Abstract
Objectives
To project the potential economic impact of pandemic influenza mitigation strategies from a societal perspective in the United States.
Methods
We use a stochastic agent-based model to simulate pandemic influenza in the community. We compare 17 strategies: targeted antiviral prophylaxis (TAP) alone and in combination with school closure as well as prevaccination.
Results
In the absence of intervention, we predict a 50% attack rate with an economic impact of $187 per capita as loss to society. Full TAP is the most effective single strategy, reducing number of cases by 54% at the lowest cost to society ($127 per capita). Prevaccination reduces number of cases by 48% and is the second least costly alternative ($140 per capita). Adding school closure to full TAP or prevaccination further improves health outcomes, but increases total cost to society by approximately $2700 per capita.
Conclusion
Full targeted antiviral prophylaxis is an effective and cost-saving measure for mitigating pandemic influenza.
Keywords: Influenza, Human Disease Outbreaks, Cost-Benefit Analysis, Economics, Pharmaceutical Models, Theoretical, Computer Simulation
Introduction
Influenza pandemic preparedness is a public health priority in light of the global epidemic of highly pathogenic H5N1 influenza infection in avian populations. Recent epidemiological models have explored various mitigation strategies for pandemic influenza in the United States. This research has shown the likely effectiveness of targeted antiviral use, low-efficacy vaccines, and non-medical interventions such as school closure, case isolation, and household quarantine in reducing peak or cumulative illness attack rates, even for highly transmissible viruses [1, 2]. Further modelling work highlights the importance of targeted antiviral use and social distancing measures [3], and has helped inform the US pandemic influenza plan [4]
However, an important missing component is a cost effectiveness analysis of proposed mitigation strategies [5]. Many economic evaluations of interpandemic influenza programmes do not take into account the dynamic, non-linear effects of interventions in infectious diseases, likely underestimating the cost effectiveness of interventions [6]
Our objective was to evaluate the cost utility of alternative pandemic influenza mitigation strategies in the US from the societal perspective using a stochastic, individual-level, microsimulation model [7]. We examined the cost utility of targeted antiviral prophylaxis (TAP), school closure, and prevaccination with low-efficacy vaccines. The time horizon of the analysis was 6 months, which reflects the time until a fully matched vaccine would be available in sufficient quantities to effectively protect the population. To our knowledge, this is the first economic evaluation of influenza pandemic mitigation strategies based on a dynamic influenza transmission model. The research also expands on current epidemiological models by incorporating severity of influenza illness, complications, mortality, and quality of life.
Methods
Strategies
This paper focuses on strategies that were shown to be the most promising ones in previously published influenza pandemic models [1, 3, 7]. We compared the economic impact of no intervention with 16 single and combination strategies (Table 1). Single prophylactic strategies included prevaccination, antiviral post-exposure prophylaxis (in combination with treatment of the index case) and school closure. TAP included household-only prophylaxis (household targeted antiviral post-exposure prophylaxis [HTAP]), and prophylaxis in the full set of contact groups for an index case (full targeted antiviral post-exposure prophylaxis [FTAP]). Oseltamivir stockpiles in varying quantities were assumed to be available from the start of a pandemic, ranging from covering 25% of the total population (a single course of oseltamivir, one pack, consists of 10 capsules, enough for 5 days of treatment or 10 days of post-exposure prophylaxis) to an “unlimited” stockpile (i.e. as much as needed). TAP was carried out by treating identified index cases (the first symptomatic illness in a contact group) and offering post-exposure prophylaxis to contacts of these index cases in households, neighbourhood clusters, large day care centres, small playgroups, schools and workgroups. We assumed that 60% of symptomatic index cases could be ascertained [8]. We also evaluated a treatment-only strategy, i.e. only individuals with symptomatic illness are treated with antivirals.
Table 1.
