Abstract
Objectives
Using a rabbit model of post-traumatic joint contractures, we investigated whether treatment with a mast cell stabilizer after joint injury would lessen the molecular manifestations of joint capsule fibrosis.
Methods
Surgical joint injury was used to create stable post-traumatic contractures of the knee in skeletally mature New Zealand white rabbits. Four groups of animals were studied: a non-operated control group (n = 8), an operated contracture group (n = 13) and two operated groups treated with the mast cell stabilizer, ketotifen, at doses of 0.5 mg/kg (n = 9) and 1.0 mg/kg (n = 9) twice daily. Joint capsule fibrosis was assessed by quantifying the mRNA and protein levels of α-SMA, tryptase, TGF-β1, collagen I and collagen III. Significance was tested using an ANOVA analysis of variance.
Results
The protein and mRNA levels of α-SMA, TGF-β1, tryptase and collagen I and III were significantly elevated in the operated contracture group compared to control (p < 0.01). In both ketotifen-treated groups, protein and mRNA levels of α-SMA, TGF-β1 and collagen I were significantly reduced compared to the operated contracture group (p < 0.01).
Conclusions
These data suggest an inflammatory pathway mediated by mast cell activation is involved in joint capsule fibrosis after traumatic injury.
Keywords: Ketotifen, Mast cells, Fibrosis, Rabbit, Post-traumatic
Introduction
Post-traumatic joint contractures are a remarkably common problem and very difficult to treat. In the setting of a congruent articular surface, the joint capsule is regarded as the critical motion-limiting anatomic structure in a developing contracture [1, 2], yet few details of the pathologic changes occurring in the joint capsule are understood.
Joint capsule specimens from contracted elbows are thicker and less compliant than unaffected elbows [2–4]. Histologic preparations demonstrate significant myofibroblast hyperplasia and increased collagen synthesis and deposition [5, 6]. These changes are characteristic of connective tissue fibrosis and are observed in numerous other human conditions such as scleroderma, hypertrophic wound healing and renal, hepatic and pulmonary fibrosis [7–9]. The myofibroblast, a differentiated lineage of fibroblasts, is viewed as an effector cell in the pathogenesis of connective tissue fibrosis [10]. The precise molecular events driving sustained myofibroblast differentiation, proliferation and activity during post-traumatic contracture formation remain obscure.
Transforming growth factor-beta1 (TGF-β1) is an important regulator of connective tissue fibrosis. In-vitro studies have demonstrated that TGF-β1 is a potent fibroblast chemoattractant and mitogen, a key signal for the differentiation of the myofibroblast phenotype and an upregulator of collagen synthesis [10, 11]. Recently, we have documented chronically elevated levels of joint capsule TGF-β1 protein and mRNA levels in both human and animal models of post-traumatic contractures [12]. The precise mechanisms responsible for this chronic increase in TGF-β1 expression have not been defined. Interestingly, increased mast cell densities are found in many fibrotic connective tissues, often in close proximity to myofibroblasts [13]. Mast cells are also a source of numerous proteases and growth factors that can induce fibroblast proliferation, collagen synthesis and myofibroblast differentiation such as TGF-β1 [14].
Ketotifen fumarate is an anti-allergic and anti-histaminic agent known to inhibit the calcium-dependent degranulation of mast cells, and non-competitively blocks histamine at the H1 receptor [15]. Ketotifen has been FDA approved as an adjuvant treatment for adult and pediatric populations with asthma for over 15 years and more recently has been FDA approved for treatment of allergic conditions of the eye [16]. Ketotifen administration has also been shown to impair abnormal wound fibrosis and hypercontractile scar formation in the red Duroc pig [17]. Recently, we have shown in a rabbit model that treatment with ketotifen significantly reduced the biomechanical severity of post-traumatic joint contractures [18], although the effects of ketotifen treatment at the molecular level in this animal model have yet to be determined. The purpose of the present study was to assess the mRNA and protein levels of key molecular markers of connective tissue fibrosis in capsular tissue following ketotifen administration in a rabbit model of post-traumatic joint contractures.
