Applying an age cutoff to a Lynch syndrome screening program has considerable potential for decreasing total screening costs and increasing efficiency, but at a loss of effectiveness.
Abstract
Purpose:
To determine the impact of applying an age cutoff to tumor-based Lynch syndrome (LS) screening, specifically focusing on changes in relative effectiveness, efficiency, and cost. The project was undertaken to answer questions about implementation of the LS screening program in an integrated health care delivery system.
Patients and Methods:
Clinical data extracted from an internal cancer registry, previous modeling efforts, published literature, and gray data were used to populate decision models designed to answer questions about the impact of age cutoffs in LS screening. Patients with colorectal cancer (CRC) were stratified at 10-year intervals from ages 50 to 80 years and compared with no age cutoff. Outcomes are reported for a cohort of 325 patients screened and includes total cost to screen, LS cases present in the cutoff category, number of LS cases expected to be identified by screening, cost per LS case detected, and total number and percentage of LS cases missed.
Conclusion:
Applying an age cutoff to an LS screening program has considerable potential for decreasing total screening costs and increasing efficiency, but at a loss of effectiveness. Imposing an age cutoff of 50 years reduces the cost of the screening program to 16% of a program with no age cutoff, but at the expense of missing more than half of the cases. Failure to identify LS cases is magnified by a cascade effect in family members. The results of this analysis influenced the final policy in our system.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States. In 2011, an estimated 141,210 people were diagnosed with CRC, and 49,380 died as a result.1 Approximately 3% of patients with CRC also have Lynch syndrome (LS), an inherited cancer syndrome caused by germline mutations that affect one of four DNA mismatch repair genes: MLH1, MSH2, MSH6, and PMS2.2–5 Individuals with LS have a substantially increased lifetime risk for developing CRC compared with population risk, ranging from 54% to 74% in males and 35% to 52% in females.2,5 In addition, women with LS will have a 28% to 60% lifetime risk of endometrial cancer.2 LS is also associated with modest increased risks (generally 10% by age 70) for other cancers, including gastric, ovarian, small bowel, urinary tract, pancreatic, and sebaceous gland.2 Increasing emphasis is directed to the identification of relatives who also carry an LS mutation and who are at significant risk (45% for men and 35% for women by age 70) to develop CRC.5
The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group in 2009 recommended screening of all CRC tumors for LS using either immunohistochemistry (IHC) or microsatellite instability.6 This recommendation was based on a systematic review of the literature that suggested such screening could reduce morbidity and mortality in relatives of patients with CRC with LS mutations. Mvundura et al,7 followed by Ladabaum et al,8 showed that an IHC-first approach was more cost effective than other screening methods. In 2010, Intermountain Healthcare implemented an IHC-first LS universal screening program for patients with CRC.
The decision to embark on LS universal screening included thorough evaluation by multiple stakeholders of potential implications within the health system, including issues such as importance for the health of the Intermountain community; the cost of universal screening; the implications of abnormal screen results; and the workflow involved to implement and carry through with the universal screening program, the results of which were published previously.9 Although five of eight hospitals in the Intermountain system readily implemented universal LS screening, representatives of the remaining three hospital pathology departments were not convinced that screening all ages represented the best approach to such a screening program. Questions centered around age cutoffs above which screening seemed unnecessary because of the associated high cost to the system of including older patients in whom the likelihood of LS is expected to be lower. In response to these questions, and the lack of data elsewhere that could be assumed to represent “our system,” this study modeled different screening options, using internal and external data to populate the decision analysis models to provide the estimates of key outcomes that could be used to answer the questions. The primary outcome of this study was to define the impact on sensitivity and cost of applying an age cutoff to our LS screening program. A secondary outcome was to model the impact of less than 100% acceptance of confirmatory testing.
Patients and Methods
From our previously published modeling efforts,9 supplemental calculations and modeling for this study, and related works by others, we established the key data parameters shown in Table 1 to populate the Excel-based models for this study. The LS prevalence value listed reflects our best estimate in a population of unselected patients with CRC and is based on the Ohio LS screening experience as published in Hampel et al.10,11 The proportion of LS cases in an unselected CRC population was taken directly from the Hampel et al10 data set, which was the most complete data set we had access to at the time of our study. We accepted these data as reasonable proxies for LS prevalence and CRC (incident) distribution in our (Utah) population. The sensitivity of the LS screening/testing algorithm is based on screening with IHC including BRAF and methylation (of the MLH1 promoter) rule-out testing, because this is the protocol available to us from our reference laboratory. The costs-per-CRC-case screened was also based on our previous modeling study, with minor adjustments to support continuity of the model by accounting for rates of acceptance of sequencing in patients eligible for this testing.9
Table 1.
