Rab predominantly expressed in the mouse brain (302), and although there are potential similarities, Carpenter syndrome phenotypes are more pleiotropic than those seen for opb mice (211). The Rab23 mutations that cause Carpenter syndrome may have uncovered novel signaling pathways involving shh that will require further attention to characterize these connections.
Rab7 is a critical regulatory component that directs traffic in the endosomal pathway to the lysosome (44, 66). Point mutations in Rab7 lead to Charcot-Marie-Tooth disease type 2B, an inherited motor and sensory neurological disorder characterized primarily by distal muscle weakness and atrophy (24, 189, 270, 447). Biochemical analysis indicates that Rab7 carrying any of the identified point mutations is preferentially GTP bound and has a slower rate of GTP hydrolysis (96, 401). Therefore, Rab7 in a prolonged “on” state may be the cause of the disease. It is interesting to note that mutations in an endocytosis-related gene, dynamin 2, that impair clathrin-mediated endocytosis also produce Charcot-Marie-Tooth disease phenotypes (33, 122, 497).
IX. MICROORGANISMS, Rabs, AND DISEASE
The above examples show how Rab-regulated pathways can be perturbed to cause disease. In a related manner, Rabs and their effectors have become targets for infectious microorganisms that have developed mechanisms to evade host defenses by hiding and replicating in an intracellular environment. To avoid the host cell degradation machinery and obtain nutrients and building blocks to multiply, such organisms manipulate several different Rabs to their advantage. The majority of intra-cellular pathogens hijack Rabs involved in the endocytic pathway, while the causative agent of Legionnaire’s disease uses a bifunctional protein to capture Rab1.
A. Salmonella enterica and Chlamydia pneumonia
Salmonella enterica and Serovar typhimurium, the cause of gastroenteritis commonly referred to as salmonellosis, are initially taken up by epithelial cells that line the gut. They reside in Salmonella-containing vacuoles (SCVs) in the cell that transition from a Rab5- to a Rab7-containing compartment (21, 173, 288, 403, 404). Rab7 effectors position the compartment at a perinuclear location close to the Golgi (39, 181). Acidification of the compartment causes release of Salmonella virulence factors that act to block the compartment from fusing with the lysosome, anchor the SCV to the Golgi, and recruit traffic from the Golgi (21, 43, 320, 403). For example, the Salmonella SopB protein, a PI phosphatase, recruits sorting nexin 1 (Snx1) to the SCV membrane for retromer-mediated removal of mannose-6-phosphate receptors from its membrane (46, 298). Mannose-6-phosphate receptors are integral membrane proteins that sort acid hydrolases from the Golgi to the vacuole/lysosome and are then recycled back to the Golgi through the action of the retromer complex (36, 37). The SopB protein, therefore, prevents maturation by enhancing recycling of unwanted lysosomal proteins from the SCV. The Salmonella Pip2B and SifA interact with the host SKIP (SifA and kinesin interacting protein) to prevent kinesin-powered movement of SCVs away from their perinuclear localization (39, 181, 205). The SCVs accumulate a variety of Rab proteins on their membranes but not those indicative of phagosomes undergoing a normal maturation process towards lysosomes (43). It is unclear how SCVs bypass this process. The SCVs also extend membranous fingers called Salmonella-induced filaments, or Sifs, that hijack traffic between endosomes and the Golgi through the recruitment of Rab9 by SKIP (205).
Chlamydia does not take advantage of the endosomal/lysosomal pathway but, like Salmonella, releases proteins to avoid being directed to the lysosome. Once inside the cell in a structure known as an inclusion, it releases effector proteins termed “integral inclusion membrane” (Inc) proteins that prevent recruitment of Rab5, Rab7, and Rab9 and recruit exocytic and Golgi-bound traffic marked by Rabs such as Rab4, Rab11, and Rab1 (320, 351, 352, 434). A key component is the Chlamydia Inc protein Cpn0585 that has similar features to Golgin proteins and interacts with Rab1, Rab10 and Rab11 (86).
B. Legionella pneumophila
Although Legionella disrupt the endosomal/lysosomal pathway, a recent discovery places it between the ER and Golgi. The SidM/DrrA protein from Legionella pneumophila, the cause of the pneumonia known as Legionnaire’s disease, is a bifunctional protein that was first characterized as both a GDF and a GEF for Rab1 (256, 257, 287). The cocrystal structure of SidM/DrrA with Rab1 indicated that the GDF activity is mediated by the region of SidM/DrrA that mediates GEF activity on Rab1. The high affinity of SidM/DrrA for GDP-bound Rab1 may account for the GDF activity demonstrated by SidM/DrrA (412). The NH2-terminal domain of SidM/DrrA mediates adenosine monophosphorylation (AMPylation) of the switch II region of Rab1, and GTP-bound Rab1 is the preferred substrate for SidM/DrrA-mediated AMPylation (289). The AMPylation activity of SidM/DrrA causes cytotoxicity in mammalian cells and reduces the interaction of Rab1 with the host effector protein MICAL-3 but not the bacterially encoded effector LidA (289). Through the function of SidM/DrrA, Legionella residing in a vacuolar-like compartment hijack traffic destined for the Golgi by recruiting and activating GDI-bound Rab1.
X. CONCLUSIONS
The volume of information describing Rab function in membrane traffic has grown dramatically in recent years. In addition to identifying the many Rab proteins, defining their subcellular localizations, and isolating their regulators and effectors, we are beginning to understand how Rabs communicate with each other to specify where their respective territories begin and end. Although we have provided a few examples of how one Rab domain might transition to another, it is presently unclear if these mechanisms are universally applicable to all Rab-regulated pathways. If not, how do these other Rabs determine the pathways that they regulate? The mechanism of Rab conversion, described above, relies on their associated GAPs and GEFs. However, do GDFs also play a role in this process? Does each pathway have a specific GDF, or are they shared among sets of pathways? How is this sharing regulated? We may have identified the major factors that regulate Rab function, but establishing how they are coordinated to achieve a common goal will require further analysis.
While several Rabs have been very intensively studied, a large fraction of the Rab proteins expressed in mammalian cells have not, and relatively little is known regarding their function and regulation. A recent study indicated that 42 Rab GTPases are expressed in COS7 cells, with the abundant Rabs being those that regulate endocytosis, secretion, and traffic to, from, and within the Golgi (295). Are these uncharacterized Rabs simply redundant with the better-known members of their branch of the Rab family or have they acquired unique functions? Do these Rabs serve tissue-specific roles? Will the same mechanisms act to control their function? How do they interact with the other Rabs found inside the cell? To understand the forces underlying the dramatic expansion of the Rab family during evolution, we must begin by describing the function of each Rab in greater detail. Knockouts and knockdowns of the less-studied Rabs, both singly and in a combinatorial fashion, will help to reveal the common and unique functions of each Rab. In vitro assays using donor and target membranes and all identified factors are now a realistic goal for many Rabs. In addition to describing Rab function at a molecular level, assays such as these can be used to identify and analyze novel factors that affect the pathway of interest.
Rabs are involved in the pathogenesis of a wide range of diseases but exactly what role they play in some of these disorders is still unclear. Analyzing the role of Rabs in the pathogenesis of Parkinson’s or Huntington’s disease provides a unique angle to approach the study of these diseases. Recent discoveries of the interaction of Rab35 with the actin bundling protein fascin to regulate intracellular actin assembly (488) or the function of Rab23 in brain and chondrocyte (477) development highlight the diverse roles of Rab proteins. Their involvement in signaling pathways outside of their stereotypical role in membrane traffic only magnifies our need to investigate in greater detail how Rabs work.
Acknowledgments
GRANTS
This study was supported by National Institute of General Medical Sciences Grants GM-35370 and GM-82861 (to P. J. Novick).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Aivazian D, Serrano R, Pfeffer S. TIP47 is a key effector for Rab9 localization. J Cell Biol. 2006;173:917–926. doi: 10.1083/jcb.200510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandrov K, Horiuchi H, Steele-Mortimer O, Seabra M, Zerial M. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J. 1994;13:5262–5273. doi: 10.1002/j.1460-2075.1994.tb06860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali B, Seabra M. Targeting of Rab GTPases to cellular membranes. Biochem Soc Trans. 2005;33:652–656. doi: 10.1042/BST0330652. [DOI] [PubMed] [Google Scholar]
- 4.Aligianis I, Johnson C, Gissen P, Chen D, Hampshire D, Hoffmann K, Maina E, Morgan N, Tee L, Morton J, Ainsworth J, Horn D, Rosser E, Cole T, Stolte-Dijkstra I, Fieggen K, Clayton-Smith J, Mégarbané A, Shield J, Newbury-Ecob R, Dobyns W, Graham JJ, Kjaer K, Warburg M, Bond J, Trembath R, Harris L, Takai Y, Mundlos S, Tannahill D, Woods C, Maher E. Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat Genet. 2005;37:221–223. doi: 10.1038/ng1517. [DOI] [PubMed] [Google Scholar]
- 5.Aligianis I, Morgan N, Mione M, Johnson C, Rosser E, Hennekam R, Adams G, Trembath R, Pilz D, Stoodley N, Moore A, Wilson S, Maher E. Mutation in Rab3 GTPase-activating protein (RAB3GAP) noncatalytic subunit in a kindred with Martsolf syndrome. Am J Hum Genet. 2006;78:702–707. doi: 10.1086/502681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan B, Moyer B, Balch W. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289:444–448. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 7.Alory C, Balch W. Molecular evolution of the Rab-escort-protein/guanine-nucleotide-dissociation-inhibitor superfamily. Mol Biol Cell. 2003;14:3857–3867. doi: 10.1091/mbc.E03-04-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alto N, Soderling J, Scott J. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amillet J, Ferbus D, Real F, Antony C, Muleris M, Gress T, Goubin G. Characterization of human Rab20 overexpressed in exocrine pancreatic carcinoma. Hum Pathol. 2006;37:256–263. doi: 10.1016/j.humpath.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 10.An Y, Chen C, Moyer B, Rotkiewicz P, Elsliger M, Godzik A, Wilson I, Balch W. Structural and functional analysis of the globular head domain of p115 provides insight into membrane tethering. J Mol Biol. 2009;391:26–41. doi: 10.1016/j.jmb.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An Y, Shao Y, Alory C, Matteson J, Sakisaka T, Chen W, Gibbs R, Wilson I, Balch W. Geranylgeranyl switching regulates GDI-Rab GTPase recycling. Structure. 2003;11:347–357. doi: 10.1016/s0969-2126(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 12.Andag U, Neumann T, Schmitt H. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J Biol Chem. 2001;276:39150–39160. doi: 10.1074/jbc.M105833200. [DOI] [PubMed] [Google Scholar]
- 13.Andag U, Schmitt H. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem. 2003;278:51722–51734. doi: 10.1074/jbc.M308740200. [DOI] [PubMed] [Google Scholar]
- 14.Andres D, Seabra M, Brown M, Armstrong S, Smeland T, Cremers F, Goldstein J. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell. 1993;73:1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- 15.Ang A, Fölsch H, Koivisto U, Pypaert M, Mellman I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J Cell Biol. 2003;163:339–350. doi: 10.1083/jcb.200307046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang A, Taguchi T, Francis S, Fölsch H, Murrells L, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai S, Noda Y, Kainuma S, Wada I, Yoda K. Ypt11 functions in bud-directed transport of the Golgi by linking Myo2 to the coatomer subunit Ret2. Curr Biol. 2008;18:987–991. doi: 10.1016/j.cub.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Babbey C, Ahktar N, Wang E, Chen C, Grant B, Dunn K. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacon R, Salminen A, Ruohola H, Novick P, Ferro-Novick S. The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J Cell Biol. 1989;109:1015–1022. doi: 10.1083/jcb.109.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahadoran P, Aberdam E, Mantoux F, Buscà R, Bille K, Yalman N, de Saint-Basile G, Casaroli-Marano R, Ortonne J, Ballotti R. Rab27a: a key to melanosome transport in human melanocytes. J Cell Biol. 2001;152:843–850. doi: 10.1083/jcb.152.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakowski M, Braun V, Brumell J. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic. 2008;9:2022–2031. doi: 10.1111/j.1600-0854.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 22.Bankaitis V, Johnson L, Emr S. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci USA. 1986;83:9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banta L, Robinson J, Klionsky D, Emr S. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988;107:1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barisic N, Claeys K, Sirotković-Skerlev M, Löfgren A, Nelis E, De Jonghe P, Timmerman V. Charcot-Marie-Tooth disease: a clinico-genetic confrontation. Ann Hum Genet. 2008;72:416–441. doi: 10.1111/j.1469-1809.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 25.Barr F. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr Biol. 1999;9:381–384. doi: 10.1016/s0960-9822(99)80167-5. [DOI] [PubMed] [Google Scholar]