Methods
Group allocation
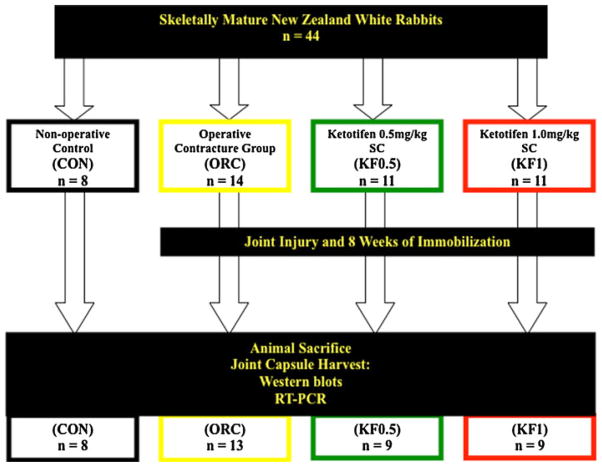
All aspects of this study were reviewed and approved by our university animal care committee. A total of 44 rabbits were procured for this study. Upon arrival at our animal care facility, rabbits were randomly assigned to one of four groups. Group allocation was considered complete at n = 8 (based on a pre-determined sample size calculation) and the remaining animals (12) were distributed in a non-randomized fashion. Figure 1 illustrates the group allocation used in the study: the first group consisted of rabbits untouched by any surgical or pharmacologic interventions (non-operated control group, “CON”). In the second group, surgical intra-articular joint injury followed by 8 weeks of immobilization was used to create stable post-traumatic joint contractures (operated contracture group, “ORC”), as previously described [19]. No pharmacologic interventions were given to this study group. The third and fourth groups (experimental #1 and experimental #2) were treated with identical surgical interventions as the operated contracture group, but additionally received a twice daily subcutaneous dose of a mast cell stabilizer, ketotifen fumarate (KF; Sigma-Aldrich, St. Louis, MO, USA), for the entire 8 weeks of immobilization. Two different doses of KF were used: 0.5 and 1.0 mg/kg twice daily (groups “KF0.5” and “KF1.0”, respectively). All rabbits were killed exactly 8 weeks from the initial day of surgery.
Fig. 1.
Overview of the study design
From the available literature, a wide range of oral, subcutaneous and intravenous doses of ketotifen have been used to inhibit histamine liberation from mast cells in response to a variety of allergic and anaphylactic stimuli. The rationale for the doses and route of administration of ketotifen used in this study is based on the following: the efficacy of subcutaneous ketotifen administration has been previously documented [20], subcutaneously injections are technically easy to administer and provide reliable dosing, and, finally, prior in-vivo studies using similar ketotifen doses have been shown to maximally inhibit allergen-induced anaphylaxis and mast cell histamine liberation [20–22].
Joint interventions
The joint procedures were performed under inhalational general anesthesia, on the right or left knee, which was randomly selected in advance. The detailed surgical procedure has been described previously [19]. Briefly, a single lateral thigh incision was used to expose the femur and the mobile skin was retracted distally to expose the medial and lateral aspects of the distal femur. Medial and lateral parapatellar arthrotomies were made, taking care to avoid the collateral ligaments. Using an osteotome, 5-mm2 cortical windows were removed from the non-articular portion of the medial and lateral femoral condyles, producing a traumatic intra-capsular hemarthrosis while preserving the integrity of the articular surfaces. The knee was then immobilized at 150° of flexion using a 1.6-mm diameter Kirschner wire (Zimmer, Mississauga, ON, Canada), which was drilled through the tibia, passed subcutaneously behind the knee and bent around the femur (Fig. 2). After each surgery, the rabbits were allowed free cage activity. All surgically manipulated rabbits were immobilized for a total of 8 weeks. A broad-spectrum antibiotic and a morphine derivative were administered for 3 days post-operatively.
Fig. 2.
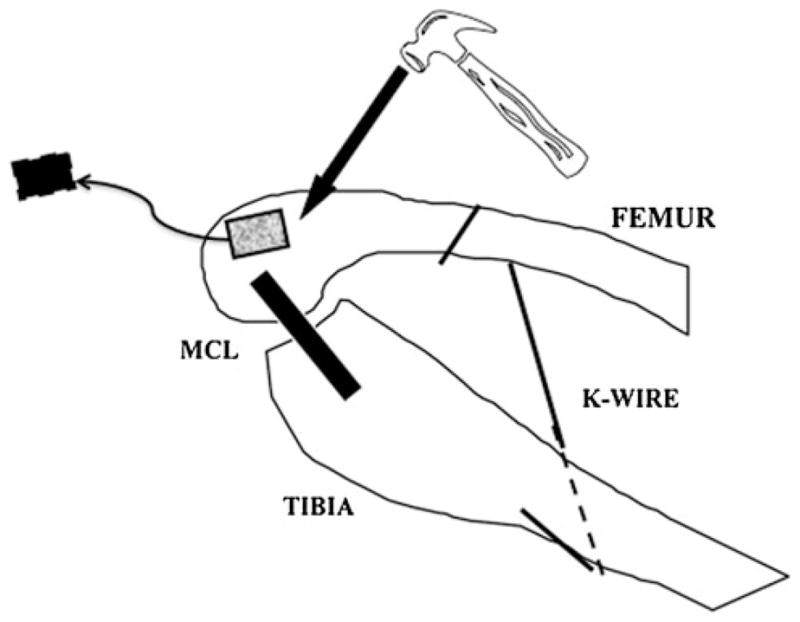
Diagram depicting the contracture surgery (Adapted with permission from Hildebrand et al. [19]
Eight weeks from the initial surgery date, rabbits were killed with a barbiturate overdose and the posterior joint capsule was harvested from the knee and partitioned into equal samples for the determination of protein quantification (Western blots) and mRNA level assessments (RT-PCR). Samples were snap frozen in liquid nitrogen and then stored at −80°C.