Description of interventions
| Intervention | Description |
|---|---|
| No intervention | No prevaccination, prophylaxis or treatment with antivirals |
| HTAP25 | Household targeted antiviral prophylaxis, stockpile for 25% of population |
| HTAP50 | Household targeted antiviral prophylaxis, stockpile for 50% of population |
| HTAP | Household targeted antiviral prophylaxis, stockpile unlimited |
| School closure | Closing all schools for 26 weeks |
| Prevaccination | Prevaccinating 70% population with low-efficacy vaccine |
| HTAP25 + school closure | Household targeted antiviral prophylaxis, stockpile for 25% of population, plus closing all schools for 26 weeks |
| HTAP50 + school closure | Household targeted antiviral prophylaxis, stockpile for 50% of population, plus closing all schools for 26 weeks |
| HTAP + school closure | Household targeted antiviral prophylaxis, stockpile unlimited, plus closing all schools for 26 weeks |
| Prevaccination + school closure | Prevaccinating 70% population with low-efficacy vaccine, plus closing all schools for 26 weeks |
| Treatment only | Treating all cases with antivirals |
| FTAP25 | Full targeted antiviral prophylaxis (household contacts and 60% of work/school contacts), stockpile for 25% of population |
| FTAP50 | Full targeted antiviral prophylaxis (household contacts and 60% of work/school contacts), stockpile for 50% of population |
| FTAP | Full targeted antiviral prophylaxis (household contacts and 60% of work/school contacts), stockpile unlimited |
| FTAP25 + school closure | Full targeted antiviral prophylaxis (household contacts and 60% of work/school contacts), stockpile for 25% of population, plus closing all schools for 26 weeks |
| FTAP50 + school closure | Full targeted antiviral prophylaxis (household contacts and 60% of work/school contacts), stockpile for 50% of population, plus closing all schools for 26 weeks |
| FTAP + school closure | Full targeted antiviral prophylaxis (household contacts and 60% of work/school contacts), stockpile unlimited, plus closing all schools for 26 weeks |
Prevaccination assumes that 70% of the population are successfully vaccinated with a low-efficacy vaccine, before the outbreak of a pandemic. We also considered school closure as a measure of social distancing alone, or in combination with pharmaceutical interventions. We modelled the impact of closing schools for the duration of the pandemic (26 weeks).
Mathematical model
We used a discrete-time, stochastic simulation model of influenza spread within a structured population to compare the effectiveness of various intervention strategies [7]. A recent publication demonstrates the comparability of our model predictions (influenza attack rate) to other published models [3]. The model simulates stochastic spread of influenza in a population of people interacting in known contact groups [7–9]. Each person is assumed to have daily contacts with household members and people in the three closest households (neighbourhood cluster), as well as with people in the larger neighbourhood and community. Preschool children attend either small playgroups or larger day-care centres, school-age children attend elementary, middle, or high school, as appropriate, and 63% of adults are in workgroups [10].
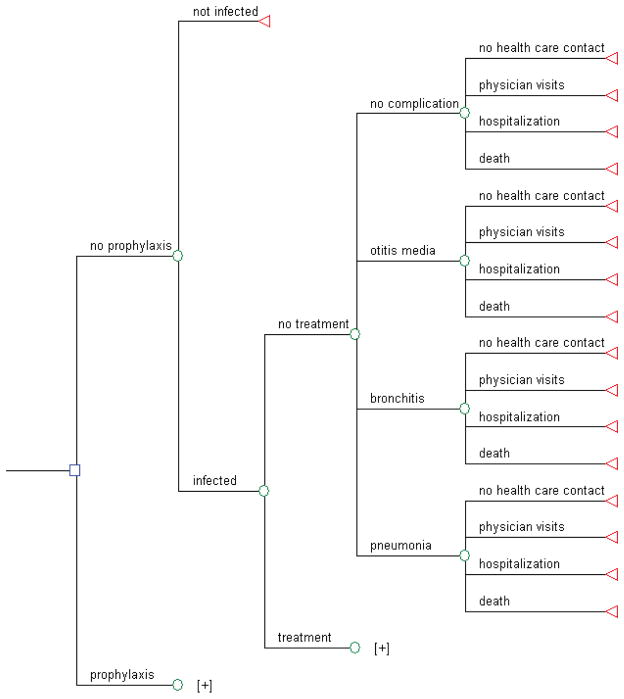
Once infected, people follow the clinical pathway as shown in figure 1. An infected person may receive treatment, which modifies health outcome (probability of otitis media, bronchitis, pneumonia, hospitalisation due to influenza, mortality) and resource use (probability of healthcare contact). Stratification of the population by age and risk status is accounted for in the model. The age groups are children 0–4 years old, children 5–18 years, younger adults (19–64 years old), and older adults (≥65 years). Younger adults are further stratified into high and low risk. High risk adults have underlying chronic conditions (e.g. cardiovascular, respiratory, or metabolic disease), which increases their risk for bronchitis, pneumonia, hospitalisations, and mortality.
Figure 1.
Simplified schematic representation of decision model
Data
Transmission
Many of the transmission parameters were adopted from previous work (7–9). The probability that an infected individual will be symptomatic is 0.67 [11]. An asymptomatic infection is assumed to be 50% as infectious as a symptomatic infection [7, 12].
One hundred runs were performed for each intervention, and the results were averaged. The average R0 was 2.0, with a range of 1.5 to 2.6. R0 is defined as the average number of secondary infections produced by a typical infected person in a fully susceptible population [13].