- 26.Barr F, Nakamura N, Warren G. Mapping the interaction between GRASP65 and GM130, components of a protein complex involved in the stacking of Golgi cisternae. EMBO J. 1998;17:3258–3268. doi: 10.1093/emboj/17.12.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayer M, Fischer J, Kremerskothen J, Ossendorf E, Matanis T, Konczal M, Weide T, Barnekow A. Identification and characterization of Iporin as a novel interaction partner for rab1. BMC Cell Biol. 2005;6:15. doi: 10.1186/1471-2121-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beard M, Satoh A, Shorter J, Warren G. A cryptic Rab1-binding site in the p115 tethering protein. J Biol Chem. 2005;280:25840–25848. doi: 10.1074/jbc.M503925200. [DOI] [PubMed] [Google Scholar]
- 29.Becker C, Creagh E, O’Neill L. RAB39A binds caspase-1 and is required for caspase-1-dependent interleukin-1beta secretion. J Biol Chem. 2009;284:34531–34537. doi: 10.1074/jbc.M109.046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benli M, Döring F, Robinson D, Yang X, Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- 31.Benmerah A, Lamaze C. Clathrin-coated pits: vive la différence? Traffic. 2007;8:970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 32.Bielli A, Thörnqvist P, Hendrick A, Finn R, Fitzgerald K, McCaffrey M. The small GTPase Rab4A interacts with the central region of cytoplasmic dynein light intermediate chain-1. Biochem Biophys Res Commun. 2001;281:1141–1153. doi: 10.1006/bbrc.2001.4468. [DOI] [PubMed] [Google Scholar]
- 33.Bitoun M, Durieux A, Prudhon B, Bevilacqua J, Herledan A, Sakanyan V, Urtizberea A, Cartier L, Romero N, Guicheney P. Dynamin 2 mutations associated with human diseases impair clath-rin-mediated receptor endocytosis. Hum Mutat. 2009;30:1419–1427. doi: 10.1002/humu.21086. [DOI] [PubMed] [Google Scholar]
- 34.Boldogh I, Ramcharan S, Yang H, Pon L. A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division. Mol Biol Cell. 2004;15:3994–4002. doi: 10.1091/mbc.E04-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifacino J. Insights into the biogenesis of lysosome-related organelles from the study of the Hermansky-Pudlak syndrome. Ann NY Acad Sci. 2004;1038:103–114. doi: 10.1196/annals.1315.018. [DOI] [PubMed] [Google Scholar]
- 36.Bonifacino J, Hurley J. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonifacino J, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 38.Bos J, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Boucrot E, Henry T, Borg J, Gorvel J, Méresse S. The intra-cellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005;308:1174–1178. doi: 10.1126/science.1110225. [DOI] [PubMed] [Google Scholar]
- 40.Brennwald P, Novick P. Interactions of three domains distinguishing the Ras-related GTP-binding proteins Ypt1 and Sec4. Nature. 1993;362:560–563. doi: 10.1038/362560a0. [DOI] [PubMed] [Google Scholar]
- 41.Brett C, Plemel R, Lobinger B, Vignali M, Fields S, Merz A. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol. 2008;182:1141–1151. doi: 10.1083/jcb.200801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brondyk W, McKiernan C, Fortner K, Stabila P, Holz R, Macara I. Interaction cloning of Rabin3, a novel protein that associates with the Ras-like GTPase Rab3A. Mol Cell Biol. 1995;15:1137–1143. doi: 10.1128/mcb.15.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brumell J, Scidmore M. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev. 2007;71:636–652. doi: 10.1128/MMBR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buffa L, Fuchs E, Pietropaolo M, Barr F, Solimena M. ICA69 is a novel Rab2 effector regulating ER-Golgi trafficking in insulinoma cells. Eur J Cell Biol. 2008;87:197–209. doi: 10.1016/j.ejcb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Bujny M, Ewels P, Humphrey S, Attar N, Jepson M, Cullen P. Sorting nexin-1 defines an early phase of Salmonella-containing vacuole-remodeling during Salmonella infection. J Cell Sci. 2008;121:2027–2036. doi: 10.1242/jcs.018432. [DOI] [PubMed] [Google Scholar]
- 47.Burton J, Burns M, Gatti E, Augustine G, De Camilli P. Specific interactions of Mss4 with members of the Rab GTPase subfamily. EMBO J. 1994;13:5547–5558. doi: 10.1002/j.1460-2075.1994.tb06892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burton J, De Camilli P. A novel mammalian guanine nucleotide exchange factor (GEF) specific for rab proteins. Adv Second Messenger Phosphoprotein Res. 1994;29:109–119. doi: 10.1016/s1040-7952(06)80010-8. [DOI] [PubMed] [Google Scholar]
- 49.Burton J, Roberts D, Montaldi M, Novick P, De Camilli P. A mammalian guanine-nucleotide-releasing protein enhances function of yeast secretory protein Sec4. Nature. 1993;361:464–467. doi: 10.1038/361464a0. [DOI] [PubMed] [Google Scholar]
- 50.Cabrera M, Ostrowicz C, Mari M, LaGrassa T, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell. 2009;20:1937–1948. doi: 10.1091/mbc.E08-09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay J, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. [DOI] [PubMed] [Google Scholar]
- 53.Cai H, Zhang Y, Pypaert M, Walker L, Ferro-Novick S. Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. J Cell Biol. 2005;171:823–833. doi: 10.1083/jcb.200505145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai Y, Chin H, Lazarova D, Menon S, Fu C, Cai H, Sclafani A, Rodgers D, De La Cruz E, Ferro-Novick S, Reinisch K. The structural basis for activation of the Rab Ypt1p by the TRAPP membrane-tethering complexes. Cell. 2008;133:1202–1213. doi: 10.1016/j.cell.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callaghan J, Nixon S, Bucci C, Toh B, Stenmark H. Direct interaction of EEA1 with Rab5b. Eur J Biochem. 1999;265:361–366. doi: 10.1046/j.1432-1327.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 56.Callaghan J, Simonsen A, Gaullier J, Toh B, Stenmark H. The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem J. 1999;338:539–543. [PMC free article] [PubMed] [Google Scholar]
- 57.Cantalupo G, Alifano P, Roberti V, Bruni C, Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 2001;20:683–693. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins–unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 59.Carr C, Grote E, Munson M, Hughson F, Novick P. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carroll K, Hanna J, Simon I, Krise J, Barbero P, Pfeffer S. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 2001;292:1373–1376. doi: 10.1126/science.1056791. [DOI] [PubMed] [Google Scholar]
- 61.Casanova J, Wang X, Kumar R, Bhartur S, Navarre J, Woodrum J, Altschuler Y, Ray G, Goldenring J. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casavola E, Catucci A, Bielli P, Di Pentima A, Porcu G, Pennestri M, Cicero D, Ragnini-Wilson A. Ypt32p and Mlc1p bind within the vesicle binding region of the class V myosin Myo2p globular tail domain. Mol Microbiol. 2008;67:1051–1066. doi: 10.1111/j.1365-2958.2008.06106.x. [DOI] [PubMed] [Google Scholar]
- 63.Caswell P, Spence H, Parsons M, White D, Clark K, Cheng K, Mills G, Humphries M, Messent A, Anderson K, McCaffrey M, Ozanne B, Norman J. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Cavanaugh L, Chen X, Richardson B, Ungar D, Pelczer I, Rizo J, Hughson F. Structural analysis of conserved oligomeric Golgi complex subunit 2. J Biol Chem. 2007;282:23418–23426. doi: 10.1074/jbc.M703716200. [DOI] [PubMed] [Google Scholar]
- 65.Chavrier P, Gorvel J, Stelzer E, Simons K, Gruenberg J, Zerial M. Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature. 1991;353:769–772. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- 66.Chavrier P, Parton R, Hauri H, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 67.Chen C, Balch W. The Hsp90 chaperone complex regulates GDI-dependent Rab recycling. Mol Biol Cell. 2006;17:3494–3507. doi: 10.1091/mbc.E05-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, Hu J, Yun Y, Wang T. Rab36 regulates the spatial distribution of late endosomes and lysosomes through a similar mechanism to Rab34. Mol Membr Biol. 2010;27:24–31. doi: 10.3109/09687680903417470. [DOI] [PubMed] [Google Scholar]
- 69.Chen S, Liang M, Chia J, Ngsee J, Ting A. Rab8b and its interacting partner TRIP8b are involved in regulated secretion in AtT20 cells. J Biol Chem. 2001;276:13209–13216. doi: 10.1074/jbc.M010798200. [DOI] [PubMed] [Google Scholar]
- 70.Chen T, Han Y, Yang M, Zhang W, Li N, Wan T, Guo J, Cao X. Rab39, a novel Golgi-associated Rab GTPase from human dendritic cells involved in cellular endocytosis. Biochem Biophys Res Commun. 2003;303:1114–1120. doi: 10.1016/s0006-291x(03)00482-0. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Stevens T. The VPS8 gene is required for localization and trafficking of the CPY sorting receptor in Saccharomyces cerevisiae. Eur J Cell Biol. 1996;70:289–297. [PubMed] [Google Scholar]
- 72.Cheng K, Lahad J, Gray J, Mills G. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–2519. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 73.Cheng K, Lahad J, Kuo W, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu K, Fishman D, Gray J, Mills G. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 74.Cheviet S, Coppola T, Haynes L, Burgoyne R, Regazzi R. The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis. Mol Endocrinol. 2004;18:117–126. doi: 10.1210/me.2003-0300. [DOI] [PubMed] [Google Scholar]
- 75.Chia W, Tang B. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795:110–116. doi: 10.1016/j.bbcan.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Christoforidis S, McBride H, Burgoyne R, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 77.Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip S, Waterfield M, Backer J, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 78.Cohen-Solal K, Sood R, Marin Y, Crespo-Carbone S, Sinsimer D, Martino J, Robbins C, Makalowska I, Trent J, Chen S. Identification and characterization of mouse Rab32 by mRNA and protein expression analysis. Biochim Biophys Acta. 2003;1651:68–75. doi: 10.1016/s1570-9639(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 79.Collins K, Wickner W. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci USA. 2007;104:8755–8760. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collins R. “Getting it on”–GDI displacement and small GTPase membrane recruitment. Mol Cell. 2003;12:1064–1066. doi: 10.1016/s1097-2765(03)00445-3. [DOI] [PubMed] [Google Scholar]
- 81.Conibear E, Cleck J, Stevens T. Vps51p mediates the association of the GARP (Vps52/53/54) complex with the late Golgi t-SNARE Tlg1p. Mol Biol Cell. 2003;14:1610–1623. doi: 10.1091/mbc.E02-10-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooper A, Gitler A, Cashikar A, Haynes C, Hill K, Bhullar B, Liu K, Xu K, Strathearn K, Liu F, Cao S, Caldwell K, Caldwell G, Marsischky G, Kolodner R, Labaer J, Rochet J, Bonini N, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R. Pancreatic beta-cell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell. 2002;13:1906–1915. doi: 10.1091/mbc.02-02-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cormont M, Mari M, Galmiche A, Hofman P, Le Marchand-Brustel Y. A FYVE-finger-containing protein, Rabip4, is a Rab4 effector involved in early endosomal traffic. Proc Natl Acad Sci USA. 2001;98:1637–1642. doi: 10.1073/pnas.031586998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cormont M, Metón I, Mari M, Monzo P, Keslair F, Gaskin C, McGraw T, Le Marchand-Brustel Y. CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic. 2003;4:97–112. doi: 10.1034/j.1600-0854.2003.40205.x. [DOI] [PubMed] [Google Scholar]
- 86.Cortes C, Rzomp K, Tvinnereim A, Scidmore M, Wizel B. Chlamydia pneumoniae inclusion membrane protein Cpn0585 interacts with multiple Rab GTPases. Infect Immun. 2007;75:5586–5596. doi: 10.1128/IAI.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cowles C, Odorizzi G, Payne G, Emr S. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 88.Coxon F, Ebetino F, Mules E, Seabra M, McKenna C, Rogers M. Phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase disrupt the prenylation and membrane localization of Rab proteins in osteoclasts in vitro and in vivo. Bone. 2005;37:349–358. doi: 10.1016/j.bone.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 89.Cremers F, Armstrong S, Seabra M, Brown M, Goldstein J. REP-2, a Rab escort protein encoded by the choroideremia-like gene. J Biol Chem. 1994;269:2111–2117. [PubMed] [Google Scholar]
- 90.Croizet-Berger K, Daumerie C, Couvreur M, Courtoy P, van den Hove M. The endocytic catalysts, Rab5a and Rab7, are tandem regulators of thyroid hormone production. Proc Natl Acad Sci USA. 2002;99:8277–8282. doi: 10.1073/pnas.122187699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.D’Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon A, Oostra B, Wu S, Tandon A, Valtorta F, Balch W, Chelly J, Toniolo D. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet. 1998;19:134–139. doi: 10.1038/487. [DOI] [PubMed] [Google Scholar]