Western blot studies
Protein levels for alpha smooth muscle actin (α-SMA), TGF-β1, tryptase, and collagen type I and III were assessed using Western blot techniques as previously described [23]. Harvested joint capsule tissue was powdered at liquid nitrogen temperatures, suspended in buffer solution and centrifuged for 15 min at 50,400g. A protease inhibitor cocktail (Sigma-Aldrich) was added to the isolated supernatant fraction, which was then concentrated 10-fold to 100 μL. Total protein content of the sample was determined with a Bio-Rad protein assay (Bio-Rad, Mississauga, ON, Canada). Aliquots of the protein samples were then boiled for 5 min in SDS sample buffer with 2-mercaptoethanol (Bio-Rad) as a reducing agent, and 20 μg of protein per lane was electrophoretically resolved on a 12.5% polyacrylamide pre-cast gel (Owl Scientific, Portsmouth, NH, USA). Fractionated proteins were transferred onto nitrocellulose membranes overnight. Non-specific binding was blocked using 5% skimmed milk in Tris buffered saline (TBS). Fractionated proteins were then incubated with the appropriate primary antibodies (Table 1) diluted to 2.5 μL in TBS for 2 h at room temperature. The blots were then incubated with the appropriate secondary antibody conjugated with horseradish peroxidase (HRP) at 1:1,000 dilution in TBS with 5% skimmed milk powder for 1 h at room temperature. Peroxidase activity was detected with ECL detection reagents (Amersham Pharmacia Biotech, Baie d’Urfe, QC, Canada). Band intensities were quantified using Quantity One image analysis software (Bio-Rad) and are reported as a ratio relative to the protein band intensity of the basal housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Negative controls omitted the primary antibodies.
Table 1.
Primary mouse antibodies used in Western blot experiments
| Antibody | Type | Source | Catalogue # |
|---|---|---|---|
| α-SMA | Monoclonal | Sigma | A2547 |
| TGF-β1 | Monoclonal | Santa Cruz | SC-81017 |
| Tryptase | Polyclonal | R&D | AF1937 |
| Col I | Monoclonal | Santa Cruz | SC-59772 |
| Col III | Monoclonal | MEDICORP | AF5810 |
| GAPDH | Monoclonal | Invitrogen | 39-8600 |
All reactions were incubated for 1 h at room temperature
Reverse transcription–polymerase chain reaction (RT-PCR)
A semiquantitative RT-PCR approach was used to assess mRNA levels in this study, as previously described using this rabbit model [12, 23]. RNA isolation was preformed using the TRIspin method [24]. Joint capsule specimens were powdered at liquid nitrogen temperatures, added to 1 mL of the TRIzol reagent (Life Technologies, Gaithesberg, MD, USA), suspended in chloroform, mixed by vortex and centrifuged. The upper aqueous phase containing the RNA was then removed and one volume of 70% ethanol was added and mixed thoroughly. Total RNA was extracted and purified by the addition of an RNase Free DNase kit (Qiagen, Chatsworth, CA, USA). The RNA yields were then quantified fluorometrically (Perkin-Elmer, Branchburg, NJ, USA) with use of SYBR Green II (FMC BioProducts, Rockland, MN, USA) and were compared to standards obtained with calf liver ribosomal RNA.