Probabilities of events
Probabilities of events used in the model are shown in table A2. The probabilities of bronchitis, pneumonia, and otitis media for an untreated population were based on a large general practice database from the UK [14]. The mortality rate is based on data from previous pandemics [15] and captures all influenza-related deaths, including those due to complications.
Effectiveness of interventions
We used current estimates of antiviral efficacy of oseltamivir (table 2) [11, 16–19]. The antiviral efficacy for symptomatic disease given exposure is 0.72, and we assumed that the antiviral efficacy for infectiousness is 0.62 [19]. Oseltamivir treatment effectively reduces incidence of otitis media, bronchitis, pneumonia, influenza-related hospitalisations, and mortality, and improves quality of life [20–23].
Table 2.
Effectiveness of interventions
| Intervention | Incidence reduction/QoL improvement | Source |
|---|---|---|
| Oseltamivir | ||
| Infection given exposure | 30% | Halloran et al, 2007 (11), Hayden et al, 1999 (18), Hayden et al 2000 (16), Welliver et al 2001 (17), Yang et al 2006 (19) |
| Symptomatic disease given infection | 60% | Halloran et al, 2007 (11), Hayden et al, 1999 (18), Hayden et al 2000 (16), Welliver et al 2001 (17), Yang et al 2006 (19) |
| Symptomatic disease given exposure | 72% | Calculated |
| Infectiousness | 62% | Yang et al 2006 (19) |
| Low-efficacy vaccine | ||
| Susceptibility to infection | 30% | Longini et al, 2005 (12) |
| Infectiousness | 50% | Longini et al, 2005 (12) |
| Bronchitis | ||
| Children <5 years | 52% | Kaiser et al, 2003 (21) |
| Children 5–18 years | 52% | Kaiser et al, 2003 (21) |
| Low risk younger adults | 60% | Kaiser et al, 2003 (21) |
| High risk younger adults | 33% | Kaiser et al, 2003 (21) |
| Older adults | 33% | Kaiser et al, 2003 (21) |
| Pneumonia | ||
| Children <5 years | 63% | Kaiser et al, 2003 (21) |
| Children 5–18 years | 63% | Kaiser et al, 2003 (21) |
| Low risk younger adults | 85% | Kaiser et al, 2003 (21) |
| High risk younger adults | 24% | Kaiser et al, 2003 (21) |
| Older adults | 24% | Kaiser et al, 2003 (21) |
| Otitis media | ||
| Children <5 years | 62% | Data on file |
| Influenza deaths (all) | Same as reduction in hospitalisations | Assumption |
| Influenza hospitalisations | ||
| Children | 61% | Kaiser et al, 2003 (21) |
| Low risk younger adults | 64% | Kaiser et al, 2003 (21) |
| High risk younger adults | 39% | Kaiser et al, 2003 (21) |
| Older adults | 39% | Kaiser et al, 2003 (21) |
| QoL improvement (influenza) | ||
| Children and low risk younger adults | 11% | Data on file |
| High risk younger adults | 4% | Data on file |
| Older adults | 5% | Data on file |
QoL=quality of life
For a low-efficacy vaccine, we assumed the vaccine efficacy for susceptibility to infection to be 0.30, and vaccine efficacy for infectiousness to be 0.50 [12]. We assumed that two doses of vaccine would be needed [24].
Utilities
We calculated quality adjusted life years (QALYs) based on quality weights between 0 (death) and 1 (perfect health). The QALY penalties for influenza were derived from clinical trial data as used and described in a recently published health technology assessment on the prevention and control of influenza [20] and for bronchitis and otitis media from the literature [25, 26] There were no quality weights published for bronchitis; we therefore assumed the same QALY penalty for bronchitis as for influenza. Future life years were discounted at 3% per annum in line with US guidelines for economic evaluations [27].
Costs
Resource use
We estimated resource use related to treatment of illness separately for children and adults, as well as resource use related to prophylaxis including school closure. We included physician visits, hospitalisations, use of antibiotics, and use of over the counter medicines. For prevaccination and TAP, we included both drug and delivery costs. For HTAP, we estimated travel and time cost to obtain prophylaxis, and assigned this cost once per household, assuming that the index case obtains prophylaxis for household members. For FTAP, we assumed three times this cost to account for prophylaxis of household members, contacts in the school or workplace, and contacts in the community.
We assume 2.5 days of work loss per week per household for children <12 years if a) the child is sick or b) schools are closed. This estimate is based on best available data from the literature [10, 20, 28, 29]. Babysitting pools or other similar arrangements should be avoided during a pandemic when school closure is in effect to minimize transmission.
For school closure, we assumed 2.5 person days per week time loss for affected households, and 5 days per week for teachers and other professionals, using a national ratio of teachers and other professionals per student [30]. If one parent stays home already because of a sick child, no additional work loss is added. For teachers and other school staff who are parents, the work loss is 5 days.