- 92.D’Adamo P, Welzl H, Papadimitriou S, Raffaele di Barletta M, Tiveron C, Tatangelo L, Pozzi L, Chapman P, Knevett S, Ramsay M, Valtorta F, Leoni C, Menegon A, Wolfer D, Lipp H, Toniolo D. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum Mol Genet. 2002;11:2567–2580. doi: 10.1093/hmg/11.21.2567. [DOI] [PubMed] [Google Scholar]
- 93.Dalfó E, Gómez-Isla T, Rosa J, Nieto Bodelón M, Cuadrado Tejedor M, Barrachina M, Ambrosio S, Ferrer I. Abnormal alpha-synuclein interactions with Rab proteins in alpha-synuclein A30P transgenic mice. J Neuropathol Exp Neurol. 2004;63:302–313. doi: 10.1093/jnen/63.4.302. [DOI] [PubMed] [Google Scholar]
- 94.De Graaf P, Zwart W, van Dijken R, Deneka M, Schulz T, Geijsen N, Coffer P, Gadella B, Verkleij A, van der Sluijs P, van Bergen en Henegouwen P. Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol Biol Cell. 2004;15:2038–2047. doi: 10.1091/mbc.E03-12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Leeuw H, Koster P, Calafat J, Janssen H, van Zonneveld A, van Mourik J, Voorberg J. Small GTP-binding proteins in human endothelial cells. Br J Haematol. 1998;103:15–19. doi: 10.1046/j.1365-2141.1998.00965.x. [DOI] [PubMed] [Google Scholar]
- 96.De Luca A, Progida C, Spinosa M, Alifano P, Bucci C. Characterization of the Rab7K157N mutant protein associated with Char-cot-Marie-Tooth type 2B. Biochem Biophys Res Commun. 2008;372:283–287. doi: 10.1016/j.bbrc.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 97.De Renzis S, Sönnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol. 2002;4:124–133. doi: 10.1038/ncb744. [DOI] [PubMed] [Google Scholar]
- 98.Dejgaard S, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig T, Pepperkok R, Simpson J, Presley J. Rab18 and Rab43 have key roles in ER-Golgi trafficking. J Cell Sci. 2008;121:2768–2781. doi: 10.1242/jcs.021808. [DOI] [PubMed] [Google Scholar]
- 99.Del Nery E, Miserey-Lenkei S, Falguières T, Nizak C, Johannes L, Perez F, Goud B. Rab6A and Rab6A’ GTPases play non-overlapping roles in membrane trafficking. Traffic. 2006;7:394–407. doi: 10.1111/j.1600-0854.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 100.Del Toro D, Alberch J, Lázaro-Diéguez F, Martín-Ibáñez R, Xifró X, Egea G, Canals J. Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol Biol Cell. 2009;20:1478–1492. doi: 10.1091/mbc.E08-07-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delprato A, Lambright D. Structural basis for Rab GTPase activation by VPS9 domain exchange factors. Nat Struct Mol Biol. 2007;14:406–412. doi: 10.1038/nsmb1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Desnoyers L, Anant J, Seabra M. Geranylgeranylation of Rab proteins. Biochem Soc Trans. 1996;24:699–703. doi: 10.1042/bst0240699. [DOI] [PubMed] [Google Scholar]
- 103.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 104.Di Pietro S, Dell’Angelica E. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 105.Diao A, Frost L, Morohashi Y, Lowe M. Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner. J Biol Chem. 2008;283:6957–6967. doi: 10.1074/jbc.M708401200. [DOI] [PubMed] [Google Scholar]
- 106.Diao A, Rahman D, Pappin D, Lucocq J, Lowe M. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol. 2003;160:201–212. doi: 10.1083/jcb.200207045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Díaz E, Pfeffer S. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–443. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 108.Díaz E, Schimmöller F, Pfeffer S. A novel Rab9 effector required for endosome-to-TGN transport. J Cell Biol. 1997;138:283–290. doi: 10.1083/jcb.138.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel J, Carraway R, Reeves S. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 110.Dirac-Svejstrup A, Sumizawa T, Pfeffer S. Identification of a GDI displacement factor that releases endosomal Rab GTPases from Rab-GDI. EMBO J. 1997;16:465–472. doi: 10.1093/emboj/16.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dong G, Hutagalung A, Fu C, Novick P, Reinisch K. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat Struct Mol Biol. 2005;12:1094–1100. doi: 10.1038/nsmb1017. [DOI] [PubMed] [Google Scholar]
- 112.Dong G, Medkova M, Novick P, Reinisch K. A catalytic coiled coil: structural insights into the activation of the Rab GTPase Sec4p by Sec2p. Mol Cell. 2007;25:455–462. doi: 10.1016/j.molcel.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dong J, Chen W, Welford A, Wandinger-Ness A. The proteasome alpha-subunit XAPC7 interacts specifically with Rab7 and late endosomes. J Biol Chem. 2004;279:21334–21342. doi: 10.1074/jbc.M401022200. [DOI] [PubMed] [Google Scholar]
- 114.Du L, Novick P. Purification and properties of a GTPase-activating protein for yeast Rab GTPases. Methods Enzymol. 2001;329:91–99. doi: 10.1016/s0076-6879(01)29070-3. [DOI] [PubMed] [Google Scholar]
- 115.Eathiraj S, Pan X, Ritacco C, Lambright D. Structural basis of family-wide Rab GTPase recognition by rabenosyn-5. Nature. 2005;436:415–419. doi: 10.1038/nature03798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Echard A, Jollivet F, Martinez O, Lacapère J, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- 117.Eggenschwiler J, Espinoza E, Anderson K. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- 118.Eggers C, Schafer J, Goldenring J, Taylor S. D-AKAP2 interacts with Rab4 and Rab11 through its RGS domains and regulates transferrin receptor recycling. J Biol Chem. 2009;284:32869–32880. doi: 10.1074/jbc.M109.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eitzen G, Will E, Gallwitz D, Haas A, Wickner W. Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J. 2000;19:6713–6720. doi: 10.1093/emboj/19.24.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Evans T, Ferguson C, Wainwright B, Parton R, Wicking C. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 2003;4:869–884. doi: 10.1046/j.1600-0854.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- 121.Faber P, Barnes G, Srinidhi J, Chen J, Gusella J, MacDonald M. Huntingtin interacts with a family of WW domain proteins. Hum Mol Genet. 1998;7:1463–1474. doi: 10.1093/hmg/7.9.1463. [DOI] [PubMed] [Google Scholar]
- 122.Fabrizi G, Ferrarini M, Cavallaro T, Cabrini I, Cerini R, Bertolasi L, Rizzuto N. Two novel mutations in dynamin-2 cause axonal Charcot-Marie-Tooth disease. Neurology. 2007;69:291–295. doi: 10.1212/01.wnl.0000265820.51075.61. [DOI] [PubMed] [Google Scholar]
- 123.Fagarasanu A, Rachubinski R. Orchestrating organelle inheritance in Saccharomyces cerevisiae. Curr Opin Microbiol. 2007;10:528–538. doi: 10.1016/j.mib.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 124.Fischer J, Weide T, Barnekow A. The MICAL proteins and rab1: a possible link to the cytoskeleton? Biochem Biophys Res Commun. 2005;328:415–423. doi: 10.1016/j.bbrc.2004.12.182. [DOI] [PubMed] [Google Scholar]
- 125.Fischer von Mollard G, Mignery G, Baumert M, Perin M, Hanson T, Burger P, Jahn R, Südhof T. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci USA. 1990;87:1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Forno L. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 127.Foulquier F. COG defects, birth and rise! Biochim Biophys Acta. 2008;1792:896–902. doi: 10.1016/j.bbadis.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 128.Fouraux M, Deneka M, Ivan V, van der Heijden A, Raymackers J, van Suylekom D, van Venrooij W, van der Sluijs P, Pruijn G. Rabip4′ is an effector of rab5 and rab4 and regulates transport through early endosomes. Mol Biol Cell. 2004;15:611–624. doi: 10.1091/mbc.E03-05-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fridmann-Sirkis Y, Siniossoglou S, Pelham H. TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol. 2004;5:18. doi: 10.1186/1471-2121-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman E, De Camilli P, Unger V. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fuchs E, Haas A, Spooner R, Yoshimura S, Lord J, Barr F. Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J Cell Biol. 2007;177:1133–1143. doi: 10.1083/jcb.200612068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- 133.Fukuda M. Synaptotagmin-like protein (Slp) homology domain 1 of Slac2-a/melanophilin is a critical determinant of GTP-dependent specific binding to Rab27A. J Biol Chem. 2002;277:40118–40124. doi: 10.1074/jbc.M205765200. [DOI] [PubMed] [Google Scholar]
- 134.Fukuda M, Itoh T. Direct link between Atg protein and small GTPase Rab: Atg16L functions as a potential Rab33 effector in mammals. Autophagy. 2008;4:824–826. doi: 10.4161/auto.6542. [DOI] [PubMed] [Google Scholar]
- 135.Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics. 2008;7:1031–1042. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- 136.Fukuda M, Kanno E, Yamamoto A. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J Biol Chem. 2004;279:13065–13075. doi: 10.1074/jbc.M306812200. [DOI] [PubMed] [Google Scholar]
- 137.Fukuda M, Kuroda T. Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem. 2002;277:43096–43103. doi: 10.1074/jbc.M203862200. [DOI] [PubMed] [Google Scholar]
- 138.Fukuda M, Kuroda T, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem. 2002;277:12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- 139.Fukui K, Tamura S, Wada A, Kamada Y, Igura T, Kiso S, Hayashi N. Expression of Rab5a in hepatocellular carcinoma: possible involvement in epidermal growth factor signaling. Hepatol Res. 2007;37:957–965. doi: 10.1111/j.1872-034X.2007.00143.x. [DOI] [PubMed] [Google Scholar]
- 140.Lee M, Mishra A, Lambright D. Structural mechanisms for regulation of membrane traffic by Rab GTPases. Traffic. 2009;10:1377–1389. doi: 10.1111/j.1600-0854.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gasser T. Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med. 2009;11:e22. doi: 10.1017/S1462399409001148. [DOI] [PubMed] [Google Scholar]
- 142.Gedeon A, Colley A, Jamieson R, Thompson E, Rogers J, Sillence D, Tiller G, Mulley J, Gécz J. Identification of the gene (SEDL) causing X-linked spondyloepiphyseal dysplasia tarda. Nat Genet. 1999;22:400–404. doi: 10.1038/11976. [DOI] [PubMed] [Google Scholar]
- 143.Gedeon A, Tiller G, Le Merrer M, Heuertz S, Tranebjaerg L, Chitayat D, Robertson S, Glass I, Savarirayan R, Cole W, Rimoin D, Kousseff B, Ohashi H, Zabel B, Munnich A, Gecz J, Mulley J. The molecular basis of X-linked spondyloepiphyseal dysplasia tarda. Am J Hum Genet. 2001;68:1386–1397. doi: 10.1086/320592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Geppert M, Bolshakov V, Siegelbaum S, Takei K, De Camilli P, Hammer R, Südhof T. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- 145.Geppert M, Goda Y, Stevens C, Südhof T. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 146.Gil J, Rego A. Mechanisms of neurodegeneration in Huntington’s disease. Eur J Neurosci. 2008;27:2803–2820. doi: 10.1111/j.1460-9568.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- 147.Giovedì S, Darchen F, Valtorta F, Greengard P, Benfenati F. Synapsin is a novel Rab3 effector protein on small synaptic vesicles. II. Functional effects of the Rab3A-synapsin I interaction. J Biol Chem. 2004;279:43769–43779. doi: 10.1074/jbc.M404168200. [DOI] [PubMed] [Google Scholar]
- 148.Girod A, Storrie B, Simpson J, Johannes L, Goud B, Roberts L, Lord J, Nilsson T, Pepperkok R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat Cell Biol. 1999;1:423–430. doi: 10.1038/15658. [DOI] [PubMed] [Google Scholar]
- 149.Gitler A, Bevis B, Shorter J, Strathearn K, Hamamichi S, Su L, Caldwell K, Caldwell G, Rochet J, McCaffery J, Barlowe C, Lindquist S. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goedert M. Neurofibrillary pathology of Alzheimer’s disease and other tauopathies. Prog Brain Res. 1998;117:287–306. doi: 10.1016/s0079-6123(08)64022-4. [DOI] [PubMed] [Google Scholar]
- 151.Goldenring J, Shen K, Vaughan H, Modlin I. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, lung. J Biol Chem. 1993;268:18419–18422. [PubMed] [Google Scholar]
- 152.Gomes A, Ali B, Ramalho J, Godfrey R, Barral D, Hume A, Seabra M. Membrane targeting of Rab GTPases is influenced by the prenylation motif. Mol Biol Cell. 2003;14:1882–1899. doi: 10.1091/mbc.E02-10-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goody R, Rak A, Alexandrov K. The structural and mechanistic basis for recycling of Rab proteins between membrane compartments. Cell Mol Life Sci. 2005;62:1657–1670. doi: 10.1007/s00018-005-4486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goud B, Salminen A, Walworth N, Novick P. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- 155.Goud B, Zahraoui A, Tavitian A, Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990;345:553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- 156.Gournier H, Stenmark H, Rybin V, Lippé R, Zerial M. Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion. EMBO J. 1998;17:1930–1940. doi: 10.1093/emboj/17.7.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Graham M, Handley M, Barclay J, Ciufo L, Barrow S, Morgan A, Burgoyne R. A gain-of-function mutant of Munc18–1 stimulates secretory granule recruitment and exocytosis and reveals a direct interaction of Munc18–1 with Rab3. Biochem J. 2008;409:407–416. doi: 10.1042/BJ20071094. [DOI] [PubMed] [Google Scholar]