Model Variables
| Variable | Best Estimate | Source |
|---|---|---|
| Prevalence of LS in unselected CRC patients | 3.6% | Data from IH's first 18 months of LS screening, and Hampel et al (2008)11 |
| Test costs | Provided by our reference laboratory | |
| Immunohistochemistry | $230 | |
| BRAF V600E | $305 | |
| Methylation of MLH1 promoter | $295 | |
| Proportion of total LS cases by age cohorts | Hampel et al (2005) data set10 | |
| < 50 years | 50.0% | |
| < 60 years | 77.3% | |
| < 70 years | 86.4% | |
| < 80 years | 95.5% | |
| Sensitivity of the screening/testing protocol | 91.5% | Gudgeon et al (2011)9 |
| Cost per CRC case screened | Unpublished internal modeling data | |
| At 100% acceptance for sequencing | $348 | |
| At 50% acceptance for sequencing | $310 |
Abbreviations: CRC, colorectal cancer; IH, Intermountain Healthcare; LS, Lynch syndrome.
The structure of the population on which all calculations were based was a cohort of patients with CRC treated within the Intermountain (IH) delivery system in 2010 for whom adequate surgical specimens were available for LS screening, as identified in a query of our electronic medical record (EMR). Query methods have been previously reported.9
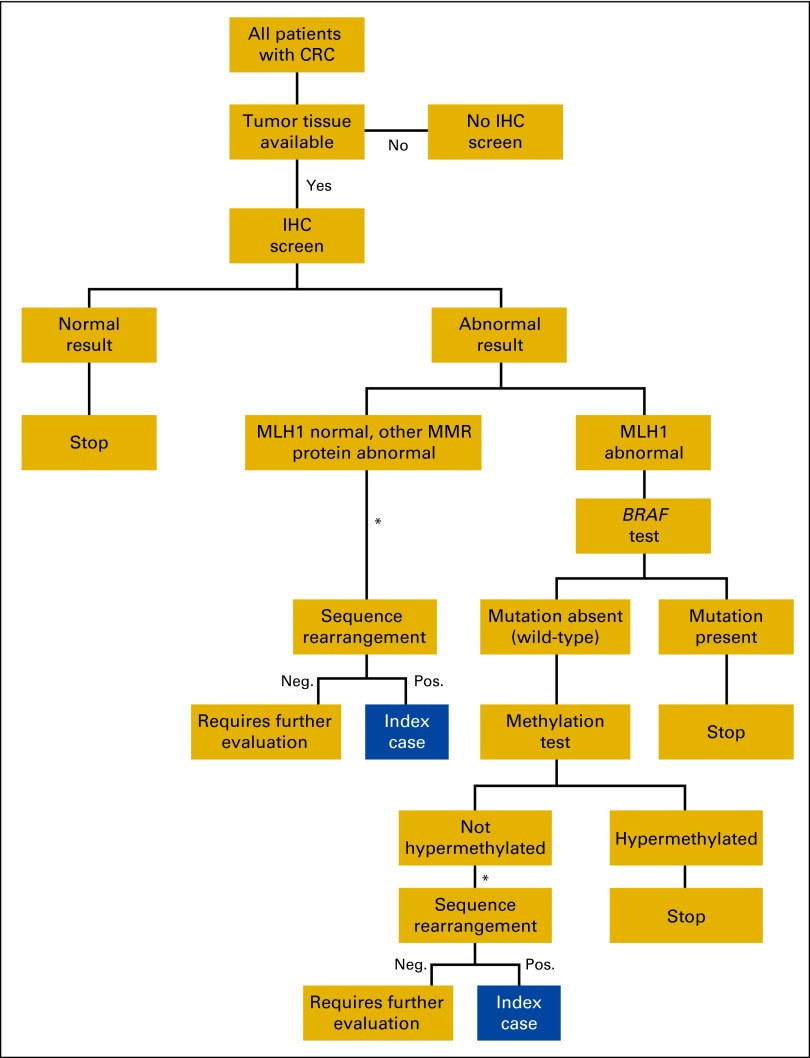
In the preliminary modeling for this study, we assumed 100% acceptance and completion of testing throughout the screening protocol, including confirmatory (ie, sequencing and rearrangement analyses), as illustrated in Figure 1. Because of known challenges in achieving 100% acceptance in confirmatory testing, we also modeled a 50% acceptance rate for confirmatory testing. We acknowledge that genetic counseling is an important service before confirmatory testing, and has a cost that we did not include in our calculations.
Figure 1.