Unit costs
Unit medical cost estimates were based on US fee and price schedules [27, 31]. Oseltamivir is priced at the stockpile acquisition cost for adults and children. Oseltamivir costs were converted from Euros to US dollars using the Interbank rate as of 5 July 2006 [32]. The low-efficacy vaccine is priced at one third of the current price per dose [31]. As mass vaccination is anticipated to be less costly than current vaccination practices, we assumed only 50% of the usual cost for vaccine delivery. We added 20% to both oseltamivir and vaccine cost, to reflect the cost incurred by the government for storage and distribution. Hospitalisation costs were derived from Diagnosis Related Group (DRG) codes [33] for children (0–17 years) and adults (≥17 years) with (used for high risk adults and older adults) and without complications (used for low risk adults). In the absence of a DRG code for influenza, we assumed hospitalisation costs for influenza to be similar to bronchitis.
We valued productivity loss using the human capital approach by applying average compensation (salary plus fringe benefits) [34] to days of work lost for sick adults and caregivers of sick children, as well as caregivers for households affected by school closure. We used average earnings for teachers [35] to value work loss for teachers due to school closure. Productivity loss due to premature mortality was not included, since this is reflected in the measure of health outcomes (QALYs) [33].
Because resource use and cost data on health care services used during an influenza pandemic are not readily available, some of the estimates are assumptions based on the available literature on annual influenza and expert opinion.
Analyses
Base case
In the base case analysis, we estimated the expected health outcomes (number of cases, number of deaths, QALYs) and costs from the societal perspective for one pandemic wave, assuming a death rate of 2.5% per influenza case. We chose the societal perspective to capture productivity loss due to potentially very high absenteeism rates and the potential impact due to school closure, which do not incur any costs to the healthcare payer, but may cause substantial disruption. Because quality of life is important to patients and decision makers, we ranked strategies by expected QALYs and performed a cost utility analysis calculating costs per QALY gained. This approach also enables comparison with other public health interventions. In the base case, costs were not discounted because all costs occur within 1 year.
Sensitivity analyses
As the sensitivity related to the effectiveness of oseltamivir has been tested and reported previously [1, 7, 12] we focused our analysis on a number of other key variables in the model (R0, mortality, school closure, and probability of a pandemic). We explored the lower end of the possible range for the basic reproduction number by fixing R0 at 1.6, and also investigated a situation with R0 fixed at 2.0 to eliminate the effects of uncertainty surrounding R0.
To assess the sensitivity of results to variations in health care resource use we define a low intensity and high intensity resource use scenario, varying a number of resource use parameters at the same time.
Severity of a pandemic is difficult to predict; we therefore tested a 5% mortality rate. To minimise social disruption due to school closure, staff—i.e. teachers and other professionals—may be assigned to different tasks, such as teaching by distance or supporting healthcare workers and other essential services. We assumed 50% productivity loss for teachers and other staff during school closure instead of 100% in the base case.
There is an additional sensitivity analysis, assuming a pandemic occurs within 33 years, and that stockpiles need to be renewed.
Results
Base case
All base case results are shown in table 3. In the absence of any intervention, we projected an illness attack rate of 50%, resulting in 13 deaths per 1000 population. All interventions reduced the illness attack rate, and hence morbidity and mortality. Many interventions are also cost saving compared with no intervention, meaning that additional costs of intervention (antivirals, vaccine) are offset by the lower number of cases requiring treatment and incurring productivity loss. FTAP is the most effective single strategy, reducing the attack rate by 54%. If a low-efficacy vaccine is available and administered before the onset of the pandemic, then prevaccinating 70% of the population is expected to reduce the number of cases by 48% and is the second least costly strategy. However, FTAP dominates (i.e. reduces morbidity, mortality, and costs) all single strategies and most combination strategies, which are therefore eliminated from further analysis. The expected illness attack rate is smallest (6 and 4%, respectively) if either 60% of close contacts of ascertained index cases receive prophylaxis (FTAP), or 70% of the population is prevaccinated with a low-efficacy vaccine, and schools are closed for the duration of the outbreak. However, school closure incurs high costs to society (about $2.7 million per 1000 population). Total costs are therefore much higher than for FTAP or prevaccination alone. Strategies involving school closure are approximately 14- to 21-times as costly as single intervention strategies with antivirals or prevaccination.
Table 3.