- 158.Grigoriev I, Splinter D, Keijzer N, Wulf P, Demmers J, Ohtsuka T, Modesti M, Maly I, Grosveld F, Hoogenraad C, Akh-manova A. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 159.Grosshans B, Andreeva A, Gangar A, Niessen S, Yates Brennwald P, Jr, Novick P. The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. J Cell Biol. 2006;172:55–66. doi: 10.1083/jcb.200510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Grosshans B, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grote E, Carr C, Novick P. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grunebaum E, Arpaia E, MacKenzie J, Fitzpatrick J, Ray P, Roifman C. A missense mutation in the SEDL gene results in delayed onset of X linked spondyloepiphyseal dysplasia in a large pedigree. J Med Genet. 2001;38:409–411. doi: 10.1136/jmg.38.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guignot J, Caron E, Beuzón C, Bucci C, Kagan J, Roy C, Holden D. Microtubule motors control membrane dynamics of Salmonella-containing vacuoles. J Cell Sci. 2004;117:1033–1045. doi: 10.1242/jcs.00949. [DOI] [PubMed] [Google Scholar]
- 164.Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guo W, Tamanoi F, Novick P. Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol. 2001;3:353–360. doi: 10.1038/35070029. [DOI] [PubMed] [Google Scholar]
- 166.Günther T, Struwe M, Aguzzi A, Schughart K. Open brain, a new mouse mutant with severe neural tube defects, shows altered gene expression patterns in the developing spinal cord. Development. 1994;120:3119–3130. doi: 10.1242/dev.120.11.3119. [DOI] [PubMed] [Google Scholar]
- 167.Haas A, Fuchs E, Kopajtich R, Barr F. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat Cell Biol. 2005;7:887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- 168.Hagiwara M, Shirai Y, Nomura R, Sasaki M, Kobayashi K, Tadokoro T, Yamamoto Y. Caveolin-1 activates Rab5 and enhances endocytosis through direct interaction. Biochem Biophys Res Commun. 2009;378:73–78. doi: 10.1016/j.bbrc.2008.10.172. [DOI] [PubMed] [Google Scholar]
- 169.Halachmi N, Lev Z. The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- 170.Hales C, Griner R, Hobdy-Henderson K, Dorn M, Hardy D, Kumar R, Navarre J, Chan E, Lapierre L, Goldenring J. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 171.Hales C, Vaerman J, Goldenring J. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 172.Hanna J, Carroll K, Pfeffer S. Identification of residues in TIP47 essential for Rab9 binding. Proc Natl Acad Sci USA. 2002;99:7450–7454. doi: 10.1073/pnas.112198799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harrison R, Brumell J, Khandani A, Bucci C, Scott C, Jiang X, Finlay B, Grinstein S. Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol Biol Cell. 2004;15:3146–3154. doi: 10.1091/mbc.E04-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hattula K, Furuhjelm J, Arffman A, Peränen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13:3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hattula K, Peränen J. FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr Biol. 2000;10:1603–1606. doi: 10.1016/s0960-9822(00)00864-2. [DOI] [PubMed] [Google Scholar]
- 176.Hayakawa A, Hayes S, Leonard D, Lambright D, Corvera S. Evolutionarily conserved structural and functional roles of the FYVE domain. Biochem Soc Symp. 2007:95–105. doi: 10.1042/BSS0740095. [DOI] [PubMed] [Google Scholar]
- 177.Hayes G, Brown F, Haas A, Nottingham R, Barr F, Pfeffer S. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol Biol Cell. 2009;20:209–217. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haynes L, Evans G, Morgan A, Burgoyne R. A direct inhibitory role for the Rab3-specific effector, Noc2, in Ca2+-regulated exocytosis in neuroendocrine cells. J Biol Chem. 2001;276:9726–9732. doi: 10.1074/jbc.M006959200. [DOI] [PubMed] [Google Scholar]
- 179.Heidrych P, Zimmermann U, Bress A, Pusch C, Ruth P, Pfister M, Knipper M, Blin N. Rab8b GTPase, a protein transport regulator, is an interacting partner of otoferlin, defective in a human autosomal recessive deafness form. Hum Mol Genet. 2008;17:3814–3821. doi: 10.1093/hmg/ddn279. [DOI] [PubMed] [Google Scholar]
- 180.Hennies H, Kornak U, Zhang H, Egerer J, Zhang X, Seifert W, Kühnisch J, Budde B, Nätebus M, Brancati F, Wilcox W, Müller D, Kaplan P, Rajab A, Zampino G, Fodale V, Dallapiccola B, Newman W, Metcalfe K, Clayton-Smith J, Tassabehji M, Steinmann B, Barr F, Nürnberg P, Wieacker P, Mundlos S. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008;40:1410–1412. doi: 10.1038/ng.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Henry T, Couillault C, Rockenfeller P, Boucrot E, Dumont A, Schroeder N, Hermant A, Knodler L, Lecine P, Steele-Mortimer O, Borg J, Gorvel J, Méresse S. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc Natl Acad Sci USA. 2006;103:13497–13502. doi: 10.1073/pnas.0605443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hickson G, Matheson J, Riggs B, Maier V, Fielding A, Prekeris R, Sullivan W, Barr F, Gould G. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell. 2003;14:2908–2920. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hidestrand P, Vasconez H, Cottrill C. Carpenter syndrome. J Craniofac Surg. 2009;20:254–256. doi: 10.1097/SCS.0b013e318184357a. [DOI] [PubMed] [Google Scholar]
- 184.Hill E, Clarke M, Barr F. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 2000;19:5711–5719. doi: 10.1093/emboj/19.21.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ho L, Carmichael J, Swartz J, Wyttenbach A, Rankin J, Rubinsztein D. The molecular biology of Huntington’s disease. Psychol Med. 2001;31:3–14. doi: 10.1017/s0033291799002871. [DOI] [PubMed] [Google Scholar]
- 186.Horazdovsky B, Cowles C, Mustol P, Holmes M, Emr S. A novel RING finger protein, Vps8p, functionally interacts with the small GTPase, Vps21p, to facilitate soluble vacuolar protein localization. J Biol Chem. 1996;271:33607–33615. doi: 10.1074/jbc.271.52.33607. [DOI] [PubMed] [Google Scholar]
- 187.Horazdovsky B, Davies B, Seaman M, McLaughlin S, Yoon S, Emr S. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell. 1997;8:1529–1541. doi: 10.1091/mbc.8.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Horiuchi H, Lippé R, McBride H, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 189.Houlden H, King R, Muddle J, Warner T, Reilly M, Orrell R, Ginsberg L. A novel RAB7 mutation associated with ulcero-mutilating neuropathy. Ann Neurol. 2004;56:586–590. doi: 10.1002/ana.20281. [DOI] [PubMed] [Google Scholar]
- 190.Hsu S, Hazuka C, Roth R, Foletti D, Heuser J, Scheller R. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–1122. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 191.Huber L, Pimplikar S, Parton R, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hume A, Collinson L, Rapak A, Gomes A, Hopkins C, Seabra M. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 2001;152:795–808. doi: 10.1083/jcb.152.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hunziker W, Peters P. Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem. 1998;273:15734–15741. doi: 10.1074/jbc.273.25.15734. [DOI] [PubMed] [Google Scholar]
- 194.Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J. 2006;25:3750–3761. doi: 10.1038/sj.emboj.7601274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Höning S, Ricotta D, Krauss M, Späte K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen D. Phosphatidyl-inositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 196.Ibáñez P, Bonnet A, Débarges B, Lohmann E, Tison F, Pollak P, Agid Y, Dürr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 197.Ignatev A, Kravchenko S, Rak A, Goody R, Pylypenko O. A structural model of the GDP dissociation inhibitor rab membrane extraction mechanism. J Biol Chem. 2008;283:18377–18384. doi: 10.1074/jbc.M709718200. [DOI] [PubMed] [Google Scholar]
- 198.Iida H, Wang L, Nishii K, Ookuma A, Shibata Y. Identification of rab12 as a secretory granule-associated small GTP-binding protein in atrial myocytes. Circ Res. 1996;78:343–347. doi: 10.1161/01.res.78.2.343. [DOI] [PubMed] [Google Scholar]
- 199.Ikonomov O, Sbrissa D, Mlak K, Deeb R, Fligger J, Soans A, Finley RJ, Shisheva A. Active PIKfyve associates with and promotes the membrane attachment of the late endosome-to-trans-Golgi network transport factor Rab9 effector p40. J Biol Chem. 2003;278:50863–50871. doi: 10.1074/jbc.M307260200. [DOI] [PubMed] [Google Scholar]
- 200.Itoh T, Fujita N, Kanno E, Yamamoto A, Yoshimori T, Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol Biol Cell. 2008;19:2916–2925. doi: 10.1091/mbc.E07-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Itoh T, Watabe A, Toh-EA, Matsui Y. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22:7744–7757. doi: 10.1128/MCB.22.22.7744-7757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Itzen A, Pylypenko O, Goody R, Alexandrov K, Rak A. Nucleotide exchange via local protein unfolding: structure of Rab8 in complex with MSS4. EMBO J. 2006;25:1445–1455. doi: 10.1038/sj.emboj.7601044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Itzen A, Rak A, Goody R. Sec2 is a highly efficient exchange factor for the Rab protein Sec4. J Mol Biol. 2007;365:1359–1367. doi: 10.1016/j.jmb.2006.10.096. [DOI] [PubMed] [Google Scholar]
- 204.Jackson A, Flett A, Smythe C, Hufton L, Wettey F, Smythe E. Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase. J Cell Biol. 2003;163:231–236. doi: 10.1083/jcb.200304079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jackson L, Nawabi P, Hentea C, Roark E, Haldar K. The Salmonella virulence protein SifA is a G protein antagonist. Proc Natl Acad Sci USA. 2008;105:14141–14146. doi: 10.1073/pnas.0801872105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Janoueix-Lerosey I, Jollivet F, Camonis J, Marche P, Goud B. Two-hybrid system screen with the small GTP-binding protein Rab6. Identification of a novel mouse GDP dissociation inhibitor isoform and two other potential partners of Rab6. J Biol Chem. 1995;270:14801–14808. doi: 10.1074/jbc.270.24.14801. [DOI] [PubMed] [Google Scholar]
- 207.Jasmin B, Goud B, Camus G, Cartaud J. The low molecular weight guanosine triphosphate-binding protein Rab6p associates with distinct post-Golgi vesicles in Torpedo marmorata electrocytes. Neuroscience. 1992;49:849–855. doi: 10.1016/0306-4522(92)90361-5. [DOI] [PubMed] [Google Scholar]
- 208.Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jeffries T, Morgan G, Field M. TbRAB18, a developmentally regulated Golgi GTPase from Trypanosoma brucei. Mol Biochem Parasitol. 2002;121:63–74. doi: 10.1016/s0166-6851(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 211.Jenkins D, Seelow D, Jehee F, Perlyn C, Alonso L, Bueno D, Donnai D, Josifova D, Josifiova D, Mathijssen I, Morton J, Orstavik K, Sweeney E, Wall S, Marsh J, Nurnberg P, Passos-Bueno M, Wilkie A. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Johansson M, Lehto M, Tanhuanpää K, Cover T, Olkkonen V. The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol Biol Cell. 2005;16:5480–5492. doi: 10.1091/mbc.E05-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen V, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 215.Junutula J, De Maziére A, Peden A, Ervin K, Advani R, van Dijk S, Klumperman J, Scheller R. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218–2229. doi: 10.1091/mbc.E03-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jäger D, Stockert E, Jäger E, Güre A, Scanlan M, Knuth A, Old L, Chen Y. Serological cloning of a melanocyte rab guanosine 5′-triphosphate-binding protein and a chromosome condensation protein from a melanoma complementary DNA library. Cancer Res. 2000;60:3584–3591. [PubMed] [Google Scholar]
- 217.Kail M, Barnekow A. Identification and characterization of interacting partners of Rab GTPases by yeast two-hybrid analyses. Methods Mol Biol. 2008;440:111–125. doi: 10.1007/978-1-59745-178-9_9. [DOI] [PubMed] [Google Scholar]
- 218.Kanno E, Ishibashi K, Kobayashi H, Matsui T, Ohbayashi N, Fukuda M. Comprehensive screening for novel rab-binding proteins by GST pull-down assay using 60 different mammalian Rabs. Traffic. 2010;11:491–507. doi: 10.1111/j.1600-0854.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 219.Karki S, Holzbaur E. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 220.Kauppi M, Simonsen A, Bremnes B, Vieira A, Callaghan J, Stenmark H, Olkkonen V. The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. J Cell Sci. 2002;115:899–911. doi: 10.1242/jcs.115.5.899. [DOI] [PubMed] [Google Scholar]
- 221.Kelly E, Horgan C, Adams C, Patzer T, Ní Shúilleabháin D, Norman J, McCaffrey M. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol Cell. 2009;102:51–62. doi: 10.1042/BC20090068. [DOI] [PubMed] [Google Scholar]
- 222.Kim D, Sacher M, Scarpa A, Quinn A, Ferro-Novick S. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol Biol Cell. 1999;10:3317–3329. doi: 10.1091/mbc.10.10.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim Y, Raunser S, Munger C, Wagner J, Song Y, Cygler M, Walz T, Oh B, Sacher M. The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell. 2006;127:817–830. doi: 10.1016/j.cell.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 224.Kloer D, Rojas R, Ivan V, Moriyama K, van Vlijmen T, Murthy N, Ghirlando R, van der Sluijs P, Hurley J, Bonifacino J. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J Biol Chem. 2010;285:7794–7804. doi: 10.1074/jbc.M109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 226.Kraynack B, Chan A, Rosenthal E, Essid M, Umansky B, Waters M, Schmitt H. Dsl1p, Tip20p, and the novel Dsl3(Sec39) protein are required for the stability of the Q/t-SNARE complex at the endoplasmic reticulum in yeast. Mol Biol Cell. 2005;16:3963–3977. doi: 10.1091/mbc.E05-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kuroda T, Fukuda M, Ariga H, Mikoshiba K. Synaptotagmin-like protein 5: a novel Rab27A effector with C-terminal tandem C2 domains. Biochem Biophys Res Commun. 2002;293:899–906. doi: 10.1016/S0006-291X(02)00320-0. [DOI] [PubMed] [Google Scholar]