Intermountain Healthcare Lynch syndrome screening/testing protocol. CRC, colorectal cancer; IHC, immunohistochemistry; MMR, mismatch repair; Neg., negative; Pos., positive. (*) Genetic counselor contacts patient for counseling and consent.
Results
Table 2 presents results from our models, detailing the impact of age cutoffs with respect to test outcomes at both 100% and 50% acceptance rates of sequencing in eligible patients. This represents the expected impact of LS screening in a population of patients with CRC in our system who were expected to be treated for CRC within a 1-year period and for whom a surgical specimen would be expected to be available (n = 325) given our best estimates of the test performance and costs available to us at the time of this study.
Table 2.
Impact of Age Cutoffs on LS Screen/Test Outcomes, at 100% and 50% Confirmatory Testing Acceptance Rates (N = 325 patients with CRC)
| Variable | No Age Cutoff |
80 Years |
70 Years |
60 Years |
50 Years |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100% Acceptance Rate | 50% Acceptance Rate | 100% Acceptance Rate | 50% Acceptance Rate | 100% Acceptance Rate | 50% Acceptance Rate | 100% Acceptance Rate | 50% Acceptance Rate | 100% Acceptance Rate | 50% Acceptance Rate | |
| Total cost to screen and test this age cohort | $113,123 | $100,711 | $97,808 | $87,076 | $72,747 | $64,765 | $46,293 | $41,214 | $17,752 | $15,804 |
| No. of LS cases present in entire CRC cohort | 11.7 | 11.7 | 11.7 | 11.7 | 11.7 | 11.7 | 11.7 | 11.7 | 11.7 | 11.7 |
| No. of LS cases expected in screened cohort | 11.7 | 11.7 | 11.2 | 11.2 | 10.1 | 10.1 | 9.0 | 9.0 | 5.9 | 5.9 |
| Mean No. of LS cases expected to be identified in screened cohort | 10.7 | 5.4 | 10.2 | 5.1 | 9.2 | 4.6 | 8.3 | 4.1 | 5.4 | 2.7 |
| Cost per LS case detected in screened cohort | $10,567 | $18,794 | $9567 | $17,016 | $7865 | $3989 | $5594 | $9950 | $3316 | $5899 |
| No. of LS cases missed if screening cut off at this age | 1.0 | 6.3 | 1.5 | 6.6 | 2.5 | 7.1 | 3.4 | 7.6 | 6.3 | 9.0 |
| No. of LS cases expected to be missed in screened cohort | 1.0 | 6.3 | 0.9 | 6.1 | 0.9 | 5.5 | 0.8 | 4.9 | 0.5 | 3.2 |
| LS cases that would be missed with this strategy, % | 8.5 | 54.2 | 12.6 | 56.3 | 20.9 | 60.4 | 29.3 | 64.6 | 54.3 | 77.1 |
NOTE. Boldface indicates parameters the authors believe to be most important in the age cutoffs reviewed in the Discussion section.
Abbreviations: CRC, colorectal cancer; LS, Lynch syndrome.
For example, in the screening population of 325 patients with CRC with no age cutoff, 11.7 individuals with LS are expected to be present according to the assumed prevalence. Because the screening program does not have 100% sensitivity, our models indicate that 10.7 individuals with LS would be identified in this cohort when the acceptance rate of confirmatory testing is at 100%; at 50%, 5.4 individuals would be expected to be detected. The cost-per-case-detected (CpCD) given a 100% acceptance rate is $10,567; a 50% rate yields a CpCD of $18,794. In this age cohort, 1 case (approximately 8.5%) of LS is expected to be missed when 100% of confirmatory testing is completed. LS is missed in 6.3 patients (54%) when the acceptance rate for confirmatory testing is 50%.
If only patients with CRC less than 70 years of age are screened (the proposed cutoff by some of our pathologists), the models estimate 10.1 individuals with LS will be present among those screened. At 100% acceptance of confirmatory testing, 9.2 individuals would be expected to be identified; at 50% acceptance, 4.6 individuals would be expected to be detected. The CpCD given a 100% acceptance rate would be $7,865; a 50% rate yields a CpCD of $13,989. Moreover, 2.5 (approximately 21%) and 7.1 (approximately 60%) patients with LS would be expected to be missed when the acceptance rate is 100% and 50%, respectively.