Base case results (ranked by expected QALYs)
| Intervention | Illness attack rate (%) | Deaths per 1000 | QALYs* per 1000 | Incremental QALYs† per 1000 | Courses per 1000 | Total cost in million $ per 1000 |
|---|---|---|---|---|---|---|
| No intervention | 50 | 13 | 21 141 | – | – | 0.19 |
| FTAP25 | 48 | 12 | 21 157 | 16 | 246 | 0.18 |
| FTAP50 | 45 | 11 | 21 175 | 34 | 481 | 0.18 |
| HTAP25 | 48 | 11 | 21 181 | 40 | 250 | 0.19 |
| School closure | 39 | 10 | 21 210 | 69 | – | 2.72 |
| HTAP50 | 42 | 8 | 21 239 | 98 | 498 | 0.17 |
| Treatment only | 49 | 8 | 21 241 | 100 | 243 | 0.19 |
| HTAP | 41 | 7 | 21 264 | 123 | 651 | 0.17 |
| Prevaccination | 26 | 6 | 21 271 | 130 | – | 0.14 |
| HTAP25 and school closure | 31 | 7 | 21 273 | 132 | 204 | 2.70 |
| FTAP25 and school closure | 23 | 6 | 21 300 | 159 | 150 | 2.66 |
| FTAP50 and school closure | 22 | 5 | 21 310 | 169 | 279 | 2.66 |
| HTAP50 and school closure | 27 | 5 | 21 316 | 175 | 374 | 2.68 |
| HTAP and school closure | 24 | 4 | 21 330 | 189 | 395 | 2.67 |
| FTAP | 23 | 5 | 21 351 | 210 | 2447 | 0.12 |
| FTAP and school closure | 6 | 1 | 21 403 | 262 | 640 | 2.61 |
| Prevaccination and school closure | 4 | 1 | 21 403 | 262 | – | 2.62 |
HTAP=household targeted antiviral prophylaxis; FTAP=full targeted antiviral prophylaxis; QALY=quality adjusted life year.
Expected average quality-adjusted life expectancy;
Compared to no intervention.
Note: QALY ranking differs slightly from illness attack rate ranking because QALYs take into account the differences in morbidity and mortality (life expectancy) across age groups, i.e. it is important in which age groups cases and deaths occur.
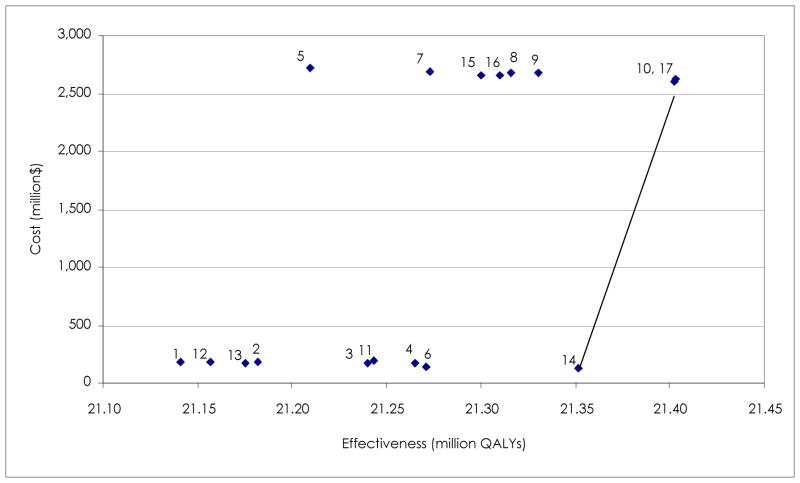
Table 4 shows the results for the incremental cost-utility analysis. Eliminating all dominated interventions leaves only three strategies for comparison: FTAP, FTAP in combination with school closure, and prevaccination in combination with school closure. Compared to FTAP not involving school closure, FTAP plus school closure or prevaccination plus school closure gains 51 QALYs, but increases total cost by approximately $2.5 million for a population of 1000. School closure incurs substantial costs to society, driven by extensive work loss for carers and teachers. The incremental cost utility ratio (ICUR) for either strategy compared to FTAP is $48,500/QALY gained. Figure 2 shows the cost effective frontier. The options connected by a line are the set of potentially optimal choices. All other options are dominated, i.e. not as effective and more costly.
Table 4.
Incremental cost-utility for non-eliminated strategies (pandemic occurs within 1 year)
| Intervention | Total Cost in Million $ Per 1000 | Incremental Cost in Million $ Per 1000 | QALYs Per 1000 | Incremental QALYs Per 1000 | Incremental Cost-Utility Ratio ($) |
|---|---|---|---|---|---|
| FTAP | 0.12 | – | 21 352 | – | - |
| FTAP and school closure | 2.73 | 2.48 | 21 403 | 51 | 48 472 |
| Prevaccination and school closure | 2.73 | 2.50 | 21 403 | 51 | 48 638 |
FTAP=full targeted antiviral prophylaxis; QALY=quality adjusted life year.