- 228.Kuroda T, Fukuda M, Ariga H, Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1–4 and Slac2 functions as a novel Rab27A binding domain. J Biol Chem. 2002;277:9212–9218. doi: 10.1074/jbc.M112414200. [DOI] [PubMed] [Google Scholar]
- 229.Köhler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 2004;165:175–180. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lapierre L, Kumar R, Hales C, Navarre J, Bhartur S, Burnette J, Provance DJ, Mercer J, Bähler M, Goldenring J. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Larance M, Ramm G, Stöckli J, van Dam E, Winata S, Wasinger V, Simpson F, Graham M, Junutula J, Guilhaus M, James D. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 232.Laufman O, Kedan A, Hong W, Lev S. Direct interaction between the COG complex and the SM protein, Sly1, is required for Golgi SNARE pairing. EMBO J. 2009;28:2006–2017. doi: 10.1038/emboj.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lawe D, Patki V, Heller-Harrison R, Lambright D, Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J Biol Chem. 2000;275:3699–3705. doi: 10.1074/jbc.275.5.3699. [DOI] [PubMed] [Google Scholar]
- 234.Lawson M, Coulton L, Ebetino F, Vanderkerken K, Croucher P. Geranylgeranyl transferase type II inhibition prevents myeloma bone disease. Biochem Biophys Res Commun. 2008;377:453–457. doi: 10.1016/j.bbrc.2008.09.157. [DOI] [PubMed] [Google Scholar]
- 235.Leaf D, Blum L. Analysis of rab10 localization in sea urchin embryonic cells by three-dimensional reconstruction. Exp Cell Res. 1998;243:39–49. doi: 10.1006/excr.1997.3917. [DOI] [PubMed] [Google Scholar]
- 236.Lee R, Iioka H, Ohashi M, Iemura S, Natsume T, Kinoshita N. XRab40 and XCullin5 form a ubiquitin ligase complex essential for the noncanonical Wnt pathway. EMBO J. 2007;26:3592–3606. doi: 10.1038/sj.emboj.7601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li L, Omata W, Kojima I, Shibata H. Direct interaction of Rab4 with syntaxin 4. J Biol Chem. 2001;276:5265–5273. doi: 10.1074/jbc.M003883200. [DOI] [PubMed] [Google Scholar]
- 238.Li X, Sapp E, Chase K, Comer-Tierney L, Masso N, Alexander J, Reeves P, Kegel K, Valencia A, Esteves M, Aronin N, Difiglia M. Disruption of Rab11 activity in a knock-in mouse model of Huntington’s disease. Neurobiol Dis. 2009;36:374–383. doi: 10.1016/j.nbd.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li X, Sapp E, Valencia A, Kegel K, Qin Z, Alexander J, Masso N, Reeves P, Ritch J, Zeitlin S, Aronin N, Difiglia M. A function of huntingtin in guanine nucleotide exchange on Rab11. Neuroreport. 2008;19:1643–1647. doi: 10.1097/WNR.0b013e328315cd4c. [DOI] [PubMed] [Google Scholar]
- 240.Li X, Standley C, Sapp E, Valencia A, Qin Z, Kegel K, Yoder J, Comer-Tierney L, Esteves M, Chase K, Alexander J, Masso N, Sobin L, Bellve K, Tuft R, Lifshitz L, Fogarty K, Aronin N, DiFiglia M. Mutant huntingtin impairs vesicle formation from recycling endosomes by interfering with Rab11 activity. Mol Cell Biol. 2009;29:6106–6116. doi: 10.1128/MCB.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li Y, Meng X, Feng H, Zhang G, Liu C, Li P. Over-expression of the RAB5 gene in human lung adenocarcinoma cells with high metastatic potential. Chin Med Sci J. 1999;14:96–101. [PubMed] [Google Scholar]
- 242.Li Y, Wandinger-Ness A, Goldenring J, Cover T. Clustering and redistribution of late endocytic compartments in response to Helicobacter pylori vacuolating toxin. Mol Biol Cell. 2004;15:1946–1959. doi: 10.1091/mbc.E03-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lian J, Stone S, Jiang Y, Lyons P, Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature. 1994;372:698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- 244.Liewen H, Meinhold-Heerlein I, Oliveira V, Schwarzenbacher R, Luo G, Wadle A, Jung M, Pfreundschuh M, Stenner-Liewen F. Characterization of the human GARP (Golgi associated retrograde protein) complex. Exp Cell Res. 2005;306:24–34. doi: 10.1016/j.yexcr.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 245.Lindsay A, Hendrick A, Cantalupo G, Senic-Matuglia F, Goud B, Bucci C, McCaffrey M. Rab coupling protein (RCP), a novel Rab4 and Rab11 effector protein. J Biol Chem. 2002;277:12190–12199. doi: 10.1074/jbc.M108665200. [DOI] [PubMed] [Google Scholar]
- 246.Lindsay A, McCaffrey M. Rab11-FIP2 functions in transferrin recycling and associates with endosomal membranes via its COOH-terminal domain. J Biol Chem. 2002;277:27193–27199. doi: 10.1074/jbc.M200757200. [DOI] [PubMed] [Google Scholar]
- 247.Lipatova Z, Tokarev A, Jin Y, Mulholland J, Weisman L, Segev N. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol Biol Cell. 2008;19:4177–4187. doi: 10.1091/mbc.E08-02-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lippé R, Miaczynska M, Rybin V, Runge A, Zerial M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell. 2001;12:2219–2228. doi: 10.1091/mbc.12.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Loftus S, Larson D, Baxter L, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer, Pavan W. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci USA. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lombardi D, Soldati T, Riederer M, Goda Y, Zerial M, Pfeffer S. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Losev E, Reinke C, Jellen J, Strongin D, Bevis B, Glick B. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- 252.Lynch-Day M, Bhandari D, Menon S, Huang J, Cai H, Bartholomew C, Brumell J, Ferro-Novick S, Klionsky D. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci USA. 2010;107:7811–7816. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lütcke A, Jansson S, Parton R, Chavrier P, Valencia A, Huber L, Lehtonen E, Zerial M. Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell polarization. J Cell Biol. 1993;121:553–564. doi: 10.1083/jcb.121.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lütcke A, Olkkonen V, Dupree P, Lütcke H, Simons K, Zerial M. Isolation of a murine cDNA clone encoding Rab19, a novel tissue-specific small GTPase. Gene. 1995;155:257–260. doi: 10.1016/0378-1119(94)00931-h. [DOI] [PubMed] [Google Scholar]
- 255.Lütcke A, Parton R, Murphy C, Olkkonen V, Dupree P, Valencia A, Simons K, Zerial M. Cloning and subcellular localization of novel rab proteins reveals polarized and cell type-specific expression. J Cell Sci. 1994;107:3437–3448. doi: 10.1242/jcs.107.12.3437. [DOI] [PubMed] [Google Scholar]
- 256.Machner M, Isberg R. A bifunctional bacterial protein links GDI displacement to Rab1 activation. Science. 2007;318:974–977. doi: 10.1126/science.1149121. [DOI] [PubMed] [Google Scholar]
- 257.Machner M, Isberg R. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 258.MacKenzie J, Fitzpatrick J, Babyn P, Ferrero G, Ballabio A, Billingsley G, Bulman D, Strasberg P, Ray P, Costa T. X linked spondyloepiphyseal dysplasia: a clinical, radiological, molecular study of a large kindred. J Med Genet. 1996;33:823–828. doi: 10.1136/jmg.33.10.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mallard F, Tang B, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–664. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mammoto A, Ohtsuka T, Hotta I, Sasaki T, Takai Y. Rab11BP/Rabphilin-11, a downstream target of rab11 small G protein implicated in vesicle recycling. J Biol Chem. 1999;274:25517–25524. doi: 10.1074/jbc.274.36.25517. [DOI] [PubMed] [Google Scholar]
- 261.Martinez O, Antony C, Pehau-Arnaudet G, Berger E, Salamero J, Goud B. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc Natl Acad Sci USA. 1997;94:1828–1833. doi: 10.1073/pnas.94.5.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Martinez O, Schmidt A, Salaméro J, Hoflack B, Roa M, Goud B. The small GTP-binding protein rab6 functions in intra-Golgi transport. J Cell Biol. 1994;127:1575–1588. doi: 10.1083/jcb.127.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Masuda E, Luo Y, Young C, Shen M, Rossi A, Huang B, Yu S, Bennett M, Payan D, Scheller R. Rab37 is a novel mast cell specific GTPase localized to secretory granules. FEBS Lett. 2000;470:61–64. doi: 10.1016/s0014-5793(00)01288-6. [DOI] [PubMed] [Google Scholar]
- 264.Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw C, Barnekow A, Hoogenraad C. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- 265.Matesic L, Yip R, Reuss A, Swing D, O’Sullivan T, Fletcher C, Copeland N, Jenkins N. Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc Natl Acad Sci USA. 2001;98:10238–10243. doi: 10.1073/pnas.181336698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 267.Matteoli M, Takei K, Cameron R, Hurlbut P, Johnston P, Südhof T, Jahn R, De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J Cell Biol. 1991;115:625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.McGrail M, Gepner J, Silvanovich A, Ludmann S, Serr M, Hays T. Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex. J Cell Biol. 1995;131:411–425. doi: 10.1083/jcb.131.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Medkova M, France Y, Coleman J, Novick P. The rab exchange factor Sec2p reversibly associates with the exocyst. Mol Biol Cell. 2006;17:2757–2769. doi: 10.1091/mbc.E05-10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Meggouh F, Bienfait H, Weterman M, de Visser M, Baas F. Charcot-Marie-Tooth disease due to a de novo mutation of the RAB7 gene. Neurology. 2006;67:1476–1478. doi: 10.1212/01.wnl.0000240068.21499.f5. [DOI] [PubMed] [Google Scholar]
- 271.Menon S, Cai H, Lu H, Dong G, Cai Y, Reinisch K, Ferro-Novick S. mBET3 is required for the organization of the TRAPP complexes. Biochem Biophys Res Commun. 2006;350:669–677. doi: 10.1016/j.bbrc.2006.09.096. [DOI] [PubMed] [Google Scholar]
- 272.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton R, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 273.Mills I, Jones A, Clague M. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- 274.Mills I, Jones A, Clague M. Regulation of endosome fusion. Mol Membr Biol. 1999;16:73–79. doi: 10.1080/096876899294788. [DOI] [PubMed] [Google Scholar]
- 275.Mir A, Kaufman L, Noor A, Motazacker M, Jamil T, Azam M, Kahrizi K, Rafiq M, Weksberg R, Nasr T, Naeem F, Tzschach A, Kuss A, Ishak G, Doherty D, Ropers H, Barkovich A, Najmabadi H, Ayub M, Vincent J. Identification of mutations in TRAPPC9, which encodes the NIK- and IKK-beta-binding protein, in nonsyndromic autosomal-recessive mental retardation. Am J Hum Genet. 2009;85:909–915. doi: 10.1016/j.ajhg.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Miserey-Lenkei S, Waharte F, Boulet A, Cuif M, Tenza D, El Marjou A, Raposo G, Salamero J, Héliot L, Goud B, Monier S. Rab6-interacting protein 1 links Rab6 and Rab11 function. Traffic. 2007;8:1385–1403. doi: 10.1111/j.1600-0854.2007.00612.x. [DOI] [PubMed] [Google Scholar]
- 277.Mizuno K, Kitamura A, Sasaki T. Rabring7, a novel Rab7 target protein with a RING finger motif. Mol Biol Cell. 2003;14:3741–3752. doi: 10.1091/mbc.E02-08-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mizuta R, LaSalle J, Cheng H, Shinohara A, Ogawa H, Copeland N, Jenkins N, Lalande M, Alt F. RAB22 and RAB163/mouse BRCA2: proteins that specifically interact with the RAD51 protein. Proc Natl Acad Sci USA. 1997;94:6927–6932. doi: 10.1073/pnas.94.13.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mochida G, Mahajnah M, Hill A, Basel-Vanagaite L, Gleason D, Hill R, Bodell A, Crosier M, Straussberg R, Walsh C. A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly. Am J Hum Genet. 2009;85:897–902. doi: 10.1016/j.ajhg.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Monier S, Jollivet F, Janoueix-Lerosey I, Johannes L, Goud B. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3:289–297. doi: 10.1034/j.1600-0854.2002.030406.x. [DOI] [PubMed] [Google Scholar]
- 281.Moore B, Robinson H, Xu Z. The crystal structure of mouse Exo70 reveals unique features of the mammalian exocyst. J Mol Biol. 2007;371:410–421. doi: 10.1016/j.jmb.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mosesson Y, Mills G, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 283.Moya M, Roberts D, Novick P. DSS4–1 is a dominant suppressor of sec4–8 that encodes a nucleotide exchange protein that aids Sec4p function. Nature. 1993;361:460–463. doi: 10.1038/361460a0. [DOI] [PubMed] [Google Scholar]
- 284.Moyer B, Allan B, Balch W. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic. 2001;2:268–276. doi: 10.1034/j.1600-0854.2001.1o007.x. [DOI] [PubMed] [Google Scholar]
- 285.Mu F, Callaghan J, Steele-Mortimer O, Stenmark H, Parton R, Campbell P, McCluskey J, Yeo J, Tock E, Toh B. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 286.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 287.Murata T, Delprato A, Ingmundson A, Toomre D, Lambright D, Roy C. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- 288.Méresse S, Steele-Mortimer O, Finlay B, Gorvel J. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 1999;18:4394–4403. doi: 10.1093/emboj/18.16.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Müller M, Peters H, Blümer J, Blankenfeldt W, Goody R, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- 290.Nachury M, Loktev A, Zhang Q, Westlake C, Peränen J, Merdes A, Slusarski D, Scheller R, Bazan J, Sheffield V, Jackson P. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 291.Nagelkerken B, Van Anken E, Van Raak M, Gerez L, Mohrmann K, Van Uden N, Holthuizen J, Pelkmans L, Van Der Sluijs P. Rabaptin4, a novel effector of the small GTPase rab4a, is recruited to perinuclear recycling vesicles. Biochem J. 2000;346:593–601. [PMC free article] [PubMed] [Google Scholar]