If the cutoff was applied at age 50, the models suggest there will be 5.9 patients with LS present among the screened subpopulation, or 50% of all patients with LS present in the entire unselected CRC cohort. At 100% acceptance of confirmatory testing, 5.4 (46%) and 2.7 (23%) individuals with LS would be expected to be identified by our protocol at 100% and 50% acceptance rates, respectively. The CpCD given a 100% acceptance rate would be $3,316; a 50% rate results in a CpCD of $5,899. Finally, 6.3 (approximately 54%) and 9.0 (approximately 77%) patients with LS would be expected to be missed when the acceptance rate is 100% and 50%, respectively. Calculations applied to the other two age cutoffs yield intermediate results (Table 2)
Discussion
In our system, a group of pathologists maintained reservations about screening all patients with CRC despite a central decision to implement screening for patients of all ages. Reasons included beliefs about the low prevalence of LS in older patients; systematic exclusion of such patients from screening would substantially reduce total costs and improve efficiency of screening, with “negligible” loss of LS case detection. In addition, some believed there might be minimal implications for the proband. Pathologists also commented on suspected potential for lower penetrance in family members of probands diagnosed at older age of onset of first CRC.
Our models suggest that applying age cutoffs to a LS screening program would reduce the costs of an LS screening and testing program and increase its efficiency as measured by CpCD, at the expense of decreased sensitivity of case detection, which approaches 50% as the cutoff is lowered to age 50. In addition, decreased case detection would have a cascade effect. For each nonscreened individual with an LS mutation, there are potentially multiple at-risk relatives, as 50% of first-degree and 25% of second-degree relatives are expected to carry the mutation. Thus, the failure to begin risk-reducing interventions in these individuals represents a missed opportunity to decrease morbidity and mortality from LS-associated cancers. Similarly, relatives who are aware of increased risk for CRC in the family but who do not pursue confirmatory testing may undergo early and frequent colonoscopy when, in fact, they do not carry the LS mutation. These individuals will be exposed to the small but real risks associated with colonoscopy performed more frequently than recommended for the normal-risk population without meaningful benefit while incurring substantial and unnecessary costs.
Benefits for individuals identified at younger ages might also translate to older populations, such as recognition of significant risk for a second primary CRC and other LS-associated cancers. Some older LS probands detected by a universal LS screening program may have had other LS-associated cancers that were not recognized as such. Furthermore, there is emerging evidence that the abnormalities of mismatch repair proteins detectable by IHC in the tumor are a positive prognostic factor and may predict response to fluorouracil and other chemotherapeutic agents.12,13 This has relevance to patients of all ages, but could be of particular importance to older patients who are more susceptible to adverse effects from treatment.
As experience has grown with universal screening for LS, a confounding challenge to evaluating effectiveness involves the rate of acceptance for confirmatory testing. Confirmatory diagnostic testing consists of molecular sequencing and/or duplication-deletion testing of genes known to be associated with LS. Whereas screening is performed on tumor resection specimens, confirmatory testing is performed on a blood or buccal specimen collected later in the clinical process and involves genetic counseling and informed consent before proceeding with germline testing.
The ability to account for the acceptance rate of confirmatory testing is important to assess screening cost and efficiency in the real world, regardless of age cutoff. Modeling previously reported by our group assumed a 100% acceptance rate.9 The current models offer a look at the downstream effect of two widely divergent acceptance rates, for the purpose of illustrating the impact. As can be seen in Table 2, even when no age cutoff is applied, the impact of a 50% acceptance rate is dramatic and costly. The EGAPP report suggested that acceptance rates vary widely, from 19% to 75%.6
Barriers exist in clinical care to an individual's follow through with confirmatory testing. Although not formally studied, they are thought to include the current stage in cancer treatment in the screened individual, insurance coverage of molecular testing, genetic discrimination concerns, and so on. The lack of a confirmed diagnosis of LS in the proband may lead to reduced recognition of the increased risk for a second primary CRC and other LS-associated cancers and decreased access to recommended surveillance. Adverse effects of nonadherence are amplified when diagnostic testing is not offered or provided to at-risk family members. Often family members are not informed of a screening result indicating LS, and if they are informed of the screen positive status, their options for confirmatory testing are limited. Without a known LS-associated mutation, confirmatory testing would require molecular analysis for multiple genes at significant cost. In addition, it is unlikely that testing would be covered by insurance for the relatives who do not have a personal history of cancer and for whom a known mutation in the family has not been identified. The cost effectiveness of LS screening as determined by the EGAPP working group and subsequent economic studies is predicated on the identification of at-risk relatives. It is important to continue to evaluate barriers to the acceptance of confirmatory testing in the probands identified through LS screening.