Note: FTAP plus school closure and prevaccination plus school closure are individually compared to the same baseline (FTAP)
Figure 2.
Cost effectiveness frontier base case
1=no intervention; 2=HTAP25; 3=HTAP50; 4=HTAP; 5=school closure; 6=prevaccination; 7=HTAP25 and school closure; 8=HTAP50 and school closure; 9=HTAP and school closure; 10=prevaccination and school closure; 11=treatment only; 12=FTAP25; 13=FTAP50; 14=FTAP; 15=FTAP25 and school closure: 16=FTAP50 and school closure.
HTAP=household targeted antiviral prophylaxis; FTAP=full targeted antiviral prophylaxis; QALYs=quality adjusted life year
Sensitivity analyses
The basic reproductive number is a key driver in the model, as it determines the number of influenza cases, and therefore the subsequent impact on the economy. It also affects the relative effect of the different interventions. Fixing R0 at 2.0 does not change the ranking of strategies compared to the base case. FTAP remains the most effective (26 cases/100) and least costly single strategy $140/capita). This is despite the fact that it is estimated to consume almost three packs on average per capita. As in the base case, the school closure strategies are very expensive from the society’s perspective, but adding school closure to any FTAP strategy or to prevaccination effectively eliminates the pandemic (0.2 to 7 cases per 100). If school closure is added to FTAP, no more than about 50% antiviral stockpiling is needed in order to effectively control the pandemic. For a low R0 of 1.6, a pandemic can be effectively controlled with FTAP25. The cost savings are also highest for this scenario, with a cost of $3/capita compared with $130/capita for baseline.
Variations in health care resource use have some impact on the cost-utility ratios but nit the ranking of strategies. In the best case scenario (low resource use for treatment of influenza cases), the ICUR for FTAP plus school closure, and vaccination plus school closure compared to FTAP alone is just below $28,000 per QALY gained. For the worst case scenario (high resource use for treatment of influenza cases), the ICUR for FTAP plus school closure, and vaccination plus school closure compared to FTAP alone is below $83,000/QALY.
The ranking of strategies is unaffected when changing assumptions about mortality and school closure. Assuming a higher case fatality rate of 5%, the incremental cost-utility ratios for FTAP plus school closure, and vaccination plus school closure compared to FTAP reduces from $48 500/QALY to $18,500/QALY gained, making these strategies more attractive at higher mortality rates. When teachers and professionals incur only half the productivity loss, ICURs are only slightly lower than in the base case ($41,500/QALY for FTAP/vaccination plus school closure compared to FTAP). This is because most of the productivity loss (60%) during school closure can be attributed to parents (carers) being unable to work.
Our analysis indicates that the higher the attack rate, the more worthwhile are interventions providing broad coverage, such as school closure, FTAP, and prevaccination. At low attack rates, targeted strategies provide similar effects, but at lower cost.
Discussion
The base case analysis clearly demonstrates that both FTAP and prepandemic vaccination effectively reduce the burden of pandemic influenza. In comparison with no intervention, both are cost saving from a societal perspective, the costs of the intervention (i.e. stockpiling up to 2.5 courses of antivirals per capita or prevaccinating 70% of the population) being more than offset by the substantial savings made in terms of both healthcare costs and productivity losses. Further reductions in infection rate, morbidity, and mortality can be achieved by the addition of school closure to these strategies, but at a much higher cost to society (approximately 14- to 21-times that of a single intervention). However, due to the further benefits realised in terms of health outcomes, with the addition of school closure in this setting, this approach could still be cost effective (~$48,500/QALY gained) from a societal perspective.
To our knowledge, this study represents the first economic analysis of pandemic mitigation strategies in a dynamic, non-linear model. Although the analysis has a number of limitations due to uncertainties about factors such as the characteristics (infectivity and associated morbidity/mortality) of the pandemic strain and the current feasibility of some of the mitigation strategies evaluated (e.g. timely availability/efficacy of a pandemic vaccine), this analysis provides an important economic evaluation of a number of relevant mitigation strategies that may be considered in the event of a pandemic.
Because the severity of a future pandemic is unknown, we used a distribution for R0 (~1.5 to 2.6), the basic reproduction number, to account for this uncertainty. Our results, therefore, reflect what to expect on average. There is a strong R0 threshold just under 2.0, below which interventions aimed at the population at large (prevaccination, school closure) are less valuable. In addition, R0 also has an impact on the quantity of antivirals required to mitigate a pandemic outbreak, the number of doses used exhibiting a highly non-linear dynamic threshold. Thus, given the uncertainty regarding R0, our base case analysis best captures the information required for pandemic planning.