- 292.Nakada-Tsukui K, Saito-Nakano Y, Ali V, Nozaki T. A retro-merlike complex is a novel Rab7 effector that is involved in the transport of the virulence factor cysteine protease in the enteric protozoan parasite Entamoeba histolytica. Mol Biol Cell. 2005;16:5294–5303. doi: 10.1091/mbc.E05-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Nakajima H, Hirata A, Ogawa Y, Yonehara T, Yoda K, Yamasaki M. A cytoskeleton-related gene, uso1, is required for intra-cellular protein transport in Saccharomyces cerevisiae. J Cell Biol. 1991;113:245–260. doi: 10.1083/jcb.113.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nakamura N, Lowe M, Levine T, Rabouille C, Warren G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89:445–455. doi: 10.1016/s0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 295.Nguyen U, Guo Z, Delon C, Wu Y, Deraeve C, Fränzel B, Bon R, Blankenfeldt W, Goody R, Waldmann H, Wolters D, Alexandrov K. Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat Chem Biol. 2009;5:227–235. doi: 10.1038/nchembio.149. [DOI] [PubMed] [Google Scholar]
- 296.Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nishimura N, Araki K, Shinahara W, Nakano Y, Nishimura K, Higashio H, Sasaki T. Interaction of Rab3B with microtubule-binding protein Gas8 in NIH 3T3 cells. Arch Biochem Biophys. 2008;474:136–142. doi: 10.1016/j.abb.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 298.Norris F, Wilson M, Wallis T, Galyov E, Majerus P. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc Natl Acad Sci USA. 1998;95:14057–14059. doi: 10.1073/pnas.95.24.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nuoffer C, Wu S, Dascher C, Balch W. Mss4 does not function as an exchange factor for Rab in endoplasmic reticulum to Golgi transport. Mol Biol Cell. 1997;8:1305–1316. doi: 10.1091/mbc.8.7.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Oka T, Ungar D, Hughson F, Krieger M. The COG and COPI complexes interact to control the abundance of GEARs, a subset of Golgi integral membrane proteins. Mol Biol Cell. 2004;15:2423–2435. doi: 10.1091/mbc.E03-09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Olkkonen V, Dupree P, Killisch I, Lütcke A, Zerial M, Simons K. Molecular cloning and subcellular localization of three GTP-binding proteins of the rab subfamily. J Cell Sci. 1993;106:1249–1261. doi: 10.1242/jcs.106.4.1249. [DOI] [PubMed] [Google Scholar]
- 302.Olkkonen V, Peterson J, Dupree P, Lütcke A, Zerial M, Simons K. Isolation of a mouse cDNA encoding Rab23, a small novel GTPase expressed predominantly in the brain. Gene. 1994;138:207–211. doi: 10.1016/0378-1119(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 303.Opdam F, Echard A, Croes H, van den Hurk J, van de Vorstenbosch R, Ginsel L, Goud B, Fransen J. The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J Cell Sci. 2000;113:2725–2735. doi: 10.1242/jcs.113.15.2725. [DOI] [PubMed] [Google Scholar]
- 304.Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles: evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ostermeier C, Brunger A. Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin-3A. Cell. 1999;96:363–374. doi: 10.1016/s0092-8674(00)80549-8. [DOI] [PubMed] [Google Scholar]
- 306.Ostrowicz C, Bröcker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, Perz A, Auffarth K, Engelbrecht-Vandré S, Ungermann C. Defined subunit arrangement and Rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010 doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 307.Overmeyer J, Wilson A, Maltese W. Membrane targeting of a Rab GTPase that fails to associate with Rab escort protein (REP) or guanine nucleotide dissociation inhibitor (GDI) J Biol Chem. 2001;276:20379–20386. doi: 10.1074/jbc.M101511200. [DOI] [PubMed] [Google Scholar]
- 308.Owen D, Collins B, Evans P. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 309.Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci. 2005;118:2601–2611. doi: 10.1242/jcs.02401. [DOI] [PubMed] [Google Scholar]
- 310.Pal A, Severin F, Lommer B, Shevchenko A, Zerial M. Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington’s disease. J Cell Biol. 2006;172:605–618. doi: 10.1083/jcb.200509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Pan X, Eathiraj S, Munson M, Lambright D. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature. 2006;442:303–306. doi: 10.1038/nature04847. [DOI] [PubMed] [Google Scholar]
- 312.Panic B, Perisic O, Veprintsev D, Williams R, Munro S. Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol Cell. 2003;12:863–874. doi: 10.1016/s1097-2765(03)00356-3. [DOI] [PubMed] [Google Scholar]
- 313.Park M, Serpinskaya A, Papalopulu N, Gelfand V. Rab32 regulates melanosome transport in Xenopus melanophores by protein kinase a recruitment. Curr Biol. 2007;17:2030–2034. doi: 10.1016/j.cub.2007.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen J, Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Peplowska K, Markgraf D, Ostrowicz C, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endolysosomal biogenesis. Dev Cell. 2007;12:739–750. doi: 10.1016/j.devcel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 316.Pereira-Leal J, Seabra M. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 317.Pereira-Leal J, Seabra M. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol. 2000;301:1077–1087. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- 318.Pfeffer S. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- 319.Pfeffer S. Structural clues to Rab GTPase functional diversity. J Biol Chem. 2005;280:15485–15488. doi: 10.1074/jbc.R500003200. [DOI] [PubMed] [Google Scholar]
- 320.Pierini R, Cottam E, Roberts R, Wileman T. Modulation of membrane traffic between endoplasmic reticulum, ERGIC and Golgi to generate compartments for the replication of bacteria and viruses. Semin Cell Dev Biol. 2009;20:828–833. doi: 10.1016/j.semcdb.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Pind S, Nuoffer C, McCaffery J, Plutner H, Davidson H, Farquhar M, Balch W. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Plutner H, Cox A, Pind S, Khosravi-Far R, Bourne J, Schwaninger R, Der C, Balch W. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Prekeris R, Davies J, Scheller R. Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J Biol Chem. 2001;276:38966–38970. doi: 10.1074/jbc.M106133200. [DOI] [PubMed] [Google Scholar]
- 324.Prekeris R, Klumperman J, Scheller R. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6:1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 325.Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol. 2000;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pylypenko O, Rak A, Durek T, Kushnir S, Dursina B, Thomae N, Constantinescu A, Brunsveld L, Watzke A, Waldmann H, Goody R, Alexandrov K. Structure of doubly prenylated Ypt1:GDI complex and the mechanism of GDI-mediated Rab recycling. EMBO J. 2006;25:13–23. doi: 10.1038/sj.emboj.7600921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Pérez-Victoria F, Abascal-Palacios G, Tascón I, Kajava A, Magadán J, Pioro E, Bonifacino J, Hierro A. Structural basis for the wobbler mouse neurodegenerative disorder caused by mutation in the Vps54 subunit of the GARP complex. Proc Natl Acad Sci USA. 2010;107:12860–12865. doi: 10.1073/pnas.1004756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Pérez-Victoria F, Bonifacino J. Dual roles of the mammalian GARP Complex in tethering and SNARE complex assembly at the trans-Golgi network. Mol Cell Biol. 2009 doi: 10.1128/MCB.00495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Rak A, Fedorov R, Alexandrov K, Albert S, Goody R, Gallwitz D, Scheidig A. Crystal structure of the GAP domain of Gyp1p: first insights into interaction with Ypt/Rab proteins. EMBO J. 2000;19:5105–5113. doi: 10.1093/emboj/19.19.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Rak A, Pylypenko O, Durek T, Watzke A, Kushnir S, Brunsveld L, Waldmann H, Goody R, Alexandrov K. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science. 2003;302:646–650. doi: 10.1126/science.1087761. [DOI] [PubMed] [Google Scholar]
- 331.Rak A, Pylypenko O, Niculae A, Goody R, Alexandrov K. Crystallization and preliminary X-ray diffraction analysis of mono-prenylated Rab7 GTPase in complex with Rab escort protein 1. J Struct Biol. 2003;141:93–95. doi: 10.1016/s1047-8477(02)00634-2. [DOI] [PubMed] [Google Scholar]
- 332.Rak A, Pylypenko O, Niculae A, Pyatkov K, Goody R, Alexandrov K. Structure of the Rab7:REP-1 complex: insights into the mechanism of Rab prenylation and choroideremia disease. Cell. 2004;117:749–760. doi: 10.1016/j.cell.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 333.Ramirez I, de Graffenried C, Ebersberger I, Yelinek J, He C, Price A, Warren G. TbG63, a golgin involved in Golgi architecture in Trypanosoma brucei. J Cell Sci. 2008;121:1538–1546. doi: 10.1242/jcs.014324. [DOI] [PubMed] [Google Scholar]
- 334.Reddy J, Burguete A, Sridevi K, Ganley I, Nottingham R, Pfeffer S. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell. 2006;17:4353–4363. doi: 10.1091/mbc.E06-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rehling P, Darsow T, Katzmann D, Emr S. Formation of AP-3 transport intermediates requires Vps41 function. Nat Cell Biol. 1999;1:346–353. doi: 10.1038/14037. [DOI] [PubMed] [Google Scholar]
- 336.Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini D. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Richardson B, Smith R, Ungar D, Nakamura A, Jeffrey P, Lupashin V, Hughson F. Structural basis for a human glycosylation disorder caused by mutation of the COG4 gene. Proc Natl Acad Sci USA. 2009;106:13329–13334. doi: 10.1073/pnas.0901966106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Richardson P, Zon L. Molecular cloning of a cDNA with a novel domain present in the tre-2 oncogene and the yeast cell cycle regulators BUB2 and cdc16. Oncogene. 1995;11:1139–1148. [PubMed] [Google Scholar]
- 339.Rieder S, Emr S. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 341.Rivera-Molina F, Novick P. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci USA. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Robinson J, Klionsky D, Banta L, Emr S. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Rodriguez-Gabin A, Cammer M, Almazan G, Charron M, Larocca J. Role of rRAB22b, an oligodendrocyte protein, in regulation of transport of vesicles from trans Golgi to endocytic compartments. J Neurosci Res. 2001;66:1149–1160. doi: 10.1002/jnr.1253. [DOI] [PubMed] [Google Scholar]
- 344.Rodriguez-Gabin A, Ortiz E, Demoliner K, Si Q, Almazan G, Larocca J. Interaction of Rab31 and OCRL-1 in oligodendrocytes: its role in transport of mannose 6-phosphate receptors. J Neurosci Res. 2010;88:589–604. doi: 10.1002/jnr.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Rojas R, van Vlijmen T, Mardones G, Prabhu Y, Rojas A, Mohammed S, Heck A, Raposo G, van der Sluijs P, Bonifacino J. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rosing M, Ossendorf E, Rak A, Barnekow A. Giantin interacts with both the small GTPase Rab6 and Rab1. Exp Cell Res. 2007;313:2318–2325. doi: 10.1016/j.yexcr.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 347.Rossi G, Kolstad K, Stone S, Palluault F, Ferro-Novick S. BET3 encodes a novel hydrophilic protein that acts in conjunction with yeast SNAREs. Mol Biol Cell. 1995;6:1769–1780. doi: 10.1091/mbc.6.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Rothman J, Howald I, Stevens T. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J. 1989;8:2057–2065. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Rothman J, Stevens T. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986;47:1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- 350.Russell R. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150–162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 351.Rzomp K, Moorhead A, Scidmore M. The GTPase Rab4 interacts with Chlamydia trachomatis inclusion membrane protein CT229. Infect Immun. 2006;74:5362–5373. doi: 10.1128/IAI.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Rzomp K, Scholtes L, Briggs B, Whittaker G, Scidmore M. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun. 2003;71:5855–5870. doi: 10.1128/IAI.71.10.5855-5870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, Schieltz D, Yates Abeliovich H, Jr, Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sacher M, Kim Y, Lavie A, Oh B, Segev N. The TRAPP complex: insights into its architecture and function. Traffic. 2008;9:2032–2042. doi: 10.1111/j.1600-0854.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sahlender D, Roberts R, Arden S, Spudich G, Taylor M, Luzio J, Kendrick-Jones J, Buss F. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Salminen A, Novick P. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987;49:527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- 357.Salminen A, Novick P. The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J Cell Biol. 1989;109:1023–1036. doi: 10.1083/jcb.109.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sano H, Roach W, Peck G, Fukuda M, Lienhard G. Rab10 in insulin-stimulated GLUT4 translocation. Biochem J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- 359.Sapperstein S, Walter D, Grosvenor A, Heuser J, Waters M. p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc Natl Acad Sci USA. 1995;92:522–526. doi: 10.1073/pnas.92.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Sasaki T, Kikuchi A, Araki S, Hata Y, Isomura M, Kuroda S, Takai Y. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265:2333–2337. [PubMed] [Google Scholar]