RT-PCR was performed on all mRNA samples simultaneously to minimize variability of the results. Reverse transcription (RT) was performed using aliquots containing 1 μg of total RNA and the Qiagen Omniscript RT kit (Qiagen GmbH, Hilden, Germany). Random primers (0.5 μmol/L) were added to the RNA and first-strand cDNA was synthesized. Reactions were incubated at 37°C for 1 h, followed by a 5-min incubation at 93°C. Aliquots of cDNA were amplified in a mixture of polymerase chain reaction buffer, 10 mmol/L D-nucleoside triphosphate mixture, 50 mmol/L MgCl2, 0.5 μmol/L of each primer and 1.25 U of Taq DNA polymerase (Rose Scientific, Edmonton, AB, Canada). Rabbit-specific primers and optimal cycle conditions (Table 2) were used as previously published [12, 25]. All no-RT controls were negative, confirming that no detectable genomic DNA was present in the RNA samples. RT-PCR was performed on all mRNA samples simultaneously to minimize potential variability.
Table 2.
RT-PCR primers and conditions
| Primer | Forward sequence 5′ → 3′ | Reverse sequence 3′ → 5′ | Primer length | Transcript size (bp) | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|---|---|
| α-SMA | GTG TGA GGA AGA GGA CAG CA | TAC GTC CAG AGG CAT AGA GG | 20 | 446 | 55 | 32 |
| Col I | GAT GCG TTC CAG TTC GAG TA | GGT CTT CCG GTG GTC TTG TA | 20 | 312 | 55 | 22 |
| Col III | TTA TAA ACC AAC CTC TTC CT | TAT TAT AGC ACC ATT GAG AC | 20 | 255 | 55 | 28 |
| TGF-β1 | CGG CAG CTG TAC ATT GAC TT | AGC GCA CGA TCA TGT TGG AC | 20 | 271 | 60 | 32 |
| GAPDH | TCA CCA TCT TCC AGG AGC AG | CAC AAT GCC GAA GTG GTC GT | 20 | 293 | 55 | 22 |
PCR products were separated by electrophoresis on 2% agarose gel and visualized with ethidium bromide, with comparison to a standard 1-kb DNA ladder (Life Technologies). Images were captured using the Gel-Doc image analysis system (Bio-Rad). Relative band intensities were quantified by densitometric scanning of negatives using the Masterscan Interpretive Densitometer (CSPI, Billerica, MA, USA). The mRNA levels for all molecules were normalized to those for the housekeeping gene, GAPDH.
Recent studies have directly compared the results from this semi-quantitative PCR method to those generated by real-time quantitative PCR (qPCR) and found them to be very similar [26]. Thus, these well controlled semi-quantitative assessments are comparable to qPCR and the findings can likely be compared to future studies using qPCR methods.
Statistics
Data are presented as mean ± standard deviation. All data was analyzed using an ANOVA analysis of variance with a post-hoc Bonferroni test for comparison between individual groups. Significance is defined as p < 0.05. Animal numbers were determined using a sample size calculation based on a t-test, using data from previous biomechanical studies testing this animal model [19]. Measurements of joint stiffness are felt to represent the most clinically relevant manifestation of joint capsule fibrosis and the most clinically relevant outcome of pharmaceutical interventions. Using this information it was determined that with seven animals in each study arm, the power to detect a difference of two standard deviations of the biomechanical means, at a significance level of 95%, was 85–90%. Given an assumed 10% failure rate (tibia fracture, animal illness, post-operative failure to thrive) a minimum of eight animals was deemed necessary for each study arm.
Results
Of the 44 rabbits included in the study, five animals were ultimately excluded from the final analysis for reasons comprising post-operative failure to thrive (n = 3; ORC, KF0.5 and KF1.0), iatrogenic tibia fracture (n = 1; KF0.5) or premature hardware failure (n = 1; KF1.0). No significant adverse events from the administration of ketotifen were observed such as significant weight loss, infection, failure to thrive or wound dehiscence.
Figures 3 and 4 illustrate the average protein levels for α-SMA, TGF-β1, tryptase, collagen I and collagen III relative to the average band intensity of GAPDH in the four animal groups. In the operated contracture (ORC) group, the protein levels for α-SMA, TGF-β1, tryptase, collagen type I and collagen type III were all significantly elevated compared to the non-operated control group (CON). In both ketotifen-treated groups, the protein levels for α-SMA, TGF-β1 and collagen type I were significantly reduced compared to the ORC group (p < 0.001). Collagen III protein levels were reduced in both ketotifen groups compared to the ORC group, but failed to reach statistical significance in the KF1.0 group. Similarly, tryptase levels were reduced in both ketotifen groups relative to the ORC group (Fig. 3), but failed to reach statistical significance in the KF0.5 group. Tryptase levels were significantly less in the KF1.0 group compared to the ORC group (p < 0.001) and, furthermore, tryptase levels in this group were actually less than the non-operated control group, although they did not reach statistical significance.