The current analysis is based on the assumption that the required quantity of either pandemic vaccine, or oseltamivir, is available for timely use. This requires adequate stockpiling in advance of an epidemic. For prevaccination in the model, it is assumed that 70% of the population are vaccinated with a low-efficacy vaccine at least 14 days before exposure to the virus. Although vaccination would, in principle, be a very effective intervention in the event of a pandemic, significant limitations to this approach exist in terms of the degree of virus strain match, production capacity and shelf life. These, together with the constantly changing antigenic nature of the virus, would adversely affect both the opportunity for advanced stockpiling and the required rapid availability of vaccines at the onset of a pandemic. In contrast, oseltamivir is not strain dependent and has a much longer shelf life than pandemic vaccines. Although the emergence of antiviral-resistant pandemic strains has been identified as a potential issue, development of resistance to oseltamivir over 7 years of use in epidemic influenza setting has been very low. In addition, it has been suggested that based on the reduced fitness and thus low transmissibility of resistant strains [36], the benefits of oseltamivir are highly unlikely to be offset by drug resistance.
To provide a national aggregate perspective on our estimates, it is useful to compare them with estimates produced from aggregate economic models. The Congressional Budget Office estimated that the impact of severe pandemic would reduce Gross Domestic Product (GDP) by 4.25%, equivalent to a typical business cycle recession [37]. With a projected GDP in the order of $14 trillion, this would imply a loss of $595 billion. However, this “severe” scenario assumed an attack rate of 30% and 2 million deaths. Our base case scenario generates an attack rate of 50% and a projected 3.9 million deaths. We estimate only the direct and indirect costs related to medical treatment in this scenario, and they amount to a projected $59 billion. School closure dramatically increases the costs to $840 billion, reflecting the broader economic impact of parents missing work to care for their children at home. By comparison, stockpiling one course of antiviral treatment for every American would cost $7 billion for the first 5 years of coverage. FTAP alone would cost 2.5-times this for the stockpile, and FTAP plus school closure would cost 64% of this for the stockpile.
Conclusion
All interventions reduce the illness attack rate, morbidity and mortality. Many interventions are also cost saving compared to no intervention. Stockpiling TAP in the event of a pandemic is cost saving to the society, and will avoid loss of life. Adding school closure provides the greatest benefit and is likely to be an attractive strategy if transmission and mortality rates are high.
Supplementary Material
Acknowledgments
Source of financial support: This work was partially supported by the National Institute of General Medical Sciences MIDAS grant U01-GM070749. Beate Sander, Azhar Nizam, Louis P. Garrison Jr., Maarten J Postma, M. Elizabeth Halloran, and Ira M. Longini Jr. received a consultancy fee from F. Hoffmann-La Roche, Ltd, Basel, Switzerland. None of the funding sources had any role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Supplemental material for this article can be found at: http://www.ispor.org/publications/value/ViHsupplementary.asp
References
- 1.Germann TC, Kadau K, Longini IM, Jr, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA. 2006;103:5935–40. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson NM, Cummings DA, Fraser C, et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halloran ME, Ferguson NM, Eubank S, Longini IM, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proceedings of the National Academy of Sciences. 2008;105:4639–44. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. HHS Pandemic Influenza Plan [monograph on the Internet] Washington: 2005. [cited 2007 Jul 2]. Available from: http://www.hhs.gov/pandemicflu/plan/ [Google Scholar]
- 5.Committee on Modeling Community Containment for Pandemic Influenza. Modeling community containing pandemic influenza: a letter report 2006 [monograph on the Internet] Washington: The National Academies Press; 2006. [cited 2007 Jul 2]. Available from: http://www.nap.edu/catalog/11800.html. [Google Scholar]
- 6.Edmunds WJ, Medley GF, Nokes DJ. Evaluating the cost-effectiveness of vaccination programmes: a dynamic perspective. Stat Med. 1999;18:3263–82. doi: 10.1002/(sici)1097-0258(19991215)18:23<3263::aid-sim315>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159:623–33. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 8.Halloran ME, Longini IM, Cowart DM, Nizam A. Community interventions and the epidemic prevention potential. Vaccine. 2002;20:3254–62. doi: 10.1016/s0264-410x(02)00316-x. [DOI] [PubMed] [Google Scholar]
- 9.Elveback LR, Fox JP, Ackerman E, et al. An influenza simulation model for immunization studies. Am J Epidemiol. 1976;103:152–65. doi: 10.1093/oxfordjournals.aje.a112213. [DOI] [PubMed] [Google Scholar]