- 361.Satoh A, Wang Y, Malsam J, Beard M, Warren G. Golgin-84 is a rab1 binding partner involved in Golgi structure. Traffic. 2003;4:153–161. doi: 10.1034/j.1600-0854.2003.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Schardt A, Brinkmann B, Mitkovski M, Sereda M, Werner H, Nave K. The SNARE protein SNAP-29 interacts with the GTPase Rab3A: implications for membrane trafficking in myelinating glia. J Neurosci Res. 2009;87:3465–3479. doi: 10.1002/jnr.22005. [DOI] [PubMed] [Google Scholar]
- 363.Schnatwinkel C, Christoforidis S, Lindsay M, Uttenweiler-Joseph S, Wilm M, Parton R, Zerial M. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Schuck S, Gerl M, Ang A, Manninen A, Keller P, Mellman I, Simons K. Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells. Traffic. 2007;8:47–60. doi: 10.1111/j.1600-0854.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 365.Seabra M, Brown M, Goldstein J. Retinal degeneration in choroideremia: deficiency of rab geranylgeranyl transferase. Science. 1993;259:377–381. doi: 10.1126/science.8380507. [DOI] [PubMed] [Google Scholar]
- 366.Seabra M, Ho Y, Anant J. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J Biol Chem. 1995;270:24420–24427. doi: 10.1074/jbc.270.41.24420. [DOI] [PubMed] [Google Scholar]
- 367.Seabra M, Mules E, Hume A. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- 368.Seachrist J, Laporte S, Dale L, Babwah A, Caron M, Anborgh P, Ferguson S. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem. 2002;277:679–685. doi: 10.1074/jbc.M109022200. [DOI] [PubMed] [Google Scholar]
- 369.Seals D, Eitzen G, Margolis N, Wickner W, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Seaman M, McCaffery J, Emr S. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Segev N. Mediation of the attachment or fusion step in vesicular transport by the GTP-binding Ypt1 protein. Science. 1991;252:1553–1556. doi: 10.1126/science.1904626. [DOI] [PubMed] [Google Scholar]
- 372.Segev N. Ypt/rab GTPases: regulators of protein trafficking. Sci STKE. 2001;2001:RE11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- 373.Segev N, Mulholland J, Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- 374.Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, Schiavo G, Grant B, Smythe E. Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J Cell Biol. 2008;183:499–511. doi: 10.1083/jcb.200806016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Seog D, Kito M, Igarashi K, Yoda K, Yamasaki M. Molecular characterization of the USO1 gene product which is essential for vesicular transport in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1994;200:647–653. doi: 10.1006/bbrc.1994.1497. [DOI] [PubMed] [Google Scholar]
- 376.Seog D, Kito M, Yoda K, Yamasaki M. Uso1 protein contains a coiled-coil rod region essential for protein transport from the ER to the Golgi apparatus in Saccharomyces cerevisiae. J Biochem. 1994;116:1341–1345. doi: 10.1093/oxfordjournals.jbchem.a124685. [DOI] [PubMed] [Google Scholar]
- 377.Shen J, Tareste D, Paumet F, Rothman J, Melia T. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 378.Shestakova A, Suvorova E, Pavliv O, Khaidakova G, Lupashin V. Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J Cell Biol. 2007;179:1179–1192. doi: 10.1083/jcb.200705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Shin H, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk M, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, Frankel W, Solimena M, De Camilli P, Zerial M. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Shintani M, Tada M, Kobayashi T, Kajiho H, Kontani K, Katada T. Characterization of Rab45/RASEF containing EF-hand domain and a coiled-coil motif as a self-associating GTPase. Biochem Biophys Res Commun. 2007;357:661–667. doi: 10.1016/j.bbrc.2007.03.206. [DOI] [PubMed] [Google Scholar]
- 381.Shirakawa R, Higashi T, Tabuchi A, Yoshioka A, Nishioka H, Fukuda M, Kita T, Horiuchi H. Munc13–4 is a GTP-Rab27-binding protein regulating dense core granule secretion in platelets. J Biol Chem. 2004;279:10730–10737. doi: 10.1074/jbc.M309426200. [DOI] [PubMed] [Google Scholar]
- 382.Shirane M, Nakayama K. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314:818–821. doi: 10.1126/science.1134027. [DOI] [PubMed] [Google Scholar]
- 383.Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Short B, Haas A, Barr F. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744:383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 385.Short B, Preisinger C, Körner R, Kopajtich R, Byron O, Barr F. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J Cell Biol. 2001;155:877–883. doi: 10.1083/jcb.200108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Short B, Preisinger C, Schaletzky J, Kopajtich R, Barr F. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr Biol. 2002;12:1792–1795. doi: 10.1016/s0960-9822(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 387.Simonsen A, Gaullier J, D’Arrigo A, Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem. 1999;274:28857–28860. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- 388.Simonsen A, Lippé R, Christoforidis S, Gaullier J, Brech A, Callaghan J, Toh B, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 389.Simpson J, Griffiths G, Wessling-Resnick M, Fransen J, Bennett H, Jones A. A role for the small GTPase Rab21 in the early endocytic pathway. J Cell Sci. 2004;117:6297–6311. doi: 10.1242/jcs.01560. [DOI] [PubMed] [Google Scholar]
- 390.Singleton A, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson M, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 391.Siniossoglou S. Affinity purification of Ypt6 effectors and identification of TMF/ARA160 as a Rab6 interactor. Methods Enzymol. 2005;403:599–607. doi: 10.1016/S0076-6879(05)03052-1. [DOI] [PubMed] [Google Scholar]
- 392.Siniossoglou S, Pelham H. An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 2001;20:5991–5998. doi: 10.1093/emboj/20.21.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Sinka R, Gillingham A, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol. 2008;183:607–615. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Sivaram M, Furgason M, Brewer D, Munson M. The structure of the exocyst subunit Sec6p defines a conserved architecture with diverse roles. Nat Struct Mol Biol. 2006;13:555–556. doi: 10.1038/nsmb1096. [DOI] [PubMed] [Google Scholar]
- 395.Sivaram M, Saporita J, Furgason M, Boettcher A, Munson M. Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry. 2005;44:6302–6311. doi: 10.1021/bi048008z. [DOI] [PubMed] [Google Scholar]
- 396.Sivars U, Aivazian D, Pfeffer S. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature. 2003;425:856–859. doi: 10.1038/nature02057. [DOI] [PubMed] [Google Scholar]
- 396a.Sönnichsen B, Lowe M, Levine T, Jämsä E, Dirac-Svejstrup B, Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140:1013–1021. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Sorkin A. Cargo recognition during clathrin-mediated endocytosis: a team effort. Curr Opin Cell Biol. 2004;16:392–399. doi: 10.1016/j.ceb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 398.Speight P, Silverman M. Diacylglycerol-activated Hmunc13 serves as an effector of the GTPase Rab34. Traffic. 2005;6:858–865. doi: 10.1111/j.1600-0854.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 399.Spillantini M, Crowther R, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Spillantini M, Goedert M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann NY Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 401.Spinosa M, Progida C, De Luca A, Colucci A, Alifano P, Bucci C. Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. J Neurosci. 2008;28:1640–1648. doi: 10.1523/JNEUROSCI.3677-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Starai V, Hickey C, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Steele-Mortimer O. The Salmonella-containing vacuole: moving with the times. Curr Opin Microbiol. 2008;11:38–45. doi: 10.1016/j.mib.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Steele-Mortimer O, Méresse S, Gorvel J, Toh B, Finlay B. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1:33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 405.Stein M, Feng Y, Cooper K, Welford A, Wandinger-Ness A. Human VPS34 and p150 are Rab7 interacting partners. Traffic. 2003;4:754–771. doi: 10.1034/j.1600-0854.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 406.Stenmark H, Aasland R, Toh B, D’Arrigo A. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J Biol Chem. 1996;271:24048–24054. doi: 10.1074/jbc.271.39.24048. [DOI] [PubMed] [Google Scholar]
- 407.Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 408.Strick D, Elferink L. Rab15 effector protein: a novel protein for receptor recycling from the endocytic recycling compartment. Mol Biol Cell. 2005;16:5699–5709. doi: 10.1091/mbc.E05-03-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Strick D, Francescutti D, Zhao Y, Elferink L. Mammalian suppressor of Sec4 modulates the inhibitory effect of Rab15 during early endocytosis. J Biol Chem. 2002;277:32722–32729. doi: 10.1074/jbc.M205101200. [DOI] [PubMed] [Google Scholar]
- 410.Strom M, Hume A, Tarafder A, Barkagianni E, Seabra M. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 411.Stroupe C, Collins K, Fratti R, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411a.Südhof T, Rothman J. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Suh H, Lee D, Lee K, Ku B, Choi S, Woo J, Kim Y, Oh B. Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA. EMBO J. 2010;29:496–504. doi: 10.1038/emboj.2009.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Sumakovic M, Hegermann J, Luo L, Husson S, Schwarze K, Olendrowitz C, Schoofs L, Richmond J, Eimer S. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J Cell Biol. 2009;186:897–914. doi: 10.1083/jcb.200902096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Sun P, Yamamoto H, Suetsugu S, Miki H, Takenawa T, Endo T. Small GTPase Rah/Rab34 is associated with membrane ruffles and macropinosomes and promotes macropinosome formation. J Biol Chem. 2003;278:4063–4071. doi: 10.1074/jbc.M208699200. [DOI] [PubMed] [Google Scholar]
- 415.Suvorova E, Duden R, Lupashin V. The Sec34/Sec35p complex, a Ypt1p effector required for retrograde intra-Golgi trafficking, interacts with Golgi SNAREs and COPI vesicle coat proteins. J Cell Biol. 2002;157:631–643. doi: 10.1083/jcb.200111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Sweet D, Pelham H. The TIP1 gene of Saccharomyces cerevisiae encodes an 80 kDa cytoplasmic protein that interacts with the cytoplasmic domain of Sec20p. EMBO J. 1993;12:2831–2840. doi: 10.1002/j.1460-2075.1993.tb05944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol Biol Cell. 2009;20:2900–2908. doi: 10.1091/mbc.E08-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Teber I, Nagano F, Kremerskothen J, Bilbilis K, Goud B, Barnekow A. Rab6 interacts with the mint3 adaptor protein. Biol Chem. 2005;386:671–677. doi: 10.1515/BC.2005.078. [DOI] [PubMed] [Google Scholar]
- 421.Terai T, Nishimura N, Kanda I, Yasui N, Sasaki T. JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin. Mol Biol Cell. 2006;17:2465–2475. doi: 10.1091/mbc.E05-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.TerBush D, Maurice T, Roth D, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 423.Terebiznik M, Vazquez C, Torbicki K, Banks D, Wang T, Hong W, Blanke S, Colombo M, Jones N. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect Immun. 2006;74:6599–6614. doi: 10.1128/IAI.01085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Thomas C, Rousset R, Noselli S. JNK signalling influences intracellular trafficking during Drosophila morphogenesis through regulation of the novel target gene Rab30. Dev Biol. 2009;331:250–260. doi: 10.1016/j.ydbio.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 425.Tiller G, Hannig V, Dozier D, Carrel L, Trevarthen K, Wilcox W, Mundlos S, Haines J, Gedeon A, Gecz J. A recurrent RNA-splicing mutation in the SEDL gene causes X-linked spondylo-epiphyseal dysplasia tarda. Am J Hum Genet. 2001;68:1398–1407. doi: 10.1086/320594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Tisdale E. Rab2 interacts directly with atypical protein kinase C (aPKC) iota/lambda and inhibits aPKCiota/lambda-dependent glyceraldehyde-3-phosphate dehydrogenase phosphorylation. J Biol Chem. 2003;278:52524–52530. doi: 10.1074/jbc.M309343200. [DOI] [PubMed] [Google Scholar]
- 427.Tisdale E, Kelly C, Artalejo C. Glyceraldehyde-3-phosphate dehydrogenase interacts with Rab2 and plays an essential role in endoplasmic reticulum to Golgi transport exclusive of its glycolytic activity. J Biol Chem. 2004;279:54046–54052. doi: 10.1074/jbc.M409472200. [DOI] [PubMed] [Google Scholar]