Fig. 3.
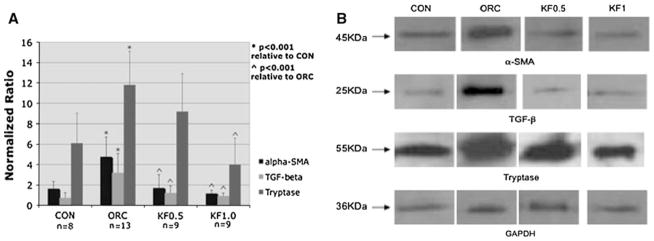
Graphical representation of joint capsule α-SMA, TGF-β1 and tryptase protein levels relative to GAPDH (a) and a photograph of α-SMA, TGF-β1, tryptase and GAPDH protein band intensities (b)
Fig. 4.
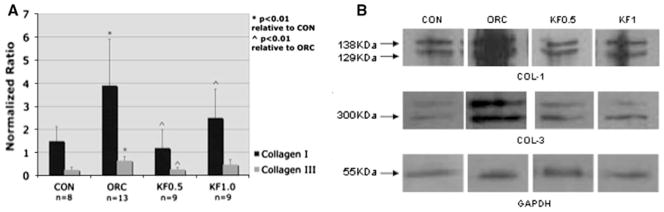
Graphical representation of joint capsule collagen I and collagen III protein levels relative to GAPDH (a) and a photograph of collagen I, collagen III and GAPDH protein band intensities (b)
Similar to the observed protein expression, the mRNA levels for α-SMA, TGF-β1, collagen I and collagen III were significantly elevated in the ORC group compared to the CON group (Fig. 5). The mRNA levels for all but collagen III were significantly reduced in both groups treated with ketotifen compared to the ORC group (p < 0.01). Collagen III mRNA levels were reduced in both ketotifen groups, but again failed to reach statistical significance in the KF1.0 group when compared to the ORC group.
Fig. 5.
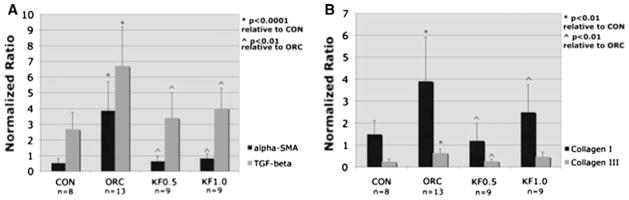
Joint capsule mRNA levels of α-SMA and TGF-β1 (a), and collagen I and collagen III (b) relative to GAPDH
Discussion
The purpose of the present study was to determine if in-vivo exposure to a known mast cell stabilizer would lessen the molecular manifestations of joint capsule fibrosis during post-traumatic contracture formation. Using this rabbit model, we have recently shown that ketotifen treatment reduced biomechanical contracture severity by 50% and joint capsule myofibroblast hyperplasia by up to 65% [18]. The effect of systemic ketotifen treatment on the expression of key molecular markers of joint capsule fibrosis has not been previously assessed. The results presented here show reductions in the protein and mRNA levels of joint capsule TGF-β1, α-SMA, collagen I and collagen III in both ketotifen-treated groups (KF0.5 and KF1.0) compared to the operated contracture group (ORC). These molecular findings and the biomechanical data support the hypothesis that mast cell contributions after joint injury are important in the molecular pathogenesis of joint capsule fibrosis and subsequent motion loss. Furthermore, these findings demonstrate that the systemic administration of a mast cell stabilizer after joint injury can lessen the inflammatory-mediated mechanisms involved in post-traumatic fibrogenesis.
Mast cells are connective tissue residents, they have been observed throughout the early inflammatory phases of fracture healing, and mast cell activation has been shown to promote myofibroblast proliferation and increased collagen synthesis [9, 13, 27–29]. Mast cell hyperplasia has more recently been observed within affected joint capsules harvested from post-traumatic contractures of the human elbow and the rabbit knee [30]. Despite evidence linking mast cell activation to fibroproliferative disorders, very few studies have assessed mast cell modulation with ketotifen in animal models of fibrosis. A recent study by Gallant-Behm et al. [17] similarly looked at ketotifen administration in the red Duroc pig. This breed of pig is known to heal full-thickness skin wounds with abnormally fibrotic, hypercontractile scars, characterized by myofibroblast and mast cell hyperplasia [9, 17]. The oral administration of ketotifen after dorsal skin wound induction significantly reduced the densities of both myofibroblasts and mast cells and collagen deposition within scar tissue compared to placebo-treated red Duroc pig scar [17]. In the present study, ketotifen treatment significantly reduced joint capsule α-SMA (specific for the myofibroblast phenotype), tryptase and collagen type I protein and mRNA levels compared to the operated contracture group. These findings parallel the observations noted in the ketotifen-treated red Duroc pig model. Interestingly, in the pig study, ketotifen treatment of Yorkshire pigs, a breed that heals skin wounds with a normal phenotype, did not affect wound healing. This would imply that ketotifen treatment ameliorates abnormal healing pathways but does not adversely affect normal healing. Similarly, no significant post-operative healing complications such as systemic infection, wound infection or wound dehiscence were observed in the ketotifen-treated rabbits.