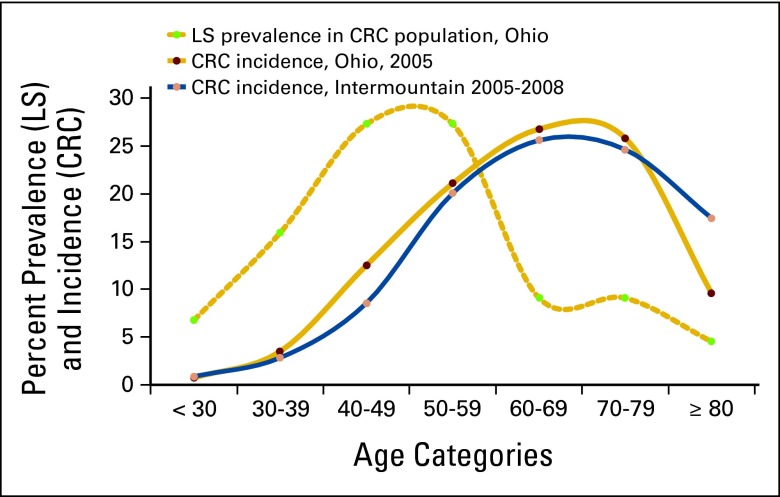
Limitations of this study include the use of an LS population structure taken from a different population (Ohio) than ours (Utah). However, we compared the two population structures on the basis of age-adjusted incidence of CRC cases (unpublished data analysis) and found them to be quite similar, and both are consistent with national CRC data.14 Furthermore, the LS population structure from Ohio was limited to the 44 patients identified at the time of this study, which yielded relatively small numbers of patients in each group. Appendix Figure A1 illustrates the distribution of LS cases among an unselected population of patients with CRC in the Ohio population,11 as well as distributions of CRC cases in both the Ohio10 and Intermountain population. These data are unadjusted (for age structure). This introduces uncertainty into our calculations of the various metrics, which is not reflected in Table 2. The reported metrics are sensitive to test performance characteristics and costs, both of which are likely to be different from other laboratories performing LS tests. Furthermore, the charges used in calculations only reflect what our reference laboratory charges our system and do not necessarily reflect the net changes in revenue to a given hospital or system.
In conclusion, we demonstrate that modeling can provide information that is useful in the design and implementation of a real-world, complex clinical process. The data presented do not define a right answer for whether or not age cutoffs should be used for a LS screening program but do illustrate their potential clinical and economic impacts. The results of these models not only shaped the age cutoff decision, but also emphasized the importance of follow-up to measure the actual impact of the decision. As our system moves next to LS screening in patients with endometrial cancer, we will perform similar analyses as decision needs arise.
Acknowledgment
Presented in part in poster format at 15th Annual Meeting of the Collaborative Group of the Americas on Inherited Colorectal Cancer, October 10-11, 2011, Montreal, Quebec, Canada.
Appendix
Figure A1.
Age distribution of patients with Lynch syndrome (LS; Ohio) and colorectal cancer (CRC; Ohio and Intermountain).
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: James M. Gudgeon, Janet L. Williams, Marc S. Williams
Financial support: Marc S. Williams
Administrative support: Marc S. Williams
Provision of study materials or patients: Thomas Belnap
Collection and assembly of data: James M. Gudgeon, Thomas Belnap, Janet L. Williams
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Centers for Disease Control and Prevention. United States Cancer Statistics: 1999-2008 incidence and mortality web-based report. 2012. http://www.cdc.gov/uscs.
- 2.Bellcross CA, Bedrosian SR, Daniels E, et al. Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: Summary of a public health/clinical collaborative meeting. Genet Med. 2012;14:152–162. doi: 10.1038/gim.0b013e31823375ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: Later age of onset. Gastroenterology. 2005;129:415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: A systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 5.Palomaki GE, McClain MR, Melillo S, et al. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11:42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evaluation of Genomic Applications in Practice and Prevention: Recommendations from the EGAPP Working Group. Genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009 Jan;11:35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12:93–104. doi: 10.1097/GIM.0b013e3181cd666c. [DOI] [PubMed] [Google Scholar]
- 8.Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: A cost-effectiveness analysis. Ann Intern Med. 2011;155:69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudgeon JM, Williams JL, Burt RW, et al. Lynch syndrome screening implementation: Business analysis by a healthcare system. Am J Manag Care. 2011;17:e288–e300. [PubMed] [Google Scholar]
- 10.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 11.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol. 2009;27:1814–1821. doi: 10.1200/JCO.2008.18.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohrling K, Edler D, Hallstrom M, et al. Mismatch repair protein expression is an independent prognostic factor in sporadic colorectal cancer. Acta Oncol. 2010;6:797–804. doi: 10.3109/02841861003705786. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Cancer of the colon and rectum (invasive) 2004-2008. http://seer.cancer.gov/csr/1975_2008/browse_csr.php?section=6&page=sect_06_table.23.html.