- 428.Tripathi A, Ren Y, Jeffrey P, Hughson F. Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex. Nat Struct Mol Biol. 2009;16:114–123. doi: 10.1038/nsmb.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Ullrich O, Reinsch S, Urbé S, Zerial M, Parton R. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Ullrich O, Stenmark H, Alexandrov K, Huber L, Kaibuchi K, Sasaki T, Takai Y, Zerial M. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993;268:18143–18150. [PubMed] [Google Scholar]
- 431.Ungar D, Oka T, Krieger M, Hughson F. Retrograde transport on the COG railway. Trends Cell Biol. 2006;16:113–120. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 432.Urbé S, Huber L, Zerial M, Tooze S, Parton R. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- 433.Utskarpen A, Slagsvold H, Iversen T, Wälchli S, Sandvig K. Transport of ricin from endosomes to the Golgi apparatus is regulated by Rab6A and Rab6A′. Traffic. 2006;7:663–672. doi: 10.1111/j.1600-0854.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 434.Valdivia R. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr Opin Microbiol. 2008;11:53–59. doi: 10.1016/j.mib.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 435.Valsdottir R, Hashimoto H, Ashman K, Koda T, Storrie B, Nilsson T. Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett. 2001;508:201–209. doi: 10.1016/s0014-5793(01)02993-3. [DOI] [PubMed] [Google Scholar]
- 436.Van der Sluijs P, Hull M, Webster P, Mâle P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- 437.Van der Sluijs P, Hull M, Zahraoui A, Tavitian A, Goud B, Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci USA. 1991;88:6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Van Gele M, Dynoodt P, Lambert J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res. 2009;22:268–282. doi: 10.1111/j.1755-148X.2009.00558.x. [DOI] [PubMed] [Google Scholar]
- 439.Van Ijzendoorn S, Tuvim M, Weimbs T, Dickey B, Mostov K. Direct interaction between Rab3b and the polymeric immunoglobulin receptor controls ligand-stimulated transcytosis in epithelial cells. Dev Cell. 2002;2:219–228. doi: 10.1016/s1534-5807(02)00115-6. [DOI] [PubMed] [Google Scholar]
- 440.Van Vlijmen T, Vleugel M, Evers M, Mohammed S, Wulf P, Heck A, Hoogenraad C, van der Sluijs P. A unique residue in rab3c determines the interaction with novel binding protein Zwint-1. FEBS Lett. 2008;582:2838–2842. doi: 10.1016/j.febslet.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 441.VanRheenen S, Cao X, Lupashin V, Barlowe C, Waters M. Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking. J Cell Biol. 1998;141:1107–1119. doi: 10.1083/jcb.141.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.VanRheenen S, Cao X, Sapperstein S, Chiang E, Lupashin V, Barlowe C, Waters M. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J Cell Biol. 1999;147:729–742. doi: 10.1083/jcb.147.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.VanRheenen S, Reilly B, Chamberlain S, Waters M. Dsl1p, an essential protein required for membrane traffic at the endoplasmic reticulum/Golgi interface in yeast. Traffic. 2001;2:212–231. doi: 10.1034/j.1600-0854.2001.020307.x. [DOI] [PubMed] [Google Scholar]
- 444.Vasan N, Hutagalung A, Novick P, Reinisch K. Structure of a C-terminal fragment of its Vps53 subunit suggests similarity of Golgi-associated retrograde protein (GARP) complex to a family of tethering complexes. Proc Natl Acad Sci USA. 2010;107:14176–14181. doi: 10.1073/pnas.1009419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Vaughan K, Vallee R. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Velier J, Kim M, Schwarz C, Kim T, Sapp E, Chase K, Aronin N, DiFiglia M. Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp Neurol. 1998;152:34–40. doi: 10.1006/exnr.1998.6832. [DOI] [PubMed] [Google Scholar]
- 447.Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon J, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, Van Gerwen V, Wagner K, Hartung H, Timmerman V. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H, Zerial M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Walch-Solimena C, Collins R, Novick P. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Wang T, Hong W. Interorganellar regulation of lysosome positioning by the Golgi apparatus through Rab34 interaction with Rab-interacting lysosomal protein. Mol Biol Cell. 2002;13:4317–4332. doi: 10.1091/mbc.E02-05-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Wang W, Ferro-Novick S. A Ypt32p exchange factor is a putative effector of Ypt1p. Mol Biol Cell. 2002;13:3336–3343. doi: 10.1091/mbc.01-12-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol. 2000;151:289–296. doi: 10.1083/jcb.151.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Wang X, Kumar R, Navarre J, Casanova J, Goldenring J. Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J Biol Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- 454.Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof T. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 455.Wanschers B, van de Vorstenbosch R, Wijers M, Wieringa B, King S, Fransen J. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell Motil Cytoskeleton. 2008;65:183–196. doi: 10.1002/cm.20254. [DOI] [PubMed] [Google Scholar]
- 456.Wasmeier C, Romao M, Plowright L, Bennett D, Raposo G, Seabra M. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wassmer T, Attar N, Harterink M, van Weering J, Traer C, Oakley J, Goud B, Stephens D, Verkade P, Korswagen H, Cullen P. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–122. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Waterman-Storer C, Karki S, Holzbaur E. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Waters M, Clary D, Rothman J. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J Cell Biol. 1992;118:1015–1026. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Weide T, Bayer M, Köster M, Siebrasse J, Peters R, Barnekow A. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2001;2:336–341. doi: 10.1093/embo-reports/kve065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Weide T, Teuber J, Bayer M, Barnekow A. MICAL-1 isoforms, novel rab1 interacting proteins. Biochem Biophys Res Commun. 2003;306:79–86. doi: 10.1016/s0006-291x(03)00918-5. [DOI] [PubMed] [Google Scholar]
- 462.Westlake C, Junutula J, Simon G, Pilli M, Prekeris R, Scheller R, Jackson P, Eldridge A. Identification of Rab11 as a small GTPase binding protein for the Evi5 oncogene. Proc Natl Acad Sci USA. 2007;104:1236–1241. doi: 10.1073/pnas.0610500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, Stelzer E. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Whyte J, Munro S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev Cell. 2001;1:527–537. doi: 10.1016/s1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]
- 465.Wiederkehr A, De Craene J, Ferro-Novick S, Novick P. Functional specialization within a vesicle tethering complex: bypass of a subset of exocyst deletion mutants by Sec1p or Sec4p. J Cell Biol. 2004;167:875–887. doi: 10.1083/jcb.200408001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Williams C, Choudhury R, McKenzie E, Lowe M. Targeting of the type II inositol polyphosphate 5-phosphatase INPP5B to the early secretory pathway. J Cell Sci. 2007;120:3941–3951. doi: 10.1242/jcs.014423. [DOI] [PubMed] [Google Scholar]
- 467.Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Wu M, Lu L, Hong W, Song H. Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat Struct Mol Biol. 2004;11:86–94. doi: 10.1038/nsmb714. [DOI] [PubMed] [Google Scholar]
- 469.Wu S, Mehta S, Pichaud F, Bellen H, Quiocho F. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 470.Wu S, Zeng K, Wilson I, Balch W. Structural insights into the function of the Rab GDI superfamily. Trends Biochem Sci. 1996;21:472–476. doi: 10.1016/s0968-0004(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 471.Wu X, Rao K, Bowers M, Copeland N, Jenkins N, Hammer J. Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J Cell Sci. 2001;114:1091–1100. doi: 10.1242/jcs.114.6.1091. [DOI] [PubMed] [Google Scholar]
- 472.Wu X, Rao K, Zhang H, Wang F, Sellers J, Matesic L, Copeland N, Jenkins N, Hammer J. Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 473.Wu Y, Tan K, Waldmann H, Goody R, Alexandrov K. Interaction analysis of prenylated Rab GTPase with Rab escort protein and GDP dissociation inhibitor explains the need for both regulators. Proc Natl Acad Sci USA. 2007;104:12294–12299. doi: 10.1073/pnas.0701817104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Wurmser A, Sato T, Emr S. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151:551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Yamamura R, Nishimura N, Nakatsuji H, Arase S, Sasaki T. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Mol Biol Cell. 2008;19:971–983. doi: 10.1091/mbc.E07-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Yamasaki A, Menon S, Yu S, Barrowman J, Meerloo T, Oorschot V, Klumperman J, Satoh A, Ferro-Novick S. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI coated vesicles. Mol Biol Cell. 2009;20:4205–4215. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Yang L, Clinton J, Blackburn M, Zhang Q, Zou J, Zielinska-Kwiatkowska A, Tang B, Chansky H. Rab23 regulates differentiation of ATDC5 chondroprogenitor cells. J Biol Chem. 2008;283:10649–10657. doi: 10.1074/jbc.M706795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, Takata K, Takeuchi T, Izumi T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol. 2002;22:1858–1867. doi: 10.1128/MCB.22.6.1858-1867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Yoshie S, Imai A, Nashida T, Shimomura H. Expression, characterization, and localization of Rab26, a low molecular weight GTP-binding protein, in the rat parotid gland. Histochem Cell Biol. 2000;113:259–263. doi: 10.1007/s004180000130. [DOI] [PubMed] [Google Scholar]
- 480.Yoshimura S, Egerer J, Fuchs E, Haas A, Barr F. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Young J, Stauber T, del Nery E, Vernos I, Pepperkok R, Nilsson T. Regulation of microtubule-dependent recycling at the trans-Golgi network by Rab6A and Rab6A′. Mol Biol Cell. 2005;16:162–177. doi: 10.1091/mbc.E04-03-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Yu S, Satoh A, Pypaert M, Mullen K, Hay J, Ferro-Novick S. mBet3p is required for homotypic COPII vesicle tethering in mammalian cells. J Cell Biol. 2006;174:359–368. doi: 10.1083/jcb.200603044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Zacchi P, Stenmark H, Parton R, Orioli D, Lim F, Giner A, Mellman I, Zerial M, Murphy C. Rab17 regulates membrane trafficking through apical recycling endosomes in polarized epithelial cells. J Cell Biol. 1998;140:1039–1053. doi: 10.1083/jcb.140.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Zahraoui A, Joberty G, Arpin M, Fontaine J, Hellio R, Tavitian A, Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in non polarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J Cell Biol. 1994;124:101–115. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Zeevaert R, Foulquier F, Jaeken J, Matthijs G. Deficiencies in subunits of the Conserved Oligomeric Golgi (COG) complex define a novel group of congenital disorders of glycosylation. Mol Genet Metab. 2008;93:15–21. doi: 10.1016/j.ymgme.2007.08.118. [DOI] [PubMed] [Google Scholar]
- 486.Zeng J, Ren M, Gravotta D, De Lemos-Chiarandini C, Lui M, Erdjument-Bromage H, Tempst P, Xu G, Shen T, Morimoto T, Adesnik M, Sabatini D. Identification of a putative effector protein for rab11 that participates in transferrin recycling. Proc Natl Acad Sci USA. 1999;96:2840–2845. doi: 10.1073/pnas.96.6.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 488.Zhang J, Fonovic M, Suyama K, Bogyo M, Scott M. Rab35 controls actin bundling by recruiting fascin as an effector protein. Science. 2009;325:1250–1254. doi: 10.1126/science.1174921. [DOI] [PubMed] [Google Scholar]
- 489.Zhang X, Bi E, Novick P, Du L, Kozminski K, Lipschutz J, Guo W. Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- 490.Zhang X, Ellis S, Sriratana A, Mitchell C, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 491.Zhang X, Wang P, Gangar A, Zhang J, Brennwald P, TerBush D, Guo W. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J Cell Biol. 2005;170:273–283. doi: 10.1083/jcb.200502055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Zheng J, Koda T, Fujiwara T, Kishi M, Ikehara Y, Kakinuma M. A novel Rab GTPase, Rab33B, is ubiquitously expressed and localized to the medial Golgi cisternae. J Cell Sci. 1998;111:1061–1069. doi: 10.1242/jcs.111.8.1061. [DOI] [PubMed] [Google Scholar]
- 493.Zhu H, Liang Z, Li G. Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis. Mol Biol Cell. 2009;20:4720–4729. doi: 10.1091/mbc.E09-06-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Zolov S, Lupashin V. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–759. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Zoncu R, Perera R, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli P. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Zuk P, Elferink L. Rab15 mediates an early endocytic event in Chinese hamster ovary cells. J Biol Chem. 1999;274:22303–22312. doi: 10.1074/jbc.274.32.22303. [DOI] [PubMed] [Google Scholar]
- 497.Züchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De Jonghe P, Merory J, Oliveira S, Speer M, Stenger J, Walizada G, Zhu D, Pericak-Vance M, Nicholson G, Timmerman V, Vance J. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet. 2005;37:289–294. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]