The expression of TGF-β1 was of particular interest given its widely implicated role in the development of connective tissue fibrosis. Certainly, in both human and animal models of post-traumatic contracture, joint capsule levels of TGF-β1 protein and mRNA are chronically elevated [12]. A variety of cell types have been shown to produce TGF-β1, including platelets, macrophages, neutrophils, activated fibroblasts and mast cells [31, 32]. Compared to unaffected joint capsule specimens, which are sparsely populated by fibroblasts, numerous studies from our research group have proven the fibrotic post-traumatic joint capsule to be quite hypercellular, with mast cells and myofibroblasts representing roughly 90% of the total cell population [18, 30]. Mast cells are a known source of TGF-β1 and given the high densities of this cell type in contracted joint capsules it seems quite plausible that elevated TGF-β1 gene and protein expression may be a product of increased mast cell activation. The significant reduction in TGF-β1 protein and mRNA levels observed in the ketotifen-treated rabbits supports this hypothesis. Furthermore, the observed reductions of protein and mRNA levels for α-SMA and collagen I in both ketotifen-treated groups may be a manifestation of decreased TGF-β1 expression in affected joint capsules. Findings from previous work in this rabbit model documented significant reductions in joint capsule myofibroblast numbers in rabbits treated with ketotifen [18], which is consistent with the observed reduction of α-SMA gene and protein expression. It is possible that the reduced TGF-β1 expression associated with ketotifen treatment lessens subsequent joint capsule myofibroblast hyperplasia and fibrogenesis. However, these results do not prove a direct cause-and-effect relationship between mast cell activation, TGF-β1 levels and the development of fibrosis.
There are limitations in this study that warrant mention. Firstly, ketotifen is not entirely specific for mast cells and has been shown to inhibit basophil degranulation and impair neutrophil migration [33, 34], although these cell types have not been identified or implicated in fibroproliferative conditions. Furthermore, despite the reductions in TGF-β1 protein and mRNA levels in animals treated with ketotifen, it cannot be assumed that TGF-β1 independently mediates joint capsule fibrogenesis. Other mast cell mediators such as tryptase, the most abundant mast cell synthetic product, have been shown to induce similar profibrotic events such as myofibroblast differentiation, matrix contraction and collagen synthesis [35, 36]. Studies designed to selectively augment or inhibit TGF-β1 and tryptase and their respective receptors are needed to determine the relative importance of these two mediators during the induction of joint capsule fibrosis after traumatic injury. Finally, the different ketotifen doses chosen for this study were an attempt to potentially demonstrate a dose response but, in retrospect, both the 0.5 and 1.0 mg/kg doses are at the upper end of the dosing range of ketotifen based on previous in-vivo and in-vitro studies [20, 21, 37, 38]. Ideally, doses should have been staggered by an order of 10 or 100, yet despite this it was encouraging to note that both ketotifen 0.5 and 1.0 mg/kg groups provided similar results.
In conclusion, the results of this pre-clinical study are quite encouraging, as we have shown that the administration of an FDA-approved medication known to inhibit mast cell degranulation can significantly reduce the expression of molecules associated with the periarticular fibrotic changes observed in a rabbit model of post-traumatic joint contracture formation. The precise mast cell mediators involved in the induction of joint capsule fibrosis after joint injury remain to be identified as we search for novel strategies focused toward the prevention and treatment of post-traumatic joint contractures.
Acknowledgments
We would like to thank the Canadian Orthopaedic Foundation for their generous funding of this project via the Robert Salter Award. D.A.H. is the Calgary Foundation–Grace Glaum Professor. All authors have no financial affiliations to disclose regarding this research.