- 10.Weycker D, Edelsberg J, Halloran ME, et al. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23:1284–93. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Halloran ME, Hayden FG, Yang Y, et al. Antiviral effects on influenza viral transmission and pathogenicity: observations from household-based trials. Am J Epidemiol. 2007;165:212–21. doi: 10.1093/aje/kwj362. [DOI] [PubMed] [Google Scholar]
- 12.Longini IM, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005;309:1083–87. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 13.Diekmann O, Heesterbeek JA, Metz JA. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J Math Biol. 1990;28:365–82. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 14.Meier CR, Napalkov PN, Wegmuller Y, et al. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis. 2000;19:834–42. doi: 10.1007/s100960000376. [DOI] [PubMed] [Google Scholar]
- 15.Mills CE, Robins JM, Lipsitch M. Transmissibility of 1918 pandemic influenza. Nature. 2004;432:904–6. doi: 10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden FG, Gubareva LV, Monto AS, et al. Zanamivir Family Study Group. Inhaled zanamivir for the prevention of influenza in families. N Engl J Med. 2000;343:1282–9. doi: 10.1056/NEJM200011023431801. [DOI] [PubMed] [Google Scholar]
- 17.Welliver R, Monto AS, Carewicz O, et al. Oseltamivir Post Exposure Prophylaxis Investigator Group. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285:748–54. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 18.Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–43. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Longini IM, Halloran ME. Design and evaluation of prophylactic interventions using infectious disease incidence data from close contact groups. Appl Statist. 2006;55:317–30. doi: 10.1111/j.1467-9876.2006.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner D, Wailoo A, Nicholson K, et al. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess. 2003;7:1–170. doi: 10.3310/hta7350. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–72. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- 22.Bettis R, Iacuzio D, Jung T, et al. Impact of influenza treatment with oseltamivir on health, sleep and daily activities of otherwise healthy adults and adolescents. Clin Drug Invest. 2006;26:329–40. doi: 10.2165/00044011-200626060-00004. [DOI] [PubMed] [Google Scholar]
- 23.Sander B, Gyldmark M, Aultman R, Aoki FY. Impact on health outcomes and costs of influenza treatment with oseltamivir in elderly and high-risk patients. J Med Econom. 2004;7:67–83. [Google Scholar]
- 24.Denis M. Pandemic vaccines: development status [abstract]. Presented at: Pandemic Influenza Vaccines. Building a Platform for Global Collaboration; 28–30 January 2007; Beijing, China. [Google Scholar]
- 25.Lee GM, Salomon JA, LeBaron CW, Lieu TA. Health-state valuations for pertussis: methods for valuing short-term health states. Health Qual Life Outcomes. 2005;3:17. doi: 10.1186/1477-7525-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prosser LA, Ray GT, O’Brien M, et al. Preferences and willingness to pay for health states prevented by pneumococcal conjugate vaccine. Pediatrics. 2004;113:283–90. doi: 10.1542/peds.113.2.283. [DOI] [PubMed] [Google Scholar]
- 27.Gold MR, Siegel JR, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 28.Nettleman MD, White T, Lavoie S, Chafin C. School absenteeism, parental work loss, and acceptance of childhood influenza vaccination. Am J Med Sci. 2001;321:178–80. doi: 10.1097/00000441-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Rothberg MB, Fisher D, Kelly B, Rose DN. Management of influenza symptoms in healthy children: cost effectiveness of rapid testing and antiviral therapy. Archives of Pediatric Adolescent Medicine. 2005;159:1055–62. doi: 10.1001/archpedi.159.11.1055. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Education Institute of Education Sciences; National Center for Educations Statistics. Digest of education statistics. 2005 http://nces.ed.gov/programs/digest/d05/tables/dt05_001.asp?referer=list.
- 31.Physicians Desk Reference. Red Book: Pharmacy’s Fundamental Reference (Red Book Drug Topics) Montvale: Thomson PDR; 2006. [Google Scholar]
- 32.OANDA. The Currency Site. 2006 http://www.oanda.com/
- 33.Schulpher M. The role and estimation of productivity costs in economic evaluation. In: Drummond M, editor. Economic evaluation in health care: merging theory with practice. New York: Oxford University Press; 2001. [Google Scholar]
- 34.US Department of Labor: Bureau of Labor Statistics. Compensation cost trends. 2006 http://www.bls.gov/ncs/ect/home.htm.
- 35.US Department of Education Institute of Education Sciences; National Center for Educations Statistics. Projections of education statistics to 2015. http://nces.ed.gov/programs/projections/
- 36.Herlocher ML, Truscon R, Elias S, et al. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis. 2004;190:1627–30. doi: 10.1086/424572. [DOI] [PubMed] [Google Scholar]
- 37.Congressional Budget Office. A potential influenza pandemic: an update on possible macroeconomic effects and policy issues. 2006 http://www.cbo.gov/showdoc.cfm?index=7214&sequence=0.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.