Contributor Information
Michael J. Monument, Division of Orthopaedic Surgery, Faculty of Medicine, McCaig Institute for Bone and Joint Health, University of Calgary, 3330 Hospital Drive NW, Calgary, AB T2N 4N1, Canada
David A. Hart, Division of Orthopaedic Surgery, Faculty of Medicine, McCaig Institute for Bone and Joint Health, University of Calgary, 3330 Hospital Drive NW, Calgary, AB T2N 4N1, Canada
A. Dean Befus, Department of Medicine, Pulmonary Research Group, University of Alberta, Edmonton, AB, Canada.
Paul T. Salo, Division of Orthopaedic Surgery, Faculty of Medicine, McCaig Institute for Bone and Joint Health, University of Calgary, 3330 Hospital Drive NW, Calgary, AB T2N 4N1, Canada
Mei Zhang, Division of Orthopaedic Surgery, Faculty of Medicine, McCaig Institute for Bone and Joint Health, University of Calgary, 3330 Hospital Drive NW, Calgary, AB T2N 4N1, Canada.
Kevin A. Hildebrand, Division of Orthopaedic Surgery, Faculty of Medicine, McCaig Institute for Bone and Joint Health, University of Calgary, 3280 Hospital Drive NW, Calgary, AB T2N 4Z6, Canada
References
- 1.Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg Am. 2007;32:1605–23. doi: 10.1016/j.jhsa.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Hastings IH. Post-traumatic contracture of the elbow. Operative release using a lateral collateral ligament sparing approach. J Bone Joint Surg Br. 1998;80B:805–12. doi: 10.1302/0301-620x.80b5.8528. [DOI] [PubMed] [Google Scholar]
- 3.Gallay SH, Richards RR, O’Driscoll SW. Intraarticular capacity and compliance of stiff and normal elbows. Arthroscopy. 1993;9:9–13. doi: 10.1016/s0749-8063(05)80336-6. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Schimmel DR, Masuda K, Hastings H, 2nd, Muehleman C. Structural and biochemical evaluation of the elbow capsule after trauma. J Shoulder Elbow Surg. 2007;16:484–90. doi: 10.1016/j.jse.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildebrand KA, Zhang M, Hart DA. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop Relat Res. 2005;439:228–34. doi: 10.1097/01.blo.0000177718.78028.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hildebrand KA, Zhang M, van Snellenberg W, King GJ, Hart DA. Myofibroblast numbers are elevated in human elbow capsules after trauma. Clin Orthop Relat Res. 2004;419:189–97. doi: 10.1097/00003086-200402000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirk TZ, Mark ME, Chua CC, Chua BH, Mayes MD. Myofibroblasts from scleroderma skin synthesize elevated levels of collagen and tissue inhibitor of metalloproteinase (TIMP-1) with two forms of TIMP-1. J Biol Chem. 1995;270:3423–8. doi: 10.1074/jbc.270.7.3423. [DOI] [PubMed] [Google Scholar]
- 8.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, et al. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–8. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 9.Harunari N, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669–77. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 11.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–9. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand KA, Zhang M, Hart DA. Myofibroblast upregulators are elevated in joint capsules in posttraumatic contractures. Clin Orthop Relat Res. 2007;456:85–91. doi: 10.1097/BLO.0b013e3180312c01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heard BE, Dewar A, Corrin B. Apposition of fibroblasts to mast cells and lymphocytes in normal human lung and in cryptogenic fibrosing alveolitis. Ultrastructure and cell perimeter measurements. J Pathol. 1992;166:303–10. doi: 10.1002/path.1711660314. [DOI] [PubMed] [Google Scholar]
- 14.Gruber BL. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5:147–53. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- 15.Grant SM, Goa KL, Fitton A, Sorkin EM. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. 1990;40:412–48. doi: 10.2165/00003495-199040030-00006. [DOI] [PubMed] [Google Scholar]
- 16.Torkildsen GL, Abelson MB, Gomes PJ. Bioequivalence of two formulations of ketotifen fumarate ophthalmic solution: a single-center, randomized, double-masked conjunctival allergen challenge investigation in allergic conjunctivitis. Clin Ther. 2008;30:1272–82. doi: 10.1016/s0149-2918(08)80051-3. [DOI] [PubMed] [Google Scholar]
- 17.Gallant-Behm CL, Hildebrand KA, Hart DA. The mast cell stabilizer ketotifen prevents development of excessive skin wound contraction and fibrosis in red Duroc pigs. Wound Repair Regen. 2008;16:226–33. doi: 10.1111/j.1524-475X.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 18.Monument MJ, Hart DA, Befus AD, Salo PT, Zhang M, Hildebrand KA. The mast cell stabilizer ketotifen fumarate lessens contracture severity and myofibroblast hyperplasia: a study of a rabbit model of posttraumatic joint contractures. J Bone Joint Surg Am. 2010;92:1468–77. doi: 10.2106/JBJS.I.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–20. doi: 10.1016/j.orthres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Martin U, Romer D. The pharmacological properties of a new, orally active antianaphylactic compound: ketotifen, a benzocycloheptathiophene. Arzneimittelforschung. 1978;28:770–82. [PubMed] [Google Scholar]
- 21.Craps LP, Ney UM. Ketotifen: current views on its mechanism of action and their therapeutic implications. Respiration. 1984;45:411–21. doi: 10.1159/000194648. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CH, Chen JL, Liu YT, Wang DP. Therapeutic effect and pharmacokinetics of ketotifen transdermal delivery system. Drug Dev Ind Pharm. 1998;24:213–7. doi: 10.3109/03639049809085612. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrand KA, Zhang M, Germscheid NM, Wang C, Hart DA. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop. 2008;79:116–25. doi: 10.1080/17453670710014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082–6. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- 25.Hildebrand KA, Zhang M, Hart DA. Joint capsule matrix turnover in a rabbit model of chronic joint contractures: correlation with human contractures. J Orthop Res. 2006;24:1036–43. doi: 10.1002/jor.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parreno J, Buckley-Herd G, de-Hemptinne I, Hart DA. Osteoblastic MG-63 cell differentiation, contraction, and mRNA expression in stress-relaxed 3D collagen I gels. Mol Cell Biochem. 2008;317:21–32. doi: 10.1007/s11010-008-9801-x. [DOI] [PubMed] [Google Scholar]
- 27.Banovac K, Renfree K, Makowski AL, Latta LL, Altman RD. Fracture healing and mast cells. J Orthop Trauma. 1995;9:482–90. doi: 10.1097/00005131-199509060-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lindholm R, Lindholm S, Liukko P, Paasimaki J, Isokaanta S, Rossi R, et al. The mast cell as a component of callus in healing fractures. J Bone Joint Surg Br. 1969;51:148–55. [PubMed] [Google Scholar]
- 29.Trabucchi E, Radaelli E, Marazzi M, Foschi D, Musazzi M, Veronesi AM, et al. The role of mast cells in wound healing. Int J Tissue React. 1988;10:367–72. [PubMed] [Google Scholar]
- 30.Hildebrand KA, Zhang M, Hart DA. Joint capsule mast cells and neuropeptides are increased within 4 weeks of injury and remain elevated in chronic stages of posttraumatic contractures. J Orthop Res. 2008;26:1313–9. doi: 10.1002/jor.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennington D, Thomas P, Lopez A, Gold W. Transforming growth factor-beta production by dog mastocytoma cells. Storage and release from mast cell granules. Chest. 1991;99:66S. doi: 10.1378/chest.99.3_supplement.66s. [DOI] [PubMed] [Google Scholar]
- 32.Kanbe N, Kurosawa M, Nagata H, Yamashita T, Kurimoto F, Miyachi Y. Production of fibrogenic cytokines by cord blood-derived cultured human mast cells. J Allergy Clin Immunol. 2000;106:S85–90. doi: 10.1067/mai.2000.106777. [DOI] [PubMed] [Google Scholar]
- 33.Gushchin IS, Zebrev AI. Ketotifen-induced histamine release, inhibition of histamine secretion and modulation of immune response. Agents Actions. 1986;18:92–5. doi: 10.1007/BF01987992. [DOI] [PubMed] [Google Scholar]
- 34.Shiratori Y, Takada H, Hai K, Kiriyama H, Mawet E, Komatsu Y, et al. Effect of anti-allergic agents on chemotaxis of neutrophils by stimulation of chemotactic factor released from hepatocytes exposed to ethanol. Dig Dis Sci. 1994;39:1569–75. doi: 10.1007/BF02088066. [DOI] [PubMed] [Google Scholar]
- 35.Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2000;278:L193–201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- 36.Gailit J, Marchese MJ, Kew RR, Gruber BL. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Invest Dermatol. 2001;117:1113–9. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- 37.Sekardi L, Friedberg KD. Inhibition of immunological histamine release from guinea pig lungs and other organs by mepyramine, ketotifen, and picumast in vivo. Arzneimittelforschung. 1989;39:1331–5. [PubMed] [Google Scholar]
- 38.Radermecker M. Inhibition of allergen-mediated histamine release from human cells by ketotifen and oxatomide. Comparison with other H1 antihistamines. Respiration. 1981;41:45–55. doi: 10.1159/000194358. [DOI] [PubMed] [Google Scholar]